化工进展 ›› 2020, Vol. 39 ›› Issue (1): 329-340.DOI: 10.16085/j.issn.1000-6613.2019-0607
偶联尿苷二磷酸循环体系的天然产物体外糖基化修饰
- 北京理工大学化学与化工学院,北京100081
-
收稿日期:2019-04-16出版日期:2020-01-05发布日期:2020-01-14 -
通讯作者:冯旭东 -
作者简介:刘潇斐(1995—),女,硕士研究生,研究方向为酶工程。E-mail:675789414@qq.com 。 -
基金资助:国家自然科学基金面上项目(21878021);国家自然科学基金重点项目(21736002)
UDP regeneration system for in vitro glycosylation modification of natural products
Xiaofei LIU( ),Liang ZHANG,Xudong FENG(
),Liang ZHANG,Xudong FENG( ),Chun LI
),Chun LI
- School of Chemistry and Chemical Engineering, Beijng Institute of Technology, Beijing 100081,China
-
Received:2019-04-16Online:2020-01-05Published:2020-01-14 -
Contact:Xudong FENG
摘要:
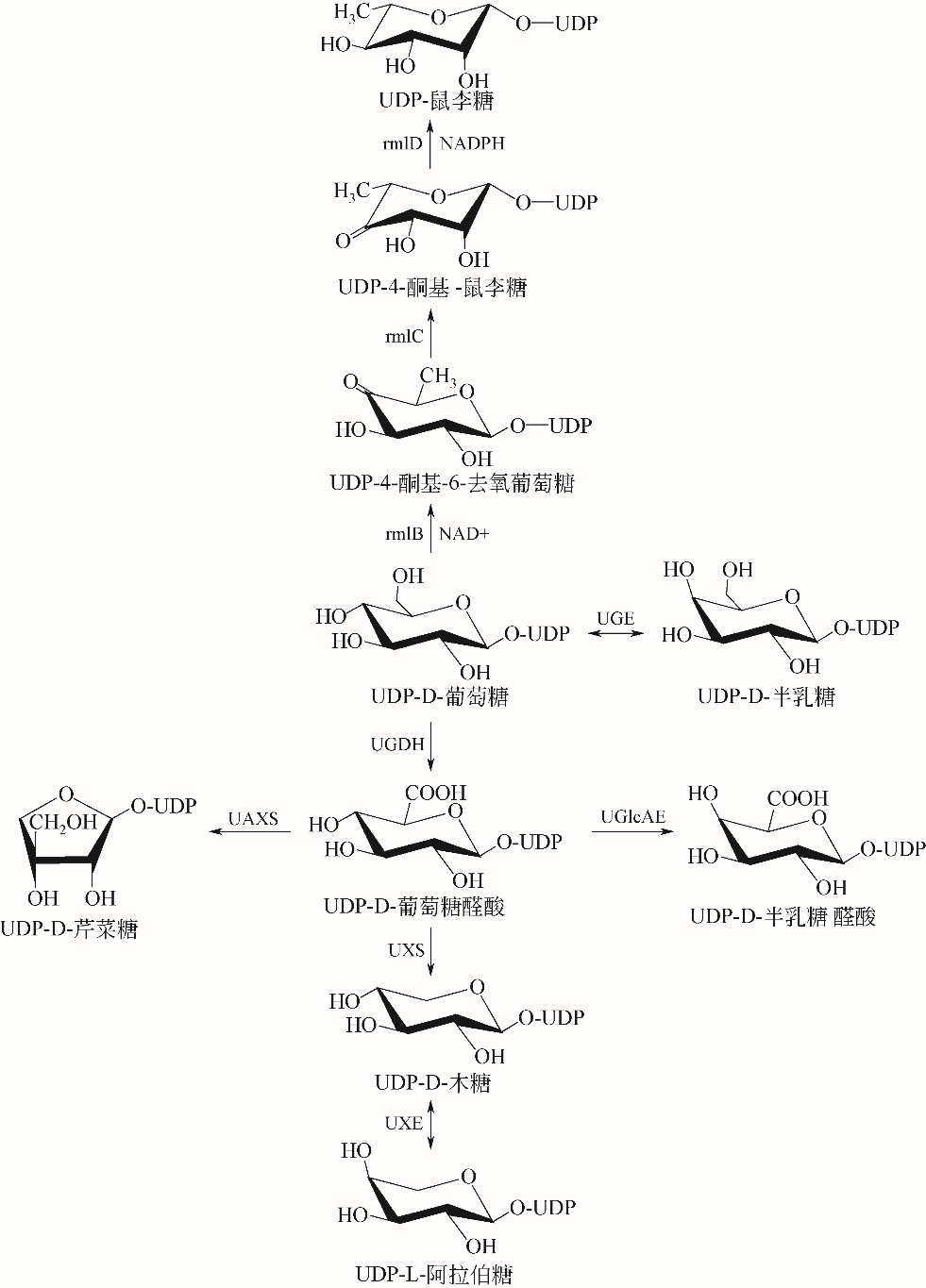
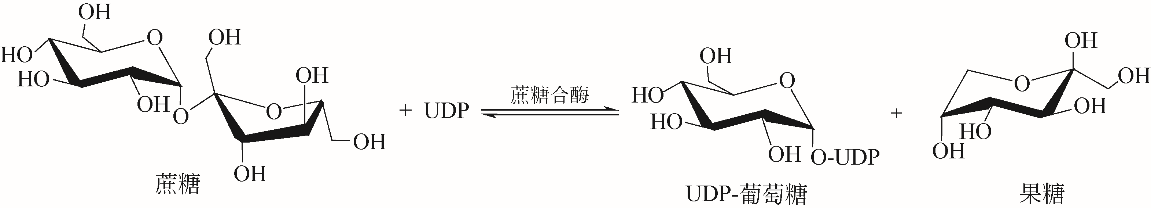
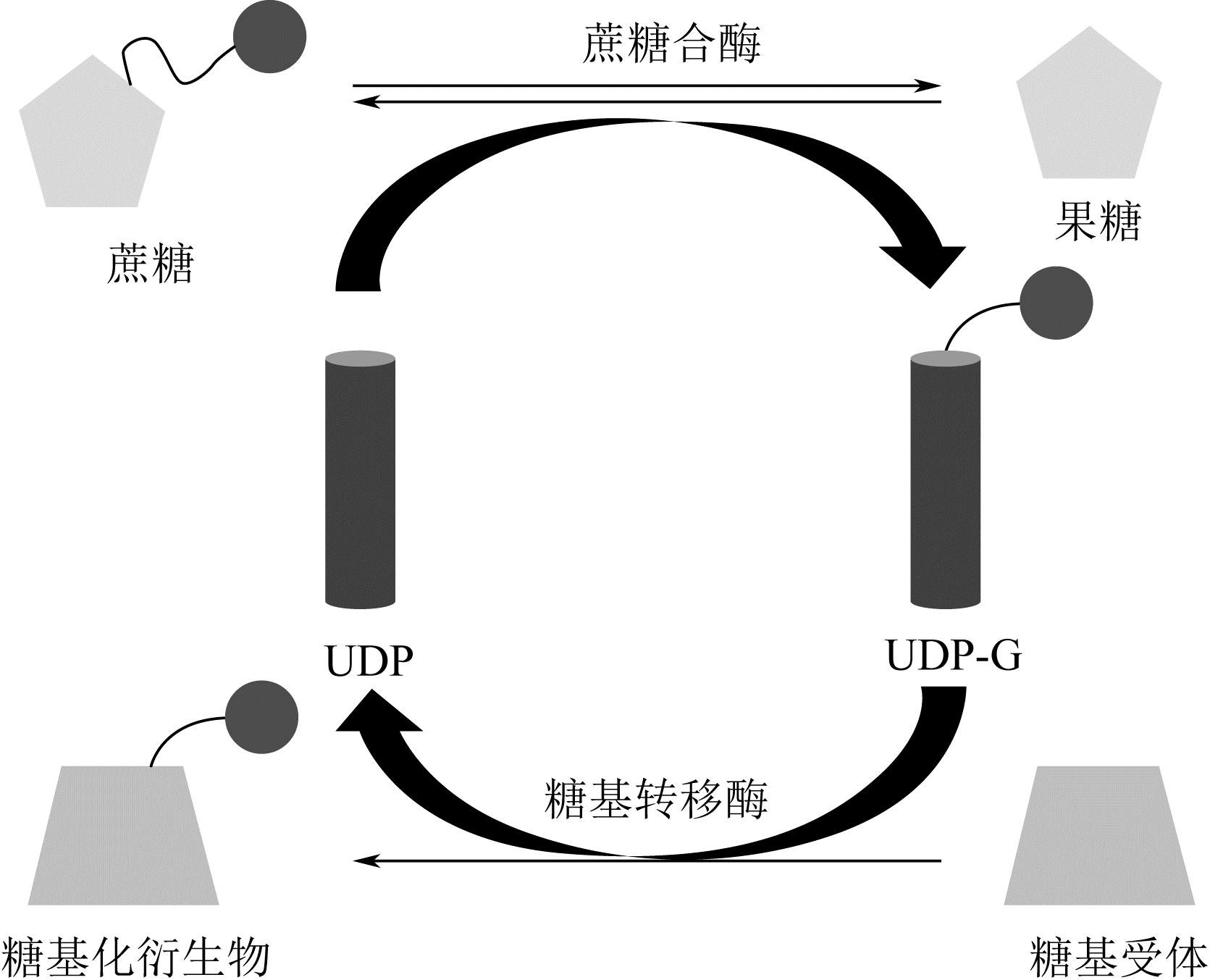
糖基化是天然产物的一种重要基团修饰,主要通过尿苷二磷酸(UDP)-糖基转移酶催化实现,而UDP-糖基供体价格昂贵限制了其应用,构建UDP-糖基供体再生系统可以有效解决该问题。本文从天然产物的体外糖基化修饰入手,阐述了糖基化修饰对天然产物功能的调控作用,比较了目前的糖基化修饰方法,其中基于UDP-糖基转移酶的生物法具备了重要的产业化应用前景。接着总结了各种UDP-糖基供体的合成方式,概述了偶联蔗糖合酶或海藻糖合酶的基于UDP循环的UDP-糖基供体再生系统,重点描述了UDP循环体系在萜类、黄酮类及其他化合物体外糖基化修饰中的应用,从而高效合成具有高附加值的天然产物糖苷化合物。指出偶联蔗糖合酶和UDP-糖基转移酶的循环体系是今后天然产物糖苷化合物合成的重要方式。
中图分类号:
引用本文
刘潇斐,张良,冯旭东,李春. 偶联尿苷二磷酸循环体系的天然产物体外糖基化修饰[J]. 化工进展, 2020, 39(1): 329-340.
Xiaofei LIU,Liang ZHANG,Xudong FENG,Chun LI. UDP regeneration system for in vitro glycosylation modification of natural products[J]. Chemical Industry and Engineering Progress, 2020, 39(1): 329-340.
| 1 | LIANG D M, LIU J H, WU H, et al. Glycosyltransferases: mechanisms and applications in natural product development[J]. Chemical Society Reviews, 2015, 44(22): 8350-8374. |
| 2 | HANSEN S F, BETTLER E, RINNAN A, et al. Exploring genomes for glycosyltransferases[J]. Molecular BioSystems, 2010, 6(10): 1773-1781. |
| 3 | JAYAPRAKASH NG, SUROLIA A. Role of glycosylation in nucleating protein folding and stability[J]. Biochemical Journal, 2017, 474(14):2333-2347. |
| 4 | GACHON C M, LANGLOIS-MEURINNE M, SAINDRENAN P. Plant secondary metabolism glycosyltransferases: the emerging functional analysis[J]. Trends in Plant Science, 2005, 10(11): 542-549. |
