化工进展 ›› 2021, Vol. 40 ›› Issue (1): 259-272.DOI: 10.16085/j.issn.1000-6613.2019-0461
硫杂杯芳烃-金属离子的新型配位化合物
程衔锟1( ), 熊延杭1, 侯雪1, 赵卓1(
), 熊延杭1, 侯雪1, 赵卓1( ), 徐亮1,2, 田勇攀1
), 徐亮1,2, 田勇攀1
- 1.安徽工业大学冶金工程学院,安徽 马鞍山 243000
2.省部共建高品质特殊钢冶金与制备国家重点实验室,上海大学,上海 200444
-
收稿日期:2020-03-25出版日期:2021-01-05发布日期:2021-01-12 -
通讯作者:赵卓 -
作者简介:程衔锟(1995—),男,硕士研究生,研究方向为超分子材料的合成及对贵金属离子的选择性分离。E-mail:m18860407943@163.com 。 -
基金资助:国家自然科学基金(U1703130)
Thiacalixarene-novel coordination compounds for metal ions
Xiankun CHENG1( ), Yanhang XIONG1, Xue HOU1, Zhuo ZHAO1(
), Yanhang XIONG1, Xue HOU1, Zhuo ZHAO1( ), Liang XU1,2, Yongpan TIAN1
), Liang XU1,2, Yongpan TIAN1
- 1.School of Metallurgical Engineering, Anhui University of Technology, Ma’anshan 243000, Anhui, China
2.State Key Laboratory of Advanced Special Steel, Shanghai University, Shanghai 200444, China
-
