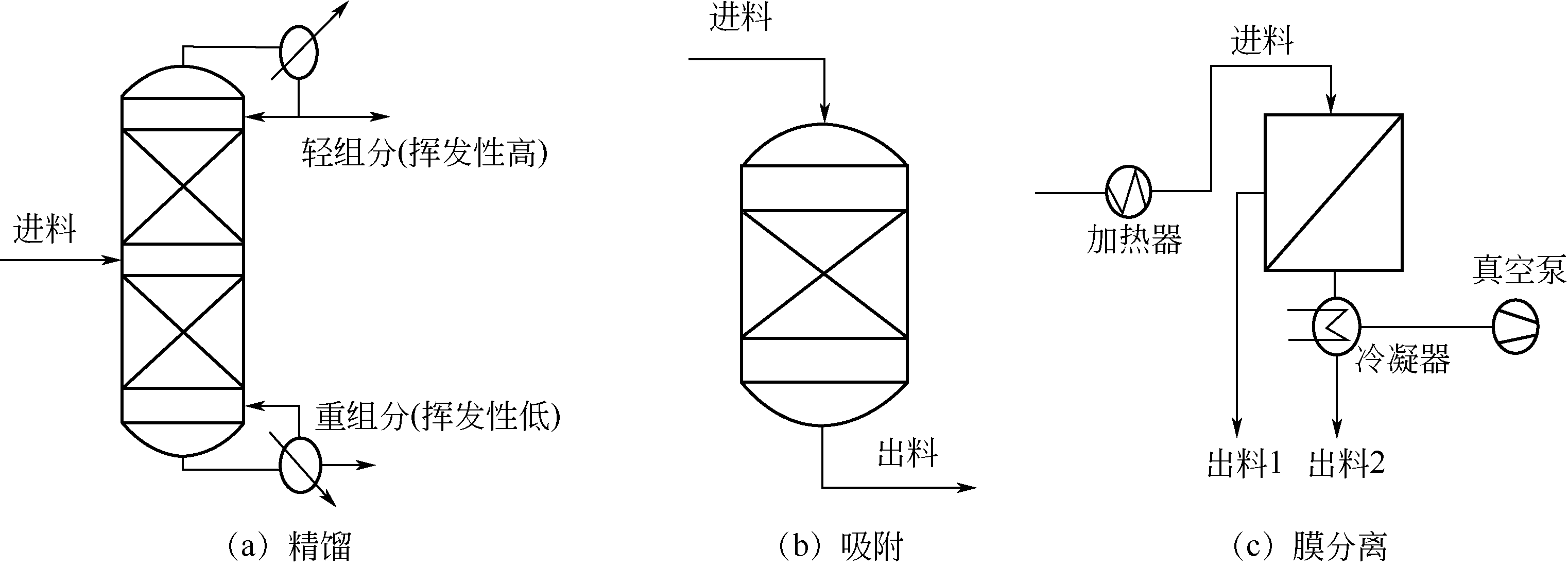
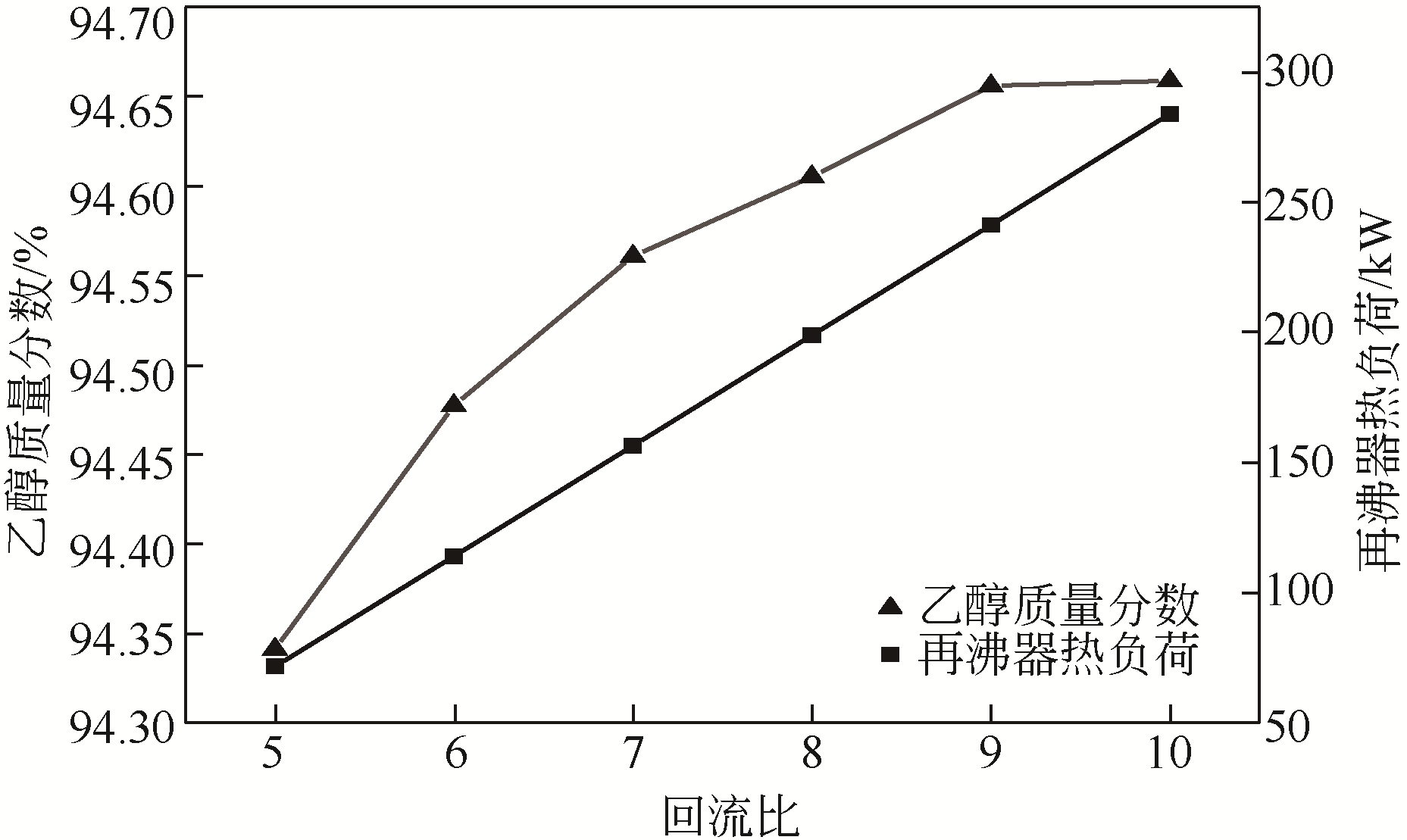
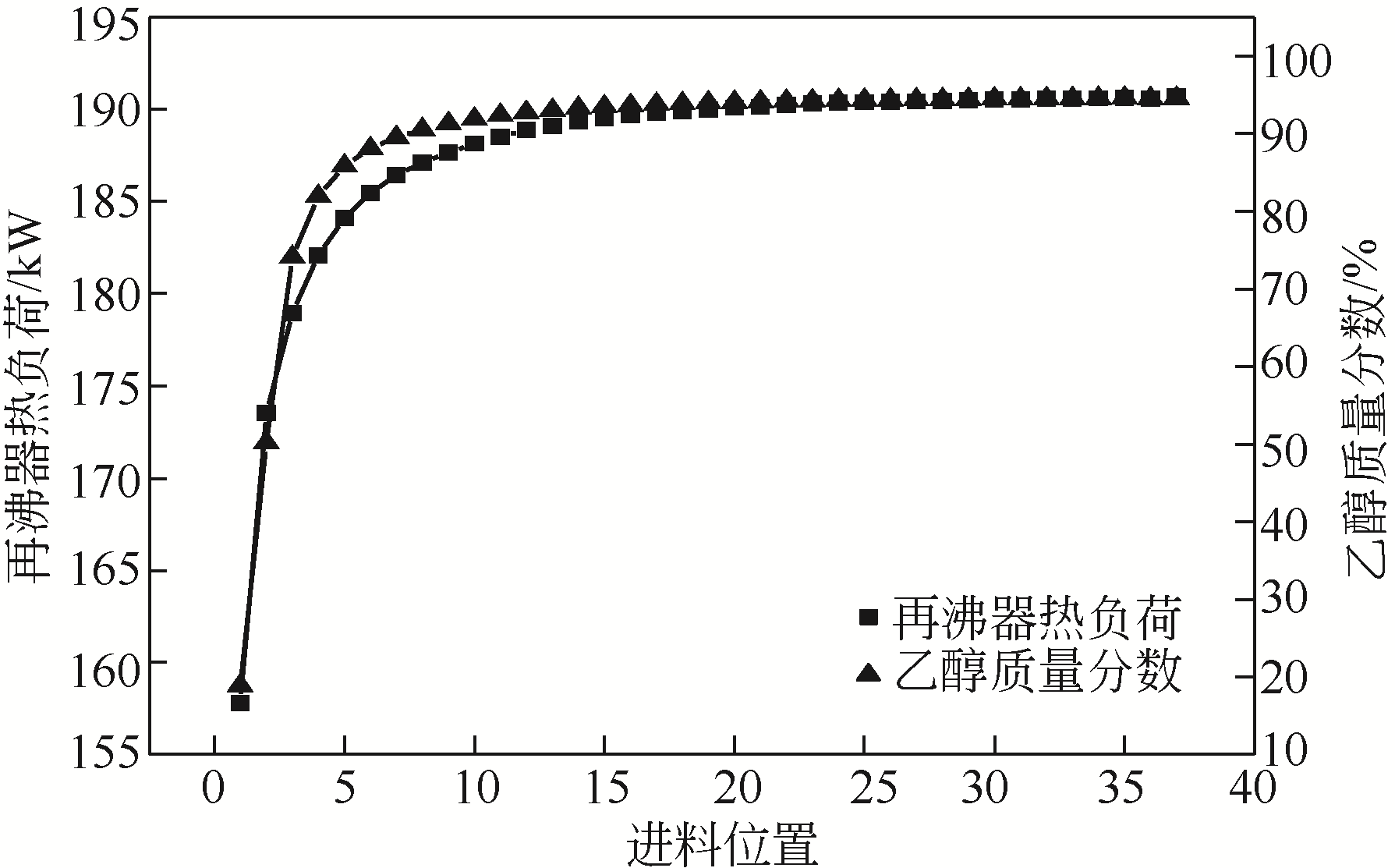
Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (3): 1354-1361.DOI: 10.16085/j.issn.1000-6613.2020-0850
• Chemical processes and equipment • Previous Articles Next Articles
Simulation of high pure alcohol preparation by distillation-adsorption-membrane separation coupling process
LI Chunli1,2( ), CHENG Yonghui1, LI Hao1,2(
), CHENG Yonghui1, LI Hao1,2( )
)
- 1.School of Chemical Engineering and Technology, Hebei University of Technology, Tianjin 300130, China
2.National-Local Joint Engineering Laboratory for Energy Conservation in Chemical Process Integration and Resources Utilization, Tianjin 300130, China
-
Received:2020-05-18Online:2021-03-17Published:2021-03-05 -
Contact:LI Hao
精馏-吸附-膜分离耦合工艺制备高纯度酒精流程模拟
- 1.河北工业大学化工学院,天津 300130
2.化工节能过程集成与资源利用国家地方联合工程实验室,天津 300130
-
通讯作者:李浩 -
作者简介:李春利(1963—),男,教授,研究方向为化工传递过程与设备。E-mail:lichunli_hebut@126.com 。
CLC Number:
Cite this article
LI Chunli, CHENG Yonghui, LI Hao. Simulation of high pure alcohol preparation by distillation-adsorption-membrane separation coupling process[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1354-1361.