| 5 | FUKUCHI-MIZUTANI MASAKO, OKUHARA HIROAKI, TANAKA Y. Biochemical and molecular characterization of a novel UDP-glucose:anthocyanin 3-O-glucosyltransferase, a key enzyme for blue anthocyanin biosynthesis, from gentian[J]. Plant Physiology, 2003, 132(3): 1652-1663. |
| 6 | YOSHIDA K, TOYAMA Y, KAMEDA K, et al. Contribution of each caffeoyl residue of the pigment molecule of gentiodelphin to blue color development[J]. Phytochemistry, 2000, 54(1): 85-92. |
| 7 | KRAMER C M, PRATA R T, WILLITS M G, et al. Cloning and regiospecificity studies of two flavonoid glucosyltransferases from Allium cepa[J]. Phytochemistry, 2003, 64(6): 1069-1076. |
| 8 | BONISCH F, FROTSCHER J, STANITZEK S, et al. A UDP-glucose:monoterpenol glucosyltransferase adds to the chemical diversity of the grapevine metabolome[J]. Plant Physiology, 2014, 165(2): 561-581. |
| 9 | MOON H I, LEE J H. Neuroprotective effects of triterpene glycosides from glycine max against glutamate induced toxicity in primary cultured rat cortical cells[J]. International Journal of Molecular Sciences, 2012, 13(8): 9642-9648. |
| 10 | RÜFER C E, BUB A, MÖSENEDER, et al. Pharmacokinetics of the soybean isoflavone daidzein in its aglycone and glucoside form: a randomized, double-blind, crossover study[J]. American Journal of Clinical Nutrition, 2008, 87(5): 1314-1323. |
| 11 | WILLIAMS G J, GANTT R W, THORSON J S. The impact of enzyme engineering upon natural product glycodiversification[J]. Current Opinion in Chemical Biology, 2008, 12(5): 556-564. |
| 12 | DE BRUYN F, MAERTENS J, BEAUPREZ J, et al. Biotechnological advances in UDP-sugar based glycosylation of small molecules[J]. Biotechnology Advances, 2015, 33(2): 288-302. |
| 13 | HODGMAN C E, JEWETT M C. Cell-free synthetic biology: thinking outside the cell[J]. Metabolic Engineering, 2012, 14(3): 261-269. |
