Chemical Industry and Engineering Progress ›› 2020, Vol. 39 ›› Issue (4): 1414-1421.DOI: 10.16085/j.issn.1000-6613.2019-1232
• Materials science and technology • Previous Articles Next Articles
Preparation of silver doped Ti/PbO2 electrode and its application in phenol degradation
Junze ZHANG( ),Hongning WANG,Ruoyu CHEN(
),Hongning WANG,Ruoyu CHEN( )
)
- College of Petrochemical Engineering, Changzhou University, Changzhou 213164, Jiangsu, China
-
Received:2019-07-31Online:2020-04-28Published:2020-04-05 -
Contact:Ruoyu CHEN
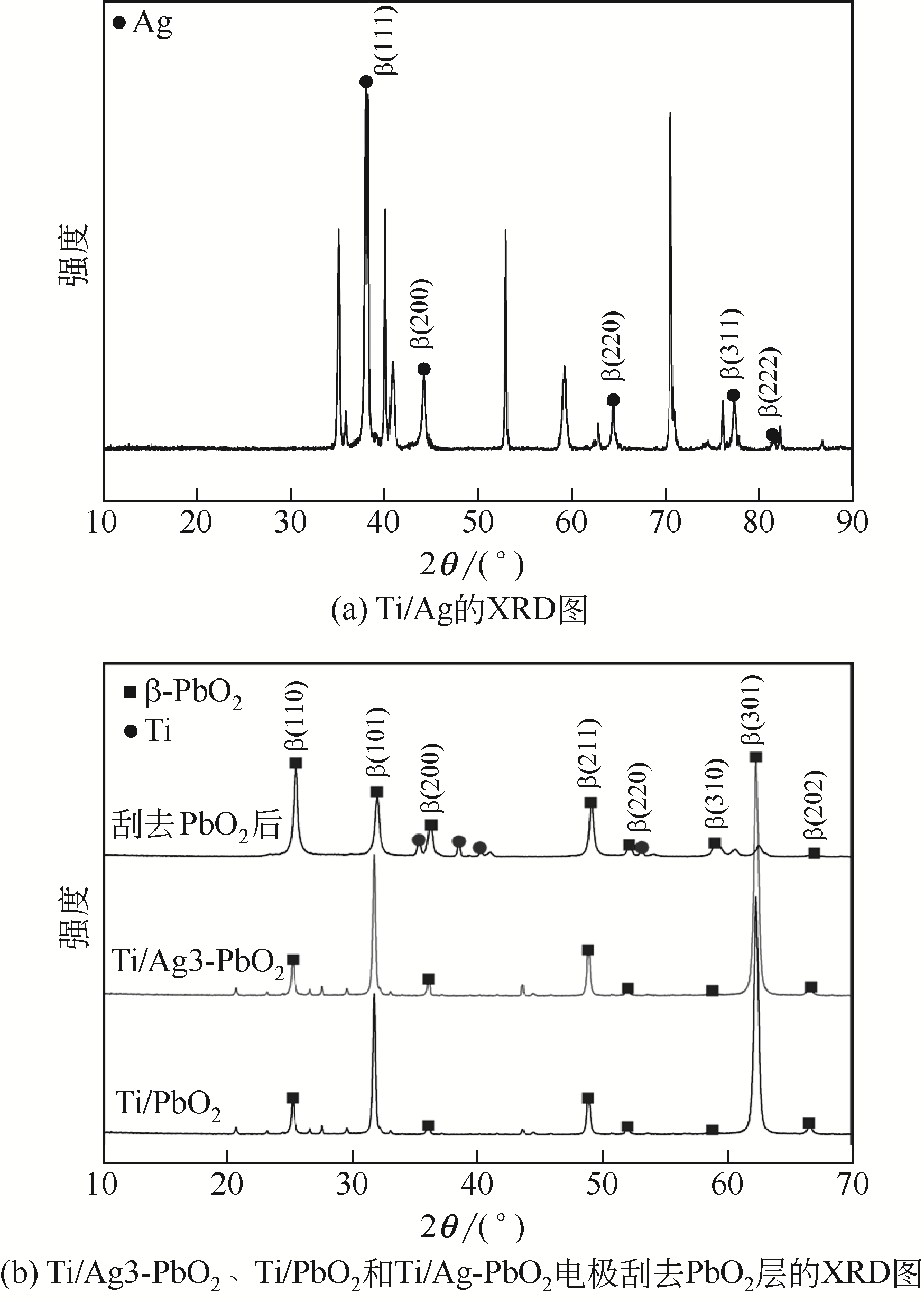
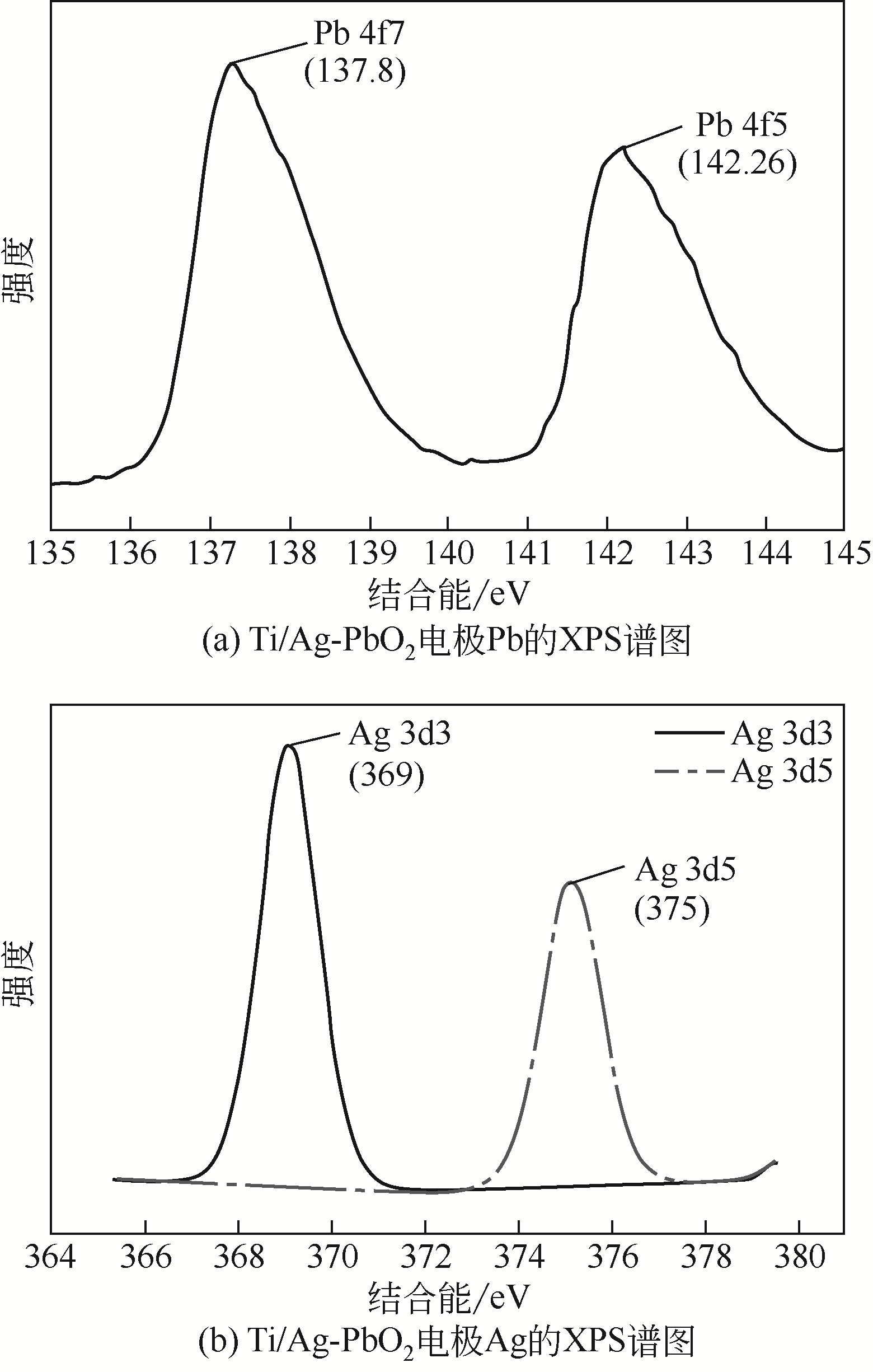
Ag掺杂Ti/PbO2电极的制备及其在苯酚降解中的应用
- 常州大学石油化工学院,江苏 常州 213164
-
通讯作者:陈若愚 -
作者简介:张君泽(1994—),男,硕士研究生,研究方向为无机材料。E-mail:1982346715@qq.com 。
CLC Number:
Cite this article
Junze ZHANG,Hongning WANG,Ruoyu CHEN. Preparation of silver doped Ti/PbO2 electrode and its application in phenol degradation[J]. Chemical Industry and Engineering Progress, 2020, 39(4): 1414-1421.
