Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (3): 1506-1516.DOI: 10.16085/j.issn.1000-6613.2020-0808
• Materials science and technology • Previous Articles Next Articles
Lithium-ion batteries with nickel-rich oxide concentration gradient cathode materials
ZHANG Shan1( ), WANG Shan1, CHEN Weixiao1, GAO Peng1(
), WANG Shan1, CHEN Weixiao1, GAO Peng1( ), ZHU Yongming1,2(
), ZHU Yongming1,2( )
)
- 1.Department of Applied Chemistry, Harbin Institute of Technology, Weihai 264209, Shandong, China
2.Songshan Lake Materials Laboratory, Dongguan 523808, Guangdong, China
-
Received:2020-05-12Online:2021-03-17Published:2021-03-05 -
Contact:GAO Peng,ZHU Yongming
浓度梯度型锂离子电池富镍氧化物正极材料
张珊1( ), 王珊1, 陈卫晓1, 高鹏1(
), 王珊1, 陈卫晓1, 高鹏1( ), 朱永明1,2(
), 朱永明1,2( )
)
- 1.哈尔滨工业大学(威海)应用化学系,山东 威海 264209
2.松山湖材料实验室,广东 东莞 523808
-
通讯作者:高鹏,朱永明 -
作者简介:张珊(1995—),女,硕士研究生,研究方向为锂离子电池富镍正极材料。E-mail:453031181@qq.com 。 -
基金资助:国家重点研发计划(2019YFA0705100);威海市科研创新基金(2019KYCXJJYB12)
CLC Number:
Cite this article
ZHANG Shan, WANG Shan, CHEN Weixiao, GAO Peng, ZHU Yongming. Lithium-ion batteries with nickel-rich oxide concentration gradient cathode materials[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1506-1516.
张珊, 王珊, 陈卫晓, 高鹏, 朱永明. 浓度梯度型锂离子电池富镍氧化物正极材料[J]. 化工进展, 2021, 40(3): 1506-1516.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-0808
| 掺杂对象 | 掺杂元素 | 掺杂方式 | 掺杂效果 | 参考文献 |
|---|---|---|---|---|
| LiNi0.8Co0.2O2 | Sn4+ | 以LiOH、Ni(OH)2、Co2O3和SnO2为原料,用流变相反应法合成 | 改善了可逆容量以及循环性能,有利于材料结构的稳定、电阻的降低和导电性的提高 | [ |
| LiNi0.8Co0.1Mn0.1O2 | Al3+、Mg2+ | 以NiSO4、CoSO4、MnSO4、MgSO4和Al(NO3)3为原料,采用共沉淀法合成 | 减少了阳离子混排,改善了结构稳定性,增强了循环稳定性和热稳定性 | [ |
| LiNi0.8Co0.2O2 | Nb5+ | 共沉淀法合成前体,然后加LiOH和Nb2O5混合研磨、高温煅烧 | 减少了Li/Ni混排,减小极化,加速了Li+的迁移速率,提高倍率性能和循环稳定性 | [ |
| LiNi0.815Co0.15Al0.035O2 | Zr4+ | 将商品化前体、LiOH和纳米ZrO2混合研磨、高温煅烧 | 抑制Li/Ni混排,减缓极化和阻抗的增长,增强了暴露在空气中的存储性能 | [ |
| LiNi0.6Co0.2Mn0.2O2 | Mo6+ | 水热法制备前体,然后加钼酸铵和Li(NO)3混合球磨、高温煅烧 | 增大晶胞参数,增强阳离子有序度并拓宽锂离子迁移通道 | [ |
| 掺杂对象 | 掺杂元素 | 掺杂方式 | 掺杂效果 | 参考文献 |
|---|---|---|---|---|
| LiNi0.8Co0.2O2 | Sn4+ | 以LiOH、Ni(OH)2、Co2O3和SnO2为原料,用流变相反应法合成 | 改善了可逆容量以及循环性能,有利于材料结构的稳定、电阻的降低和导电性的提高 | [ |
| LiNi0.8Co0.1Mn0.1O2 | Al3+、Mg2+ | 以NiSO4、CoSO4、MnSO4、MgSO4和Al(NO3)3为原料,采用共沉淀法合成 | 减少了阳离子混排,改善了结构稳定性,增强了循环稳定性和热稳定性 | [ |
| LiNi0.8Co0.2O2 | Nb5+ | 共沉淀法合成前体,然后加LiOH和Nb2O5混合研磨、高温煅烧 | 减少了Li/Ni混排,减小极化,加速了Li+的迁移速率,提高倍率性能和循环稳定性 | [ |
