Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (11): 6133-6143.DOI: 10.16085/j.issn.1000-6613.2024-1671
• Chemical processes and equipment • Previous Articles
Research progress on preparation of high purity lithium carbonate through hydrogenation-decomposition technology
LI Yahui( ), LIU Taoran, ZHU Ruisong, CAO Jing, LIU Minghui, LI Yingwen, HU Xuesheng(
), LIU Taoran, ZHU Ruisong, CAO Jing, LIU Minghui, LI Yingwen, HU Xuesheng( ), GAO Fei
), GAO Fei
- Petrochemical Research Institute, PetroChina, Beijing 102206, China
-
Received:2024-10-17Revised:2024-11-21Online:2025-12-08Published:2025-11-25 -
Contact:HU Xuesheng
氢化热解法制备高纯碳酸锂的研究进展
李亚辉( ), 刘陶然, 朱瑞松, 曹靖, 刘铭晖, 李应文, 胡雪生(
), 刘陶然, 朱瑞松, 曹靖, 刘铭晖, 李应文, 胡雪生( ), 高飞
), 高飞
- 中国石油天然气股份有限公司石油化工研究院,北京 102206
-
通讯作者:胡雪生 -
作者简介:李亚辉(1997—),女,博士,研究方向为石油化工绿色分离过程。E-mail:liyahui@petrochina.com.cn。 -
基金资助:中国石油天然气股份有限公司科学研究与技术开发项目(23-LH-39-05)
CLC Number:
Cite this article
LI Yahui, LIU Taoran, ZHU Ruisong, CAO Jing, LIU Minghui, LI Yingwen, HU Xuesheng, GAO Fei. Research progress on preparation of high purity lithium carbonate through hydrogenation-decomposition technology[J]. Chemical Industry and Engineering Progress, 2025, 44(11): 6133-6143.
李亚辉, 刘陶然, 朱瑞松, 曹靖, 刘铭晖, 李应文, 胡雪生, 高飞. 氢化热解法制备高纯碳酸锂的研究进展[J]. 化工进展, 2025, 44(11): 6133-6143.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1671
| 方法名称 | 方法描述 | 方法评价 |
|---|---|---|
| Zintl-Harder-Dauth法 | 将粗碳酸锂溶于醋酸中,以(NH4)2C2O4沉淀Ca2+,以Ba(OH)2沉淀Mg2+,再以H2SO4除去Ba2+,所得混合液经过滤干燥后灼热除去铵盐,剩余固体溶于盐酸后碳化得到精制Li2CO3[ | 该法需要投加大量化学试剂且伴产固废,增加了固废处置的费用,环境友好性差 |
| 苛化法 | 向粗碳酸锂浆料中加入精制石灰乳,将Ca2+、Mg2+等杂质沉淀后过滤去除,碳化 | 收率较低、产生固废 |
| 电解法 | 以粗碳酸锂氢化或酸溶后的溶液为阳极液,LiOH溶液为阴极液,两者间以离子选择性渗透膜隔开,电解 | 所需膜材料价格昂贵,工业应用前景较差 |
| 重结晶法 | 利用碳酸锂在水中的溶解度随温度的升高而降低、大部分杂质溶解度随温度升高而增大的差异,通过调控温度,反复进行溶解-结晶过程提升产品纯度 | 该方法对产品纯度的提升程度非常有限,且由于碳酸锂具有一定的水溶性,收率普遍低于75%[ |
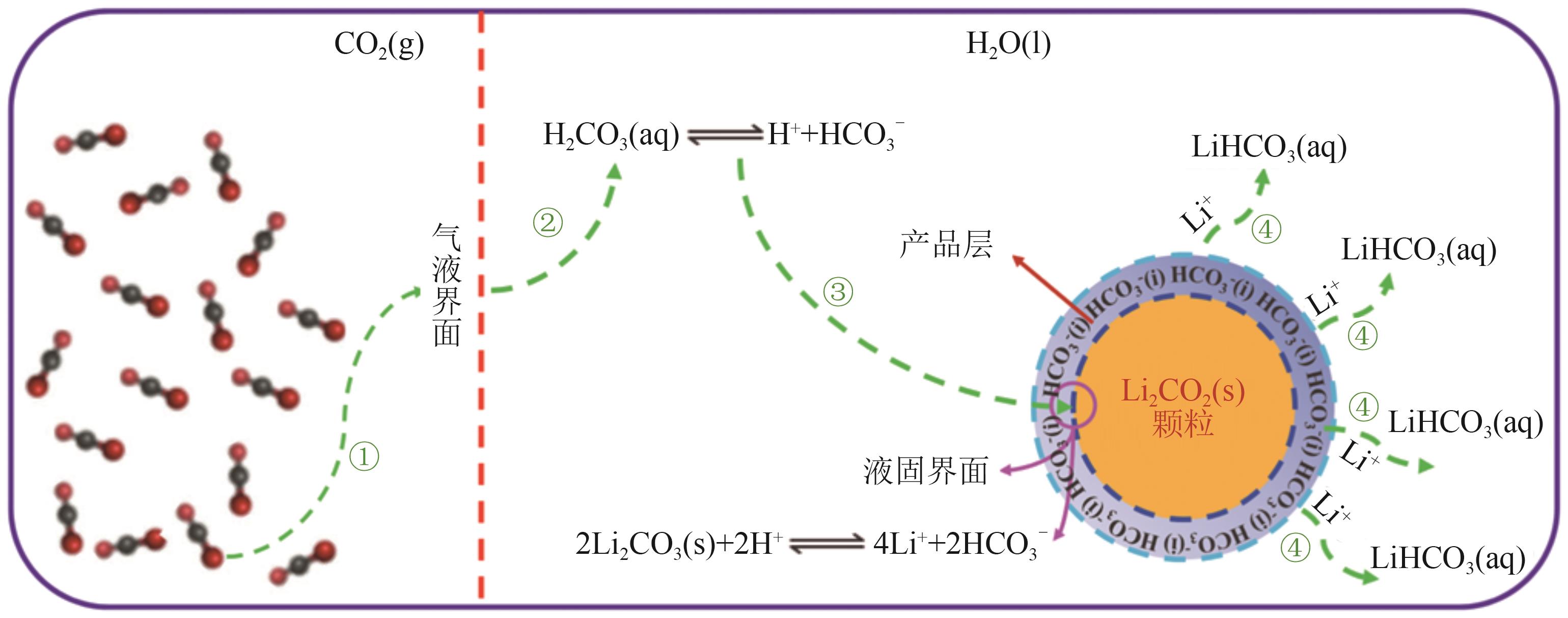
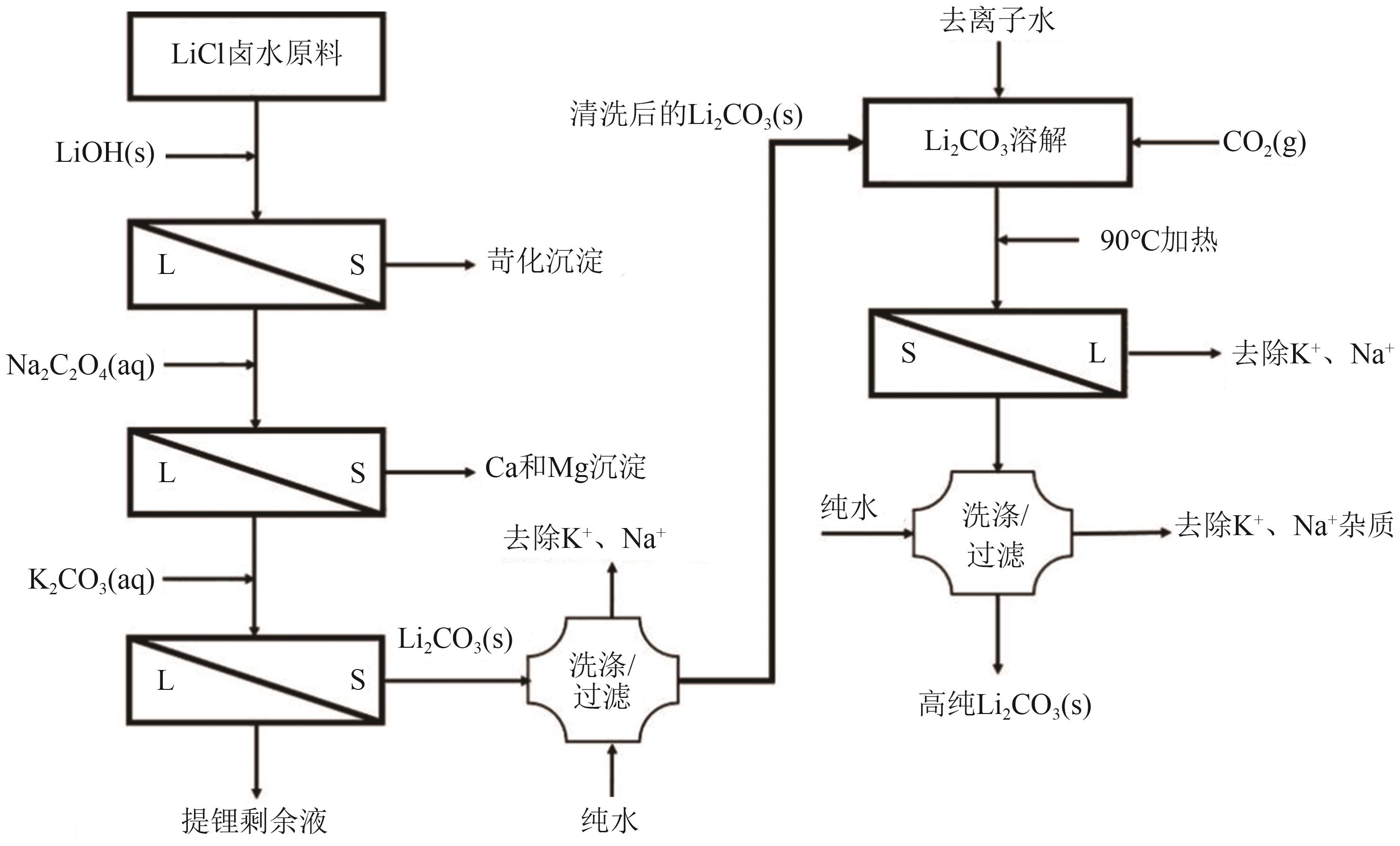
| 氢化热解法 | 以粗碳酸锂料浆为原料,通入CO2将Li2CO3氢化,使微溶的Li2CO3转变为可溶性的LiHCO3,将杂质去除后升温热解制备高纯碳酸锂 | 流程简单,CO2的利用和回收是关键问题,应用前景广阔 |
| 方法名称 | 方法描述 | 方法评价 |
|---|---|---|
| Zintl-Harder-Dauth法 | 将粗碳酸锂溶于醋酸中,以(NH4)2C2O4沉淀Ca2+,以Ba(OH)2沉淀Mg2+,再以H2SO4除去Ba2+,所得混合液经过滤干燥后灼热除去铵盐,剩余固体溶于盐酸后碳化得到精制Li2CO3[ | 该法需要投加大量化学试剂且伴产固废,增加了固废处置的费用,环境友好性差 |
| 苛化法 | 向粗碳酸锂浆料中加入精制石灰乳,将Ca2+、Mg2+等杂质沉淀后过滤去除,碳化 | 收率较低、产生固废 |
| 电解法 | 以粗碳酸锂氢化或酸溶后的溶液为阳极液,LiOH溶液为阴极液,两者间以离子选择性渗透膜隔开,电解 | 所需膜材料价格昂贵,工业应用前景较差 |
| 重结晶法 | 利用碳酸锂在水中的溶解度随温度的升高而降低、大部分杂质溶解度随温度升高而增大的差异,通过调控温度,反复进行溶解-结晶过程提升产品纯度 | 该方法对产品纯度的提升程度非常有限,且由于碳酸锂具有一定的水溶性,收率普遍低于75%[ |