Received:2020-03-25Online:2021-01-05Published:2021-01-12 -
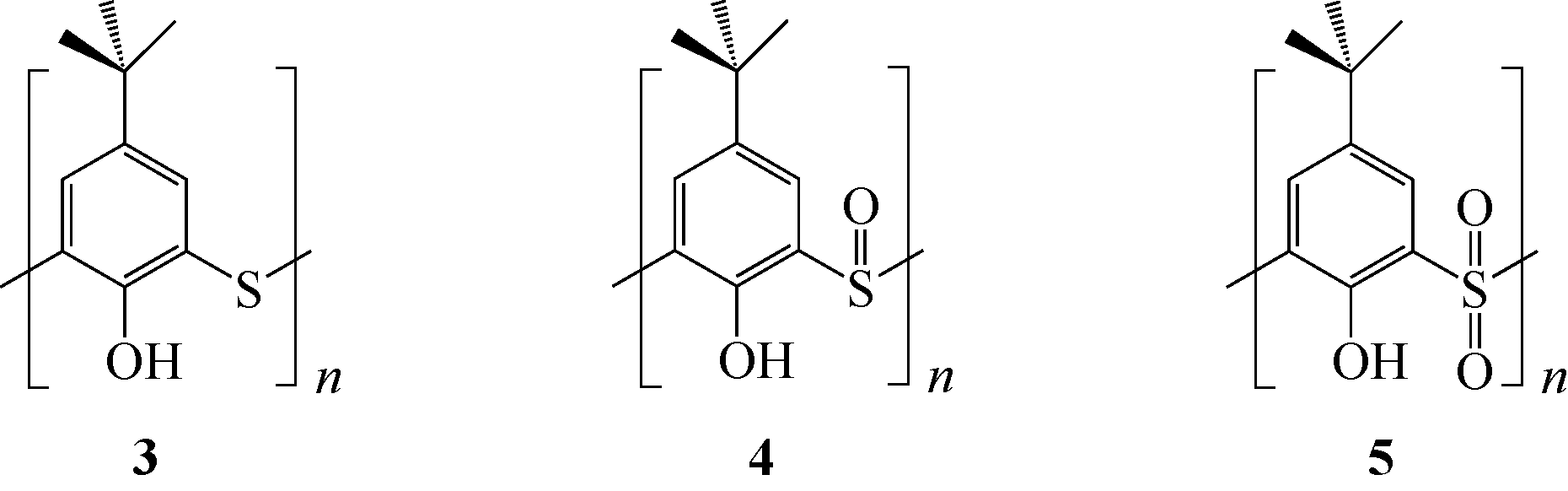
Contact:Zhuo ZHAO
摘要:
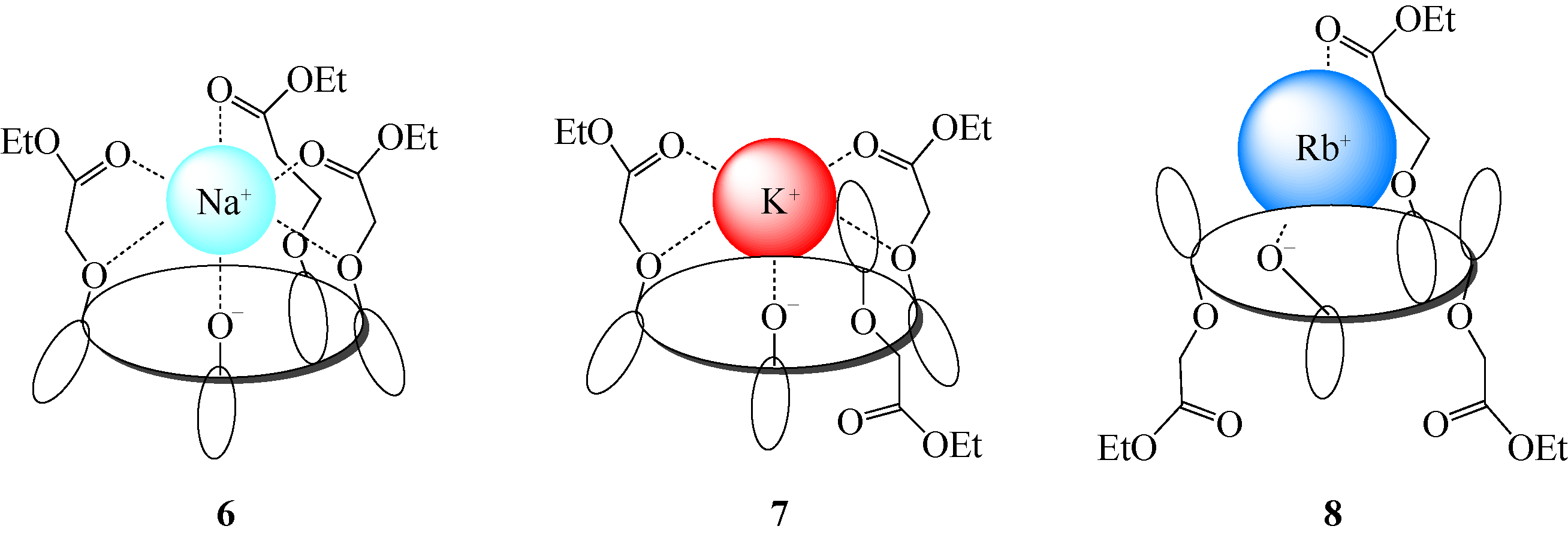
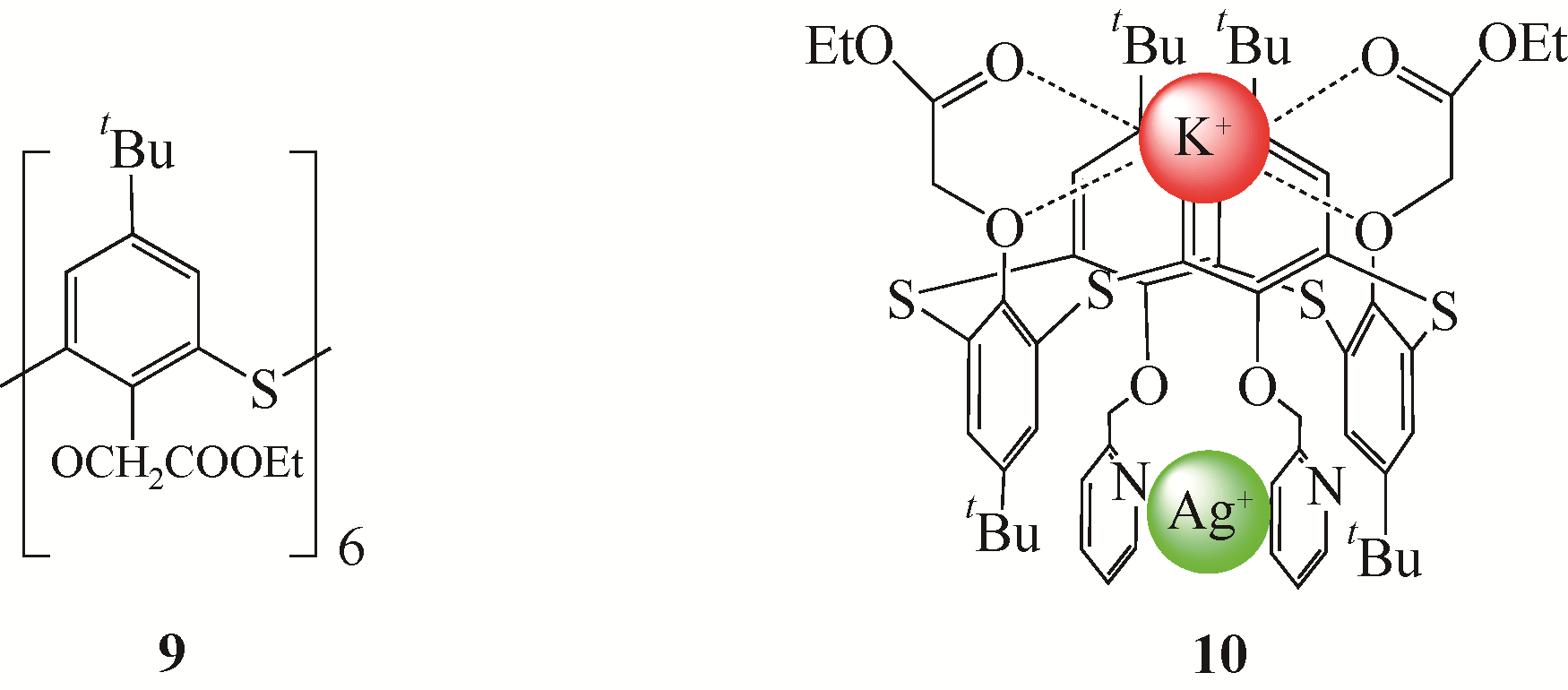
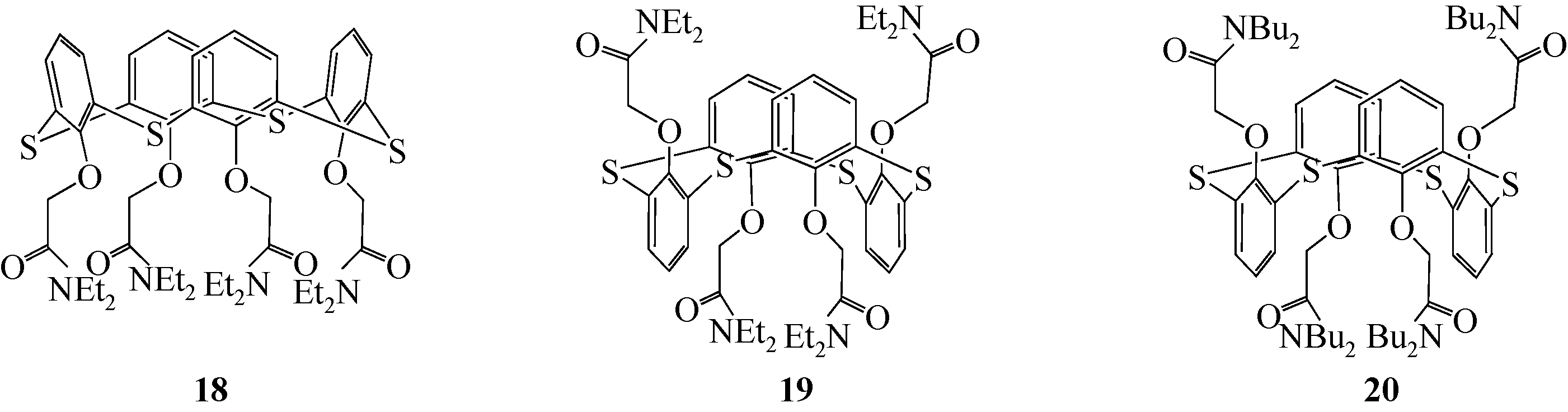
选择性分离贵金属、放射金属、碱金属等金属离子是重要研究方向之一。硫杂杯芳烃是一类由硫原子桥链苯酚单元构成的大环化合物。作为第三代超分子材料,硫杂杯芳烃反应位点丰富,上边缘、下边缘以及桥链的硫原子上都有进行功能化的可能性,而功能化后硫杂杯芳烃能够表现出与金属离子优异的配位性能,从而达到选择性分离金属离子的目的。本文介绍了硫杂杯芳烃的合成历史、自身结构特点以及配位机理。在此基础上分析了在硫杂杯芳烃的上缘、下缘引入酯基、酰胺、亚胺/胺等官能团后与碱金属、碱土金属、放射金属、贵金属离子等的配位情况,总结了硫杂杯芳烃自身构型、杯环的大小、溶剂类型等对其配位性能的影响,并深入分析了与金属离子的不同配位机理。本文可以为开发高效选择性分离提取各种金属离子的技术提供理论依据。
中图分类号:
引用本文
程衔锟, 熊延杭, 侯雪, 赵卓, 徐亮, 田勇攀. 硫杂杯芳烃-金属离子的新型配位化合物[J]. 化工进展, 2021, 40(1): 259-272.
Xiankun CHENG, Yanhang XIONG, Xue HOU, Zhuo ZHAO, Liang XU, Yongpan TIAN. Thiacalixarene-novel coordination compounds for metal ions[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 259-272.