| LiNi0.815Co0.15Al0.035O2 | Zr4+ | 将商品化前体、LiOH和纳米ZrO2混合研磨、高温煅烧 | 抑制Li/Ni混排,减缓极化和阻抗的增长,增强了暴露在空气中的存储性能 | [ |
| LiNi0.6Co0.2Mn0.2O2 | Mo6+ | 水热法制备前体,然后加钼酸铵和Li(NO)3混合球磨、高温煅烧 | 增大晶胞参数,增强阳离子有序度并拓宽锂离子迁移通道 | [ |
| 包覆对象 | 包覆物质 | 包覆方式 | 包覆效果 | 参考文献 |
|---|---|---|---|---|
| LiNi0.6Co0.2Mn0.2O2 | Al2O3 | 正极材料粉体和纳米级Al2O3在乙醇中超声分散,待溶剂蒸发后,在500℃下煅烧得到 | 包覆层不仅可以改善材料的结构稳定性,而且可以抑制活性材料与电解质之间的反应。在循环过程中,包覆层显著降低了阻抗 | [ |
| LiNi0.8Co0.1Mn0.1O2 | Ag | 正极材料粉体和Ag(OH)2粉体在乙醇中超声分散,用氨水控制pH,待溶剂蒸发后,在600℃下煅烧得到 | Ag包覆层的存在有助于减少电极极化、增强电子传导性并加速Li+扩散。同时能降低Li/Ni混排程度,有助于形成稳定的结构 | [ |
| LiNi0.6Co0.2Mn0.2O2 | ZrO2 | 正极材料粉体和多孔Zr基MOF材料球磨混合,然后在600℃下煅烧得到 | 包覆层抑制了由与电解质的有害副反应引起的结构劣化,从而保持了结构的完整性,同时包覆层的多孔框架也有利于Li+扩散 | [ |
| LiNi0.8Co0.1Mn0.1O2 | Li3PO4 | 将商品化前体与(NH4)2HPO4、LiOH和PVA进行水热反应,然后在800℃下煅烧得到 | 包覆层抑制了活性物质与空气和电解液的反应,并且可以为锂离子迁移提供快速通道,有效地抑制了SEI膜的生长 | [ |
| LiNi0.8Co0.1Mn0.1O2 | MoS2 | 正极材料粉体和(NH4)2 MoS4在乙醇中搅拌分散,待溶剂完全蒸发,在500℃氩气气氛下煅烧得到 | 具有化学稳定性的MoS2可以防止与HF的副反应,从而提高正极材料结构的稳定性。同时包覆层可为Li+的嵌入和脱出提供稳定的通道 | [ |
| LiNi0.8Co0.15Al0.05O2 | SnO2 | 将锂、镍、钴、铝的硝酸盐和SnCl2溶解在乙醇中,滴加到含有草酸的乙醇中,溶剂蒸发后在800℃煅烧得到 | 包覆层会抑制活性物质和电解液之间的副反应,同时可防止高温煅烧时层状氧化物颗粒的团聚,提高材料循环稳定性 | [ |
| 包覆对象 | 包覆物质 | 包覆方式 | 包覆效果 | 参考文献 |
|---|---|---|---|---|
| LiNi0.6Co0.2Mn0.2O2 | Al2O3 | 正极材料粉体和纳米级Al2O3在乙醇中超声分散,待溶剂蒸发后,在500℃下煅烧得到 | 包覆层不仅可以改善材料的结构稳定性,而且可以抑制活性材料与电解质之间的反应。在循环过程中,包覆层显著降低了阻抗 | [ |
| LiNi0.8Co0.1Mn0.1O2 | Ag | 正极材料粉体和Ag(OH)2粉体在乙醇中超声分散,用氨水控制pH,待溶剂蒸发后,在600℃下煅烧得到 | Ag包覆层的存在有助于减少电极极化、增强电子传导性并加速Li+扩散。同时能降低Li/Ni混排程度,有助于形成稳定的结构 | [ |
| LiNi0.6Co0.2Mn0.2O2 | ZrO2 | 正极材料粉体和多孔Zr基MOF材料球磨混合,然后在600℃下煅烧得到 | 包覆层抑制了由与电解质的有害副反应引起的结构劣化,从而保持了结构的完整性,同时包覆层的多孔框架也有利于Li+扩散 | [ |
| LiNi0.8Co0.1Mn0.1O2 | Li3PO4 | 将商品化前体与(NH4)2HPO4、LiOH和PVA进行水热反应,然后在800℃下煅烧得到 | 包覆层抑制了活性物质与空气和电解液的反应,并且可以为锂离子迁移提供快速通道,有效地抑制了SEI膜的生长 | [ |
| LiNi0.8Co0.1Mn0.1O2 | MoS2 | 正极材料粉体和(NH4)2 MoS4在乙醇中搅拌分散,待溶剂完全蒸发,在500℃氩气气氛下煅烧得到 | 具有化学稳定性的MoS2可以防止与HF的副反应,从而提高正极材料结构的稳定性。同时包覆层可为Li+的嵌入和脱出提供稳定的通道 | [ |
| LiNi0.8Co0.15Al0.05O2 | SnO2 | 将锂、镍、钴、铝的硝酸盐和SnCl2溶解在乙醇中,滴加到含有草酸的乙醇中,溶剂蒸发后在800℃煅烧得到 | 包覆层会抑制活性物质和电解液之间的副反应,同时可防止高温煅烧时层状氧化物颗粒的团聚,提高材料循环稳定性 | [ |
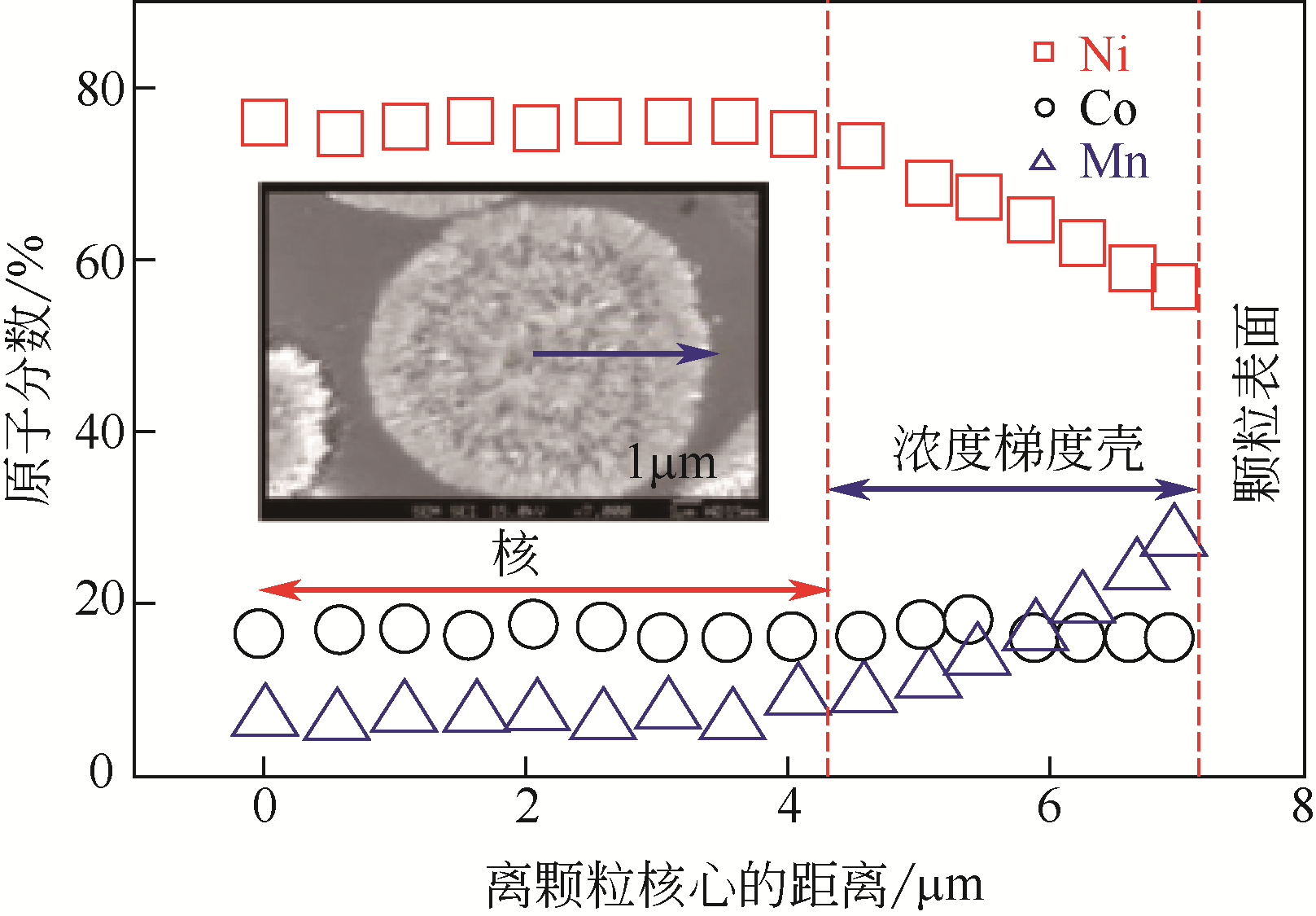
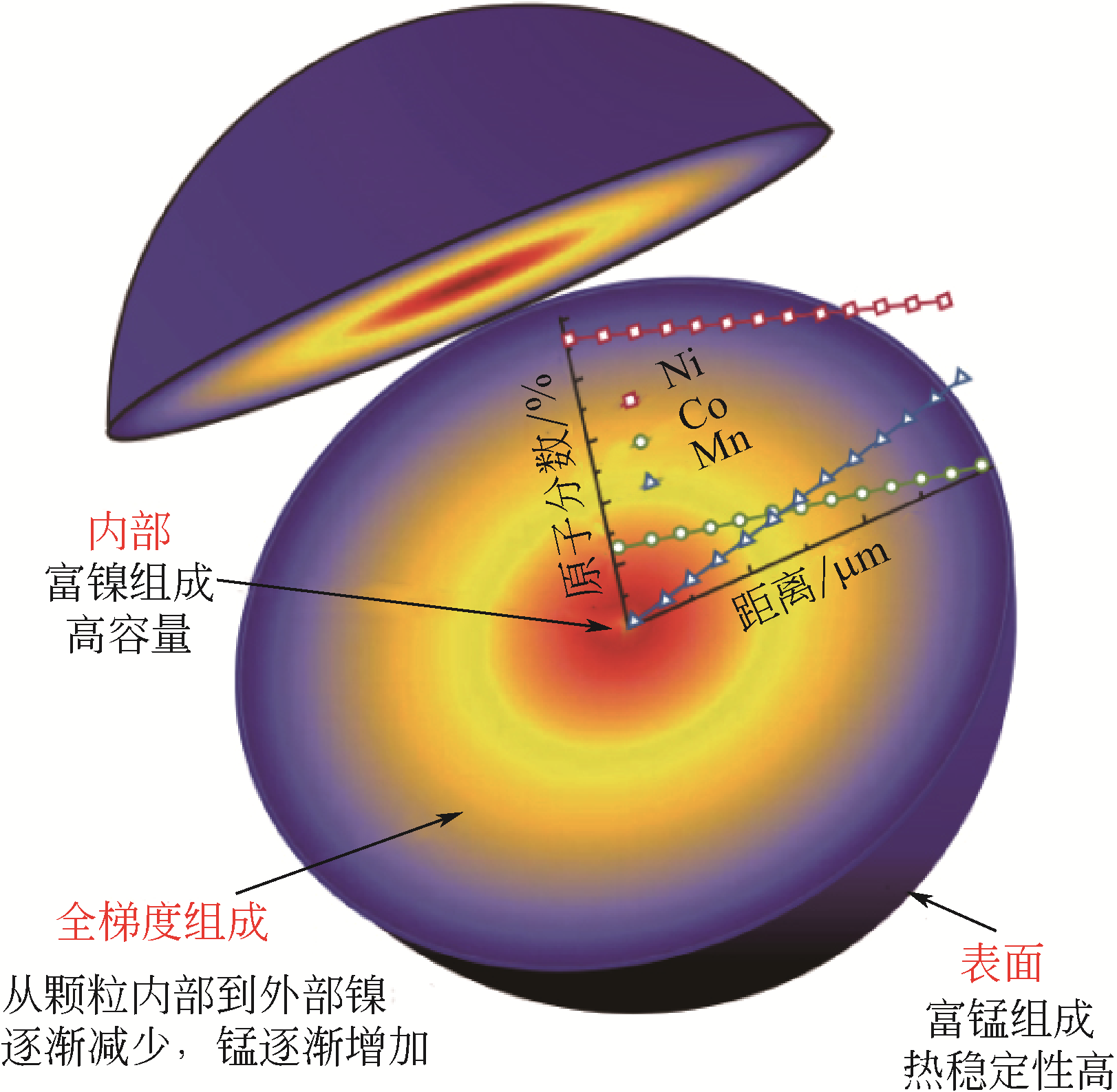
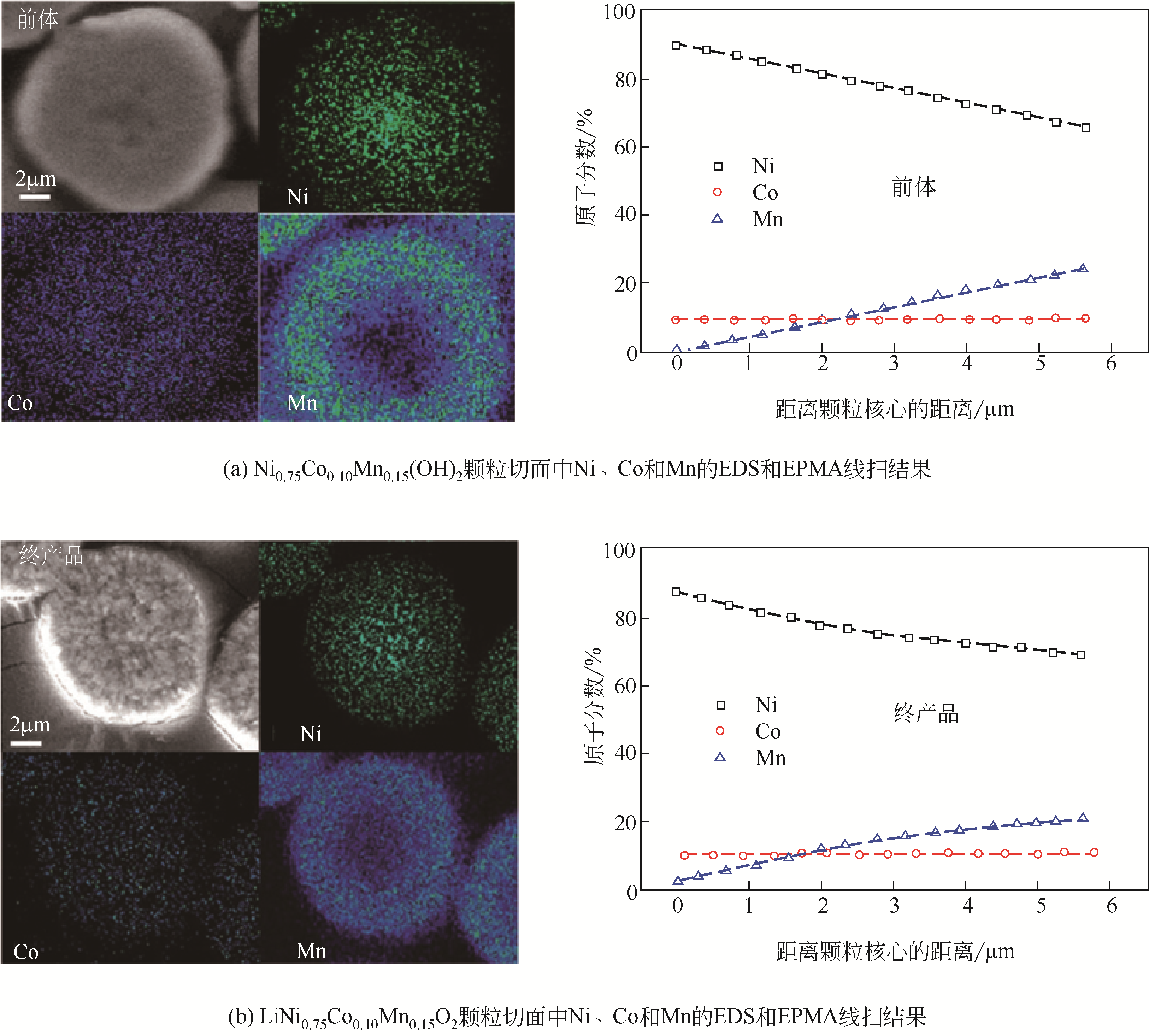
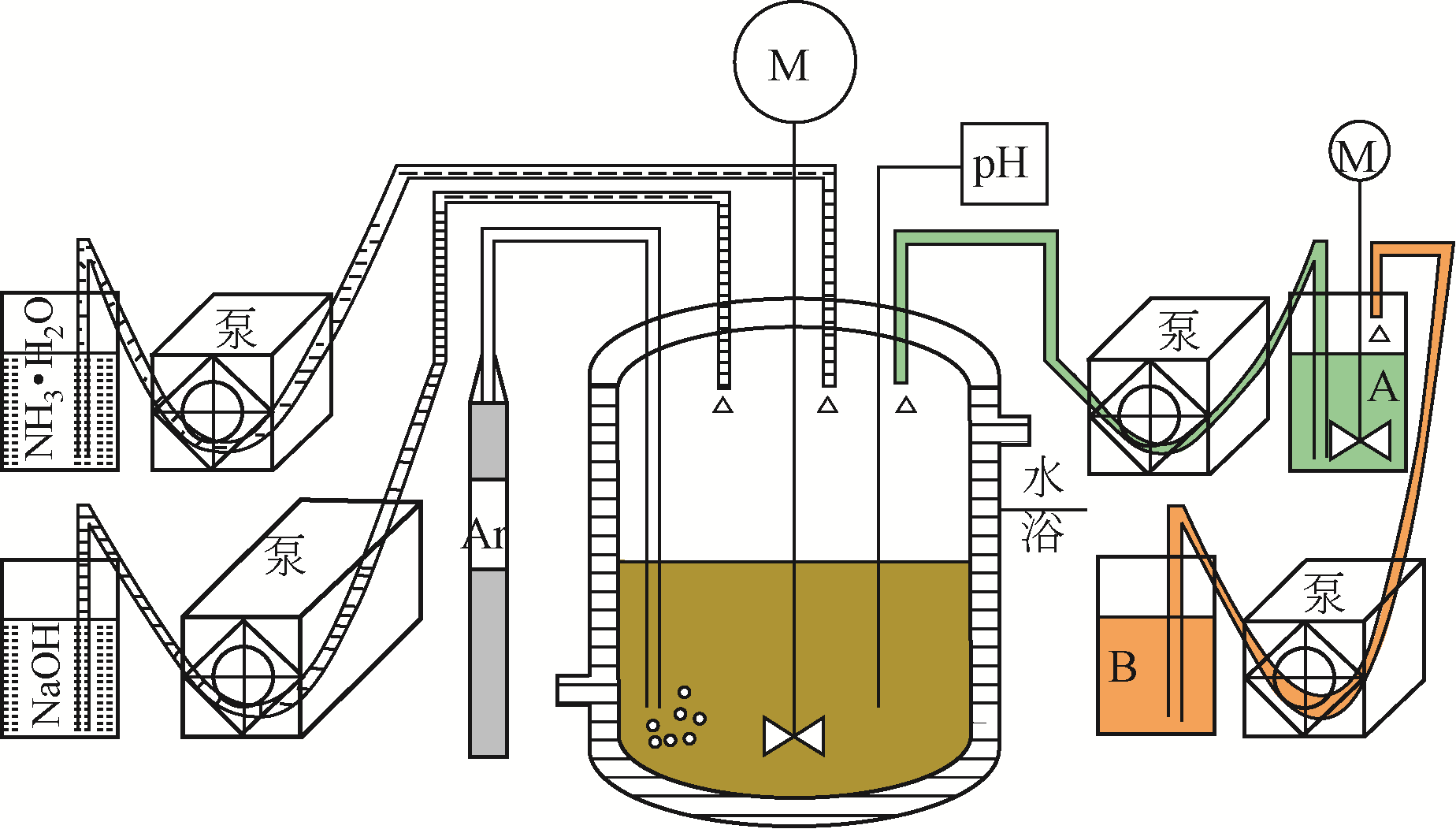
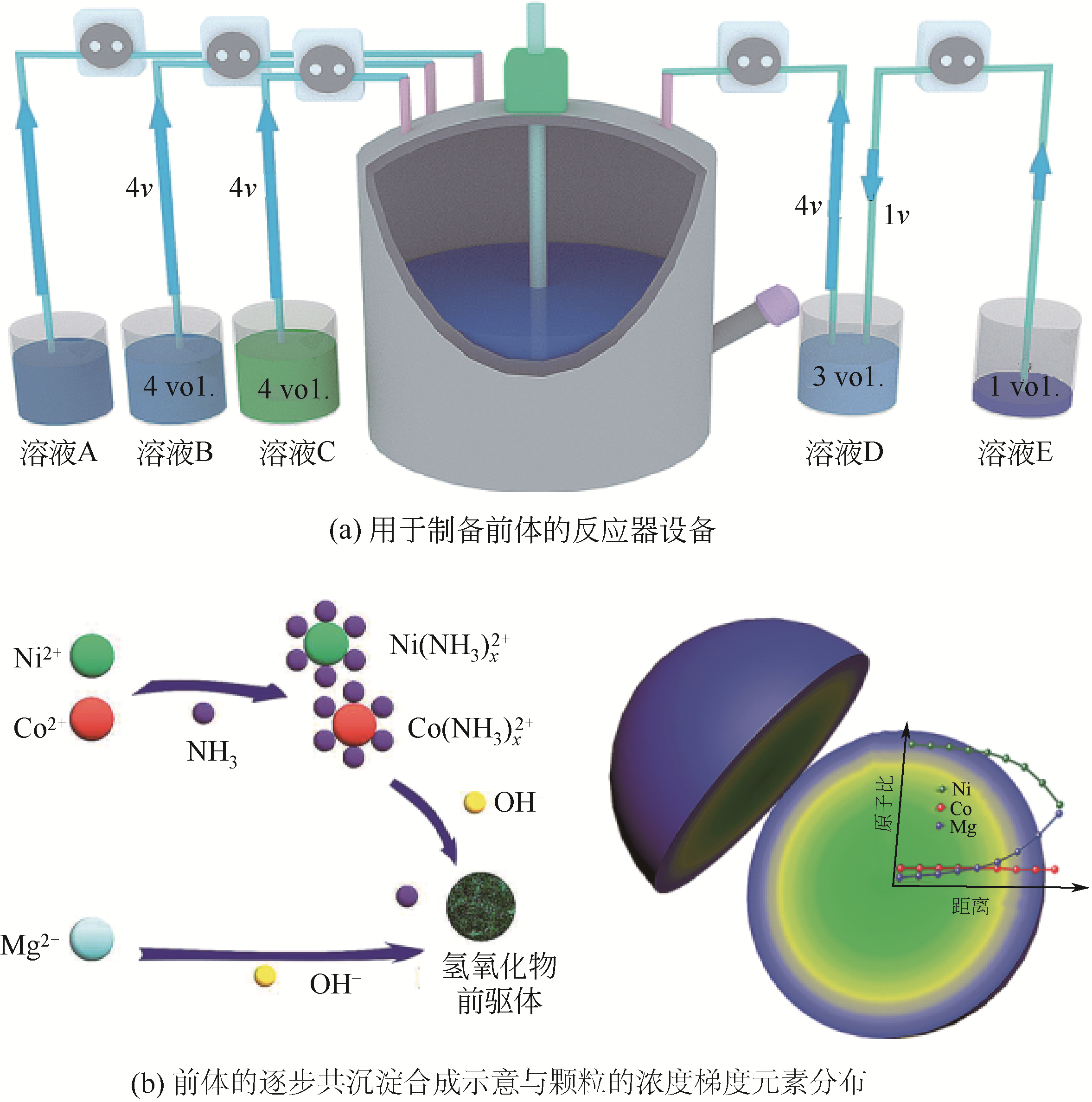
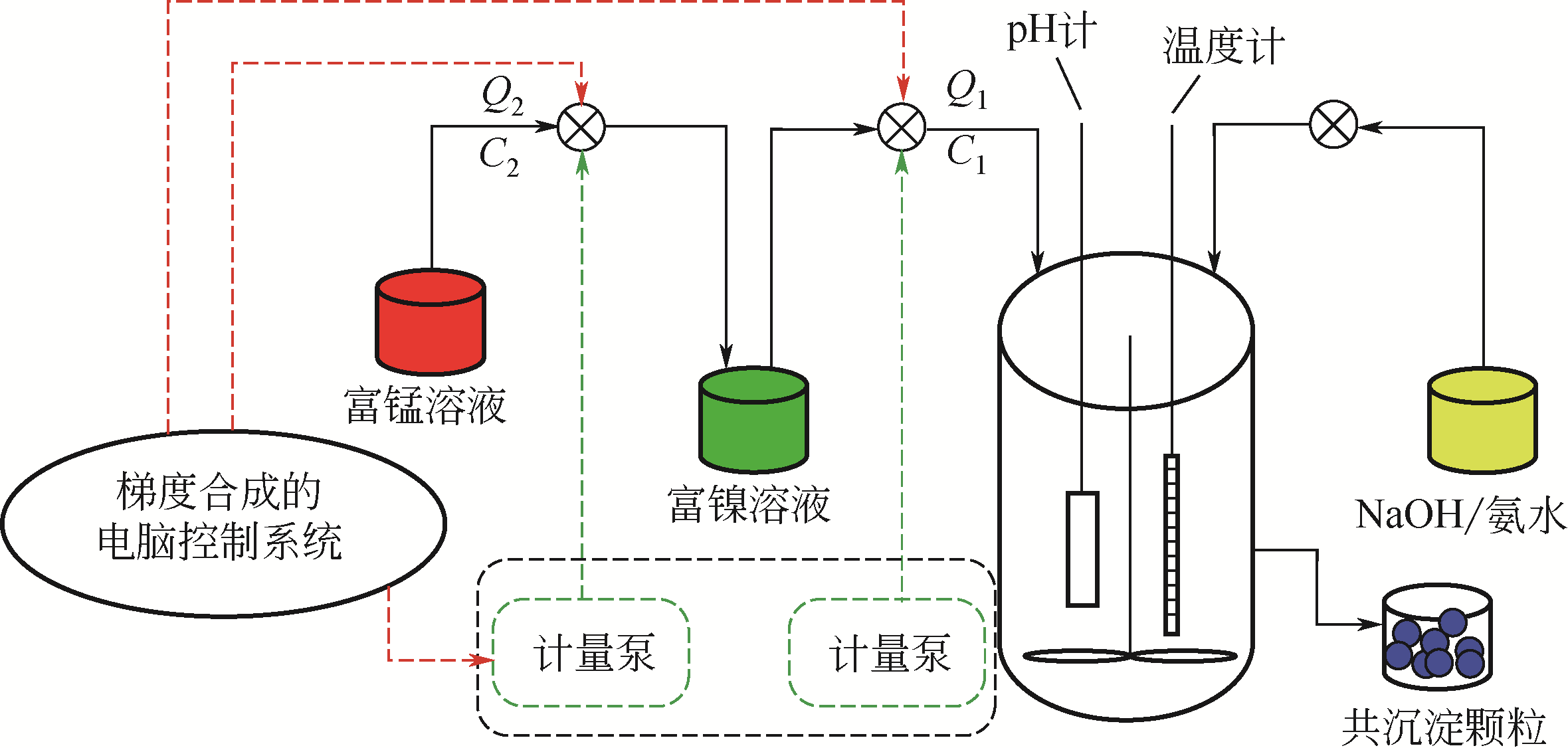
| 浓度梯度结构 | 共沉淀控制方式 | 梯度结构特点 | 优点 | 缺点 |
|---|---|---|---|---|