李春利, 程永辉, 李浩. 精馏-吸附-膜分离耦合工艺制备高纯度酒精流程模拟[J]. 化工进展, 2021, 40(3): 1354-1361.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-0850
| 组成 | 质量分数/% | 密度/g·cm-3 | 沸点/℃ |
|---|---|---|---|
| 水 | 89.70 | 1.00 | 100 |
| 乙醇 | 10.00 | 0.789 | 78.3 |
| 甲醇 | 0.10 | 0.792 | 64.7 |
| 乙酸乙酯 | 0.05 | 0.902 | 77 |
| 正丙醇 | 0.03 | 0.803 | 97.1 |
| 异丁醇 | 0.02 | 0.81 | 107.9 |
| 异戊醇 | 0.06 | 0.81 | 132.5 |
| 乙酸 | 0.02 | 1.05 | 117.9 |
| 辛酸 | 0.01 | 0.91 | 237 |
| 己酸 | 0.01 | 0.93 | 205.4 |
| 组成 | 质量分数/% | 密度/g·cm-3 | 沸点/℃ |
|---|---|---|---|
| 水 | 89.70 | 1.00 | 100 |
| 乙醇 | 10.00 | 0.789 | 78.3 |
| 甲醇 | 0.10 | 0.792 | 64.7 |
| 乙酸乙酯 | 0.05 | 0.902 | 77 |
| 正丙醇 | 0.03 | 0.803 | 97.1 |
| 异丁醇 | 0.02 | 0.81 | 107.9 |
| 异戊醇 | 0.06 | 0.81 | 132.5 |
| 乙酸 | 0.02 | 1.05 | 117.9 |
| 辛酸 | 0.01 | 0.91 | 237 |
| 己酸 | 0.01 | 0.93 | 205.4 |
| 项目 | T1 (粗馏塔) | T2 (水洗塔) | T3 (精馏塔) | T4 (脱甲醇塔) | T5 (回收塔) |
|---|---|---|---|---|---|
| 塔板数 | 25 | 50 | 67 | 51 | 50 |
| 进料位置 | 18 | 28 | 50 | 26 | 43 |
| 塔压降/kPa | 13 | 20 | 60 | 30 | 30 |
| 加热模式 | 再沸器 | 直接蒸汽 | 直接蒸汽 | 再沸器 | 直接蒸汽 |
| 再沸比 | 0.385 | 0.134 | 0.521 | 0.400 | 1.090 |
| 项目 | T1 (粗馏塔) | T2 (水洗塔) | T3 (精馏塔) | T4 (脱甲醇塔) | T5 (回收塔) |
|---|---|---|---|---|---|
| 塔板数 | 25 | 50 | 67 | 51 | 50 |
| 进料位置 | 18 | 28 | 50 | 26 | 43 |
| 塔压降/kPa | 13 | 20 | 60 | 30 | 30 |
| 加热模式 | 再沸器 | 直接蒸汽 | 直接蒸汽 | 再沸器 | 直接蒸汽 |
| 再沸比 | 0.385 | 0.134 | 0.521 | 0.400 | 1.090 |
| 精馏-吸附-膜分离耦合工艺 | 五塔精馏工艺 |
|---|---|
| 质量分数 | |
| 水 0.22% | 水 16.42% |
| 乙醇 99.2% | 乙醇 83.4% |
| 甲醇 0.03% | 甲醇 0.075% |
| 乙酸乙酯 0.054% | 乙酸乙酯 0 |
| 正丙醇 0 | 正丙醇 0.004% |
| 异丁醇 0 | 异丁醇 0.0027% |
| 乙酸 0 | 乙酸 0.007% |
| 辛酸 0 | 辛酸 4.74×10-8 |
| 己酸 0 | 己酸 1.88×10-8 |
| 异戊醇 0 | 异戊醇 0.0081% |
| 流量 132.254kg/h | 流量 148.5kg/h |
| 精馏-吸附-膜分离耦合工艺 | 五塔精馏工艺 |
|---|---|
| 质量分数 | |
| 水 0.22% | 水 16.42% |
| 乙醇 99.2% | 乙醇 83.4% |
| 甲醇 0.03% | 甲醇 0.075% |
| 乙酸乙酯 0.054% | 乙酸乙酯 0 |
| 正丙醇 0 | 正丙醇 0.004% |
| 异丁醇 0 | 异丁醇 0.0027% |
| 乙酸 0 | 乙酸 0.007% |
| 辛酸 0 | 辛酸 4.74×10-8 |
| 己酸 0 | 己酸 1.88×10-8 |
| 异戊醇 0 | 异戊醇 0.0081% |
| 流量 132.254kg/h | 流量 148.5kg/h |
| 吸附剂 | 脱除前甲醇质量分数 /% | 脱除前水质量分数 /% | 脱除后甲醇质量分数 /% | 脱除后水质量分数 /% | 甲醇脱除率 /% | 水脱除率 /% | 处理合格酒精 /mL |
|---|---|---|---|---|---|---|---|
| 732树脂 | 0.3 | 4.9 | 0.15 | 0.4 | 50 | 91.8 | |
| 天然沸石 | 0.3 | 4.9 | <0.02 | <0.2 | 93 | >96 | 142 |
| 4A分子筛 | 0.3 | 4.9 | <0.0018 | <0.15 | 96 | >96 | 302 |
| 吸附剂 | 脱除前甲醇质量分数 /% | 脱除前水质量分数 /% | 脱除后甲醇质量分数 /% | 脱除后水质量分数 /% | 甲醇脱除率 /% | 水脱除率 /% | 处理合格酒精 /mL |
|---|---|---|---|---|---|---|---|
| 732树脂 | 0.3 | 4.9 | 0.15 | 0.4 | 50 | 91.8 | |
| 天然沸石 | 0.3 | 4.9 | <0.02 | <0.2 | 93 | >96 | 142 |
| 4A分子筛 | 0.3 | 4.9 | <0.0018 | <0.15 | 96 | >96 | 302 |
| 膜材料 | 渗透通量/g·m-2·h-1 | 分离因子 | 温度/℃ |
|---|---|---|---|
| PDMS | 200 | 4.4~10.8 | 66 |
| PTFE | 620 | 2.4 | 40 |
| PVDF | 1125 | 13.1 | 60 |
| 膜材料 | 渗透通量/g·m-2·h-1 | 分离因子 | 温度/℃ |