| 氢化热解法 | 以粗碳酸锂料浆为原料,通入CO2将Li2CO3氢化,使微溶的Li2CO3转变为可溶性的LiHCO3,将杂质去除后升温热解制备高纯碳酸锂 | 流程简单,CO2的利用和回收是关键问题,应用前景广阔 |
| 序号 | 氢化时间/min | 氢化温度/℃ | 液固比/mL·g-1 | pH | CO2流速/L·min-1 | 搅拌转速/r·min-1 | 有无树脂纯化 | 产品纯度/% | 单级收率/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | — | 30 | — | 3.0 | 600 | 无 | 99.64 | — | [ |
| 2 | 80 | — | >23 | 7.6 | — | — | 无 | — | — | [ |
| 3 | 60 | 20 | 25 | — | 4.0 | — | 无 | ≥99.5 | >74 | [ |
| 4 | 120 | 25 | 25 | — | — | 400 | 无 | 99.6 | 70 | [ |
| 5 | 120~180 | 30~40 | — | — | — | — | 有 | 99.991 | — | [ |
| 6 | — | 27.5 | — | — | — | — | 有 | 99.9 | 87.6 | [ |
| 7 | 120 | 25 | — | — | 1.0 | 400 | 有 | 99.99 | 84.13 | [ |
| 8 | 150 | 20 | 20 | — | 1.0 | — | 无 | ≥99 | >75 | [ |
| 9 | 50 | 20 | 40 | — | 5.0 | — | 无 | 99.70 | 74.5 | [ |
| 10 | 50 | 20 | 25 | — | 1.0 | 300 | 无 | 99.80 | 71.92 | [ |
| 序号 | 氢化时间/min | 氢化温度/℃ | 液固比/mL·g-1 | pH | CO2流速/L·min-1 | 搅拌转速/r·min-1 | 有无树脂纯化 | 产品纯度/% | 单级收率/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | — | 30 | — | 3.0 | 600 | 无 | 99.64 | — | [ |
| 2 | 80 | — | >23 | 7.6 | — | — | 无 | — | — | [ |
| 3 | 60 | 20 | 25 | — | 4.0 | — | 无 | ≥99.5 | >74 | [ |
| 4 | 120 | 25 | 25 | — | — | 400 | 无 | 99.6 | 70 | [ |
| 5 | 120~180 | 30~40 | — | — | — | — | 有 | 99.991 | — | [ |
| 6 | — | 27.5 | — | — | — | — | 有 | 99.9 | 87.6 | [ |
| 7 | 120 | 25 | — | — | 1.0 | 400 | 有 | 99.99 | 84.13 | [ |
| 8 | 150 | 20 | 20 | — | 1.0 | — | 无 | ≥99 | >75 | [ |
| 9 | 50 | 20 | 40 | — | 5.0 | — | 无 | 99.70 | 74.5 | [ |
| 10 | 50 | 20 | 25 | — | 1.0 | 300 | 无 | 99.80 | 71.92 | [ |
| 名称 | 类型 | 官能团 | 颗粒尺寸/mm | 交换容量 | 参考文献 |
|---|---|---|---|---|---|
| TOKEM 308 | 强酸性磺酸阳离子交换 | —SO3H | 0.2~0.3 | 1.9mg/cm3 | [ |
| TOKEM 200 | 弱酸性羧酸阳离子交换 | —COOH | 0.3~1.6 | 4.3mg/cm3 | [ |
| Purolite S930 | 亚氨基羧基阳离子交换剂 | —N(CH2COOH)2 | 0.4~1.0 | 1.5mg/cm3 | [ |
| Amberlite IRC 748 | 亚氨基羧基阳离子交换剂 | —N(CH2COOH)2 | 0.5~0.65 | ≥1.35mg/cm3 | [ |
| AXIONIT 3S | 亚氨基羧基阳离子交换剂 | —N(CH2COOH)2 | 0.3~0.8 | 1.3~1.4mg/cm3 | [ |
| D412 | — | — | — | 0.014mg/cm3(以Ca计) | [ |
| Dowex G26 | 强酸性阳离子交换树脂 | — | — | 80mg/g(吸附容量) | [ |
| Amberlite IRC 747 | 螯合树脂 | —CH2NHCH2PO3Na2 | 0.52~0.66 | ≥1.75×10-3mg/cm3(以Na计) | [ |
| AG 50W-X8 | 磺化聚苯乙烯树脂 | —SO3H | — | — | [ |
| 名称 | 类型 | 官能团 | 颗粒尺寸/mm | 交换容量 | 参考文献 |
|---|---|---|---|---|---|
| TOKEM 308 | 强酸性磺酸阳离子交换 | —SO3H | 0.2~0.3 | 1.9mg/cm3 | [ |
| TOKEM 200 | 弱酸性羧酸阳离子交换 | —COOH | 0.3~1.6 | 4.3mg/cm3 | [ |
| Purolite S930 | 亚氨基羧基阳离子交换剂 | —N(CH2COOH)2 | 0.4~1.0 | 1.5mg/cm3 | [ |