| 浓度梯度壳加富镍核 | 先用正常比例的富镍溶液制备富镍核,然后将贫镍富锰溶液通入富镍溶液继续反应制备浓度梯度壳 | 内部核无梯度,外部壳层Ni的浓度逐渐下降,Mn的浓度逐渐增加 | 核壳之间元素浓度可以形成一个过渡,不会因为核壳界面处的体积变化而导致核壳结构失配 | 终产品元素比例变化,不再是正常比例,且浓度梯度范围较小 |
| 线性浓度梯度 | 通过比例设计,将富锰含钴低镍的溶液连续泵入富镍含钴无锰的溶液中进行反应 | Ni的浓度从颗粒中心向外层持续线性下降,Mn的浓度持续线性增加,Co的浓度不变 | 能够利用富镍核的高能量密度和富锰外层的高稳定性 | 结构设计复杂,不利于减轻Li+嵌入和脱出过程中晶格变化引起的内应力 |
| 渐进式浓度梯度 | 将锰溶液连续泵入镍钴溶液中进行反应 | Ni和Co的浓度从颗粒中心向外层缓慢下降,Mn的浓度缓慢增加 | 能够利用富镍核的高能量密度和富锰外层的高稳定性,且可以显著缓解材料的内应力,有效提高循环后的机械稳定性 | 无 |
| 浓度梯度结构 | 共沉淀控制方式 | 梯度结构特点 | 优点 | 缺点 |
|---|---|---|---|---|
| 浓度梯度壳加富镍核 | 先用正常比例的富镍溶液制备富镍核,然后将贫镍富锰溶液通入富镍溶液继续反应制备浓度梯度壳 | 内部核无梯度,外部壳层Ni的浓度逐渐下降,Mn的浓度逐渐增加 | 核壳之间元素浓度可以形成一个过渡,不会因为核壳界面处的体积变化而导致核壳结构失配 | 终产品元素比例变化,不再是正常比例,且浓度梯度范围较小 |
| 线性浓度梯度 | 通过比例设计,将富锰含钴低镍的溶液连续泵入富镍含钴无锰的溶液中进行反应 | Ni的浓度从颗粒中心向外层持续线性下降,Mn的浓度持续线性增加,Co的浓度不变 | 能够利用富镍核的高能量密度和富锰外层的高稳定性 | 结构设计复杂,不利于减轻Li+嵌入和脱出过程中晶格变化引起的内应力 |
| 渐进式浓度梯度 | 将锰溶液连续泵入镍钴溶液中进行反应 | Ni和Co的浓度从颗粒中心向外层缓慢下降,Mn的浓度缓慢增加 | 能够利用富镍核的高能量密度和富锰外层的高稳定性,且可以显著缓解材料的内应力,有效提高循环后的机械稳定性 | 无 |
| 1 | KONISHI H, HIRANO T, TAKAMATSU D, et al. Improvement of electrochemical performance of nickel-manganese-based lithium-rich layer-structured cathode material by controlling lithium/transition-metal ratio[J]. Solid State Ionics, 2018, 327: 39-46. |
| 2 | 肖忠良, 周乘风, 宋刘斌, 等. 富镍锂离子电池三元材料NCM的研究进展[J]. 化工进展, 2020, 39(1): 216-223. |
| XIAO Zhongliang, ZHOU Chengfeng, SONG Liubin, et al. Research progress of ternary material NCM for nickel-rich lithium ion battery[J]. Chemical Industry and Engineering Progress, 2020, 39(1): 216-223. | |
| 3 | GUAN P Y, ZHOU L, YU Z L, et al. Recent progress of surface coating on cathode materials for high-performance lithium-ion batteries[J]. Journal of Energy Chemistry, 2020, 43: 220-235. |
| 4 | HRYU H H, PARK N Y, SEO J H, et al. A highly stabilized Ni-rich NCA cathode for high-energy lithium-ion batteries[J]. Materials Today, 2020, 36: 73-82. |
| 5 | 陈鹏, 肖冠, 廖世军. 具有不同组成的镍钴锰三元材料的最新研究进展[J]. 化工进展, 2016, 35(1): 166-174. |
| CHEN Peng, XIAO Guan, LIAO Shijun. Recent progress in the cobalt-nickel-manganese ternary cathode materials with various proportions of nickel to cobalt and manganese[J]. Chemical Industry and Engineering Progress, 2016, 35(1): 166-174. | |
| 6 | MYUNG S T, MAGLIA F, PARK K J, et al. Nickel-rich layered cathode materials for automotive lithium-ion batteries: achievements and perspectives[J]. ACS Energy Lett., 2017, 2: 196-223. |