| 萃取剂 | E/% | 参考文献 | |||
|---|---|---|---|---|---|
| Li(Ⅰ) | Na(Ⅰ) | K(Ⅰ) | Cs(Ⅰ) | ||
| 14 | 62 | 78 | 80 | 45 | [ |
| 15 | 89 | 99 | 94 | 99 | [ |
| 18 | 95 | 70 | 23 | 14 | [ |
| 19 | 11 | 81 | 98 | 96 | [ |
| 20 | 10 | 76 | 98 | 96 | [ |
表1 14~18对碱金属苦味酸盐的萃取率
| 萃取剂 | E/% | 参考文献 | |||
|---|---|---|---|---|---|
| Li(Ⅰ) | Na(Ⅰ) | K(Ⅰ) | Cs(Ⅰ) | ||
| 14 | 62 | 78 | 80 | 45 | [ |
| 15 | 89 | 99 | 94 | 99 | [ |
| 18 | 95 | 70 | 23 | 14 | [ |
| 19 | 11 | 81 | 98 | 96 | [ |
| 20 | 10 | 76 | 98 | 96 | [ |
| 硫杂杯芳烃 | 构象 | 阳离子 | n | lg Kex |
|---|---|---|---|---|
| 23 | 锥形 | Li(Ⅰ) | 0.78±0.01 | 3.23±0.04 |
| Na(Ⅰ) | 0.92±0.02 | 4.03±0.06 | ||
| K(Ⅰ) | 0.80±0.07 | 3.05±0.19 | ||
| Cs(Ⅰ) | — | — | ||
| Ag(Ⅰ) | 0.73±0.03 | 4.20±0.10 | ||
| 部分锥型 | Li(Ⅰ) | 0.62±0.01 | 1.97±0.02 | |
| Na(Ⅰ) | 0.82±0.01 | 3.53±0.04 | ||
| K(Ⅰ) | 0.65±0.02 | 3.35±0.05 | ||
| Cs(Ⅰ) | 0.73±0.01 | 2.45±0.02 | ||
| Ag(Ⅰ) | 1.56±0.06 | 8.37±0.23 | ||
| 1,3-交替型 | Li(Ⅰ) | 0.64±0.03 | 1.99±0.06 | |
| Na(Ⅰ) | 1.22±0.03 | 5.74±0.10 | ||
| K(Ⅰ) | 2.14±0.12 | 9.47±0.47 | ||
| Cs(Ⅰ) | 1.35±0.18 | 6.28±0.18 | ||
| Ag(Ⅰ) | 2.01±0.11 | 9.93±0.42 | ||
| 24 | 锥形 | Li(Ⅰ) | 1.91±0.13 | 8.43±0.52 |
| Na(Ⅰ) | 1.79±0.10 | 8.03±0.41 | ||
| K(Ⅰ) | 1.15±0.03 | 5.43±0.10 | ||
| Cs(Ⅰ) | 0.62±0.01 | 2.27±0.04 | ||
| Ag(Ⅰ) | 1.06±0.12 | 6.08±0.49 | ||
| 部分锥型 | Li(Ⅰ) | 0.98±0.03 | 4.51±0.08 | |
| Na(Ⅰ) | 1.36±0.02 | 6.44±0.09 | ||
| K(Ⅰ) | 1.57±0.07 | 7.32±0.28 | ||
| Cs(Ⅰ) | 0.91±0.01 | 3.95±0.04 | ||
| Ag(Ⅰ) | 1.57±0.13 | 8.18±0.55 | ||
| 1,3-交替型 | Li(Ⅰ) | 0.80±0.03 | 3.28±0.09 | |
| Na(Ⅰ) | 1.87±0.12 | 8.33±0.46 | ||
| K(Ⅰ) | 1.99±0.16 | 9.06±0.63 | ||
| Cs(Ⅰ) | 1.93±0.14 | 8.52±0.53 | ||
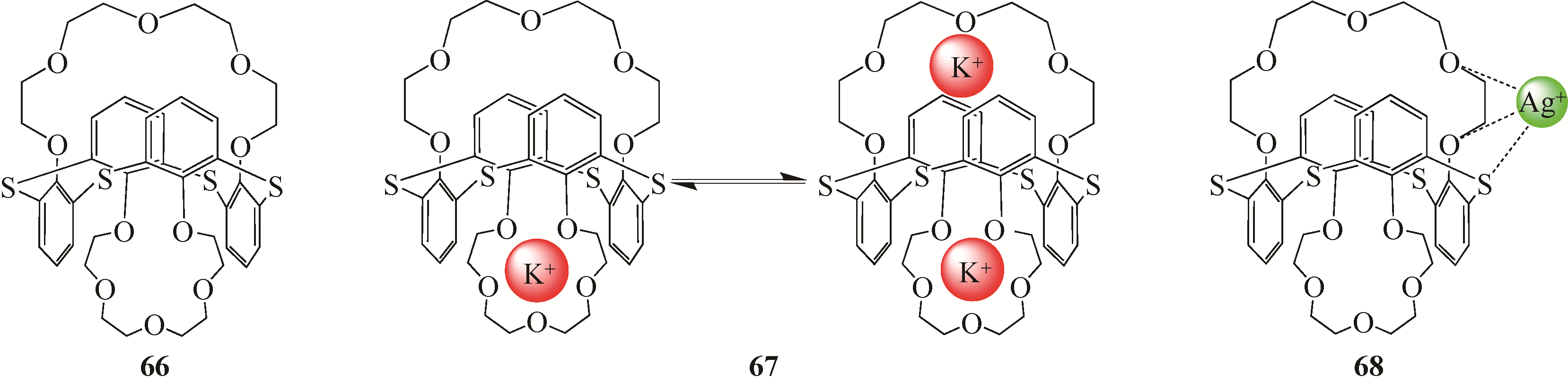
| Ag(Ⅰ) | 1.87±0.22 | 9.83±0.92 | ||
| 25 | 锥形 | Fe(Ⅲ) | 0.48±0.02 | 4.99±0.06 |
| Ni(Ⅱ) | 1.12±0.08 | 8.05±0.31 | ||
| Al(Ⅲ) | 1.61±0.10 | 7.17±0.21 | ||
| Pb(Ⅱ) | 1.56±0.12 | 8.44±0.04 | ||
| Cd(Ⅱ) | 1.88±0.08 | 10.90±0.33 | ||
| Co(Ⅱ) | 1.88±0.10 | 10.73±0.36 | ||
| Cu(Ⅱ) | 2.30±0.28 | 13.36±1.17 | ||
| Ag(Ⅰ) | 1.99±0.15 | 10.01±0.60 | ||
| 部分锥型 | Al(Ⅲ) | 0.93±0.05 | 4.91±0.11 | |
| Fe(Ⅲ) | 0.81±0.05 | 5.13±0.11 | ||
| Ag(Ⅰ) | 1.11±0.04 | 6.06±0.15 | ||
| Ni(Ⅱ) | 1.15±0.08 | 7.09±0.23 | ||
| Cu(Ⅱ) | 1.23±0.11 | 7.48±0.37 | ||
| Pb(Ⅱ) | 1.17±0.07 | 5.94±0.17 | ||
| 1,3-交替型 | Cu(Ⅱ) | 0.42±0.03 | 3.68±0.08 | |
| Ag(Ⅰ) | 1.22±0.06 | 5.78±0.21 | ||
| Fe(Ⅲ) | 0.43±0.14 | 3.82±0.29 |
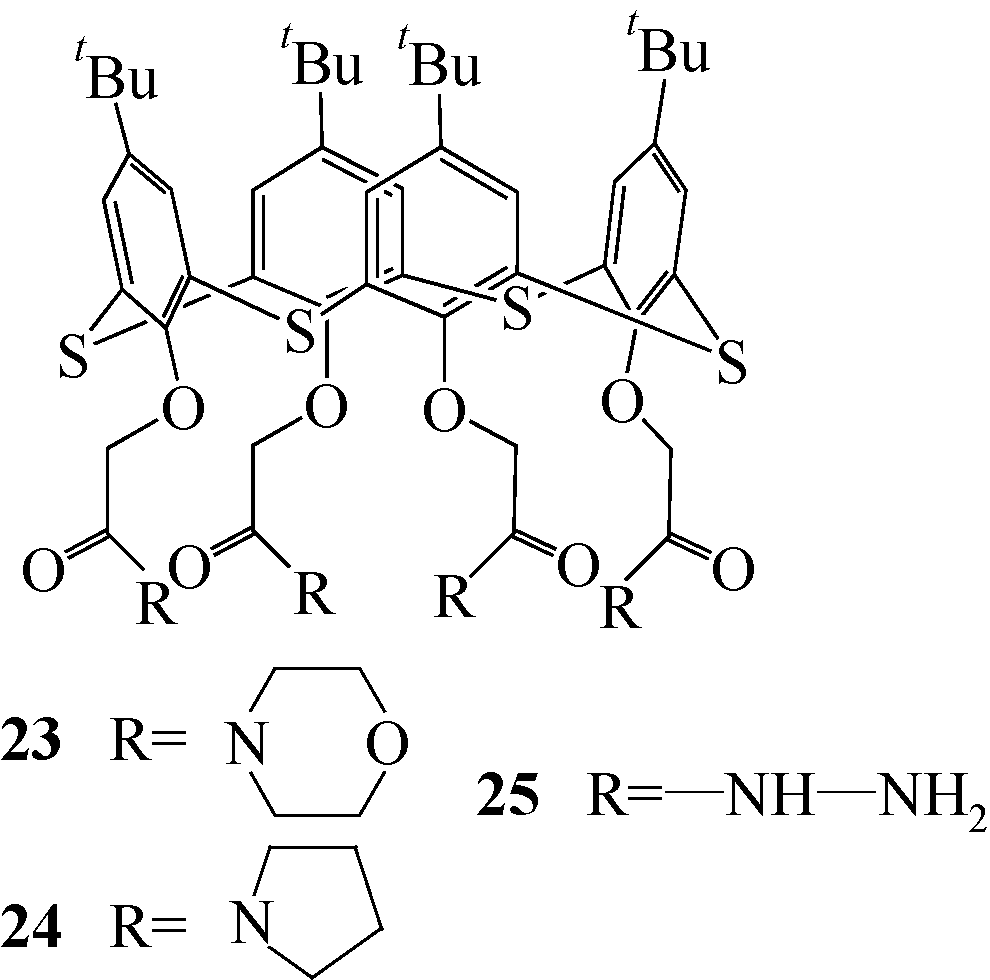
表2 23~25的lg Kex和化学计量比
| 硫杂杯芳烃 | 构象 | 阳离子 | n | lg Kex |
|---|---|---|---|---|
| 23 | 锥形 | Li(Ⅰ) | 0.78±0.01 | 3.23±0.04 |
| Na(Ⅰ) | 0.92±0.02 | 4.03±0.06 | ||
| K(Ⅰ) | 0.80±0.07 | 3.05±0.19 | ||
| Cs(Ⅰ) | — | — | ||
| Ag(Ⅰ) | 0.73±0.03 | 4.20±0.10 | ||
| 部分锥型 | Li(Ⅰ) | 0.62±0.01 | 1.97±0.02 | |
| Na(Ⅰ) | 0.82±0.01 | 3.53±0.04 | ||
| K(Ⅰ) | 0.65±0.02 | 3.35±0.05 | ||
| Cs(Ⅰ) | 0.73±0.01 | 2.45±0.02 | ||
| Ag(Ⅰ) | 1.56±0.06 | 8.37±0.23 | ||
| 1,3-交替型 | Li(Ⅰ) | 0.64±0.03 | 1.99±0.06 | |
| Na(Ⅰ) | 1.22±0.03 | 5.74±0.10 | ||
| K(Ⅰ) | 2.14±0.12 | 9.47±0.47 | ||
| Cs(Ⅰ) | 1.35±0.18 | 6.28±0.18 | ||
| Ag(Ⅰ) | 2.01±0.11 | 9.93±0.42 | ||
| 24 | 锥形 | Li(Ⅰ) | 1.91±0.13 | 8.43±0.52 |
| Na(Ⅰ) | 1.79±0.10 | 8.03±0.41 | ||
| K(Ⅰ) | 1.15±0.03 | 5.43±0.10 | ||
| Cs(Ⅰ) | 0.62±0.01 | 2.27±0.04 | ||
| Ag(Ⅰ) | 1.06±0.12 | 6.08±0.49 | ||
| 部分锥型 | Li(Ⅰ) | 0.98±0.03 | 4.51±0.08 | |
| Na(Ⅰ) | 1.36±0.02 | 6.44±0.09 | ||
| K(Ⅰ) | 1.57±0.07 | 7.32±0.28 | ||
| Cs(Ⅰ) | 0.91±0.01 | 3.95±0.04 | ||
| Ag(Ⅰ) | 1.57±0.13 | 8.18±0.55 | ||