| 14 | SUBSOONTORN P, KIM J, WINFREE E. Ensemble Bayesian analysis of bistability in a synthetic transcriptional switch[J]. ACS Synthetic Biology, 2012, 1(8): 299-316. |
| 15 | DAVIS B G. Recent developments in oligosaccharide synthesis[J]. Journal of the Chemical Society: Perkin Transactions, 2000, 14(1):2137-2160. |
| 16 | FLITSCH S L. Chemical and enzymatic synthesis of glycopolymers[J]. Current Opinion in Chemical Biology, 2000,4(6):619-625. |
| 17 | 刘志辉,李琳,钱芳. 醋酸水解三七总皂苷制备人参皂苷Rg3工艺的优选[J]. 中国医院药学杂志, 2009, 29(11): 881-884. |
| LIU Z H, LI L, QIAN F. Optimization of the preparation of ginsenoside Rg3 by acetic acid hydrolysis of Panax notoginseng saponins[J]. Chinese Journal of Hospital Pharmacy, 2009, 29(11): 881-884. | |
| 18 | PARK S E, NA C S, YOO S A, et al. Biotransformation of major ginsenosides in ginsenoside model culture by lactic acid bacteria[J]. Journal of Ginseng Research, 2017, 41(1): 36-42. |
| 19 | SINGH G, DHAR YV, ASIF MH, et al. Exploring the functional significance of sterol glycosyltransferase enzymes[J]. Progress in Lipid Research, 2018, 69:1-10. |
| 20 | SHAIKH F A, WITHERS S G. Teaching old enzymes new tricks: engineering and evolution of glycosidases and glycosyl transferases for improved glycoside synthesis[J]. Biochemical Cell Biology, 2008, 86(2): 169-177. |
| 21 | MACKENZIE L F, WANG Q P, WARREN R A, et al. Glycosynthases:mutant glycosidases for oligosaccharide synthesis[J]. Journal of the American Chemical Society, 1998, 120(22): 5583-5584. |
| 22 | YANG M, DAVIES G J, DAVIS B G. A glycosynthase catalyst for the synthesis of flavonoid glycosides[J]. Angewandte Chemie: International Edtion, 2007, 46(21): 3885-3888. |
| 23 | 张丹, 鱼闪红, 奥大介, 等. 高产人参皂甙β-葡萄糖苷酶菌种的筛选[J]. 大连轻工业学院学报, 2000, 19(3): 195-198. |
| ZHANG D, YU S H, AO D J, et al. Screening of high-yield ginsenoside β-glucosidase strains[J]. Journal of Dalian Institute of Light Industry, 2000, 19(3): 195-198. | |
| 24 | CHOI Y B, KIM K S, RHEE J S. Hydrolysis of soybean isoflavone glucosides by lactic acid bacteria[J]. Biotechnology Letters, 2002, 24(24): 2113-2116. |