| 7 | JIANG Y, BI Y, LIU M. Improved stability of Ni-rich cathode by the substitutive cations with stronger bonds[J]. Electrochim. Acta, 2018, 268: 41-48. |
| 8 | SUN S, LIU T, NIU Q, et al. Improvement of superior cycle performance of LiNi0.8Co0.15Al0.05O2 cathode for lithium-ion batteries by multiple compound modifications[J]. J. Electroanal. Chem., 2019, 838: 178-185. |
| 9 | LI W, LIU X, CELIO H, et al. Mn versus Al in layered oxide cathodes in lithium-ion batteries: a comprehensive evaluation on long-term cyclability[J]. Adv. Energy Mater., 2018, 8: 1703154. |
| 10 | LEE K S, MYUNG S T, AMINE K, et al. Structural and electrochemical properties of layered Li[Ni1-2xCoxMnx]O2 (x=0.1-0.3) positive electrode materials for Li-ion batteries[J]. Journal of the Electrochemical Society, 2007, 154: A971. |
| 11 | NOH H J, YOUN S J, YOON C S, et al. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x=1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries[J]. Journal of Power Sources, 2013, 233: 121-130. |
| 12 | YOON C S, CHOI M H, LIM B B, et al. Effect of lithium in transition metal layers of Ni-rich cathode materials on electrochemical properties[J]. Journal of the Electrochemical Society, 2015, 162(12): A2313-A2318. |
| 13 | PARK K J, JUNG H G, KUO L Y, et al. Improved cycling stability of Li[Ni0.90Co0.05Mn0.05]O2 through microstructure modification by boron doping for Li-ion batteries[J]. Advanced Energy Materials, 2018, 8(25): 1801202. |
| 14 | MA X L, WANG C W, SUN J T, et al. Effects of Sn doping on the structural and electrochemical properties of LiNi0.8Co0.2O2 cathode materials[J]. Solid State Ionics, 2007, 178: 125-129. |
| 15 | WOO S W, MYUNG S T, BANG H, et al. Improvement of electrochemical and thermal properties of Li[Ni0.8Co0.1Mn0.1]O2 positive electrode materials by multiple metal (Al, Mg) substitution[J]. Electrochim. Acta, 2009, 54: 3851-3856. |
| 16 | WU K,JIA G F, SHANGGUAN X H,et al. Improved high rate performance and cycle stability for LiNi0.8Co0.2O2 by doping of the high valence stateion Nb5+ into Li+ sites[J]. Journal of Alloys and Compounds, 2018, 765: 700-709. |
| 17 | LAI Y Q, WU J, TANG Y W, et al. Alleviating the air sensitivity of nickel-rich LiNi0.815Co0.15Al0.035O2 cathode by Zr4+-modification for Li-ion batteries[J]. Ceramics International, 2019, 45: 14270-14277. |