| Amberlite IRC 748 | 亚氨基羧基阳离子交换剂 | —N(CH2COOH)2 | 0.5~0.65 | ≥1.35mg/cm3 | [ |
| AXIONIT 3S | 亚氨基羧基阳离子交换剂 | —N(CH2COOH)2 | 0.3~0.8 | 1.3~1.4mg/cm3 | [ |
| D412 | — | — | — | 0.014mg/cm3(以Ca计) | [ |
| Dowex G26 | 强酸性阳离子交换树脂 | — | — | 80mg/g(吸附容量) | [ |
| Amberlite IRC 747 | 螯合树脂 | —CH2NHCH2PO3Na2 | 0.52~0.66 | ≥1.75×10-3mg/cm3(以Na计) | [ |
| AG 50W-X8 | 磺化聚苯乙烯树脂 | —SO3H | — | — | [ |
| 初始LiHCO3溶液浓度 | 热解温度/°C | 搅拌速率/r·min-1 | 热解时间/min | 参考文献 |
|---|---|---|---|---|
| 6.05g/100g H2O | 90 | 400 | 60 | [ |
| >18g/L | 90 | — | 60~80 | [ |
| 6.05g/100g H2O(水浴) | 90 | 400 | — | [ |
| 6.80g/100g H2O(微波) | 90 | 400 | — | [ |
| — | 90 | 400 | — | [ |
| 初始LiHCO3溶液浓度 | 热解温度/°C | 搅拌速率/r·min-1 | 热解时间/min | 参考文献 |
|---|---|---|---|---|
| 6.05g/100g H2O | 90 | 400 | 60 | [ |
| >18g/L | 90 | — | 60~80 | [ |
| 6.05g/100g H2O(水浴) | 90 | 400 | — | [ |
| 6.80g/100g H2O(微波) | 90 | 400 | — | [ |
| — | 90 | 400 | — | [ |
| [47] | 吴鉴, 戴永年, 姚耀春. 氢化条件对碳酸锂提纯的影响[J]. 材料导报, 2011, 25(14): 82-84, 102. |
| WU Jian, DAI Yongnian, YAO Yaochun. Effect of carbonation conditions on the purification of lithium carbonate[J]. Materials Review, 2011, 25(14): 82-84, 102. | |
| [48] | 陈志文, 吴宇鹏, 刘雅婷, 等. 一种碳酸锂的纯化方法、碳酸锂: CN117865191A[P]. 2024-04-12. |
| CHEN Zhiwen, WU Yupeng, LIU Yating, et al. Purification method of lithium carbonate and lithium carbonate: CN117865191A[P]. 2024-04-12. | |
| [49] | WU Jian, YAO Yaochun, DAI Yongnian, et al. Effect of cycles and ion-exchange on the purification of lithium carbonate[J]. Advanced Materials Research, 2012, 443/444: 594-600. |
| [50] | MILYUTIN V V, NEKRASOVA N A, RUDSKIKH V V, et al. Preparation of high-purity lithium carbonate using complexing ion-exchange resins[J]. Russian Journal of Applied Chemistry, 2020, 93(4): 549-553. |
| [51] | 张云河, 许开华. 脉冲式氢化工业级碳酸锂制备电池级碳酸锂的方法: CN106365182A[P]. 2017-02-01. |
| ZHANG Yunhe, XU Kaihua. Method for preparing battery grade lithium carbonate by using impulse type hydrogenated industrial grade lithium carbonate: CN106365182A[P]. 2017-02-01. | |
| [52] | 杜国山, 汪德华, 杨永亮, 等. 碳酸锂碳酸化-热析系统研究及工业应用[J]. 有色设备, 2021, 35(2): 45-48. |
| DU Guoshan, WANG Dehua, YANG Yongliang, et al. Research and industrial application of Li2CO3 carbonation-crystallization system[J]. Nonferrous Metallurgical Equipment, 2021, 35(2): 45-48. | |
| [53] | 许志中, 陶箴奇, 张志强, 等. 碳酸氢锂热分解结晶过程的研究[J]. 中小企业管理与科技(下旬刊), 2016(11): 181-183. |