|---|---|---|---|
| PDMS | 200 | 4.4~10.8 | 66 |
| PTFE | 620 | 2.4 | 40 |
| PVDF | 1125 | 13.1 | 60 |
| 名称 | T1(粗馏塔) | T2(水洗塔) | T3(精馏塔) | T4(脱甲醇塔) | T5(回收塔) | T'(精馏塔) |
|---|---|---|---|---|---|---|
| 温度/℃ | 89.201 | 76.772 | 109.066 | 94.433 | 93.715 | 94.826 |
| 热负荷/kW | 399.652 | 0 | 0 | 18.529 | 0 | 230 |
| 塔底物料流量/kg·h-1 | 1640 | 450.595 | 402.976 | 148.500 | 74.596 | 1913.550 |
| 再沸器蒸汽流量/kg·h-1 | 631.840 | 60.171 | 210.145 | 65.214 | 82.693 | 446.332 |
| 再沸比 | 0.385 | 0.134 | 0.521 | 0.439 | 1.109 | 0.233 |
| 名称 | T1(粗馏塔) | T2(水洗塔) | T3(精馏塔) | T4(脱甲醇塔) | T5(回收塔) | T'(精馏塔) |
|---|---|---|---|---|---|---|
| 温度/℃ | 89.201 | 76.772 | 109.066 | 94.433 | 93.715 | 94.826 |
| 热负荷/kW | 399.652 | 0 | 0 | 18.529 | 0 | 230 |
| 塔底物料流量/kg·h-1 | 1640 | 450.595 | 402.976 | 148.500 | 74.596 | 1913.550 |
| 再沸器蒸汽流量/kg·h-1 | 631.840 | 60.171 | 210.145 | 65.214 | 82.693 | 446.332 |
| 再沸比 | 0.385 | 0.134 | 0.521 | 0.439 | 1.109 | 0.233 |
| 工艺 | 塔身(含加工和安装费用) | 冷凝器 /再沸器 | 泵 | 换热器 | 循环水 | 汽提热蒸汽 | 再沸器消耗热蒸汽 | 其他 | 总成本 |
|---|---|---|---|---|---|---|---|---|---|
| 精馏-吸附-膜分离耦合工艺 | 8.0×103 | 1.5×104 | 1.5×104 | 2.8×104 | 1.3×104 | 6.4×105 | 2.0×104(天然沸石) 1.2×105(渗透汽化装置) 2.0×104(膜更换) 1.0×104(吸附装置) | 8.89×105 | |
| 五塔精馏工艺 | 2.0×104 | 4.2×104 | 1.0×104 | 3.6×105 | 1.0×106 | 1.432×106 |
| 工艺 | 塔身(含加工和安装费用) | 冷凝器 /再沸器 | 泵 | 换热器 | 循环水 | 汽提热蒸汽 | 再沸器消耗热蒸汽 | 其他 | 总成本 |
|---|---|---|---|---|---|---|---|---|---|
| 精馏-吸附-膜分离耦合工艺 | 8.0×103 | 1.5×104 | 1.5×104 | 2.8×104 | 1.3×104 | 6.4×105 | 2.0×104(天然沸石) 1.2×105(渗透汽化装置) 2.0×104(膜更换) 1.0×104(吸附装置) | 8.89×105 | |
| 五塔精馏工艺 | 2.0×104 | 4.2×104 | 1.0×104 | 3.6×105 | 1.0×106 | 1.432×106 |
| 1 | 赵常红. 酒精厂工艺设计中的主要节能措施[J]. 工程技术研究, 2019, 18(4): 228-230. |
| ZAHO C H. The main energy saving measures in the process design of distillery [J]. Engineering and Technological Research, 2019, 18(4): 228-230. | |
| 2 | SHINNOSUKE O, JACEK A K, WILLIAM S J, et al. Ethanol purification with ozonation, activated carbon adsorption, and gas stripping[J].Separation and Purification Technology, 2015, 151(4): 165-171. |
| 3 | AZAM M, MOHAMMAD M, RASOOL P, et al. Ethanol purification using polyamide-carbon nanotube composite membranes[J]. Polymer Engineering & Science, 2014, 54(4): 961-968. |
| 4 | 石会龙, 李成帅, 刘博文, 等. 异丁醇-乙醇-水三元体系精馏分离的模拟与优化[J]. 石油与天然气化工, 2018, 47(3): 44-48. |
| SHI H L, LI C S, LIU B W, et al. Simulation and optimization of distillation separation of isobutanol-ethanol-water three-element system[J]. Chemical Engineering of Oil & Gas, 2018, 47(3): 44-48. | |
| 5 | 王洪海, 李春利, 方静. 加盐萃取精馏制取无水乙醇过程的模拟[J]. 石油化工, 2008, 37(3): 852-855. |
| WANG H H, LI C L, FANG J. Simulation of salt extractive distillation for preparation of anhydrous ethanol [J]. Petrochemical Technology, 2008, 37(3): 852-855. | |
| 6 | 孙德芳, 邵旻, 周政, 等. 减压精馏法制备无水乙醇的研究[J]. 现代化工, 2010, 30(6): 74-77. |