| 18 | LIU Q, ZHAO Z K, WU F, et al. The effects of molybdenum doping on LiNi0.6Co0.2Mn0.2O2 cathode material[J]. Solid State Ionics, 2019, 337: 107-114. |
| 19 | CHEN Y P, ZHANG Y, WANG F, et al. Improve the structure and electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode material by nano-Al2O3 ultrasonic coating[J]. Journal of Alloys and Compounds, 2014, 611: 135-141. |
| 20 | SUN H L, ZHANG Y F, LI W, et al. Effects of Ag coating on the structural and electrochemical properties of LiNi0.8Co0.1Mn0.1O2 as cathode material for lithium ion batteries[J]. Electrochimica Acta, 2019, 32: 135054. |
| 21 | YAO L, LIANG F Q, JIN J, et al. Improved electrochemical property of Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode viain-situ ZrO2 coating for high energy density lithium ion batteries[J]. Chemical Engineering Journal, 2020, 389: 124403. |
| 22 | YUAN H, SONG W B, WANG M, et al. Lithium-ion conductive coating layer on nickel rich layered oxide cathode material with improved electrochemical properties for Li-ion battery[J]. Journal of Alloys and Compounds, 2019, 784: 1311-1322. |
| 23 | QI X Y, XUE Z C, DU K, et al. Graphene-analogous structural MoS2 modification to improve electrochemical properties of Ni-rich layered oxide cathode material for lithium-ion batteries[J]. Journal of Power Sources, 2018, 397: 288-295. |
| 24 | XIE Z C, ZHANG Y Y, YUAN A B, et al. Effects of lithium excess and SnO2 surface coating on the electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode material for Li-ion batteries[J]. Journal of Alloys and Compounds, 2019, 787: 429-439 |
| 25 | JO C H, CHO D H, NOH H J, et al. An effective method to reduce residual lithium compounds on Ni-rich Li[Ni0.6Co0.2Mn0.2]O2 active material using a phosphoric acid derived Li3PO4 nanolayer[J]. Nano Research, 2015, 8: 1464-1479. |
| 26 | LIU W, OH P, LIU X, et al. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries[J]. Angewandte Chemie: International Edition, 2015, 54: 4440-4457. |
| 27 | MYUNG S T, MAGLIA F, PARK K J, et al. Nickel-rich layered cathode materials for automotive lithium-ion batteries: achievements and perspectives[J]. ACS Energy Lett., 2016, 2(1): 196-223. |
| 28 | YAN P F, ZHENG J M, LIU J, et al. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries[J]. Nat. Energy, 2018, 3: 600-605. |