张君泽,王红宁,陈若愚. Ag掺杂Ti/PbO2电极的制备及其在苯酚降解中的应用[J]. 化工进展, 2020, 39(4): 1414-1421.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2019-1232
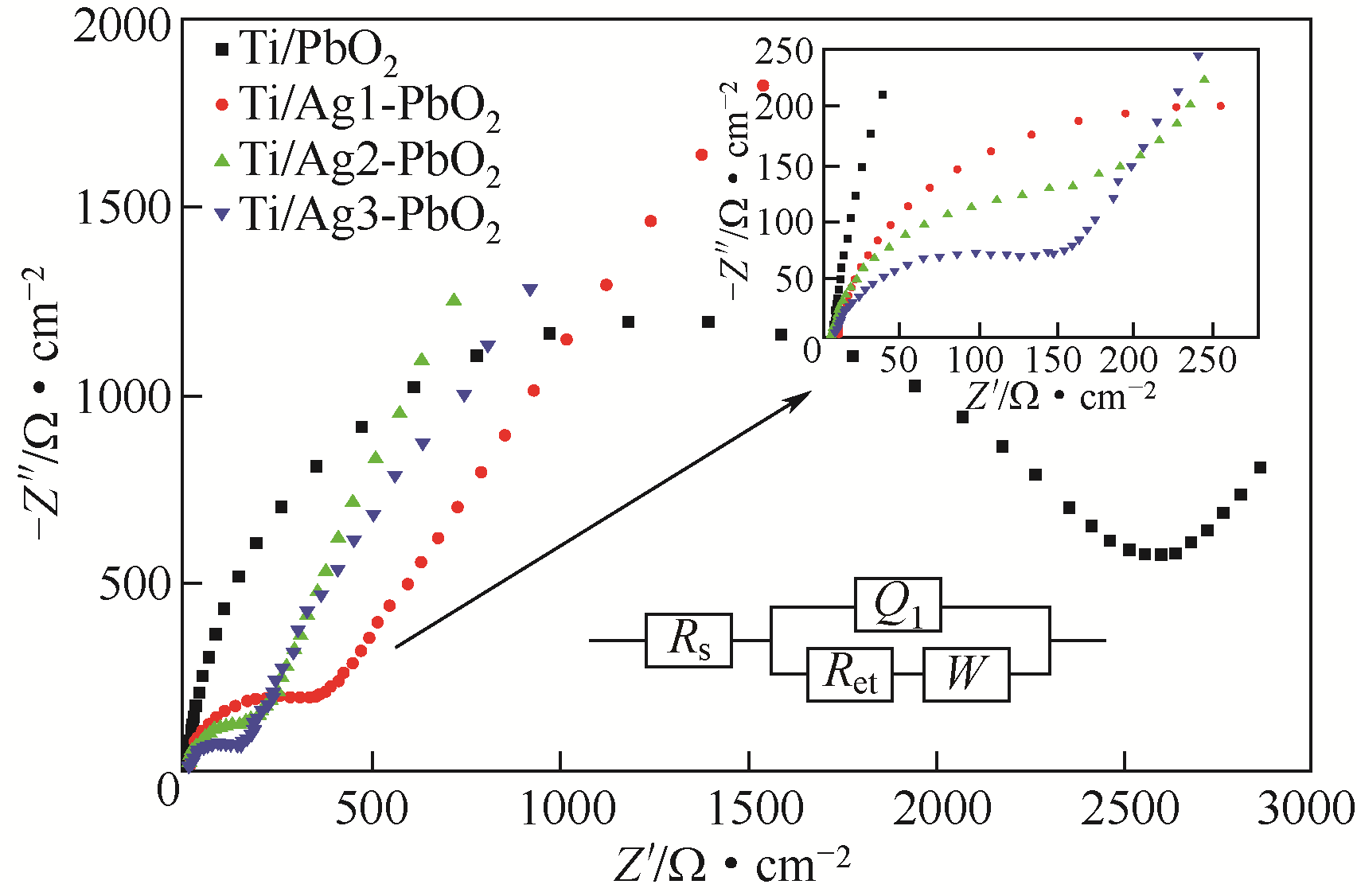
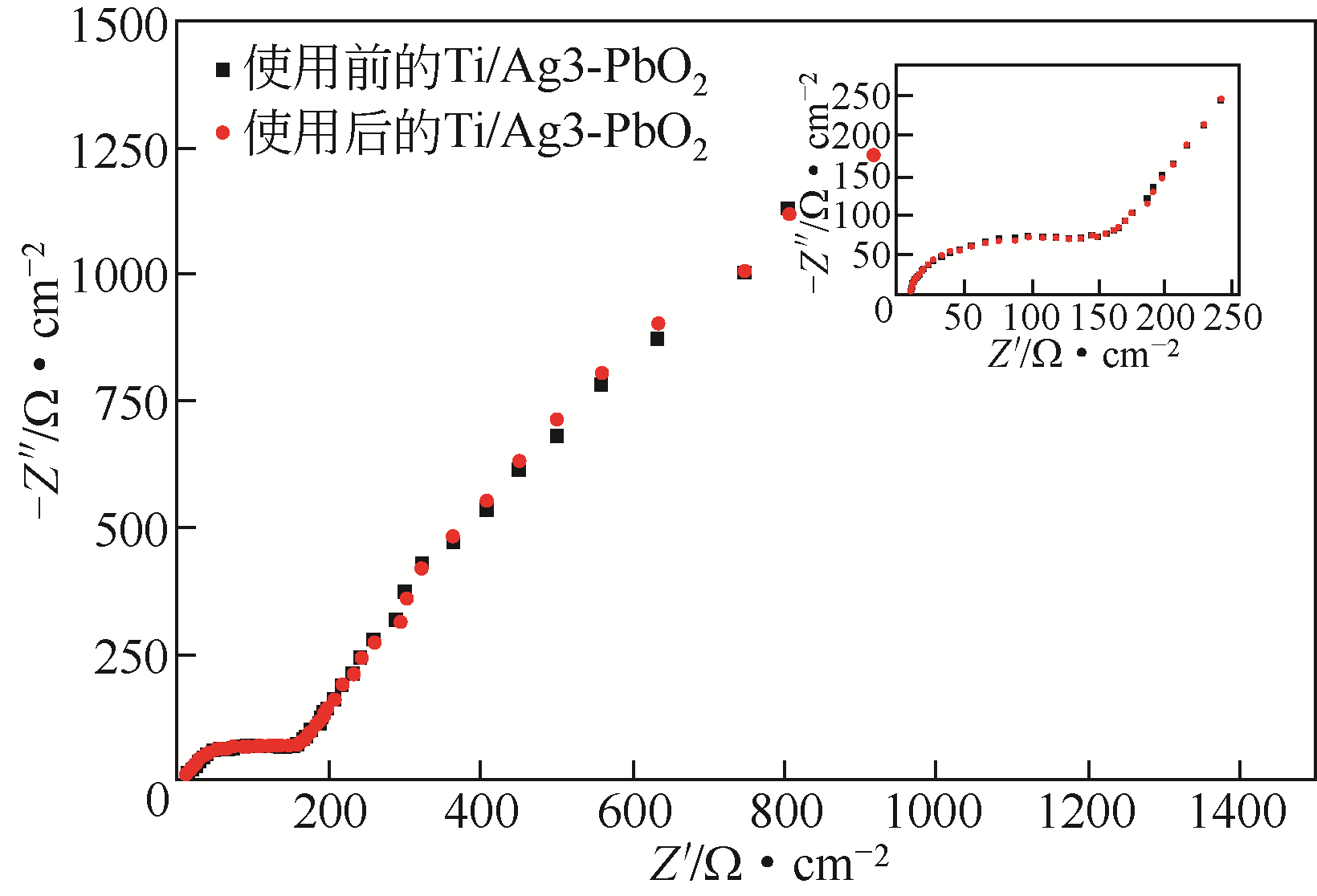
| 电极名称 | Rs/Ω·cm-2 | Rct/Ω·cm-2 |
|---|---|---|
| Ti/PbO2 | 5.754 | 2437 |
| Ti/Ag1-PbO2 | 5.037 | 246.6 |
| Ti/Ag2-PbO2 | 4.482 | 79.41 |
| Ti/Ag3-PbO2 | 4.465 | 79.25 |
| 电极名称 | Rs/Ω·cm-2 | Rct/Ω·cm-2 |
|---|---|---|
| Ti/PbO2 | 5.754 | 2437 |
| Ti/Ag1-PbO2 | 5.037 | 246.6 |
| Ti/Ag2-PbO2 | 4.482 | 79.41 |
| Ti/Ag3-PbO2 | 4.465 | 79.25 |
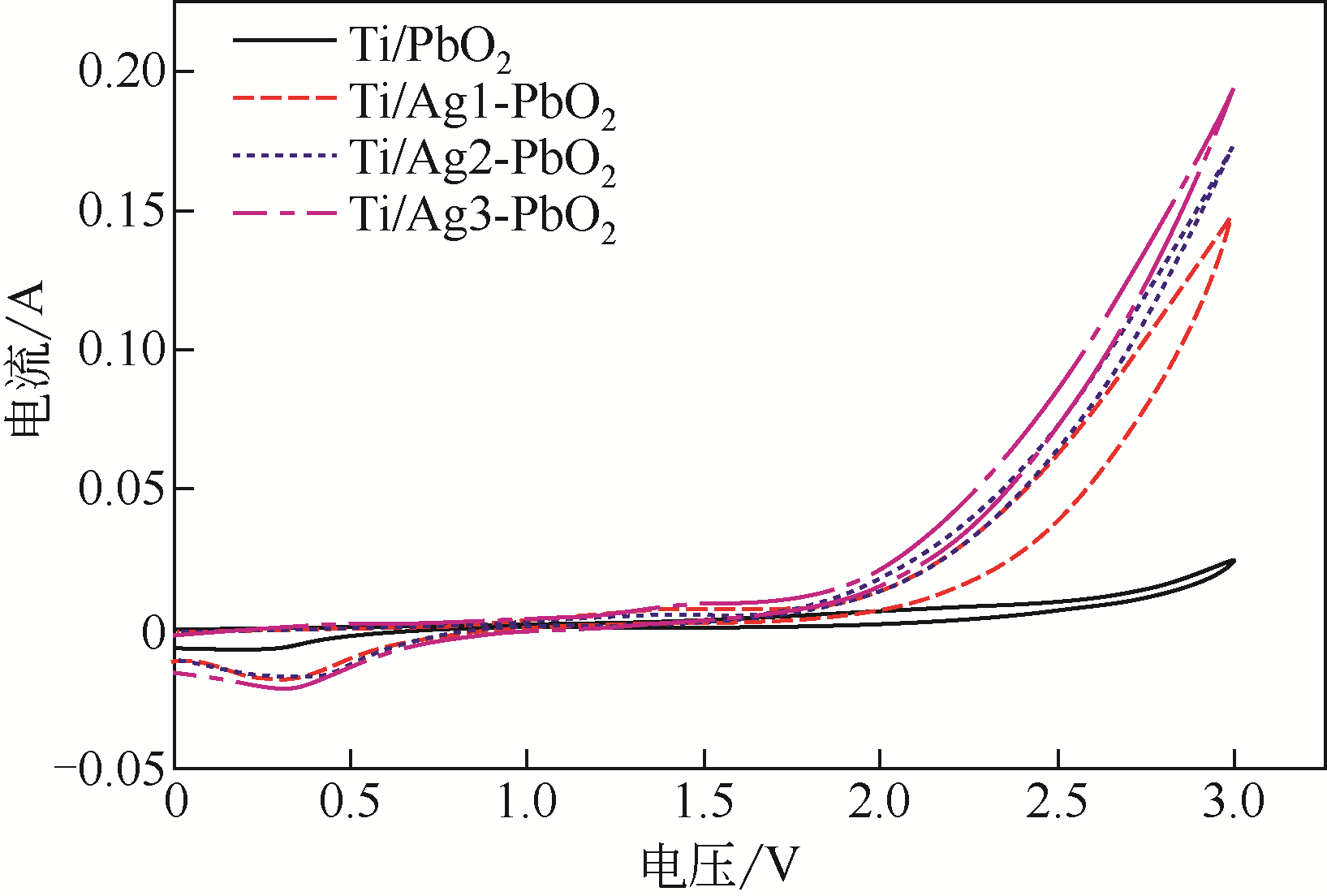
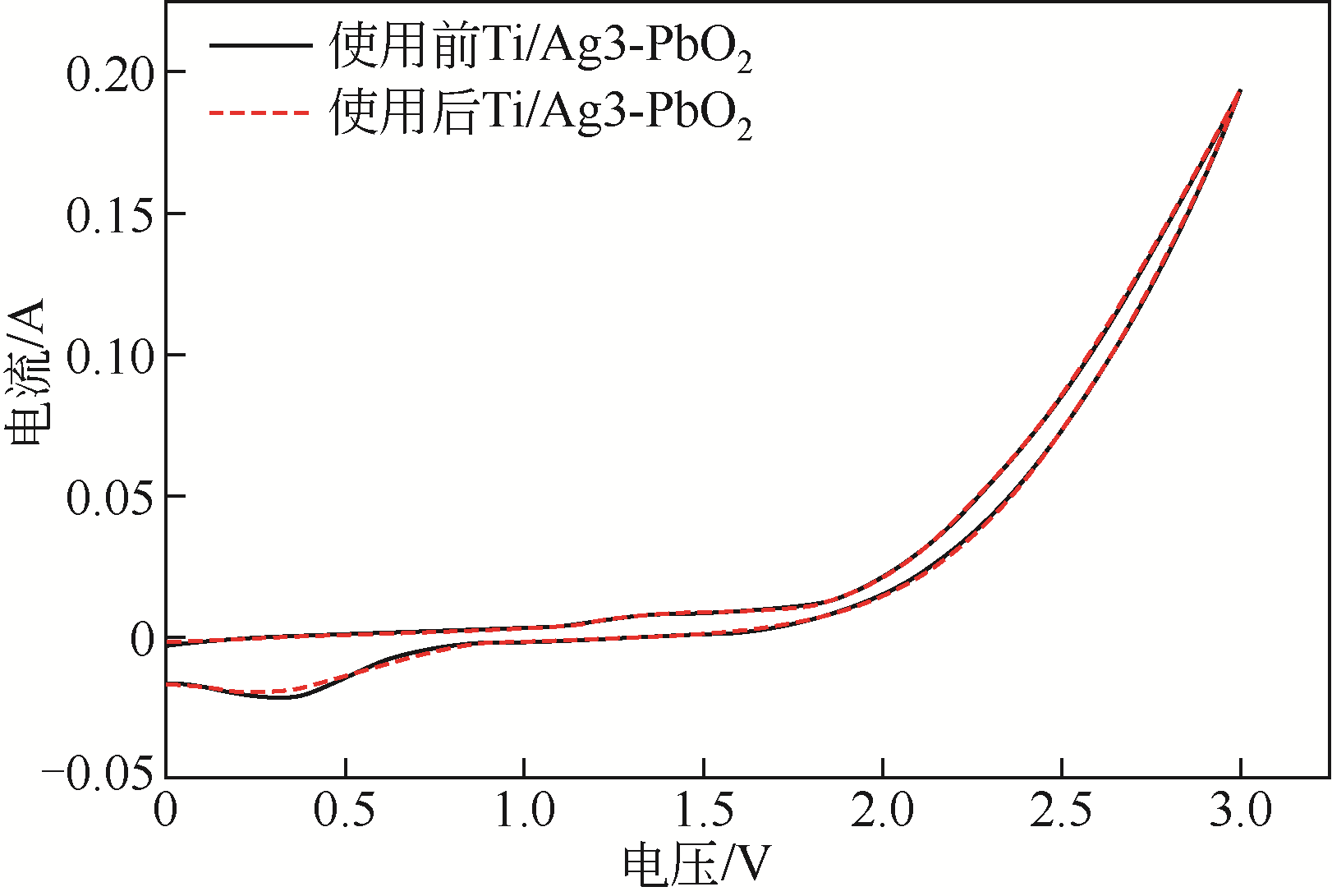
| 电极名称 | 降解电压/V | 能耗/W·h·L-1 | 节约能耗/% |
|---|---|---|---|
| Ti/PbO2 | 6.85 | 12.33 | |
| Ti/Ag1-PbO2 | 4.80 | 8.64 | 29.93 |
| Ti/Ag2-PbO2 | 4.60 | 8.28 | 32.85 |
| Ti/Ag3-PbO2 | 4.51 | 8.12 | 34.16 |
| 电极名称 | 降解电压/V | 能耗/W·h·L-1 | 节约能耗/% |
|---|---|---|---|
| Ti/PbO2 | 6.85 | 12.33 | |
| Ti/Ag1-PbO2 | 4.80 | 8.64 | 29.93 |
| Ti/Ag2-PbO2 | 4.60 | 8.28 | 32.85 |
| Ti/Ag3-PbO2 | 4.51 | 8.12 | 34.16 |
| 1 | SHMYCHKOVA O, LUK’YANENKO T, VELICHENKO A, et al. Bi-doped PbO2 anodes: electrodeposition and physico-chemical properties[J]. Electrochimica Acta, 2013, 111: 332-338. |
| 2 | YANG X, ZOU R, HUO F, et al. Preparation and characterization of Ti/SnO2-Sb2O3-Nb2O5/PbO2 thin film as electrode material for the degradation of phenol[J]. Journal of Hazardous Materials, 2009, 164(1): 367-373. |
