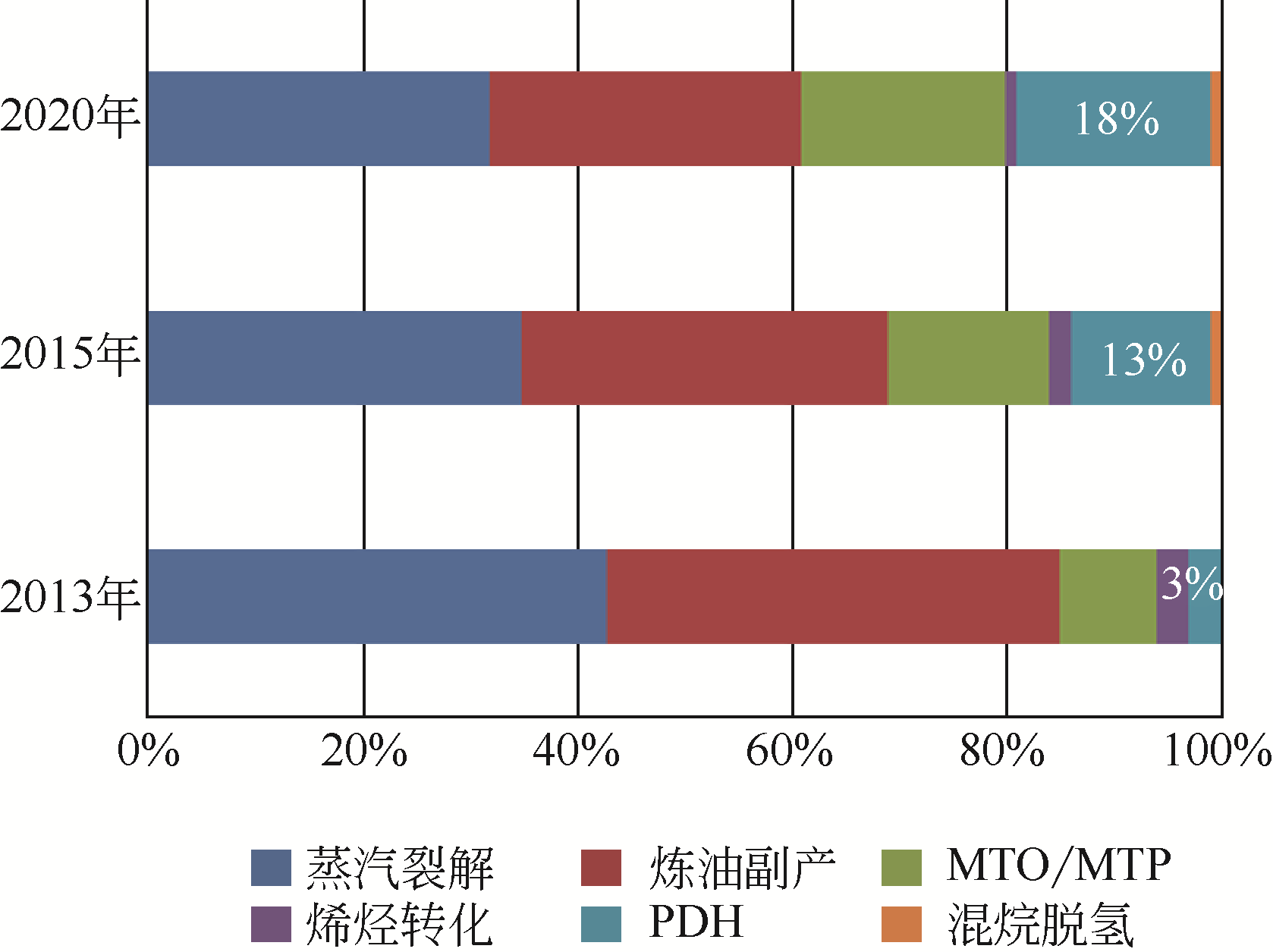
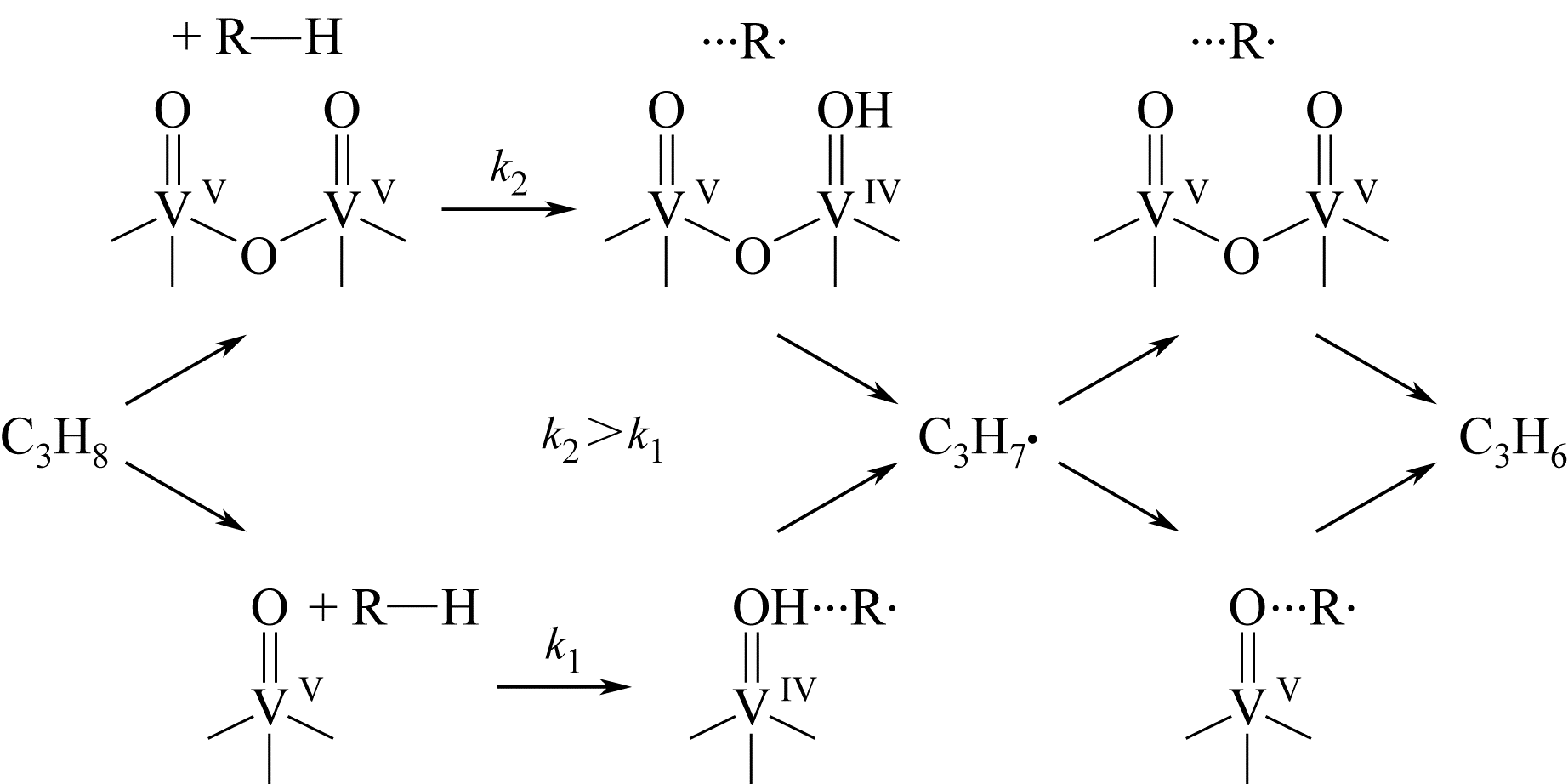
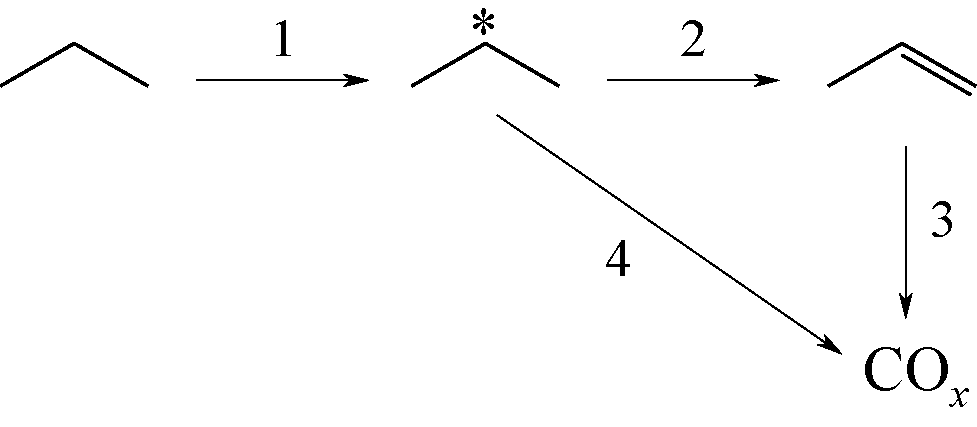
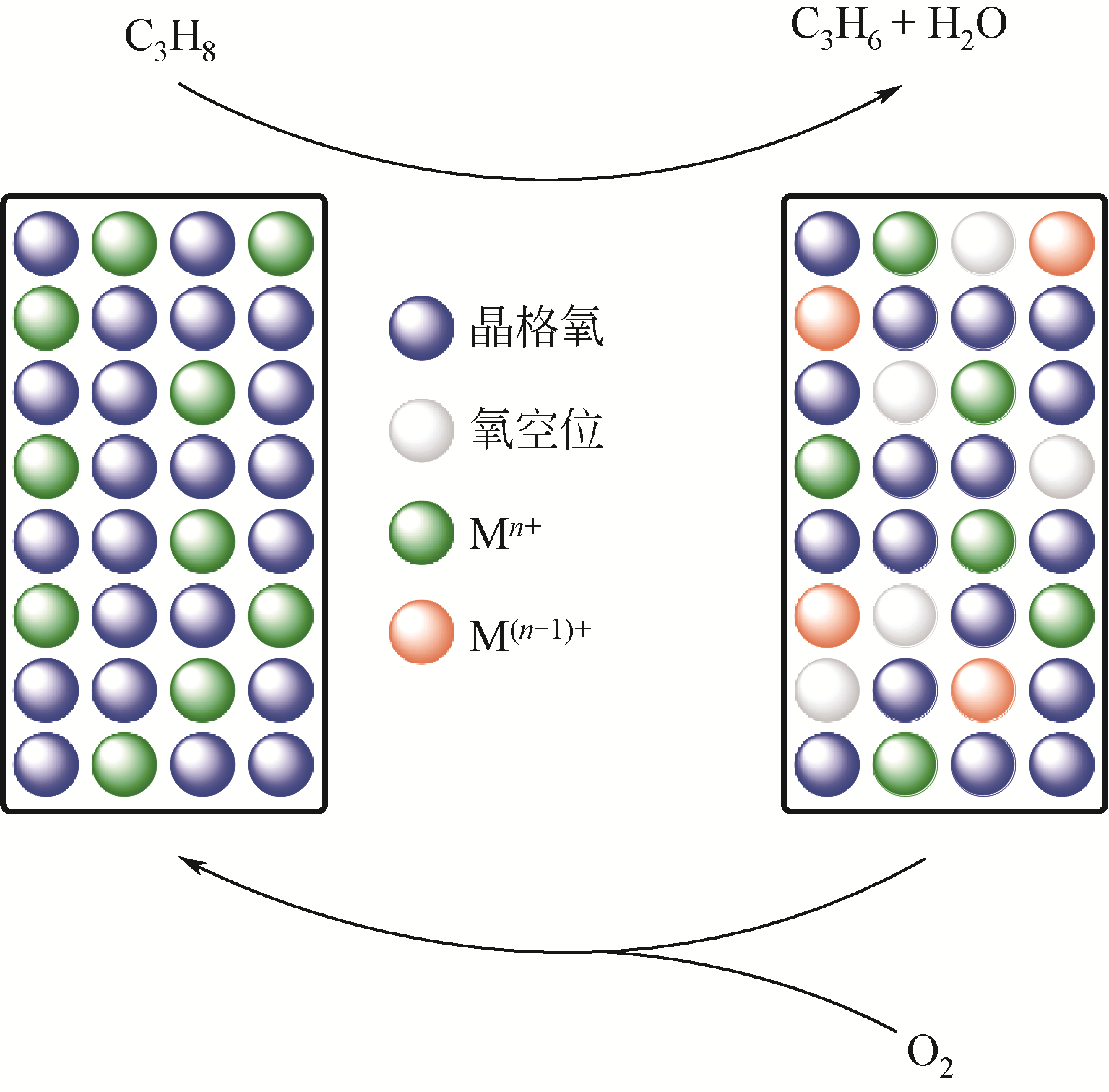
Chemical Industry and Engineering Progress ›› 2019, Vol. 38 ›› Issue (06): 2697-2706.DOI: 10.16085/j.issn.1000-6613.2018-1742
• Energy processes and technology • Previous Articles Next Articles
Research progress on oxidative dehydrogenation of propane to propene
- Department of Chemistry,Zhejiang University,Hangzhou 310027,Zhejiang,China
-
Received:2018-08-30Online:2019-06-05Published:2019-06-05 -
Contact:Jie FAN
丙烷氧化脱氢制丙烯研究进展
- 浙江大学化学系,浙江 杭州 310027
-
通讯作者:范杰 -
作者简介:杜凯敏(1993—),男,硕士研究生,研究方向为多相催化。E-mail:<email>dkmzju@163.com</email>。 -
基金资助:国家自然科学基金(91545113)
CLC Number:
Cite this article
Kaimin DU, Jie FAN. Research progress on oxidative dehydrogenation of propane to propene[J]. Chemical Industry and Engineering Progress, 2019, 38(06): 2697-2706.