| 25 | XIAOCHEN LIU, ZHANG L. Biosynthesis of glycyrrhetinic acid-3‑O‑monoglucose using glycosyltransferase UGT73C11 from Barbarea vulgaris[J]. Industrial & Engineering Chemistry Research, 2017, 56(51): 14949-14958. |
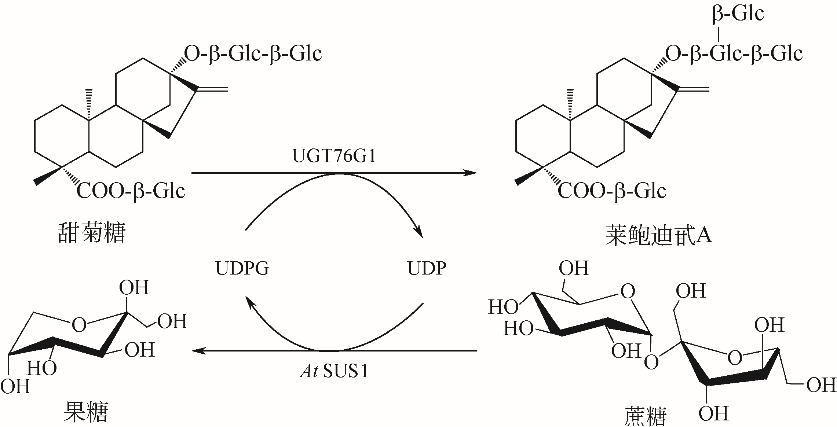
| 26 | ZHAO X, DAI X, GAO L, et al. Functional analysis of an uridine diphosphate glycosyltransferase involved in the biosynthesis of polyphenolic glucoside in tea plants (Camellia sinensis)[J]. Journal of Agricultural and Food Chemistry, 2017, 65(50): 10993-11001. |
| 27 | LUO S L, DANG L Z, ZHANG K Q, et al. Cloning and heterologous expression of UDP-glycosyltransferase genes from Bacillus subtilis and its application in the glycosylation of ginsenoside Rh1[J]. Letters in Applied Microbiology, 2015, 60(1): 72-78. |
| 28 | ZHANG Y H. Production of biocommodities and bioelectricity by cell-free synthetic enzymatic pathway biotransformations: challenges and opportunities[J]. Biotechnology and Bioengineering, 2010, 105(4): 663-677. |
| 29 | ZHANG Y H, EVANS B R, MIELENZ J R, et al. High-yield hydrogen production from starch and water by a synthetic enzymatic pathway[J]. PLoS One, 2007, 2(5): e456. |
| 30 | ZHU Z, SUN F, ZHANG X, et al. Deep oxidation of glucose in enzymatic fuel cells through a synthetic enzymatic pathway containing a cascade of two thermostable dehydrogenases[J]. Biosensors and Bioelectronics, 2012, 36(1): 110-115. |
| 31 | WANG Y, HUANG W, SATHITSUKSANOH N, et al. Biohydrogenation from biomass sugar mediated by invitro synthetic enzymatic pathways[J]. Chemical & Biology, 2011, 18(3): 372-380. |
| 32 | PANKE S, HELD M, WUBBOLTS M. Trends and innovations in industrial biocatalysis for the production of fine chemicals[J]. Current Opinion in Biotechnology, 2004, 15(4): 272-279. |
| 33 | 尹森,孔建强. UDP-糖基供体的生物合成途径分析[J].中国医药生物技术, 2016, 11(4): 355-359. |
| YIN S, KONG J Q. Analysis of biosynthesis pathway of UDP-glycosyl donor[J]. Chinese Medicine Biotechnology, 2016, 11(4): 355-359. | |