| 3 | ABACI S, TAMER U, PEKMEZ K, et al. Performance of different crystal structures of PbO2 on electrochemical degradation of phenol in aqueous solution[J]. Applied Surface Science, 2005, 240(1): 112-119. |
| 4 | KONG J, SHI S, KONG L, et al. Preparation and characterization of PbO2 electrodes doped with different rare earth oxides[J]. Electrochimica Acta, 2008, 53(4): 2048-2054. |
| 5 | 王雅琼, 顾彬, 许文林,等. 钛基PbO2电极上苯酚的电化学氧化[J]. 稀有金属材料与工程, 2007, 36(5): 874-878. |
| WANG Yaqiong, GU Bin, XU Wenlin, et al. Electrochemical oxidation of phenol on Ti-based PbO2 electrode[J]. Rare Metal Materials and Engineering, 2007, 36(5): 874-878. | |
| 6 | UEDA M, WATANABE A, SHIMAMUNE T. Lead oxide-coated electrode for use in electrolysis and process for producing the same: US4822459[P]. 1989-04-18. |
| 7 | UEDA M, WATANABE A, KAMEYAMA T, et al. Performance characteristics of a new type of lead dioxide-coated titanium anode[J]. Journal of Applied Electrochemistry, 1995, 25(9): 817-822. |
| 8 | 林祥钦, 张卫香. Mn2+在Ti/SnO2+SbOx/PbO2电极上的电氧化研究[J]. 中国科学技术大学学报, 2000, 30(1): 56-61. |
| LIN Xiangqin, ZHANG Weixiang. Electrooxidation of Mn2+ on Ti/SnO2+SbOx/PbO2 electrode[J]. Journal of University of Science and Technology of China, 2000, 30(1): 56-61. | |
| 9 | CONG Y, WU Z. Electrocatalytic generation of radical intermediates over lead dioxide electrode doped with fluoride[J]. The Journal of Physical Chemistry C, 2007, 111(8): 3442-3446. |