| 29 | QIAN G N,ZHANG Y T, LI L S, et al. Single-crystal nickel-rich layered-oxide battery cathode materials: synthesis, electrochemistry, and intra-granular fracture[J]. Energy Storage Materials, 2020, 27: 140-149. |
| 30 | WANG L, WU B R, MU D B, et al. Single-crystal LiNi0.6Co0.2Mn0.2O2 as high performance cathode materials for Li-ion batteries[J]. Journal of Alloys and Compounds, 2016, 674: 360-367. |
| 31 | LI H Y, LI J, ZAKER N, et al. Synthesis of single crystal LiNi0.88Co0.09Al0.03O2 with a two-step lithiation method[J]. Journal of the Electrochemical Society, 2019, 166: 1956-1963. |
| 32 | SUN Y K, MYUNG S T, KIM M H, et al. Synthesis and characterization of Li[(Ni0.8Co0.1Mn0.1)0.8(Ni0.5Mn0.5)0.2]O2 with the microscale core-shell structure as the positive electrode material for lithium batteries[J]. Journal of the American Chemical Society, 2005, 127: 13411-13418. |
| 33 | SUN Y K, MYUNG S T, SHIN H S, et al. Novel core-shell structured Li[(Ni0.8Co0.1Mn0.1)0.8(Ni0.5Mn0.5)0.2]O2via coprecipitation as positive electrode material for lithium secondary batteries[J]. The Journal of Physical Chemistry B, 2006, 110: 6810-6815. |
| 34 | MAENG S, CHUNG Y, MIN S, et al. Enhanced mechanical strength and electrochemical performance of core-shell structured high-nickel cathode material[J]. Journal of Power Sources, 2020, 448: 227395. |
| 35 | LI Y, XU R, REN Y, et al. Synthesis of full concentration gradient cathode studied by high energy X-ray diffraction[J]. Nano Energy, 2016, 19: 522-531. |
| 36 | SUN Y K, KIM D H, JUNG H G, et al. High-voltage performance of concentration-gradient Li[Ni0.67Co0.15Mn0.18]O2 cathode material for lithium-ion batteries[J]. Electrochimica Acta, 2010, 55: 8621-8627. |
| 37 | SUN Y K, KIM D H, YOON C S, et al. A novel cathode material with a concentration-gradient for high-energy and safe lithium-ion batteries[J]. Advanced Functional Materials, 2010, 20: 485-491. |
| 38 | SUN Y K, LEE B B, NOH H J, et al. A novel concentration-gradient Li[Ni0.83Co0.07Mn0.10]O2 cathode material for high-energy lithium-ion batteries[J]. Journal of Materials Chemistry, 2011, 21: 10108-10112. |
| 39 | SUN Y K, CHEN Z, NOH H J, et al. Nanostructured high-energy cathode materials for advanced lithium batteries[J]. Nature Materials, 2012, 11: 942-947. |
| 40 | XU X, XIANG L Z, WANG L G, et al. Progressive concentration gradient nickel-rich oxide cathode material for high-energy and long-life lithium-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7: 7728-7735. |