| 1,3-交替型 | Li(Ⅰ) | 0.80±0.03 | 3.28±0.09 | |
| Na(Ⅰ) | 1.87±0.12 | 8.33±0.46 | ||
| K(Ⅰ) | 1.99±0.16 | 9.06±0.63 | ||
| Cs(Ⅰ) | 1.93±0.14 | 8.52±0.53 | ||
| Ag(Ⅰ) | 1.87±0.22 | 9.83±0.92 | ||
| 25 | 锥形 | Fe(Ⅲ) | 0.48±0.02 | 4.99±0.06 |
| Ni(Ⅱ) | 1.12±0.08 | 8.05±0.31 | ||
| Al(Ⅲ) | 1.61±0.10 | 7.17±0.21 | ||
| Pb(Ⅱ) | 1.56±0.12 | 8.44±0.04 | ||
| Cd(Ⅱ) | 1.88±0.08 | 10.90±0.33 | ||
| Co(Ⅱ) | 1.88±0.10 | 10.73±0.36 | ||
| Cu(Ⅱ) | 2.30±0.28 | 13.36±1.17 | ||
| Ag(Ⅰ) | 1.99±0.15 | 10.01±0.60 | ||
| 部分锥型 | Al(Ⅲ) | 0.93±0.05 | 4.91±0.11 | |
| Fe(Ⅲ) | 0.81±0.05 | 5.13±0.11 | ||
| Ag(Ⅰ) | 1.11±0.04 | 6.06±0.15 | ||
| Ni(Ⅱ) | 1.15±0.08 | 7.09±0.23 | ||
| Cu(Ⅱ) | 1.23±0.11 | 7.48±0.37 | ||
| Pb(Ⅱ) | 1.17±0.07 | 5.94±0.17 | ||
| 1,3-交替型 | Cu(Ⅱ) | 0.42±0.03 | 3.68±0.08 | |
| Ag(Ⅰ) | 1.22±0.06 | 5.78±0.21 | ||
| Fe(Ⅲ) | 0.43±0.14 | 3.82±0.29 |
| 萃取剂 | CPd(aq)/mmol?L-1 | CPd(org)/mmol?L-1 | Dpd | R/% |
|---|---|---|---|---|
| 47 | 0.24 | 0.24 | >25 | >96 |
| 48 | 0.24 | 0.24 | >25 | >96 |
| 49 | 0.24 | 0.05 | 0.25 | 20 |
| 50 | 0.25 | 0.028 | 0.12 | 11 |
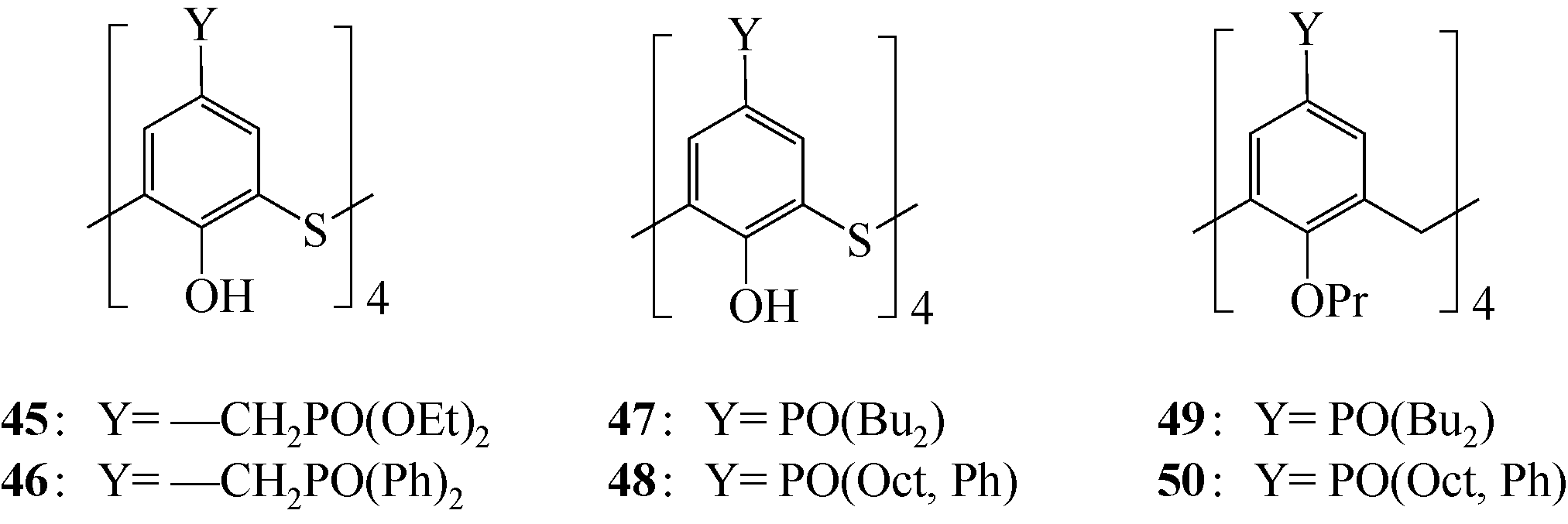
表3 47~50对Pd(Ⅱ)的萃取性能[60]
| 萃取剂 | CPd(aq)/mmol?L-1 | CPd(org)/mmol?L-1 | Dpd | R/% |
|---|---|---|---|---|
| 47 | 0.24 | 0.24 | >25 | >96 |
| 48 | 0.24 | 0.24 | >25 | >96 |
| 49 | 0.24 | 0.05 | 0.25 | 20 |
| 50 | 0.25 | 0.028 | 0.12 | 11 |
| 1 | GUTSCHE C. Calixarenes[J]. Accounts of Chemical Research, 1983, 16(5): 161-170. |
| 2 | 程衔锟, 侯雪, 田欢, 等. 大环硫杂冠醚的合成及对Ag(Ⅰ)和Tl(Ⅰ)的选择性萃取[J]. 高等学校化学学报, 2019, 40(9): 1881-1887. |
| CHENG X K, HOU X, TIAN H, et al. Synthesis of macrocyclic thiacrown ethers and their selective extraction for Ag(I) and Tl(I)[J]. Chemical Journal of Chinese Universities, 2019, 40(9): 1881-1887. | |
| 3 | SUN Y, ZHU M Y, YAO Y Y, et al. A novel approach for the selective extraction of Li+ from the leaching solution of spent lithium-ion batteries using benzo-15-crown-5 ether as extractant[J]. Separation and Purification Technology, 2020, 237: 116325. |
| 4 | 陈雅琪, 桂鑫, 段尊斌, 等. 环糊精参与的过渡金属催化有机反应[J]. 有机化学, 2019, 39(5): 1284-1292. |
| CHEN Y Q, GUI X, DUAN Z B, et al. Transition metal catalyzed organic reaction involving cyclodextrin[J]. Chinese Journal of Organic Chemistry, 2019, 39(5): 1284-1292. | |
| 5 | 夏道宏, 段尊斌, 胡尊龙, 等. β-环糊精-氧化石墨烯超分子杂化体的构筑及应用进展[J]. 化工进展, 2019, 38(4): 1823-1832. |
| XIA D H, DUAN Z B, HU Z L, et al. Progress in preparation and application of β-cyclodextrin-graphene oxide supramolecular hybrid[J]. Chemical Industry and Engineering Progress, 2019, 38(4): 1823-1832. | |
| 6 | 林集端, 赵珺. PEG/α-环糊精准聚轮烷水凝胶与BSA相互作用的光谱学研究[J]. 化工进展, 2016, 35(11): 3590-3594. |
| LIN J R, ZHAO J. Spectroscopic study of the interaction between PEG/α-cyclodextrin pseudopolyrotaxane hydrogel with BSA[J]. Chemical Industry and Engineering Progress, 2016, 35(11): 3590-3594. | |
| 7 | 严祯曦, 郭红玉, 杨发福, 等. 含硫脲基的多重氮杂杯[4]芳烃衍生物的合成与有机染料配合性能[J]. 有机化学, 2016, 36(5): 1088-1093. |
| YAN Z X, GUO H Y, YANG F F, et al. Syntheses and dyes complexation properties of multiple-azo calix[4]arene derivatives containing thiourea groups[J]. Chinese Journal of Organic Chemistry, 2016, 36(5): 1088-1093. | |
| 8 | FANG X Y, YAN D P. White-light emission and tunable room temperature phosphorescence of dibenzothiophene[J]. Science China Chemistry, 2018, 61(4): 29-33. |
| 9 | LU B, LIU S Y, YAN D P. Recent advances in photofunctional polymorphs of molecular materials[J]. Chinese Chemical Letters, 2019, 30 (11): 1908-1922. |