| SUN D F, SHAO M, ZHOU Z, et al. Study on production of anhydrous ethanol from vacuum distillation [J]. Modern Chemical Industry, 2010, 30(6):74-77. | |
| 7 | YOSHISHIGE H, SYOICHI Y, TAKUYA K, et al. An efficient ethanol concentration process by vapor permeation through asymmetric polyimide membrane[J]. Journal of Membrane Science, 2000(177): 233-239. |
| 8 | 杨座国, 许振良, 王学军. L-DBTA印迹中空纤维复合膜分离乙醇-水的研究[J]. 高分子材料科学与工程, 2006, 22(6): 220-224. |
| YANG Z G, XU Z L, WANG X J. Study on the separation of ethanol/water using L-DBTA molecular imprinted composite membranes [J]. Polymer Materials Science & Engineering, 2006, 22(6): 220-224. | |
| 9 | Al ASHEH S, BANAT F, Al LAGTAH N. Separation of ethanol water mixtures using mole cular sieves and biobased adsorbents[J].Chemical Engineering Research and Design, 2004, 82(A7): 855-864. |
| 10 | CHANG Hua, YUAN Xigang, TIAN Hua, et al. Experimental investigation and modeling of adsorption of water and ethanol on cornmeal in an ethanol water binary vapor system[J]. Chemical Engineering & Technology, 2006, 29(4): 454-461. |
| 11 | CHANG Hua, YUAN Xigang, TIAN Hua, et al. Experiment and prediction of breakthrough curves for packed bed adsorption of water vapor on cornmeal[J]. Chemical Engineering and Processing, 2006, 45(9): 747-754. |
| 12 | 贾宪勇, 汪洋, 王悦伟. Aspen Plus软件在乙醇-水分离塔设计中的应用[J]. 天津化工, 2019, 33(2): 35-39. |
| JIA X Y, WANG Y, WANG Y W. Application of Aspen Plus software in the design of ethanol-water separation column [J]. Tianjin Chemical Industry, 2019, 33(2): 35-39. | |
| 13 | 李沫林, 陈砺, 严宗诚. 燃料乙醇脱水工艺的研究与展望[J]. 食品工业科技, 2010, 31(5): 410-413. |
| LI M L, CHEN L, YAN Z C. Study and prospect of dehydration processing of fuel ethanol [J]. Science and Technology of Food Industry, 2010, 31(5): 410-413. | |
| 14 | 邵昌哲, 陈钢, 侯仲轲. 含异戊醇、乙醇废水的精馏分离模拟分析[J]. 精细化工中间体, 2017, 47(6): 33-36, 69. |
| SHAO C Z, CHEN G, HOU Z K. Simulation analysis of distillation for the separation of wastewater containing isoamyl alcohol and ethanol[J]. Fine Chemical Intermediates, 2017, 47(6): 33-36, 69. | |
| 15 | 石永胜, 王永飞, 杜娟. 精馏法处理高浓度有机废水研究进展[J]. 化工管理, 2015, 25: 212-213. |
| SHI Y S, WANG Y F, DU J. Research progress of ordinary distillation treatment on high concentration organic wastewater[J]. Chemical Enterprise Management, 2015, 25: 212-213. | |
| 16 | ROXANA A M, MICHAEL R D, MCELROY C R, et al. The role of surface functionality of sustainable mesoporous materials Starbon® on the adsorption of toxic ammonia and sulphur gasses[J]. Sustainable Chemistry and Pharmacy, 2020(15): 1-9. |
| 17 | 田俊英. 溶度积驱动循环模板法制备氧化铝空心球及其吸附性能[D]. 大连: 大连理工大学, 2018. |
| TIAN J Y. Solubility product driven preparation of Al2O3 hollow microspheres base on recyclable template and their adsorption performances[D]. Dalian: Dalian University of Technology, 2018. | |