| XU Zhizhong, TAO Zhenqi, ZHANG Zhiqiang, et al. Study on thermal decomposition and crystallization process of lithium bicarbonate[J]. Management & Technology of SME, 2016(11): 181-183. | |
| [54] | YI Wentao, YAN Chunyan, MA Peihua. Crystallization kinetics of Li2CO3 from LiHCO3 solutions[J]. Journal of Crystal Growth, 2010, 312(16/17): 2345-2350. |
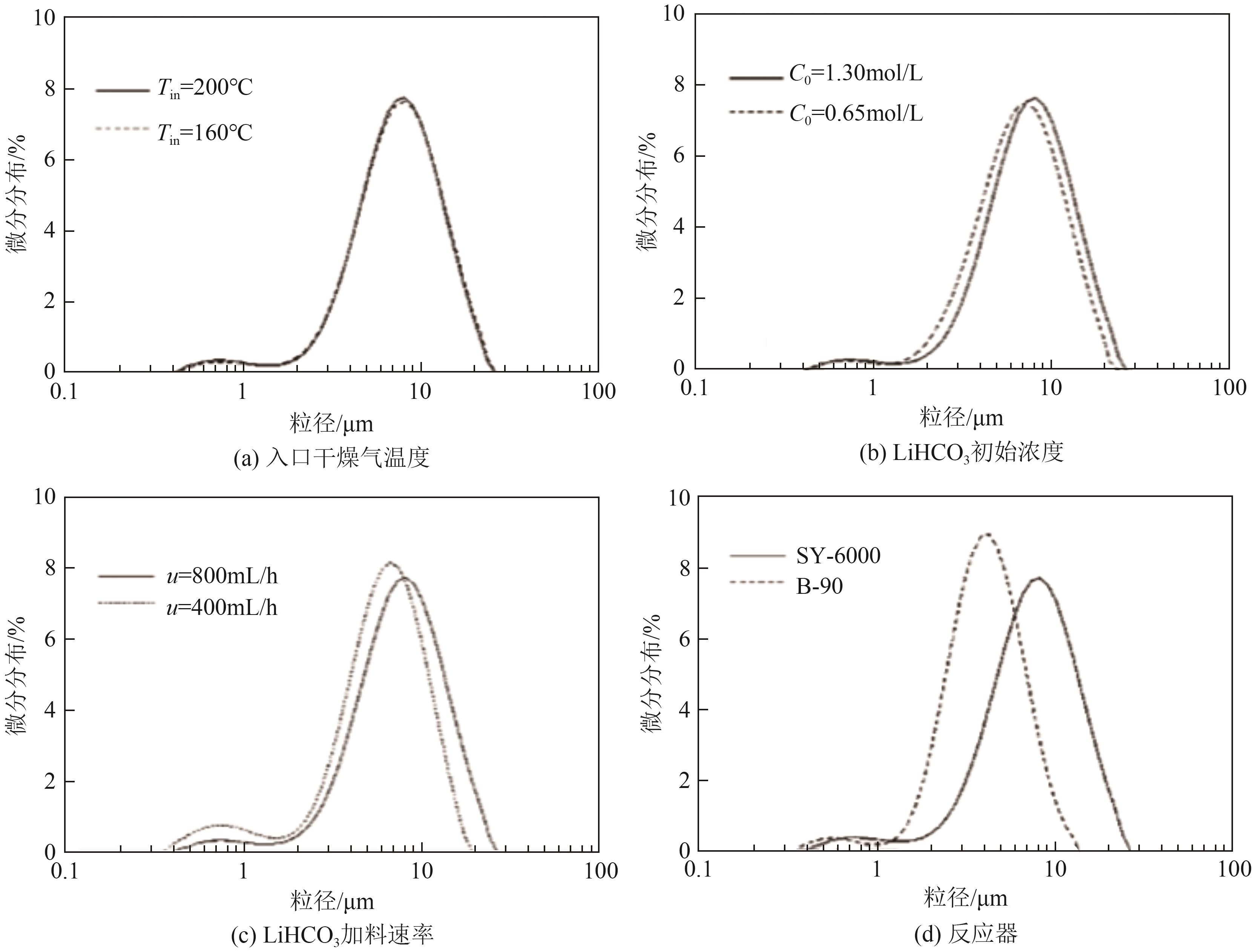
| [55] | SUN Yuzhu, SONG Xingfu, WANG Jin, et al. Preparation of lithium carbonate hollow spheres by spray pyrolysis[J]. Crystal Research and Technology, 2011, 46(2): 173-177. |
| [56] | 中华人民共和国工业和信息化部. 高纯碳酸锂: [S]. 北京: 冶金工业出版社, 2022. |
| Ministry of Industry and Information of the People’s Republic of China. High purity lithium carbonate: [S]. Beijing: Metallurgical Industry Press, 2022. | |
| [57] | WANG Shen, PEI Xiaokang, LUO Yong, et al. Preparation of lithium carbonate by microwave assisted pyrolysis[J]. Chinese Journal of Chemical Engineering, 2022, 52: 146-153. |
| [58] | KUROTANI Masahiro, MIYASAKA Etsuko, EBIHARA Satomi, et al. Effect of ultrasonic irradiation on the behavior of primary nucleation of amino acids in supersaturated solutions[J]. Journal of Crystal Growth, 2009, 311(9): 2714-2721. |
| [59] | Martijn WAGTERVELD R, MIEDEMA Henk, WITKAMP Geert-Jan. Effect of ultrasonic treatment on early growth during CaCO3 precipitation[J]. Crystal Growth and Design, 2012, 12(9): 4403-4410. |
| [60] | TAN Jianghao, WANG Qi, LIN Yanjun, et al. Direct preparation of battery-grade lithium carbonate via a nucleation-crystallization isolating process intensified by a micro-liquid film reactor[J]. The Canadian Journal of Chemical Engineering, 2023, 101(2): 870-882. |
| [61] | LIU Wei, CHU Guangwen, LI Shaochen, et al. Preparation of lithium carbonate by thermal decomposition in a rotating packed bed reactor[J]. Chemical Engineering Journal, 2019, 377: 119929. |
| [62] | 王人杰, 李明兴, 李新义. 基于氢化分解法的粗碳酸锂制备电池级碳酸锂工艺研究[J]. 化工设计通讯, 2020, 46(7): 140-142. |
| WANG Renjie, LI Mingxing, LI Xinyi. Preparation of battery grade lithium carbonate from crude lithium carbonate by hydrogenolysis[J]. Chemical Engineering Design Communications, 2020, 46(7): 140-142. | |
| [63] | CAI Wei, CHEN Ruosong, YANG Yurong, et al. Removal of SO4 2- from Li2CO3 by recrystallization in Na2CO3 solution[J]. Crystals, 2018, 8(1): 19. |
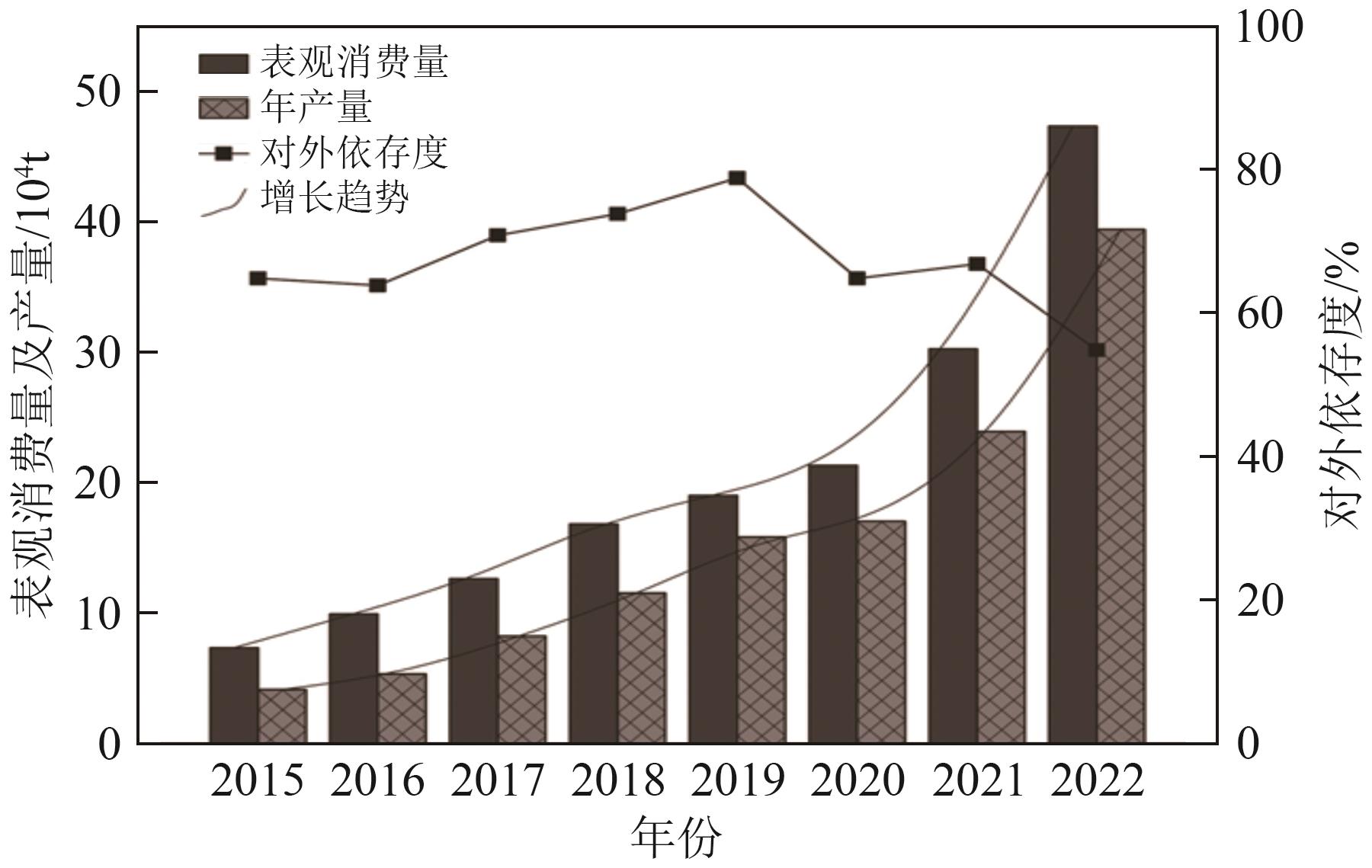
| [1] | MARTIN Gunther, RENTSCH Lars, Michael HÖCK, et al. Lithium market research—Global supply, future demand and price development[J]. Energy Storage Materials, 2017, 6: 171-179. |