| 34 | SCHWAB W, FISCHER T, WÜST M. Terpene glucoside production: Improvedbiocatalytic processes using glycosyltransferases[J]. Engineering in Life Science, 2015, 15(4): 376-386. |
| 35 | RUFFING A, CHEN R R. Metabolic engineering of microbes for oligosaccharide and polysaccharide synthesis[J]. Microbial Cell Factories, 2006, 5: 25. |
| 36 | DAUDE D, REMAUD-SIMEON M, ANDRE I. Sucrose analogs: an attractive (bio)source for glycodiversification[J]. Natural Product Reports, 2012, 29(9): 945-960. |
| 37 | SCHMOLZER K, GUTMANN A, DIRICKS M, et al. Sucrose synthase: a unique glycosyltransferase for biocatalytic glycosylation process development[J]. Biotechnology Advances, 2016, 34(2): 88-111. |
| 38 | HUANG F C, HINKELMANN J, HERMENAU A, et al. Enhanced production of beta-glucosides by in-situ UDP-glucose regeneration[J]. Journal of Biotechnology, 2016, 224: 35-44. |
| 39 | S-I RYU, KIM J-E, KIM E-J, et al. Catalytic reversibility of Pyrococcus horikoshii trehalose synthase: efficient synthesis of several nucleoside diphosphate glucoses with enzyme recycling[J]. Process Biochemistry, 2011, 46(1): 128-134. |
| 40 | CHUNG S K, RYU S I, LEE S B. Characterization of UDP-glucose 4-epimerase from Pyrococcus horikoshii: regeneration of UDP to produce UDP-galactose using two-enzyme system with trehalose[J]. Bioresource Technology, 2012, 110: 423-429. |
| 41 | SCHMOLZER K, GUTMANN A, DIRICKS M, et al. Sucrose synthase: a unique glycosyltransferase for biocatalytic glycosylation process development[J]. Biotechnology Advances, 2016, 34(2): 88-111. |
| 42 | MODZELEWSKA A, SUR S, KUMAR SK, et al. Sesquiterpenes: natural products that decrease cancer growth[J]. Current Medicinal Chemistry Anticancer Agents, 2005 5(5): 477-499. |
| 43 | YANG J, XIAN M, SU S, et al. Enhancing production of bio-isoprene using hybrid MVA pathway and isoprene synthase in E. coli[J]. PLoS One, 2012, 7(4): e33509. |