| 10 | AI S, GAO M, ZHANG W, et al. Preparation of fluorine-doped lead dioxide modified electrodes for electroanalytical applications[J]. Electroanalysis: An International Journal Devoted to Fundamental and Practical Aspects of Electroanalysis, 2003, 15(17): 1403-1409. |
| 11 | YEO I H, JOHNSON D C. Electrocatalysis of anodic oxygen-transfer reactions effect of groups ⅢA and ⅤA metal oxides in electrodeposited β-lead dioxide electrodes in acidic media[J]. Journal of The Electrochemical Society, 1987, 134(8): 1973-1977. |
| 12 | FENG J, JOHNSON D C. Electrocatalysis of anodic oxygen-transfer reactions Fe-doped beta-lead dioxide electrodeposited on noble metals[J]. Journal of The Electrochemical Society, 1990, 137(2): 507-510. |
| 13 | AI S, GAO M, ZHANG W, et al. Preparation of Ce-PbO2 modified electrode and its application in detection of anilines[J]. Talanta, 2004, 62(3): 445-450. |
| 14 | MUSIANI M, FURLANETTO F, BERTONCELLO R. Electrodeposited PbO2+RuO2: a composite anode for oxygen evolution from sulphuric acid solution[J]. Journal of Electroanalytical Chemistry, 1999, 465(2): 160-167. |
| 15 | BERTONCELLO R, CATTARIN S, FRATEUR I, et al. Preparation of anodes for oxygen evolution by electrodeposition of composite oxides of Pb and Ru on Ti[J]. Journal of Electroanalytical chemistry, 2000, 492(2): 145-149. |
| 16 | CATTARIN S, GUERRIERO P, MUSIANI M. Preparation of anodes for oxygen evolution by electrodeposition of composite Pb and Co oxides[J]. Electrochimica Acta, 2001, 46(26/27): 4229-4234. |