| [2] | LI Zehong, WANG Chunying, CHEN Jian. Supply and demand of lithium in China based on dynamic material flow analysis[J]. Renewable and Sustainable Energy Reviews, 2024, 203: 114786. |
| [3] | ANDRADE Mariana N, OLIVEIRA Glaucia C, COTRIM Marycel E B, et al. Use of the ion exchange technique for purification of lithium carbonate for nuclear industry[C]. International Nuclear Atlantic Conference, 2021. |
| [4] | APRILIANTO Doni Riski, PERDANA Indra, ROCHMADI, et al. Effect of sulfate and carbonate ions on lithium carbonate precipitation from a low concentration lithium containing solution[J]. Industrial & Engineering Chemistry Research, 2024, 63(11): 4918-4933. |
| [5] | 伊文涛, 闫春燕, 戴志锋, 等. 高纯碳酸锂的应用与制备方法评述[J]. 化工矿物与加工, 2005, 34(11): 5-8. |
| YI Wentao, YAN Chunyan, DAI Zhifeng, et al. Review of the application and preparation of high purity lithium carbonate[J]. Industrial Minerals & Processing, 2005, 34(11): 5-8. | |
| [6] | KIM Sung-hwan, YOON Hongsik, MIN Taijin, et al. Carbon dioxide utilization in lithium carbonate precipitation: A short review[J]. Environmental Engineering Research, 2024, 29(3): 230553. |
| [7] | LEI Chong, LANG Jialiang, WANG Kuangyu, et al. Extraction of LiCl from low-purity chlorides through solid electrolyte towards high-purity Li2CO3 production[J]. ChemSusChem, 2024, 17(8): e202301406. |
| [8] | 朱瑞松, 曹靖, 刘陶然, 等. 全球非常规卤水的提锂技术及产业化研究进展[J]. 无机盐工业, 2023, 55(11): 1-11. |
| ZHU Ruisong, CAO Jing, LIU Taoran, et al. Research progress of lithium extraction technology and industrialization of unconventional brines in global[J]. Inorganic Chemicals Industry, 2023, 55(11): 1-11. | |
| [9] | 文佳豪, 代鸿章, 陈翠华, 等. 中国锂资源供需现状与资源保障程度研究[J]. 地质通报, 2025, 44(S1): 245-258. |
| WEN Jiahao, DAI Hongzhang, CHEN Cuihua, et al. Research on the supply and demand status and resource guarantee degree of lithium in China[J]. Geological Bulletin of China, 2025, 44(S1): 245-258. | |
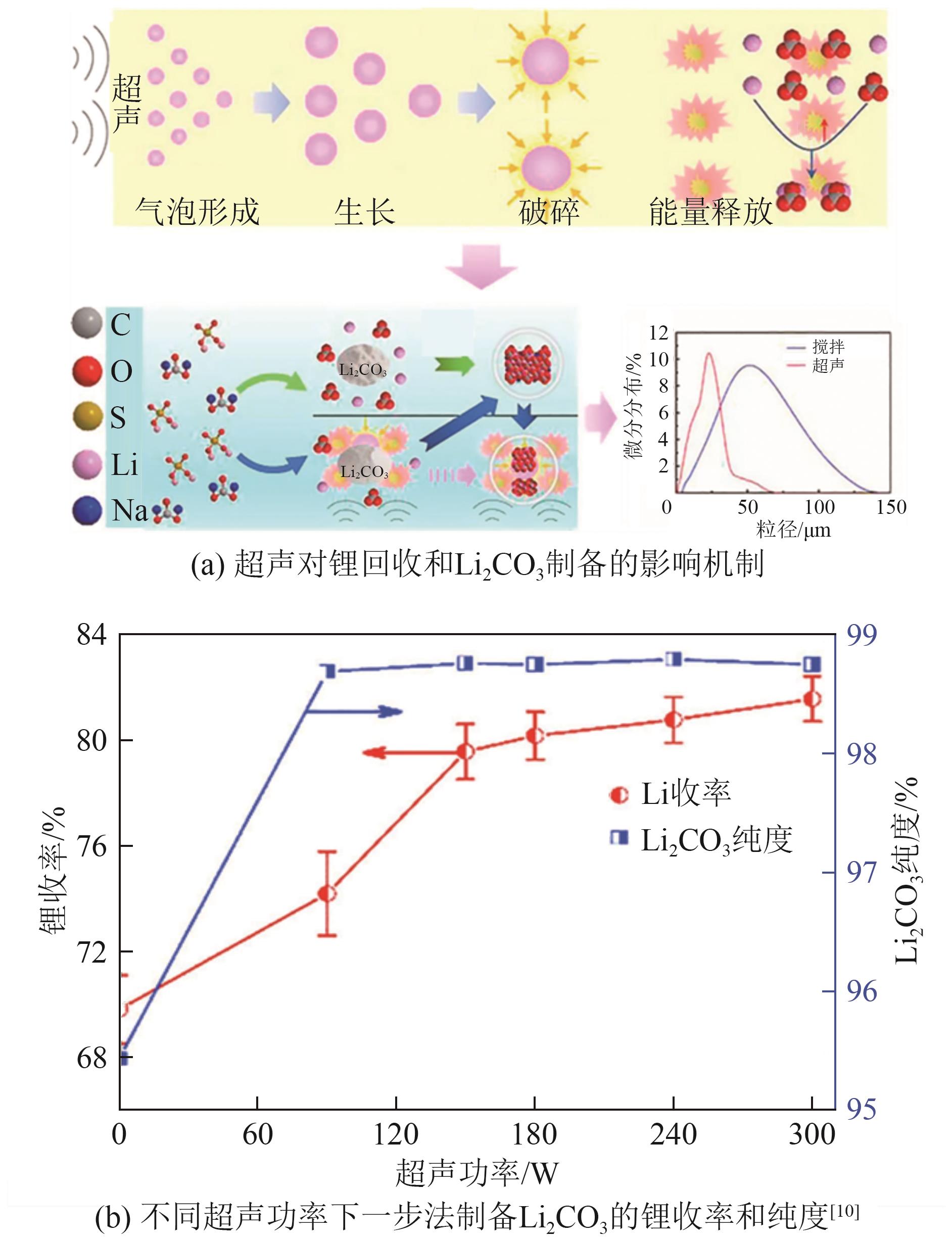
| [10] | ZHAO Chunlong, ZHANG Yanling, CAO Hongbin, et al. Lithium carbonate recovery from lithium-containing solution by ultrasound assisted precipitation[J]. Ultrasonics Sonochemistry, 2019, 52: 484-492. |
| [11] | SHIN Junho, JEONG Jae-Min, LEE Jin Bae, et al. Preparation of lithium carbonate from waste lithium solution through precipitation and wet conversion methods[J]. Hydrometallurgy, 2022, 210: 105863. |
| [12] | GILEVA Olga, ARYAL Pabitra, CHOE JunSeok, et al. Purification of lithium carbonate from radioactive contaminants using a MnO2-based inorganic sorbent[J]. Inorganics, 2023, 11(10): 410. |