| 18 | 范方舟. 化学-生物氧化组合方法处理纤维素乙醇废水的研究[D]. 天津: 天津大学, 2016. |
| FAN F Z. Chemical-biological oxidation process for treating cellulosic ethanol production wastewater[D]. Tianjin: Tianjin University, 2016. | |
| 19 | 刘帅. PDMS/PVDF复合膜制备及渗透蒸发分离水中酚的研究[D]. 哈尔滨: 哈尔滨工业大学, 2017. |
| LIU S. Research on preparation of PDMS/PVDF composite membrane and pervaporation separation properties for phenol water[D]. Harbin: Harbin Institute of Technology, 2017. | |
| 20 | 张浅, 朱华旭, 唐志书, 等. 基于蒸气渗透膜技术的中药连翘含油水体中挥发油分离工艺研究[J]. 中国中药杂志, 2018, 43(8): 1642-1648. |
| ZAHNG Q, ZHU H X, TANG Z S, et al. Study on essential oil separation from Forsythia suspensa oil-bearing water body based on vapor permeation membrane separation technology[J]. China Journal of Chinese Materia Medic, 2018, 43(8): 1642-1648. | |
| 21 | BOTOND S, TRANG D T H, DANIEL F, et al. Modelling of hybrid method for VOC removal from process wastewater: distillation and hydrophilic pervaporation[J]. Periodica Polytechnica Chemical Engineering, 2020, 64(3): 364-370. |
| 22 | 崔永芳, 凌德生, 赵蕴慧, 等. 用PVA/PAN复合膜分离乙醇水溶液[J]. 水处理技术, 1993, 19(5): 21-26. |
| CUI Y F, LING D S, ZHAO Y H, et al. Aqueous ethanol was separated by PVA/PAN membrane [J]. Technology of Water Treatment, 1993, 19(5): 21-26. | |
| 23 | 孙兰义. 化工流程模拟实训: Aspen Plus[M]. 北京: 化学工业出版社, 2012: 181-300. |
| SUN L Y. Chemical engineering process simulation using Aspen Plus[M]. Beijing: Chemical Industry Press, 2012: 181-300. | |
| 24 | 解娅男, 李复. 基于Aspen Plus的酸水汽提模拟[J]. 计算机与现代化, 2019(12): 78-82, 87. |
| XIE Y N, LI F. Simulation of acid water extraction based on Aspen Plus [J]. Computer and Modernization, 2019(12): 78-82, 87. | |
| 25 | 甄宝勤. 吸附法脱除食用酒精中的甲醇和水分研究[J]. 化工时刊, 2004, 18(10): 50-51. |
| ZHEN B Q. Study on removing methanol and water from edible alcohol with absorbent[J]. Chemical Industry Times, 2004, 18(10): 50-51. | |
| 26 | 中国科学院广州化学研究所. 用聚偏氟乙烯渗透汽化膜分离乙醇水溶液的方法: CN 99116274.9[P]. 1999-12-22. |
| Guangzhou Institute of Chemistry, Chinese Academy of Sciences. Separation of aqueous ethanol solution by pervaporation membrane of polyvinylidene fluoride: CN 99116274.9[P]. 1999-12-22. | |
| 27 | 唐俏瑜, 王莉, 展侠, 等. 高选择性PDMS/PVDF复合膜渗透汽化分离乙醇/水混合物[J]. 膜科学与技术, 2011, 31(6): 1-5. |
| TANG Q Y, WANG L, ZHAN X, et al. Highly selective PDMS/PVDF composite membrane pervaporation separation of ethanol/water mixture[J]. Membrane Science and Technology, 2011, 31(6): 1-5. | |
| 28 | SHI T, CHUN W, YANG A, et al. Optimization and control of energy saving side-stream extractive distillation with heat integration for separating ethyl acetate-ethanol azeotrope[J]. Chemical Engineering Science, 2020, 215: 1-18. |
| 29 | YANG A, SUN S R, SHI T, et al. Energy-efficient extractive pressure-swing distillation for separating binary minimum azeotropic mixture dimethyl carbonate and ethanol[J]. Separation and Purification Technology, 2019, 229: 1-11. |