| 17 | VELICHENKO A B, AMADELLI R, BARANOVA E A, et al. Electrodeposition of Co-doped lead dioxide and its physicochemical properties[J]. Journal of Electroanalytical Chemistry, 2002, 527(1/2): 56-64. |
| 18 | 贺耀华, 刘俊, 王振霞, 等. 不锈钢化学镀银速率的研究[J]. 电镀与涂饰, 2010, 29(9): 22-24. |
| HE Yaohua, LIU Jun, WANG Zhenxia, et al. Study on the rate of electroless silver plating on stainless steel[J]. Electroplating & Finishing, 2010, 29(9): 22-24. | |
| 19 | SHAO D, LIANG J, CUI X, et al. Electrochemical oxidation of lignin by two typical electrodes: Ti/SbSnO2 and Ti/PbO2[J]. Chemical Engineering Journal, 2014, 244: 288-295. |
| 20 | CHEN A C, NIGRO S. Influence of a nanoscale gold thin layer on Ti/SnO2-Sb2O5 electrodes[J]. Journal of Physical Chemistry B, 2003, 107(48): 13341-13348. |
| 21 | INIESTA J, EXPOSITO E, GONZÁLEZ-GARCIA J, et al. Electrochemical treatment of industrial wastewater containing phenols[J]. Journal of the Electrochemical Society, 2002, 149(5): D57-D62. |
| 22 | 高平. 钛基 SnO2-Sb电极的制备及电催化氧化苯酚过程的研究[D]. 哈尔滨: 哈尔滨工业大学, 2012. |
| GAO Ping. Preparation of Ti-based SnO2-Sb electrode and its electrocatalytic oxidation of phenol[D]. Harbin: Harbin Institute of Technology, 2012. | |
| 23 | 胡树兵, 李志章, 崔山昆, 等. 用划痕法测定TiN涂层结合力的影响因素[J]. 湖北汽车工业学院学报, 2001, 15(2): 25-27. |