| [13] | LINNEEN Nicholas, BHAVE Ramesh, WOERNER Douglas. Purification of industrial grade lithium chloride for the recovery of high purity battery grade lithium carbonate[J]. Separation and Purification Technology, 2019, 214: 168-173. |
| [14] | 戴立新. 超纯(5N)碳酸锂的研制及其应用前景[J]. 矿业研究与开发, 2003(S1): 192-193. |
| DAI Lixin. Development and application prospect of ultra-purified (5N) lithium carbonate [J]. Mining Research and Development, 2003(S1): 192-193. | |
| [15] | ZHENG Feifei, LIU Hong, LIU Duo, et al. Hydrothermal and wet-chemical synthesis of pure LiTaO3 powders by using commercial tantalum hydroxide as starting material[J]. Journal of Alloys and Compounds, 2009, 477(1/2): 688-691. |
| [16] | MASLOBOEVA S M, EFREMOV I N, BIRYUKOVA I V, et al. Growth and characterization of a boron-doped lithium niobate single crystal[J]. Inorganic Materials, 2020, 56(11): 1147-1152. |
| [17] | CHOE Gogwon, KIM Hyungsub, KWON Jaesub, et al. Re-evaluation of battery-grade lithium purity toward sustainable batteries[J]. Nature Communications, 2024, 15: 1185. |
| [18] | LIU Zixi. Study on preparation technology of LiNbO3 polycrystal powder[J]. Rare Metals & Cemented Carbides, 2022, 50(5): 66-69. |
| [19] | CHEN Ning, ZHOU E, DUAN Dongping, et al. Mechanochemistry synthesis of high purity lithium carbonate[J]. Korean Journal of Chemical Engineering, 2017, 34(10): 2748-2755. |
| [20] | YI Wentao, YAN Chunyan, MA Peihua. Removal of calcium and magnesium from LiHCO3 solutions for preparation of high-purity Li2CO3 by ion-exchange resin[J]. Desalination, 2009, 249(2): 729-735. |
| [21] | 戴志锋, 肖小玲, 李法强, 等. 高纯碳酸锂的制取方法探讨[J]. 盐湖研究, 2005, 13(2): 53-59. |
| DAI Zhifeng, XIAO Xiaoling, LI Faqiang, et al. Discussion on methods for the preparation for high-purity lithium carbonate[J]. Journal of Salt Lake Research, 2005, 13(2): 53-59. | |
| [22] | 米泽华. 高纯碳酸锂的制取方法探讨[J]. 新疆有色金属, 2000, 23(2): 27-29. |
| MI Zehua. Discussion on preparation method of high purity lithium carbonate[J]. XINJIANG YOUSE JINSHU, 2000, 23(2): 27-29. | |
| [23] | TORRES Walter R, DÍAZ NIETO César H, Antonin PRÉVOTEAU, et al. Lithium carbonate recovery from brines using membrane electrolysis[J]. Journal of Membrane Science, 2020, 615: 118416. |
| [24] | 赵春龙, 孙峙, 郑晓洪, 等. 碳酸锂的制备及其纯化过程的研究进展[J]. 过程工程学报, 2018, 18(1): 20-28. |