| 10 | 褚惠虹, 王谨, 潘志刚, 等. 硫杂杯芳烃及其衍生物的合成与表征[J]. 有机化学, 2003, 23(11): 1255-1259. |
| CHU H H, WANG J, PAN Z G, et al. Synthesis and characterization of p-tert-butylthiacalix[4]arenes and their derivatives[J]. Chinese Journal of Organic Chemistry, 2003, 23(11): 1255-1259. | |
| 11 | IKI N, KABUTO C, FUKUSHIMA T, et al. Synthesis of p-tert-butylthiacalix[4]arene and its inclusion property[J]. Tetrahedron, 2000, 56(11): 1437-1443. |
| 12 | SLIWA W, GIREK T. Calixarene complexes with metal ions[J]. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 2010, 66(1/2): 15-41. |
| 13 | 顾金英, 朱明莉, 施宪法. 治疗铜中毒的新型高效络合剂对叔丁基硫杂杯[4]芳烃[J]. 高等学校化学学报, 2012, 33(10): 2229-2234. |
| GU J Y, ZHU M L, SHI X F. p-tert-Butylthiacalix[4]arene—A potentially useful chelating agent for copper poisoning[J]. Chemical Journal of Chinese Universities, 2012, 33(10): 2229-2234. | |
| 14 | 尹志芳, 钟桐生, 刘蓉. 基于四甲氧基硫杂杯[4]芳烃荧光熄灭的钴离子光化学传感器[J]. 应用化学, 2013, 30(4): 464-468. |
| YING Z F, ZHONG T S, LIU R. An optical chemical sensor for cobalt ion based on fluorescence quenching of 5-tert-butyl-2-methoxy thiacalix[4]arene[J]. Chinese Journal of Applied Chemistry, 2013, 30(4): 464-468. | |
| 15 | 李燕琴,牟兰,曾晞, 等. 1,3-交替香豆素-硫杂杯[4]荧光探针对Fe3+和牛血红蛋白的光谱识别[J]. 光谱学与光谱分析, 2015, 35(11): 3111-3116. |
| LI Y Q, MOU L, ZENG X, et al. The thiacalix[4] arene-coumarin fluorescence probe recognition for Fe3+ and bovine hemoglobin[J]. Spectroscopy and Spectral Analysis, 2015, 35(11): 3111-3116. | |
| 16 | BILYK A, HALL A, HARROWFIELD J, et al. Systematic structural coordination chemistry of p-tert-butyltetrathiacalix[4]arene: 1. Group 1 elements and congeners[J]. Inorganic Chemistry, 2001, 40(4): 672-686. |
| 17 | SONE T, OHBA Y, MORIYA K, et al. Synthesis and properties of sulfur-bridged analogs of p-tert-butylcalix[4]arene[J]. Tetrahedron, 1997, 53(31): 10689-10698. |
| 18 | KUMAGAI H, HASEGAWA M, MIYANARI S, et al. Facile synthesis of p-tert-butylthiacalix[4]arene by the reaction of p-tert-butylphenol with elemental sulfur in the presence of a base[J]. Tetrahedron Letters, 1997, 38(22): 3971-3972. |
| 19 | 周乐舟, 付胜, 余克平, 等. 氧化石墨烯/硫杂杯芳烃复合材料富集分离-石墨炉原子吸收法测定痕量铊[J]. 分析测试学报, 2013, 32(10): 1242-1246. |
| ZHOU L Z, FU S, YU K P, et al. Determination of trace thallium by graphite furnace atomic absorption spectrometry after preconcentration with graphene oxide/thiacalixarene composites material[J]. Journal of Instrumental Analysis, 2013, 32(10): 1242-1246. | |
| 20 | MOROHASHI N, IKI N, SUGAWARA A, et al. Selective oxidation of thiacalix[4]arenes to the sulfinyl and sulfonyl counterparts and their complexation abilities toward metal ions as studied by solvent extraction[J]. Tetrahedron, 2001, 57(26): 5557-5563. |
| 21 | 郭倩玲, 屈一新, 马淑兰, 等. 硫酰杯[4]芳烃羧酸类衍生物及其配合物的合成、晶体结构与表征[J]. 高等学校化学学报, 2006, 27(11): 44-48. |
| GUO Q L, QU Y X, MA S L, et al. Synthesis, crystal structure and characterization of carboxylic derivatives of sulfonylcalix[4]aren[J]. Chemical Journal of Chinese Universities, 2006, 27(11): 44-48. | |
| 22 | MOROHASHI N, NARUMI F, IKI N, et al. Thiacalixarenes[J]. Chemical Reviews, 2006, 106(12): 5291-5316. |
| 23 | IKI N, MOROHASHI N, NARUMI F, et al. High complexation ability of thiacalixarene with transition metal ions. the effects of replacing methylene bridges of tetra (p-t-butyl) calix[4]arenetetrol by epithio groups[J]. Bulletin of the Chemical Society of Japan, 1998, 71(7): 1597-1603. |