| HU Shubing, LI Zhizhang, CUI Shankun, et al. Factors affecting the bonding force of TiN coating by scratch method[J]. Journal of Hubei Automotive Industry Institute, 2001, 15(2): 25-27. | |
| 24 | 王雅琼. 含Sb-SnO2中间层的钛基金属氧化物电极的结构与性能研究[D]. 南京: 南京理工大学, 2009. |
| WANG Yaqiong. Structure and properties of Ti-based metal oxide electrode containing Sb-SnO2 intermediate layer[D]. Nanjing: Nanjing University of Science and Technology, 2009. |
| [1] | WANG Fu'an. Consumption and emission reduction of the reactor of 300kt/a propylene oxide process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 213-218. |
| [2] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [3] | HU Xi, WANG Mingshan, LI Enzhi, HUANG Siming, CHEN Junchen, GUO Bingshu, YU Bo, MA Zhiyuan, LI Xing. Research progress on preparation and sodium storage properties of tungsten disulfide composites [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 344-355. |
| [4] | ZHANG Jie, BAI Zhongbo, FENG Baoxin, PENG Xiaolin, REN Weiwei, ZHANG Jingli, LIU Eryong. Effect of PEG and its compound additives on post-treatment of electrolytic copper foils [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 374-381. |
| [5] | GAO Yufei, LU Jinfeng. Mechanism of heterogeneous catalytic ozone oxidation:A review [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 430-438. |