| 44 | BUIJS N A, SIEWERS V, NIELSEN J. Advanced biofuel production by the yeast Saccharomyces cerevisiae[J]. Current Opinion in Chemical Biology, 2013, 17(3): 480-488. |
| 45 | CANE D E, IKEDA H. Exploration and mining of the bacterial terpenome[J]. Accounts of Chemical Research, 2012, 45(3): 463-473. |
| 46 | RIVAS F, PARRA A, MARTINEZ A, et al. Enzymatic glycosylation of terpenoids[J]. Phytochemistry Reviews, 2013, 12(2): 327-339. |
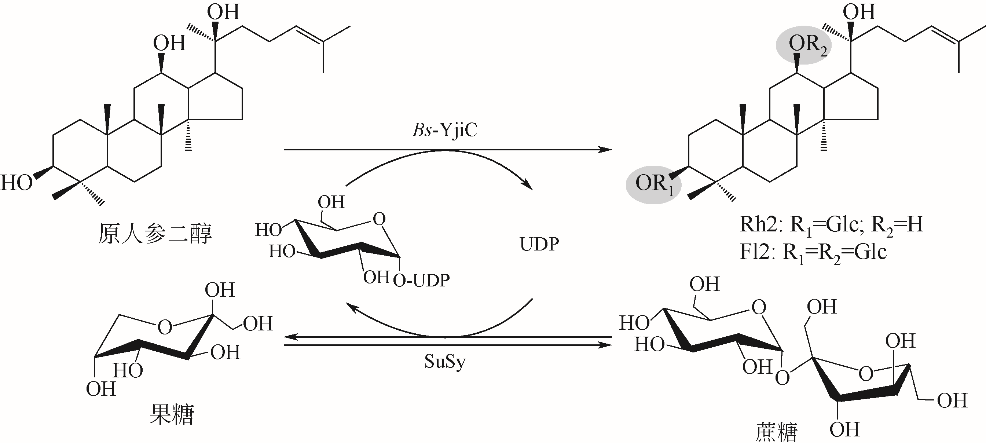
| 47 | DAI L, LIU C, LI J, et al. One-pot synthesis of ginsenoside Rh2 and bioactive unnatural ginsenoside by coupling promiscuous glycosyltransferase from Bacillus subtilis 168 to sucrose synthase[J]. Journal of Agricultural and Food Chemistry, 2018, 66(11): 2830-2837. |
| 48 | DAI L, LI J, YANG J, et al. Use of a promiscuous glycosyltransferase from Bacillus subtilis 168 for the enzymatic synthesis of novel protopanaxatriol-type ginsenosides[J]. Journal of Agricultural and Food Chemistry, 2018, 66(4): 943-949. |
| 49 | WANG Y, CHEN L, LI Y, et al. Efficient enzymatic production of rebaudioside A from stevioside[J]. Biosci. Biotechnol. Biochem., 2016, 80(1): 67-73. |
| 50 | CHEN L, SUN P, ZHOU F, et al. Synthesis of rebaudioside D, using glycosyltransferase UGTSL2 and in situ UDP-glucose regeneration[J]. Food Chemistry, 2018, 259: 286-291. |
| 51 | ZAKARYAN H, ARABYAN E, OO A, et al. Flavonoids: promising natural compounds against viral infections[J]. Archives of Virology, 2017, 162(9): 2539-2551. |
| 52 | YONEKURA-SAKAKIBARA K, TOHGE T, NIIDA R, et al. Identification of a flavonol 7-O-rhamnosyltransferase gene determining flavonoid pattern in Arabidopsis by transcriptome coexpression analysis and reverse genetics[J]. Journal of Biological Chemistry, 2007, 282(20): 14932-14941. |
| 53 | RAFFA D, MAGGIO B, RAIMONDI MV, et al. Recent discoveries of anticancer flavonoids[J]. European Journal of Medicinal Chemistry, 2017, 142: 213-228. |