| 24 | KUMAR R, LEE Y, BHALLA V, et al. Recent developments of thiacalixarene based molecular motifs[J]. Chemical Society Reviews, 2014, 43(13): 4824-4870. |
| 25 | IKI N, NARUMI F, FUJIMOTO T, et al. Selective synthesis of three conformational isomers of tetrakis [(ethoxycarbonyl) methoxy] thiacalix[4]arene and their complexation properties towards alkali metal ions[J]. Journal of the Chemical Society, Perkin Transactions2, 1998(12): 2745-2750. |
| 26 | AKAMA M, YAMADA M, SHIMAKAWA Y, et al. The effects of pH and counterion at liquid-liquid extraction of rare metals with carboxylic acid modified thiacalix[6]arene at the lower rim[J]. Journal of the Society of Materials Engineering for Resources of Japan, 2009, 22(1/2): 13-17. |
| 27 | YAMATO T, CASAS C P, ELSEGOOD M R, et al. Synthesis and inclusion properties of a novel thiacalix[4] arene-based hard-soft receptor with 1,3-alternate conformation[J]. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 2006, 55(1/2): 31-36. |
| 28 | AKGEMCI E G, BINGOL H, ERSOZ M, et al. Facilitated transfer of alkali metal ions by a tetraester derivative of thiacalix[4]arene at the liquid-liquid interface[J]. Electroanalysis, 2010, 20(12): 1354-1360. |
| 29 | OKADA Y, MIZUTANI M, NISHIMURA J. Highly selective Li+ ion transport by bridged calix[4]arenes[J]. Tetrahedron Letters, 1998, 39(46): 8467-8470. |
| 30 | SUWATTANAMALA A, APPELHANS D, WENZEL M, et al. Comparative study on the transition metal complexes of a novel thiacalix[4]arene derivative[J]. Chemical Physics, 2006, 320(2/3): 193-206. |
| 31 | CASAS C, YAMATO T. Hard-soft receptors, tetrakis[(N,N-diethylaminocarbonyl)methoxy] thiacalix[4]arene derivatives with cone and1,3-alternate conformation[J]. Journal of Inclusion Phenomena, 2005, 53(1/2): 1-8. |
| 32 | LAMARTINE R, BAVOUX C, VOCANSON F, et al. Synthesis, X-ray crystal structure and complexation properties towards metal ions of new thiacalix [4] arenes[J]. Tetrahedron Letters, 2001, 42(6): 1021-1024. |
| 33 | ARNAUD NEU F, SCHWING WEILL M J, ZIAT K, et al. Selective alkali and alkaline earth cation complexation by calixarene amides[J]. ChemInForm, 1991, 15(1): 33-37. |
| 34 | FONTÀS C, ANTICÓ E, VOCANSON F, et al. Efficient thiacalix[4] arenes for the extraction and separation of Au (Ⅲ), Pd (Ⅱ) and Pt (Ⅳ) metal ions from acidic media incorporated in membranes and solid phases[J]. Separation and Purification Technology, 2007, 54(3): 322-328. |
| 35 | SOLOVIEVA S E, KLESHNINA S R, KOZLOVA M N, et al. Synthesis and complexation properties of carbonyl-containing thiacalix[4]arenes[J]. Russian Chemical Bulletin, 2008, 57(7): 1477-1485. |
| 36 | SOLOVIEVA S E, GRÜNER M, OMRAN A O, et al. Synthesis, structure, and complexation properties of tetraamide derivatives of thiacalix[4]arene in different conformations[J]. Russian Chemical Bulletin, 2005, 54(9): 2104-2112. |
| 37 | DANIL D N, ANGELA F, PUGLIESE A, CASAL A R, et al. The various factors involved in the extraction of alkali metal picrates by calixarene ester derivatives in the mutually saturated water-dichloromethane solvent system[J]. Physical Chemistry Chemical Physics, 2000, 2(19): 4355-4360. |
| 38 |
ZAGHBANI A, TAYEB R, DHAHBI M, et al. Selective thiacalix[4] arene bearing three amide groups as ionophore of binary Pd( ) extraction by a supported liquid membrane system[J]. Separation and Purification Technology, 2007, 57(2): 374-379. ) extraction by a supported liquid membrane system[J]. Separation and Purification Technology, 2007, 57(2): 374-379.