杜凯敏, 范杰. 丙烷氧化脱氢制丙烯研究进展[J]. 化工进展, 2019, 38(06): 2697-2706.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2018-1742
| 工艺 | 反应器 | 催化剂 | 再生方式 | 寿命 /年 | 温度 /℃ | 压力 /bar | 转化率 /% | 选择性 /% |
|---|---|---|---|---|---|---|---|---|
| Catofin | 固定床 | Cr2O3/Al2O3 | 空气燃烧 | 2 | 550~620 | 0.5 | 55~60 | 90 |
| Oleflex | 移动床 | Pt-Sn/Al2O3 | 连续移出 | 4~5 | 550~600 | >1 | 35~40 | 84 |
| STAR | 固定床 | Pt-Sn/ ZnAl2O4 | 切换,空气燃烧 | 1~2 | 480~620 | 3~7 | 30~40 | 80~90 |
| LINDE | 固定床 | Pt/沸石 | 切换,空气燃烧 | >2 | 550~620 | 1~2 | 32~50 | 91~93 |
| FBD | 流化床 | Cr2O3/Al2O3 | 连续移出再生流化床,空气燃烧 | — | 540~590 | 1~2 | 40 | 84 |
| 工艺 | 反应器 | 催化剂 | 再生方式 | 寿命 /年 | 温度 /℃ | 压力 /bar | 转化率 /% | 选择性 /% |
|---|---|---|---|---|---|---|---|---|
| Catofin | 固定床 | Cr2O3/Al2O3 | 空气燃烧 | 2 | 550~620 | 0.5 | 55~60 | 90 |
| Oleflex | 移动床 | Pt-Sn/Al2O3 | 连续移出 | 4~5 | 550~600 | >1 | 35~40 | 84 |
| STAR | 固定床 | Pt-Sn/ ZnAl2O4 | 切换,空气燃烧 | 1~2 | 480~620 | 3~7 | 30~40 | 80~90 |
| LINDE | 固定床 | Pt/沸石 | 切换,空气燃烧 | >2 | 550~620 | 1~2 | 32~50 | 91~93 |
| FBD | 流化床 | Cr2O3/Al2O3 | 连续移出再生流化床,空气燃烧 | — | 540~590 | 1~2 | 40 | 84 |
| 催化剂 体系 | 代表 | 特点 | 研究 |
|---|---|---|---|
| 钒基 | V-Mg-O[ | 目前效果较好的催化体系,性能与活性相分散度及助剂酸碱性 有极大关系, 也是研究机理的主要体系,但起活温度较高(>500℃), 且长时间高温易挥发 | 多研究负载型催化剂,钒的分散性及 负载量,载体酸碱性效应及反应活性中心研究 |
| 铬基 | Cr/Al2O3 [ | 低温活性很高,多为负载型,极容易深度氧化 | 负载量及载体研究 |
| 钴基 | Co-Al-O[ | 低温活性很高,丙烯选择性较低,整体效果不佳,极容易深度氧化, 且稳定性差 | 研究较少,强调低温高收率,选择性 提高是关键 |
| 镍基 | Ni-M-O[ | 一般都有较好的低温性能,有一定的尺寸效应,收率一般都比较好, 且裂解产物较少 | 掺杂助剂,尺寸效应研究 |
| 钼基 | Mo/Al2O3 [ | 选择性较高,但低温活性差,一般低温活性低,需较高温度下才有活性(>500℃),且高温下易挥发 | 多研究非负载型催化剂 |
| 铂基 | Pt8-10/SnO/AAO[ | 一般要求Pt原子簇 | 制备方法研究 |
| 稀土铈基 | 3%Cs2O/ CeO2-2CeF3 [ | 丰富的氧空位结构,促进表面活性氧的迁移,经氟化物修饰的 稀土氧化物表现出极好的催化性能 | 助剂及氧化脱氢机理研究 |
| 非金属 | B-CNTs[ | 多以碳基为活性相,经P、B助剂的修饰可大幅提高丙烯选择性, 但稳定性不佳 | 助剂及氧化脱氢机理研究 |
| 催化剂 体系 | 代表 | 特点 | 研究 |
|---|---|---|---|
| 钒基 | V-Mg-O[ | 目前效果较好的催化体系,性能与活性相分散度及助剂酸碱性 有极大关系, 也是研究机理的主要体系,但起活温度较高(>500℃), 且长时间高温易挥发 | 多研究负载型催化剂,钒的分散性及 负载量,载体酸碱性效应及反应活性中心研究 |
| 铬基 | Cr/Al2O3 [ | 低温活性很高,多为负载型,极容易深度氧化 | 负载量及载体研究 |
| 钴基 | Co-Al-O[ | 低温活性很高,丙烯选择性较低,整体效果不佳,极容易深度氧化, 且稳定性差 | 研究较少,强调低温高收率,选择性 提高是关键 |
| 镍基 | Ni-M-O[ | 一般都有较好的低温性能,有一定的尺寸效应,收率一般都比较好, 且裂解产物较少 | 掺杂助剂,尺寸效应研究 |
| 钼基 | Mo/Al2O3 [ | 选择性较高,但低温活性差,一般低温活性低,需较高温度下才有活性(>500℃),且高温下易挥发 | 多研究非负载型催化剂 |
| 铂基 | Pt8-10/SnO/AAO[ | 一般要求Pt原子簇 | 制备方法研究 |
| 稀土铈基 | 3%Cs2O/ CeO2-2CeF3 [ | 丰富的氧空位结构,促进表面活性氧的迁移,经氟化物修饰的 稀土氧化物表现出极好的催化性能 | 助剂及氧化脱氢机理研究 |
| 非金属 | B-CNTs[ | 多以碳基为活性相,经P、B助剂的修饰可大幅提高丙烯选择性, 但稳定性不佳 | 助剂及氧化脱氢机理研究 |
| 1 | 王卅 .我国丙烯下游产业产品市场情况[J].化工进展,2014,33(9):2517-2520. |
| WANG Sa . The market of propene downstream industrial link in China[J]. Chemical Industry and Engineering Progress,2014,33(9):2517-2520. | |
| 2 | 王中银 .丙烯下游产品市场现状和产业链选择[J].煤炭加工和综合利用,2016(4):19-20. |
| WANG Zhongyin . The market and choice of propene downstream industrial link[J]. Coal Processing & Comprehensive Utilization,2016(4):19-20. | |
| 3 | 杨学萍 .国内外丙烯生产技术进展及市场分析[J].石油化工技术与经济,2017,33(6):11-15. |
| YANG Xueping . Production technology progress and market analysis of propene at home and abroad[J]. Technology & Economics in Petrochemicals,2017,33(6):11-15. | |
| 4 | 丙烷脱氢对丙烯市场的影响[J].石油化工,2016,45(6):724. |
| The effect of propane dehydrogenation on propene market[J]. Petrochemical Technology,2016,45(6):724. | |
| 5 | SATTLER J J , RUIZ-MARTINEZ J , SANTILLAN-JIMENEZ E ,et al .Catalytic dehydrogenation of light alkanes on metals and metal oxides[J]. Chemical Reviews,2014,114(20):10613-10653. |
| 6 | KUNG H H .Oxidative dehydrogenation of light (C2 to C4) alkanes[J].Advances in Catalysis,1994,40:1-38. |