| 41 | JIANG Y P, LIU Z H, ZHANG Y Z, et al. Full-gradient structured LiNi0.8Co0.1Mn0.1O2 cathode material with improved rate and cycle performance for lithium ion batteries[J]. Electrochimica Acta, 2019, 309: 74-85. |
| 42 | SUN Z, WANG D, FAN Y, et al. Improved performances of a LiNi0.6Co0.15Mn0.25O2 cathode material with full concentration-gradient for lithium ion batteries[J]. RSC Advances, 2016, 6: 103747-103753. |
| 43 | PARK K, CHOI M, MALIA F, et al. High-capacity concentration gradient Li[Ni0.865Co0.120Al0.015]O2 cathode for lithium-ion batteries[J]. Advanced Energy Materials, 2018, 8: 1703612. |
| 44 | ZHANG L P, DONG T, YU X J, et al. Synthesis and characterization of concentration-gradient LiNi0.6Co0.2Mn0.2O2 cathode material for lithium ion batteries[J]. Journalof Alloys and Compounds, 2014, 613: 296-305. |
| 45 | ZHANG Y D, LI H, LIU J X, et al. LiNi0.90Co0.07Mg0.03O2 cathode materials with Mg-concentration gradient for rechargeable lithium-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7: 20958. |
| 46 | 罗熳, 蒋文全, 韩雪, 等. 锂离子电池浓度梯度正极材料LiNi0.63Co0.055Mn0.302O2的合成和表征[J]. 高等学校化学学报, 2017, 39: 148-156. |
| LUO Man, JIANG Wenquan, HAN Xue, et al. Synthesis and characterization of full concentration-gradient LiNi0.643Co0.055Mn0.302O2 cathode material for lithium-ion batteries[J]. Chemical Journal of Chinese Universities, 2017, 39: 148-156. |
| [1] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [2] | WANG Jiaqing, SONG Guangwei, LI Qiang, GUO Shuaicheng, DAI Qingli. Rubber-concrete interface modification method and performance enhancement path [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 328-343. |
| [3] | ZHU Jie, JIN Jing, DING Zhenghao, YANG Huipan, HOU Fengxiao. Modification of CaSO4 oxygen carrier by Zhundong coal ash in chemical looping gasification and its mechanism [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4628-4635. |
| [4] | WANG Jingang, ZHANG Jianbo, TANG Xuejiao, LIU Jinpeng, JU Meiting. Research progress on modification of Cu-SSZ-13 catalyst for denitration of automobile exhaust gas [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4636-4648. |