|
| 39 | YAMADA M, GANDHI M R, KUNDA U M R, et al. Thiacalixarenes: emergent supramolecules in crystal engineering and molecular recognition[J]. Journal of Inclusion Phenomena & Macrocyclic Chemistry, 2016, 85(1/2): 1-18. |
| 40 | STOIKOV I I, YUSHKOVA E A, ZHUKOV A Y, et al. Solvent extraction and self-assembly of nanosized aggregates of p-tert-butyl thiacalix[4] arenes tetrasubstituted at the lower rim by tertiary amide groups and monocharged metal cations in the organic phase[J]. Tetrahedron, 2008, 64(32): 7489-7497. |
| 41 | STOIKOV I I, YUSHKOVA E A, ZHAROV I, et al. Supramolecular self-assemblies of stereoisomers of p-tert-butyl thiacalix[4]arenes functionalized with hydrazide groups at the lower rim with some metal cations[J]. Tetrahedron, 2009, 65(34): 7109-7114. |
| 42 | NI X L, TOMIYASU H, SHIMIZU T, et al. Synthesis and heteronuclear inclusion properties of a novel thiacalix[4] arene-based hard-soft receptor with 1,3-alternate conformation[J]. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 2010, 68(1/2): 99-108. |
| 43 | SHAMOVA L I, SHAMOV G A, ANTIPIN I S, et al. Modeling K+ and Ag+ complexation by thiacalix[4]arene amides using DFT: the role of intramolecular hydrogen bonding[J]. The Journal of Physical Chemistry A, 2009, 113(19): 5691-5699. |
| 44 | RAJIV GANDHI M, YAMADA M, KONDO Y, et al. Synthesis and characterization of dimethylthiocarbamoyl-modified thiacalix[n] arenes for selective Pd(Ⅱ)-ion extraction[J]. Industrial & Engineering Chemistry Research, 2014, 53(7): 2559-2565. |
| 45 | KUMAGAI H, HASEGAWA M, MIYANARI S, et al. Facile synthesis of p-tert-butylthiacalix[4] arene by the reaction of p-tert-butylphenol with elemental sulfur in the presence of a base[J]. Tetrahedron Letters, 1997, 38(22): 3971-3972. |
| 46 | KONDO Y, HAMADA F. Improvement method for synthesis of p-tert-butylthiacalix[n]arenes; effect of using base catalyst with carboxylic acid[J]. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 2007, 58(1/2): 123-126. |
| 47 | AKDAS H, GRAF E, HOSSEINI M W, et al. Tetramercaptotetrathiacalix [4] arene the most sulfur enriched ligand: synthesis and structural analysis[J]. Journal of Supramolecular Chemistry, 2002, 2(1/2/3): 21-28. |
| 48 | KATAGIRI H, IKI N, MATSUNAGA Y, et al. “Thiacalix[4]aniline” as a highly specific extractant for Au(Ⅲ) and Pd(Ⅱ) ions[J]. Chemical Communications, 2002, 34(18): 2080-2081. |
| 49 | KATAGIRI H, MOROHASHI N, IKI N, et al. Pd(Ⅱ) complexes with thiacalix[4]-arene and -aniline; subtle, but distinct influences of phenol and aniline units on the 3-D structure[J]. Dalton Transactions, 2003(4): 723-726. |
| 50 | YIH K H, LEE G H, WANG Y. Sulfur-assisted chloride and triphenylphosphine dissociation of palladium(Ⅱ) complex [Pd(PPh3)2(η1-SCNMe2)(Cl)]. X-ray structures of [Pd(PPh3)2(η1-SCNMe2)(Cl)], [Pd(PPh3)(Cl)]2(μ,η2-SCNMe2)2, and [Pd(PPh3)2(η2-SCNMe2)][PF6][J]. Inorganic Chemistry Communications, 2003, 6(5): 577-580. |
| 51 | BHALLA V, KUMAR M, KATAGIRI H, et al. Synthesis and binding studies of novel bisthiacalix[4]arenes with diimime linkages[J]. Tetrahedron Letters, 2005, 46(1): 121-124. |
| 52 | KUMAR M, SHARMA NEE B V, NAGENDRA B J. Synthesis and binding studies of new bis-calix[4]arenes containing aromatic and heteroaromatic units[J]. Tetrahedron, 2003, 59(18): 3267-3273. |
| 53 | KUMAR M, MAHAJAN R K, SHARMA V, et al. Synthesis of new bis-calix[4]arenes with imine linkages: a search for new silver-selective sensors[J]. Tetrahedron Letters, 2001, 42(31): 5315-5318. |
| 54 | OUESLATI F, DUMAZET BONNAMOUR I, LAMARTINE R J S C. New azothiacalix[4]arenes containing biheterocyclic subunits: extraction and complexation properties[J]. Supramolecular Chemistry, 2005, 17(3): 227-232. |
| 55 | OUESLATI F, DUMAZET BONNAMOUR I, LAMARTINE R. Synthesis of new chromogenic 2,2′-bithiazoylcalix [4] arenes[J]. Tetrahedron Letters, 2001, 42(46): 8177-8180. |
| 56 | OUESLATI F, DUMAZET BONNAMOUR I, LAMARTINE R. New chromogenic azocalix[4]arene podands incorporating 2,2′-bipyridyl subunits[J]. New Journal of Chemistry, 2003, 27(3): 644-647. |
| 57 | REGNOUF DE VAINS J B, DALBAVIE J O, LAMARTINE R, et al. Quantitative solvent extraction from neutral aqueous nitrate media of silver(Ⅰ) against lead(Ⅱ) with a new calix[4]arene-based bipyridine podand[J]. Tetrahedron Letters, 2001, 42(14): 2681-2684. |
| 58 | KUMAR M, DHIR A, BHALLA V. Regulation of metal ion recognition by allosteric effects in thiacalix[4]crown based receptors[J]. Tetrahedron, 2009, 65(36): 7510-7515. |
| 59 | FU Y, ZENG X, MU L, et al. Use of a new thiacalix[4]arene derivative bearing two 4-chloro-7-nitrobenzofurazan groups as a colorimetric and fluorescent chemosensor for Ag+ and AcO-[J]. Sensors and Actuators B: Chemical, 2012, 164(1): 69-75. |
| 60 | KUMAR M, NAGENDRA BABU J, BHALLA V, et al. Chromogenic sensing of Cu(Ⅱ) by imino linked thiacalix[4]arene in mixed aqueous environment[J]. Inorganic Chemistry Communications, 2009, 12(4): 332-335. |
| 61 | TORGOV V, US T, KORDA T, et al. Extraction of palladium with thiacalix[4]arenes from nitric acid nitrate-nitrite solutions[J]. Russian Journal of Inorganic Chemistry, 2012, 57(12): 1621-1629. |
| 62 | TORGOV V, US T, LAVRUKHINA S, et al. Separation of palladium and europium upon extraction with phosphorylated calix[4]- and thiacalix[4]arenes from nitric acid and carbonate solutions[J]. Russian Journal of Inorganic Chemistry, 2017, 62(6): 854-861. |