| 7 | 韩香莲 .丙烯生产工艺研究进展[J].山东化工,2013,42(5):60-62. |
| HAN Xianglian . Production technology progress of propene[J].Shandong Chemical Industry,2013,42(5):60-62. | |
| 8 | 盖希坤,田原宇,夏道宏 .丙烷催化脱氢制丙烯工艺分析[J].炼油技术与工程,2010,40(12):27-32. |
| GAI Xikun , TIAN Yuanyu , XIA Daohong . Technology progress analysis of the catalytic dehydrogenation of propane[J].Petroleum Refinery Engineering,2010,40(12):27-32. | |
| 9 | CAVANI F , BALLARINI N , CERICOLA A .Oxidative dehydrogenation of ethane and propane:how far from commercial implementation?[J]. Catalysis Today,2007,127(1/2/3/4):113-131. |
| 10 | GAO Xingtao , RUIZ P , GUO Xiexian , et al .Effect of coexistence of magnesium vanadate phases in the selectivity oxidation of propane to propene[J]. Journal of Catalysis, 1994,148:56-67. |
| 11 | BLASCO T , GALLI A , LOPEZ-NIETO J M ,et al .Oxidative dehydrogenation of ethane and n-butane on VO x /Al2O3 catalysts[J]. Journal of Catalysis,1997,169:203-211. |
| 12 | FRANK B , DINSE A , OVSITSER O ,et al .Mass and heat transfer effects on the oxidative dehydrogenation of propane (ODP) over a low loaded VO x /Al2O3 catalyst[J]. Applied Catalysis A:General,2007,323:66-76. |
| 13 | LUO Qunxing , ZHANG Xiaokang , HOU Bouli ,et al . Catalytic function of VO x /Al2O3 for oxidative dehydrogenation of propane: support microstructure-dependent mass transfer and diffusion[J]. Catalysis Science & Technology,2018,8:4864-4876. |
| 14 | LIU Yongmei , CAO Yong , YI Nan ,et al .Vanadium oxide supported on mesoporous SBA-15 as highly selective catalysts in the oxidative dehydrogenation of propane[J].Journal of Catalysis,2004,224(2):417-428. |
| 15 | CHAAR M A , PATEL D , KUNG H H .Selective oxidative dehydrogenation of propane over V-Mg-O catalysts[J].Journal of Catalysis,1988,109:463-467. |
| 16 | LIU Lulu , HAN Xinyou , ZHOU Juan ,et al . Oxidative dehydrogenation of propane over three-dimensionally ordered macroporous VMgO catalysts with different vanadium doping[J]. Journal of Porous Materials,2018,25(4):955-963. |
| 17 | AYANDIRAN A A , BAKARE I A , BINOUS H ,et al .Oxidative dehydrogenation of propane to propylene over VO x /CaO-γ-Al2O3 using lattice oxygen[J]. Catalysis Science & Technology,2016,6(13):5154-5167. |
| 18 | SOLSONA B , BLASCO T , LOPEZ-NIETO J M ,et al .Vanadium oxide supported on mesoporous MCM-41 as selective catalysts in the oxidative dehydrogenation of alkanes[J]. Journal of Catalysis,2001,203(2):443-452. |
| 19 | DINSE A , FRANK B , HESS C ,et al .Oxidative dehydrogenation of propane over low-loaded vanadia catalysts: impact of the support material on kinetics and selectivity[J].Journal of Molecular Catalysis A:Chemical,2008,289(1/2):28-37. |
| 20 | LIU Yongmei , FENG Weiliang , LI Tingcheng ,et al .Structure and catalytic properties of vanadium oxide supported on mesocellulous silica foams (MCF) for the oxidative dehydrogenation of propane to propylene[J]. Journal of Catalysis,2006,239(1):125-136. |
| 21 | ROZANSKA X , FORTRIE R , SAUER J .Size-dependent catalytic activity of supported vanadium oxide species: oxidative dehydrogenation of propane[J].Journal of the American Chemical Society,2014,136(21):7751-7761. |
| 22 | BARMAN S , MAITY N , BHATTE K ,et al .Single-site VO x moieties generated on silica by surface organometallic chemistry: a way to enhance the catalytic activity in the oxidative dehydrogenation of propane[J]. ACS Catalysis,2016,6(9):5908:5921. |
| 23 | JIBRIL B Y . Propane oxidative dehydrogenation over chromium oxide-based catalysts[J].Applied Catalysis A:General, 2004,264(2):193-202. |