| [5] | LI Xuejia, LI Peng, LI Zhixia, JIN Dunshang, GUO Qiang, SONG Xufeng, SONG Peng, PENG Yuelian. Experimental comparation on anti-scaling and anti-wetting ability of hydrophilic and hydrophobic modified membranes [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4458-4464. |
| [6] | CHEN Junjun, FEI Chang’en, DUAN Jintang, GU Xueping, FENG Lianfang, ZHANG Cailiang. Research progress on chemical modification of polyether ether ketone for the high bioactivity [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4015-4028. |
| [7] | TAN Lipeng, SHEN Jun, WANG Yugao, LIU Gang, XU Qingbai. Research progress on blending modification of coal tar pitch and petroleum asphalt [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3749-3759. |
| [8] | YIN Chengyang, HOU Ming, YANG Shuang, MAO Di, LIU Junyan. Research progress in transition metals modified Cu-SSZ-13 zeolite denitration catalysts [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2963-2974. |
| [9] | CHEN Mingxing, WANG Xinya, ZHANG Wei, XIAO Changfa. Development of thermally stable fiber-based air filter materials [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2439-2453. |
| [10] | YU Jie, ZHANG Wenlong. Development status and progress of lithium ion battery separator [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1760-1768. |
| [11] | YE Haixing, CHEN Yuhao, CHEN Yi, SUN Haixiang, NIU Qingshan. Research progress of composite nanofiltration membrane for magnesium and lithium separation [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1934-1943. |
| [12] | FAN Sihan, YU Guoxi, LAI Chaochao, HE Huan, HUANG Bin, PAN Xuejun. Effect of abiotic modification on photochemical activity of anaerobic microbial products [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2180-2189. |
| [13] | ZHAO Chongyang, ZHAO Lei, SHI Xiangwen, HUANG Jun, LI Zhiyao, SHEN Kai, ZHANG Yaping. Effect of O2/H2O/SO2 on the adsorption of PbCl2 by modified iron-rich attapulgite at high temperature [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2190-2200. |
| [14] | YANG Maofei, LI Jinwang, ZHOU Liuwei. Heat transfer performance of hydrophilic modified ultra-thin flat heat pipe [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 692-698. |
| [15] | HOU Limin, XU Jie, FU Shancong, WU Wenfei. Effect of Cu modification on NH3-SCR denitration of rare earth tailings catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 765-773. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||