| ZHAO Chunlong, SUN Zhi, ZHENG Xiaohong, et al. Research progress of lithium carbonate preparation and purification process[J]. The Chinese Journal of Process Engineering, 2018, 18(1): 20-28. | |
| [25] | 曹靖, 朱瑞松, 李应文, 等. 富锂卤水制备电池级碳酸锂研究进展[J]. 现代化工, 2024, 44(1): 68-73. |
| CAO Jing, ZHU Ruisong, LI Yingwen, et al. Research progress in preparation of battery-grade lithium carbonate from lithium-rich brine[J]. Modern Chemical Industry, 2024, 44(1): 68-73. | |
| [26] | 戴江洪, 王宏岩, 李平. 高纯碳酸锂制备研究进展[J]. 中国有色冶金, 2020, 49(1): 49-53. |
| DAI Jianghong, WANG Hongyan, LI Ping. Research development of preparation of high purity lithium carbonate[J]. China Nonferrous Metallurgy, 2020, 49(1): 49-53. | |
| [27] | LI Haodu, ZHANG Jie, DAI Lin, et al. Process intensification for the synthesis and purification of battery-grade Li2CO3 with microfluidics[J]. Particuology, 2024, 90: 106-117. |
| [28] | LIU Dongfu, ZHAO Zhongwei, XU Wenhua, et al. A closed-loop process for selective lithium recovery from brines via electrochemical and precipitation[J]. Desalination, 2021, 519: 115302. |
| [29] | ZHOU Ming. Preparation of battery grade Li2CO3 from defective product by the carbonation-decomposition method[J]. Crystal Research and Technology, 2023, 58(1): 2200112. |
| [30] | WANG Jinliang, HU Huazhou. Microbubble-assisted pressure carbonation for preparation of high purity lithium carbonate[J]. Journal of Materials Research and Technology, 2020, 9(5): 9498-9505. |
| [31] | 梁鑫, 洪侃, 赖耀斌, 等. 正交试验法优化碳酸锂提纯条件研究[J]. 中国资源综合利用, 2020, 38(8): 15-18. |
| LIANG Xin, HONG Kan, LAI Yaobin, et al. Study on optimization for purification technique of lithium carbonate by orthogonal experiment[J]. China Resources Comprehensive Utilization, 2020, 38(8): 15-18. | |
| [32] | DODDS W S, STUTZMAN L F, SOLLAMI B J. Carbon dioxide solubility in water[J]. Industrial & Engineering Chemistry Chemical & Engineering Data Series, 1956, 1(1): 92-95. |
| [33] | 张志月, 吴会敏, 李鹏武, 等. 碳酸锂氢化工段工艺技术研究[J]. 山东化工, 2018, 47(22): 18-20. |
| ZHANG Zhiyue, WU Huimin, LI Pengwu, et al. Research on hydrogen chemical section of lithium carbonate purification[J]. Shandong Chemical Industry, 2018, 47(22): 18-20. | |
| [34] | 孙哲. 89%级碳酸锂转电池级碳酸锂氢化工艺研究[J]. 新疆有色金属, 2016, 39(5): 82-83. |
| SUN Zhe. Study on hydrogenation process of 89% grade lithium carbonate to battery grade lithium carbonate[J]. XINJIANG YOUSE JINSHU, 2016, 39(5): 82-83. | |
| [35] | XU Zhengguo, SUN Shuying. Preparation of battery-grade lithium carbonate with lithium-containing desorption solution[J]. Metals, 2021, 11(9): 1490. |