| 24 | JIBRIL B Y , AL-ZAHRANI S M , ABASAEED A E ,et al .Effects of reducibility on propane oxidative dehydrogenation over γ-Al2O3-supported chromium oxide-based catalysts[J]. Catalysis Letters,2003,87(3/4):121-132. |
| 25 | DAVIES T E , GARCIA T , SOLSONA B ,et al .Nanocrystalline cobalt oxide: a catalyst for selective alkane oxidation under ambient conditions[J]. Chemical Communications,2006(32):3417-3419. |
| 26 | HUANG Mingxiang , WU Xin , YI Xiaodong ,et al .Highly dispersed CoO x in layered double oxides for oxidative dehydrogenation of propane: guest-host interactions[J].RSC Advance,2017,7(24):14846-14856. |
| 27 | LI Zhanyong , PETERS A W , BERNALES V ,et al .Metal-organic framework supported cobalt catalysts for the oxidative dehydrogenation of propane at low temperature[J].ACS Central Science,2017,3(1):31-38. |
| 28 | BOIZUMAULT-MORICEAU P , PENNEAUIN A , GRZYBOWSKA B ,et al .Oxidative dehydrogenation of propane on Ni-Ce-O oxide: effect of the preparation method, effect of potassium addition and physical characterization[J].Applied Catalysis A:General, 2003,245(1):55-67. |
| 29 | WU Ying , HE Yiming , CHEN Tong ,et al .Low temperature catalytic performance of nanosized Ti-Ni-O for oxidative dehydrogenation of propane to propene[J]. Applied Surface Science,2006,252(14):5220-5226. |
| 30 | HE Yiming , WU Ying , CHEN Tong ,et al .Low-temperature catalytic performance for oxidative dehydrogenation of propane on nanosized Ti(Zr)-Ni-O prepared by modified sol-gel method[J].Catalysis Communications,2006,7(5):268-271. |
| 31 | HERACLEOUS E , LEMONIDOU A A .Ni-Me-O mixed metal oxides for the effective oxidative dehydrogenation of ethane to ethylene—Effect of promoting metal Me[J]. Journal of Catalysis,2010,270:67-75. |
| 32 | ZHU Haibo , DEVON C R , MOUSSAB H ,et al .Ni-M-O(M = Sn, Ti, W) catalysts prepared by a dry mixing method for oxidative dehydrogenation of ethane[J]. ACS Catalysis,2016,6:2852-2866. |
| 33 | LI Jianhui , WANG Caicai , Chuanjing HUNG ,et al . Mesoporous nickel oxides as effective catalysts for oxidative dehydrogenation of propane to propene[J]. Applied Catalysis A:General,2010,382(1),99-105. |
| 34 | ABELLO M C , GOMEZ M F , FERRETTI O . Mo/γ-Al2O3 catalysts for the oxidative dehydrogenation of propane: effect of Mo loading[J].Applied Catalysis A:General,2001,207(1):421-431. |
| 35 | STERN D L , GRASSELLI R K .Propane oxydehydrogenation over molybdate-based catalysts[J]. Journal of Catalysis,1997,167:550-559. |
| 36 | MAZZOCCHIA C , ABOUMRAD C , DIAGNE C ,et al .On the NiMoO4 oxidative dehydrogenation of propane to propene: some physical correlations with the catalytic activity[J].Catalysis Letters,1991,10:181-192. |
| 37 | ZAVOIANU R , DIAS C R , PORTELA M F . Stabilisation of β-NiMoO4 in TiO2-supported catalysts[J].Catalysis Communications,2001,2:37-42. |
| 38 | DIAS C R , ZAVOIANU R , PORTELA M F .Isobutane oxydehydrogenation on SiO2-supported nickel molybdate catalysts: effect of the active phase loading[J].Catalysis Communications,2002,3:85-90. |
| 39 | FARIN B , DEVILLERS M , GAIGNEAUX E M . Nanostructured hybrid materials as precursors of mesoporous NiMo-based catalysts for the propane oxidative dehydrogenation[J].Microporous and Mesoporous Materials,2017,242:200-207. |
| 40 | MDERIA L M , PORTELA M F , MAZZOCCHIA C .Nickel molybdate catalysts and their use in the selective oxidation of hydrocarbons[J].Catalysis Reviews,2004,46(1):53-110. |
| 41 | SUN Mia , ZHANG Jizhe , PUTAJ P ,et al .Catalytic oxidation of light alkanes (C1-C4) by heteropoly compounds[J]. Chemical Reviews,2014,114(2):981-1019. |