| 30 | 李洪亮, 王战辉, 姚银娇. 膜分离法回收不凝气中乙醇/水蒸气的实验研究[J]. 郑州大学学报(工学版), 2012, 33(3): 91-94. |
| LI H L, WANG Z H, YAO Y J. Experimental study on recovery of ethanol/vapour from non-condensable gas by membrane separation [J]. Journal of Zhengzhou University (Engineering Science), 2012, 33(3): 91-94. | |
| 31 | VANKELECOM I F J, DEPRE D, BEUKELAER S D, et al. Influence of zeolites in PDMS membranes: pervaporation of water/alcohol mixtures[J]. The Journal of Chemical Physics, 1995, 99(35): 13193-13197. |
| [1] | LI Mengyuan, GUO Fan, LI Qunsheng. Simulation and optimization of the third and fourth distillation columns in the recovery section of polyvinyl alcohol production [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 113-123. |
| [2] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [3] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [4] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [5] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [6] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [7] | ZHANG Fengqi, CUI Chengdong, BAO Xuewei, ZHU Weixuan, DONG Hongguang. Design and evaluation of sweetening process with amine solution absorption and multiple desorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 518-528. |
| [8] | SUN Yuyu, CAI Xinlei, TANG Jihai, HUANG Jingjing, HUANG Yiping, LIU Jie. Optimization and energy-saving of a reactive distillation process for the synthesis of methyl methacrylate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 56-63. |
| [9] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [10] | ZHANG Fan, TAO Shaohui, CHEN Yushi, XIANG Shuguang. Initializing distillation column simulation based on the improved constant heat transport model [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4550-4558. |
| [11] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [12] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [13] | LI Xuejia, LI Peng, LI Zhixia, JIN Dunshang, GUO Qiang, SONG Xufeng, SONG Peng, PENG Yuelian. Experimental comparation on anti-scaling and anti-wetting ability of hydrophilic and hydrophobic modified membranes [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4458-4464. |
| [14] | JIANG Jing, CHEN Xiaoyu, ZHANG Ruiyan, SHENG Guangyao. Research progress of manganese-loaded biochar preparation and its application in environmental remediation [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4385-4397. |
| [15] | XU Jie, XIA Longbo, LUO Ping, ZOU Dong, ZHONG Zhaoxiang. Progress in preparation and application of omniphobic membranes for membrane distillation process [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3943-3955. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||