| 54 | WU X, CHU J, WU B, et al. An efficient novel glycosylation of flavonoid by beta-fructosidase resistant to hydrophilic organic solvents[J]. Bioresource Technology, 2013, 129: 659-662. |
| 55 | RUSSO P, FRUSTACI A, BUFALO A DEL, et al. Multitarget drugs of plants origin acting on Alzheimer's disease[J]. Current Medicinal Chemistry, 2013, 20(13): 1686-1693. |
| 56 | BILILIGN T, HYUN C G, WILLIAMS J S, et al. The hedamycin locus implicates a novel aromatic PKS priming mechanism[J]. Chemistry & Biology, 2004, 11(7): 959-969. |
| 57 | HARLE J, GUNTHER S, LAUINGER B, et al. Rational design of an aryl-C-glycoside catalyst from a natural product O-glycosyltransferase[J]. Chemistry & Biology, 2011, 18(4): 520-530. |
| 58 | KU S K, LEE W, KANG M, et al. Antithrombotic activities of aspalathin and nothofagin via inhibiting platelet aggregation and FIIa/FXa[J]. Archives of Pharmacal Research, 2015, 38(6): 1080-1089. |
| 59 | KU S K, KWAK S, KIM Y, et al. Aspalathin and nothofagin from rooibos (Aspalathus linearis) inhibits high glucose-induced inflammation in vitro and in vivo[J]. Inflammation, 2015, 38(1): 445-455. |
| 60 | BUNGARUANG L, GUTMANN A, NIDETZKY B. Leloir glycosyltransferases and natural product glycosylation: biocatalytic synthesis of the C-glucoside nothofagin, a major antioxidant of redbush herbal tea[J]. Advanced Synthesis & Catalysis, 2013, 355(14/15): 2757-2763. |
| 61 | SON M H, KIM B G, KIM D H, et al. Production of flavonoid O-glucoside using sucrose synthase and flavonoid O-glucosyltransferase fusion protein[J]. Journal of Microbiology and Biotechnology, 2009, 19(7): 709-712. |
| 62 | GUTMANN A, BUNGARUANG L, WEBER H, et al. Towards the synthesis of glycosylated dihydrochalcone natural products using glycosyltransferase-catalysed cascade reactions[J]. Green Chemistry, 2014, 16(9): 4417-4425. |
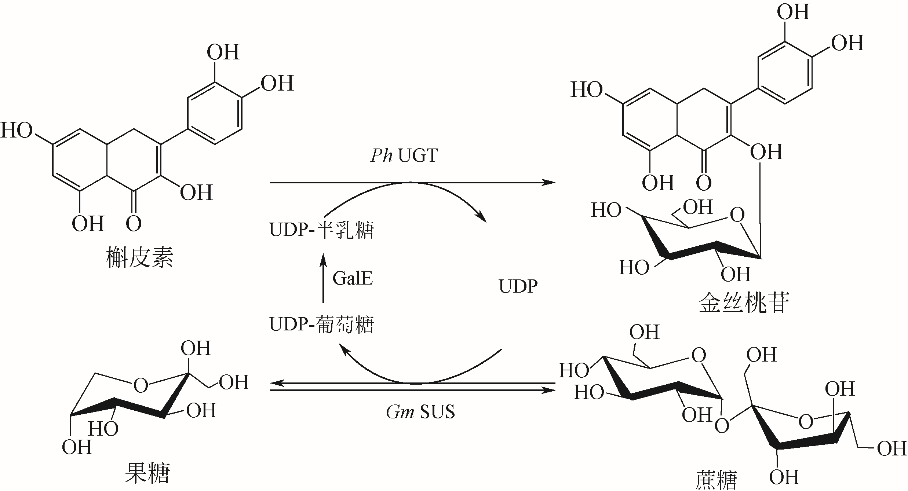
| 63 | PEI J, CHEN A, ZHAO L, et al. One-pot synthesis of hyperoside by a three-enzyme cascade using a UDP-galactose regeneration system[J]. Journal of Agricultural and Food Chemistry, 2017, 65(29): 6042-6048. |