| 42 | VAJDA S , PELLIN M J , GREELEY J P ,et al .Subnanometre platinum clusters as highly active and selective catalysts for the oxidative dehydrogenation of propane[J]. Nature Materials,2009,8(3):213-216. |
| 43 | TROVARELLI A .Catalytic properties of ceria and CeO2-containing materials[J].Catalysis Reviews,1996,38(4):439-520. |
| 44 | WAN Huilin , ZHOU Xiaoping , WENG Weizheng ,et al .Catalytic performance, structure, surface properties and activity oxygen species of the fluoride-containing rare earth(alkaine earth)-based catalysts for the oxidative coupling of methane and oxidative dehydrogenation of light alkanes[J]. Catalysis today,1999,51:161-175. |
| 45 | TROTUS L T , TEODORESCU C M , PARVULESCU V I ,et al .Enhancing oxidative dehydrogenation selectivity of ceria-based catalysts with phosphorus as additive[J].ChemCatChem,2013,5(3):757-765. |
| 46 | FRANK B , MORASSUTTO M , SCHOMACKE R ,et al .Oxidative dehydrogenation of ethane over multiwalled carbon nanotubes[J].ChemCatChem,2010,2(6):644-648. |
| 47 | FRANK B , ZHANG Jian , BLUME R ,et al .Heteroatoms increase the selectivity in oxidative dehydrogenation reactions on nanocarbons[J].Angewandte Chemie,2009,48(37):6913-6917. |
| 48 | HU Zhongpan , ZHAO Hui , CHEN Chong ,et al . Castanea mollissima shell-derived porous carbons as metal-free catalysts for highly efficient dehydrogenation of propane to propylene[J]. Catalysis Today,2018,316:214-222. |
| 49 | ZHANG Jian , LIU Xi , BLUME R ,et al .Surface-modified carbon nanotubes catalyze oxidative dehydrogenation of n-butane[J].Science,2008,322(5898):73-77. |
| 50 | ZHANG Jian , SU Dangsheng , ZHANG Aihua ,et al .Nanocarbon as robust catalyst: mechanistic insight into carbon-mediated catalysis[J].Angewandte Chemie,2007,119(38):7460-7464. |
| 51 | SUN Xiaoyan , DING Yuxiao , ZHANG Bingsen ,et al . New insights into the oxidative dehydrogenation of propane on borate-modified nanodiamond[J].Chemical Communications,2015,51(44):9145-9148. |
| 52 | GUO Xiaoling , QI Wei , LIU Wei ,et al .Oxidative dehydrogenation on nanocarbon: revealing the catalytic mechanism using model catalysts[J]. ACS Catalysis, 2017,7(2):1424-1427. |
| 53 | QI Wei , LIU Wei , ZHANG Binsen ,et al .Oxidative dehydrogenation on nanocarbon: identification and quantification of active sites by chemical titration[J].Angewandte Chemie,2013,52(52):14224:14228. |
| 54 | LI Jiaquan , YU Peng , XIE Jingxin ,et al .Improving the alkene selectivity of nanocarbon-catalyzed oxidative dehydrogenation of n-butane by refinement of oxygen species[J].ACS Catalysis,2017,7(10):7305-7311. |
| 55 | GRANT J T , CARRERO C A , GOELTL F ,et al .Selective oxidative dehydrogenation of propane to propene using boron nitride catalysts[J].Science,2016,354:1570-1573. |
| 56 | SHI Lei , WANG Dongqi , SONG Wei ,et al .Edge-hydroxylated boron nitride for oxidative dehydrogenation of propane to propylene[J].ChemCatChem,2017,9(10):1788-1793. |
| 57 | CAVANI F , TRIFIRO F .The oxidative dehydrogenation of ethane and propane as an alternative way for the production of light olefins[J].Catalysis Today,1995,24:307-313. |
| 58 | MICHALAKOS P M , BIRKELAND K , KUNG H H . Selective oxidation of pentane over Al2O3- and SiO2-supported vanadia catalysys[J].Journal of Catalysis,1996,158:349-353. |
| 59 | 陈淑 .过渡金属氧化物纳米介孔材料在丙烷选择氧化脱氢制丙烯反应中的研究[D]. 杭州:浙江大学,2015. |
| CHEN Shu . The study of transition metal oxide nano-mesoporous material in oxidative dehydrogenation of propane to propene[D]. Hangzhou:Zhejiang University,2015. | |