| [6] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [7] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [8] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [9] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [10] | LEI Wei, JIANG Weijia, WANG Yugao, HE Minghao, SHEN Jun. Synthesis of N,S co-doped coal-based carbon quantum dots by electrochemical oxidation and its application in Fe3+ detection [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4799-4807. |
| [11] | WANG Yaogang, HAN Zishan, GAO Jiachen, WANG Xinyu, LI Siqi, YANG Quanhong, WENG Zhe. Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4043-4057. |
| [12] | LYU Chengyuan, ZHANG Han, YANG Mingwang, DU Jianjun, FAN Jiangli. Recent advances of dioxetane-based afterglow system for bio-imaging [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4108-4122. |
| [13] | LIU Yi, FANG Qiang, ZHONG Dazhong, ZHAO Qiang, LI Jinping. Cu facets regulation of Ag/Cu coupled catalysts for electrocatalytic reduction of carbon dioxide [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4136-4142. |
| [14] | LI Runlei, WANG Ziyan, WANG Zhimiao, LI Fang, XUE Wei, ZHAO Xinqiang, WANG Yanji. Efficient catalytic performance of CuO-CeO2/TiO2 for CO oxidation at low-temperature [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4264-4274. |
| [15] | ZHANG Yajuan, XU Hui, HU Bei, SHI Xingwei. Preparation of NiCoP/rGO/NF electrocatalyst by eletroless plating for efficient hydrogen evolution reaction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4275-4282. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||