| 63 | SMIRNOV I, KARAVAN M, EFREMOVA T, et al. Extraction of Am, Eu, Tc, and Pd from nitric acid solutions with phosphorylated calixarenes[J]. Radiochemistry, 2007, 49(2): 482-492. |
| 64 | KHARCHENKO S, DRAPAILO A, SHISHKINA S, et al. Dibutylphosphinoylmethyloxythiacalix[4]arenes. synthesis, structure, americium, europium and technetium extraction[J]. Supramolecular Chemistry, 2014, 26(10/11/12): 864-872. |
| 65 | SMIRNOV I, KARAVAN M, BABAIN V, et al. Effect of alkyl substituents on extraction properties and solubility of calix[4]arene dialkylphosphine oxides[J]. Radiochimica Acta, 2007, 95(2): 97-102. |
| 66 | BOUHROUM S, KIM J S, LEE S W, et al. Sulphur-enriched thiacalix [4]arenes in the cone conformation: synthesis, crystal structures and cation binding properties[J]. Journal of Inclusion Phenomena, 2008, 62(3/4): 239-250. |
| 67 | FLORIANI C, JACOBY D, CHIESI VILLA A, et al. Aggregation of metal ions with functionalized, calixarenes: synthesis and structure of an, octanuclear copper (Ⅰ) chloride complex[J]. Angewandte Chemie: International Edition, 1989, 28(10): 1376-1377. |
| 68 | KIM T H, KIM J S, KIM H. Voltammetric studies for cation recognition with thiacalix[4] crown-6s[J]. Journal of Electroanalytical Chemistry, 2008, 615(2): 103-109. |
| 69 | MURAVEV A, GALIEVA F, BAZANOVA O G, et al. Thiacalix [4] monocrowns with terpyridine functional groups as new structural units for luminescent polynuclear lanthanide complexes[J]. Supramolecular Chemistry, 2016, 28(5/6): 589-600. |
| 70 | Е SOLOVIEVA S, А MURAVEV A, Т ZAKIRZYANOV R. Synthesis, structure, and extraction ability of tetrasubstituted thiacalix[4] arenes with crown ether fragments on the lower rim[J]. Macroheterocycles, 2012, 5(1): 17-22. |
| 71 |
谢迪欢, 孙春, 王晓静, 等. 基于杯[4]冠醚的1,3,4- 二唑荧光探针的合成及对金属离子的识别[J]. 高等学校化学学报, 2016, 37(11):1966-1971. 二唑荧光探针的合成及对金属离子的识别[J]. 高等学校化学学报, 2016, 37(11):1966-1971.
|
| XIE D H, SUN C, WANG X J, et al. Calix[4] crown-based 1,3,4-oxadiazoles as fluorescent chemosensors:synthesis and ion recognition[J]. Chemical Journal of Chinese Universities, 2016, 37(11):1966-1971. | |
| 72 |
ZHAO Z, XIONG Y H, CHENG X K, et al. Adsorptive removal of trace thallium( ) from wastewater: a review and new perspectives[J]. Journal of Hazardous Materials, 2020, 393: 122378. ) from wastewater: a review and new perspectives[J]. Journal of Hazardous Materials, 2020, 393: 122378.
|
| 73 | 赵卓, 田欢, 张金池, 等. 硫杂冠醚的合成及其对废水中Ag(Ⅰ)的选择性萃取研究[J]. 环境科学学报, 2019, 39(2): 417-422. |
ZHAO Z, TIAN H, ZHANG J C, et al. Synthesis of thiacrown ethers and its selective extration on Ag( ) from wastewater [J]. Acta Scientiae Circumstantiae, 2019, 39(2): 417-422. ) from wastewater [J]. Acta Scientiae Circumstantiae, 2019, 39(2): 417-422.
|
|
| 74 | TABAKCI M, MEMON S, YILMAZ M, et al. Oligomeric calix[4]arene-thiacrown ether for toxic heavy metals[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2004, 42(1): 186-193. |
| 75 |
JU H, KIM C, CHOI K S, et al. Thiacalix[4]-bis-crown with hard cavities and soft bridges exhibiting endocyclic potassium( ) complexes and exocyclic silver(Ⅰ) coordination polymers[J]. European Journal of Inorganic Chemistry, 2018, 31: 3587-3594. ) complexes and exocyclic silver(Ⅰ) coordination polymers[J]. European Journal of Inorganic Chemistry, 2018, 31: 3587-3594.
|
| 76 | LAMARE V, DOZOL J F, THUÉRY P, et al. Experimental and theoretical study of the first thiacalixcrowns and their alkali metal ion complexes[J]. Journal of the Chemical Society, Perkin Transactions2, 2001(10): 1920-1926. |
| 77 | CSOKAI V, GRÜN A, BALÁZS B, et al. Functionalized thiacalix- and calix[4]arene-based Ag+ ionophores: synthesis and comparative NMR study[J]. Tetrahedron, 2006, 62(43): 10215-10222. |
| 78 | CSOKAI V, BITTER I. Unprecedented cyclizations of calix[4]arenes with glycols under the mitsunobu protocol. Part 4. An expedient route to thiacalix[4](aza and thia)crowns[J]. Supramolecular Chemistry, 2004, 16(8): 611-619. |
| 79 | LEE J, KWON J, PARK C, et al. Calix[4]thiacrowns as ditopic hosts for homo-and heterobinuclear accommodation: first report of a chopsticks-typeπ-coordination[J]. Organic Letters, 2007, 9(3): 493-496. |
| [1] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [2] | 李化全, 王明华, 邱贵宝. 硫酸酸解钙钛矿相精矿的行为[J]. 化工进展, 2023, 42(S1): 536-541. |
| [3] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [4] | 马伊, 曹世伟, 王家骏, 林立群, 邢延, 曹腾良, 卢峰, 赵振伦, 张志军. 低共熔溶剂回收废旧锂离子电池正极材料的研究进展[J]. 化工进展, 2023, 42(S1): 219-232. |
| [5] | 廖志新, 罗涛, 王红, 孔佳骏, 申海平, 管翠诗, 王翠红, 佘玉成. 溶剂脱沥青技术应用与进展[J]. 化工进展, 2023, 42(9): 4573-4586. |
| [6] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [7] | 钱思甜, 彭文俊, 张先明. PET熔融缩聚与溶液解聚形成环状低聚物的对比分析[J]. 化工进展, 2023, 42(9): 4808-4816. |
| [8] | 朱传强, 茹晋波, 孙亭亭, 谢兴旺, 李长明, 高士秋. 固体高分子脱硝剂选择性非催化还原NO x 特性[J]. 化工进展, 2023, 42(9): 4939-4946. |
| [9] | 毛善俊, 王哲, 王勇. 基团辨识加氢:从概念到应用[J]. 化工进展, 2023, 42(8): 3917-3922. |
| [10] | 王报英, 王皝莹, 闫军营, 汪耀明, 徐铜文. 聚合物包覆膜在金属分离回收中的研究进展[J]. 化工进展, 2023, 42(8): 3990-4004. |
| [11] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [12] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [13] | 李洞, 王倩倩, 张亮, 李俊, 付乾, 朱恂, 廖强. 非水系纳米流体热再生液流电池串联堆性能特性[J]. 化工进展, 2023, 42(8): 4238-4246. |
| [14] | 王晓晗, 周亚松, 于志庆, 魏强, 孙劲晓, 姜鹏. 不同晶粒尺寸Y分子筛的合成及其加氢裂化反应性能[J]. 化工进展, 2023, 42(8): 4283-4295. |
| [15] | 李佳, 樊星, 陈莉, 李坚. 硝酸生产尾气中NO x 和N2O联合脱除技术研究进展[J]. 化工进展, 2023, 42(7): 3770-3779. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||