| 60 | GRABOWSKI R .Kinetics of oxidative dehydrogenation of C2-C3 alkanes on oxide catalysts[J].Catalysis Reviews, 2006,48(2):199-268. |
| 61 | CHRISTODOULAKIS A , MACHLI M , LEMONIDOU A A ,et al .Molecular structure and reactivity of vanadia-based catalysts for propane oxidative dehydrogenation studied by in situ Raman spectroscopy and catalytic activity measurements[J]. Journal of Catalysis,2004,222(2):293-306. |
| 62 | YOU Rui , ZHANG Xuanyu , LUO Liangfeng ,et al .NbO x /CeO2-rods catalysts for oxidative dehydrogenation of propane: Nb-CeO2 interaction and reaction mechanism[J]. Journal of Catalysis,2017,348:189-199. |
| 63 | XUE Xuliang , LANG Wanzhong , YAN Xi ,et al .Dispersed vanadium in three-dimensional dendritic mesoporous silica nanospheres: active and stable catalysts for the oxidative dehydrogenation of propane in the presence of CO2 [J]. ACS Applied Materials & Interfaces,2017,9(18):15408-15423. |
| 64 | NOWICKA E , REECE C , ALTHAHBAN S M ,et al . Elucidating the role of CO2 in the soft oxidative dehydrogenation of propane over ceria-based catalysts[J]. ACS Catalysis,2018,8(4):3454-3468. |
| 65 | 郑雯,程党国,陈丰秋,等 . N2O作为氧化脱氢剂的研究进展[J].化工进展,2006,25(12):1391-1394. |
| ZHENG Wen , CHENG Dangguo , CHEN Fengqiu ,et al . The progress of N2O as oxidant in oxidative dehydrogenation[J]. Chemical Industry and Engineering Progress,2006,25(12):1391-1394. | |
| 66 | KONDRATENKO E V , CHERIAN M , BAERNS M ,et al .Oxidative dehydrogenation of propane over V/MCM-41 catalysts: comparison of O2 and N2O as oxidants[J].Journal of Catalysis,2005,234(1):131-142. |
| 67 | GASPAR N J , PASTERNAK I S , VADEKAR M .H2S promoted oxidative dehydrogenation of hydrocarbons in molten media[J].The Canadian Journal of Chemical Engineering,1974,52:793-797. |
| 68 | PREMJI Z A ,LO J M H, CLARK P D .Experimental and ab initio investigations of H2S-assisted propane oxidative dehydrogenation reactions[J]. The Journal of Physical Chemistry A,2014,118(9):1541-1556. |
| 69 | ADAMS C T , BRANDENBERGER S G , DUBOIS J B ,et al . Dehydrogenation and coupling reactions in the presence of iodine and molten salt hydrogen iodide acceptors[J].The Journal of Organic Chemistry,1977,42(1):1-6. |
| 70 | XIE Qunhua , ZHANG Huamin , KANG Jincan ,et al . Oxidative dehydrogenation of propane to propylene in the presence of HCl catalyzed by CeO2 and NiO-modified CeO2 Nanocrystals[J]. ACS Catalysis,2018,8(6):4902-4916. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [6] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [7] | WANG Jinhang, HE Yong, SHI Lingli, LONG Zhen, LIANG Deqing. Progress of gas hydrate anti-agglomerants [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4587-4602. |
| [8] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [9] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [10] | GAO Yanjing. Analysis of international research trend of single-atom catalysis technology [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4667-4676. |
| [11] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [12] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [13] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [14] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [15] | XU Zhongshuo, ZHOU Panpan, WANG Yuhui, HUANG Wei, SONG Xinshan. Advances in sulfur iron ore mediated autotrophic denitrification [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4863-4871. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||