| 64 | ZHOU T, SONG Y C, FENG M Q, et al. Cloning, expression and biochemical characterization of UDP-glucose 6-dehydrogenase, a key enzyme in the biosynthesis of an anti-tumor polysaccharide from the marine fungus Phoma herbarum YS4108 [J]. Process Biochemistry, 2011, 46(12): 2263-2268. |
| 65 | MASADA S, KAWASE Y, NAGATOSHI M, et al. An efficient chemoenzymatic production of small molecule glucosides with insitu UDP-glucose recycling[J]. FEBS Letters, 2007, 581(13): 2562-2566. |
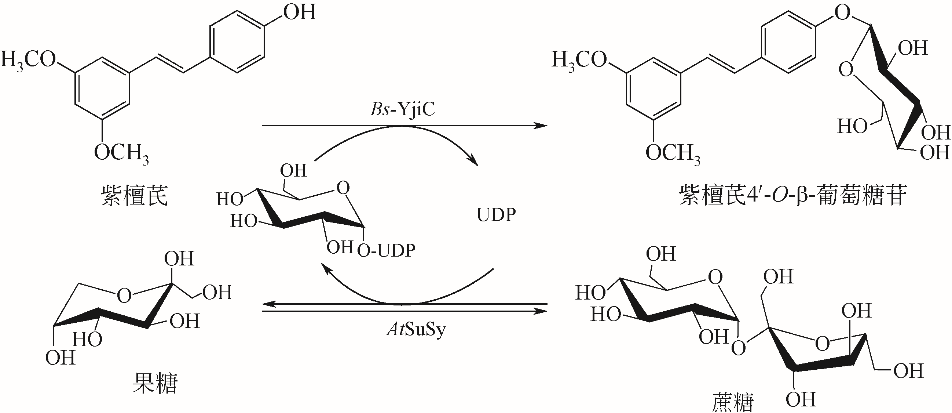
| 66 | DAI L, LI J, YAO P, et al. Exploiting the aglycon promiscuity of glycosyltransferase Bs-YjiC from Bacillus subtilis and its application in synthesis of glycosides[J]. Journal of Biotechnology, 2017, 248: 69-76. |
| 67 | MICHLMAYR H, MALACHOVA A, VARGA E, et al. Biochemical Characterization of a recombinant UDP-glucosyltransferase from rice and enzymatic production of deoxynivalenol-3-O-beta-D-glucoside[J]. Toxins (Basel), 2015, 7(7): 2685-2700. |
| 68 | KAMINAGA Y, NAGATSU A, AKIYAMA T, et al. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus[J]. FEBS Letters, 2003, 555(2): 311-316. |
| 69 | TIAN C, YANG J, ZENG Y, et al. Biosynthesis of raffinose and stachyose from sucrose via an in vitro multienzyme system[J]. Applied and Environmental Microbiology, 2019, 85(2): 6-18. |
| 70 | GUTMANN A, LEPAK A, DIRICKS M, et al. Glycosyltransferase cascades for natural product glycosylation: use of plant instead of bacterial sucrose synthases improves the UDP-glucose recycling from sucrose and UDP[J]. Biotechnology Journal, 2017, 12(7): 1600557. |
| [1] | 孙文涛, 李春. 微生物合成植物天然产物的细胞工厂设计与构建[J]. 化工进展, 2021, 40(3): 1202-1214. |
| [2] | 李瑶, 李子元, 王晓姣, 雷搏文, 赵怡, 马丽芳. 噻唑/·唑类活性天然产物及活性小分子化合物研究进展[J]. 化工进展, 2016, 35(S2): 248-257. |
| [3] | 曾维才,石 碧. 天然产物抗氧化活性的常见评价方法[J]. 化工进展, 2013, 32(06): 1205-1213. |
| [4] | 王 娟1,王 丹1,商士斌1,2. 天然产物基Gemini表面活性剂研究进展[J]. 化工进展, 2012, 31(12): 2761-2765. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||