| [36] | 周启立, 王莫飞. 碳化法制备高纯碳酸锂[J]. 无机盐工业, 2012, 44(7): 36-37, 55. |
| ZHOU Qili, WANG Mofei. Preparation of high purity lithium carbonate by carbonization method[J]. Inorganic Chemicals Industry, 2012, 44(7): 36-37, 55. | |
| [37] | CHEN Weisheng, LEE Chenghan, Hsing-Jung HO. Purification of lithium carbonate from sulphate solutions through hydrogenation using the dowex G26 resin[J]. Applied Sciences, 2018, 8(11): 2252. |
| [38] | 李燕茹, 朱亮, 袁建军, 等. 粗级碳酸锂提纯工艺过程研究[J]. 无机盐工业, 2013, 45(8): 15-17. |
| LI Yanru, ZHU Liang, YUAN Jianjun, et al. Research on purifying process of coarse lithium carbonate[J]. Inorganic Chemicals Industry, 2013, 45(8): 15-17. | |
| [39] | 郭贤慧, 王永勤, 王建萍, 等. 碳化分解法制备电池级碳酸锂的工艺研究[J]. 无机盐工业, 2019, 51(1): 50-52. |
| GUO Xianhui, WANG Yongqin, WANG Jianping, et al. Study on preparation process of battery-grade lithium carbonate by carbonation-decomposition method[J]. Inorganic Chemicals Industry, 2019, 51(1): 50-52. | |
| [40] | 赵峰, 汪胜东, 郭纵, 等. 碳化分解法制备电池级碳酸锂[J]. 有色金属工程, 2025, 15(2): 255-260. |
| ZHAO Feng, WANG Shengdong, GUO Zong, et al. Preparation of battery grade Li2CO3 by carbonation and decomposition[J]. Nonferrous Metals Engineering, 2025, 15(2): 255-260. | |
| [41] | 伊文涛, 闫春燕, 李法强, 等. 碳酸锂碳化反应过程分析与机理探讨[J]. 化工矿物与加工, 2006, 35(2): 31-34. |
| YI Wentao, YAN Chunyan, LI Faqiang, et al. Process analysis and mechanism discussion of carbonation reaction of lithium carbonate[J]. Industrial Minerals & Processing, 2006, 35(2): 31-34. | |
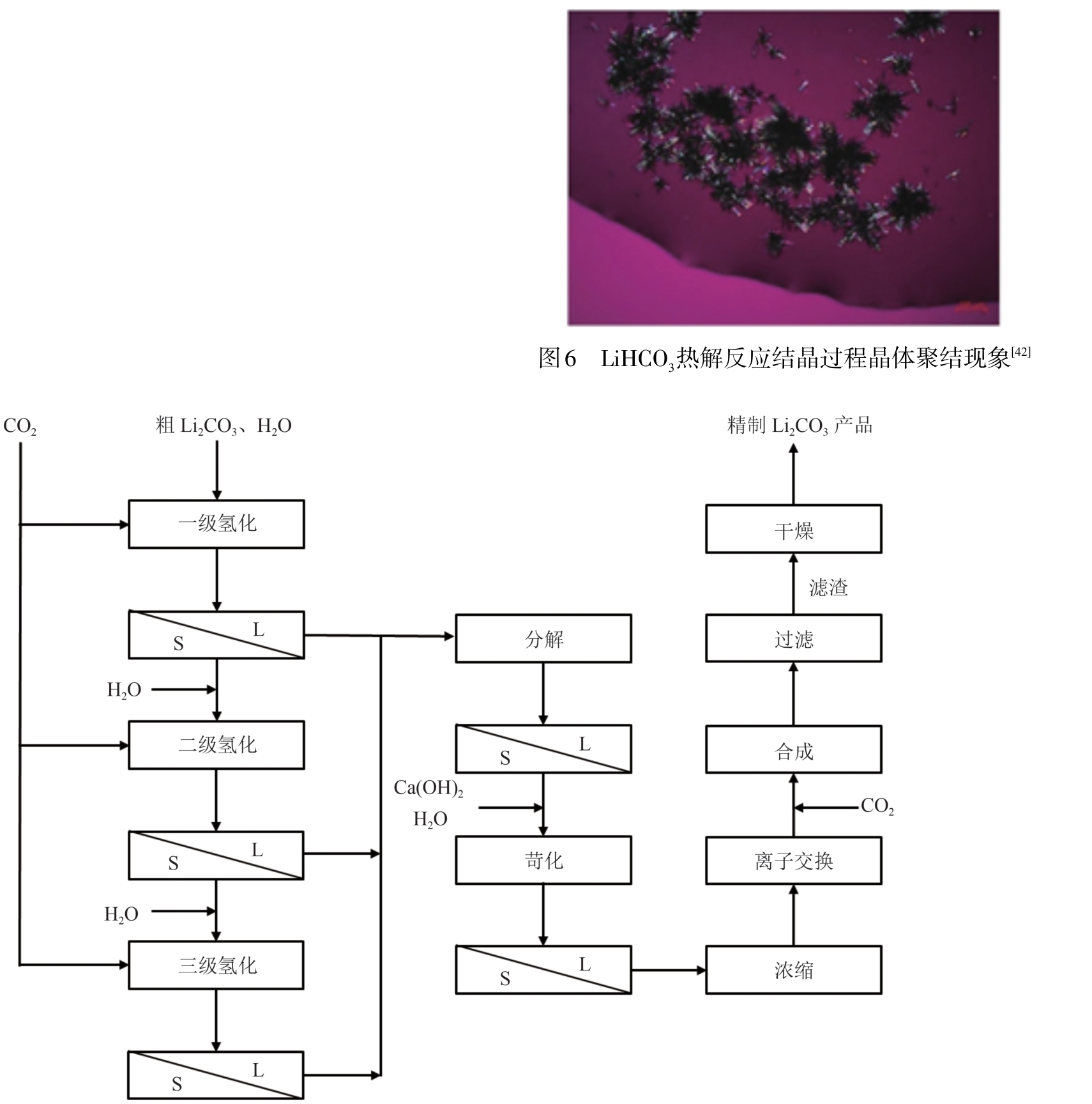
| [42] | 殷海青, 马祎明, 万旭兴, 等. 碳酸锂气液固三相反应结晶过程研究[J]. 化工学报, 2022, 73(3): 1207-1220. |
| YIN Haiqing, MA Yiming, WAN Xuxing, et al. Research of lithium carbonate three-phase reactive crystallization process [J]. CIESC Journal, 2022, 73(3): 1207-1220. | |
| [43] | 孙玉柱. 碳酸锂结晶过程研究[D]. 上海: 华东理工大学, 2010. |
| SUN Yuzhu. Study on the crystallization of lithium carbonate[D]. Shanghai: East China University of Science and Technology, 2010. | |
| [44] | 兰清泉, 赵金宇, 王瑞琦, 等. 一种制备高纯度纳米级碳酸锂的方法、系统及设备: CN117303414A[P]. 2023-12-29. |
| LAN Qingquan, ZHAO Jinyu, WANG Ruiqi, et al. A method, system and equipment for preparing high-purity nanoscale lithium carbonate: CN117303414A [P]. 2023-12-29. | |
| [45] | 钟发平, 杨建广, 张艳, 等. 一种由粗碳酸锂制备高纯碳酸锂的方法: CN116903005A[P]. 2023-10-20. |
| ZHONG Faping, YANG Jianguang, ZHANG Yan, et al. A method for preparing high-purity lithium carbonate from crude lithium carbonate: CN116903005A[P]. 2023-10-20. | |
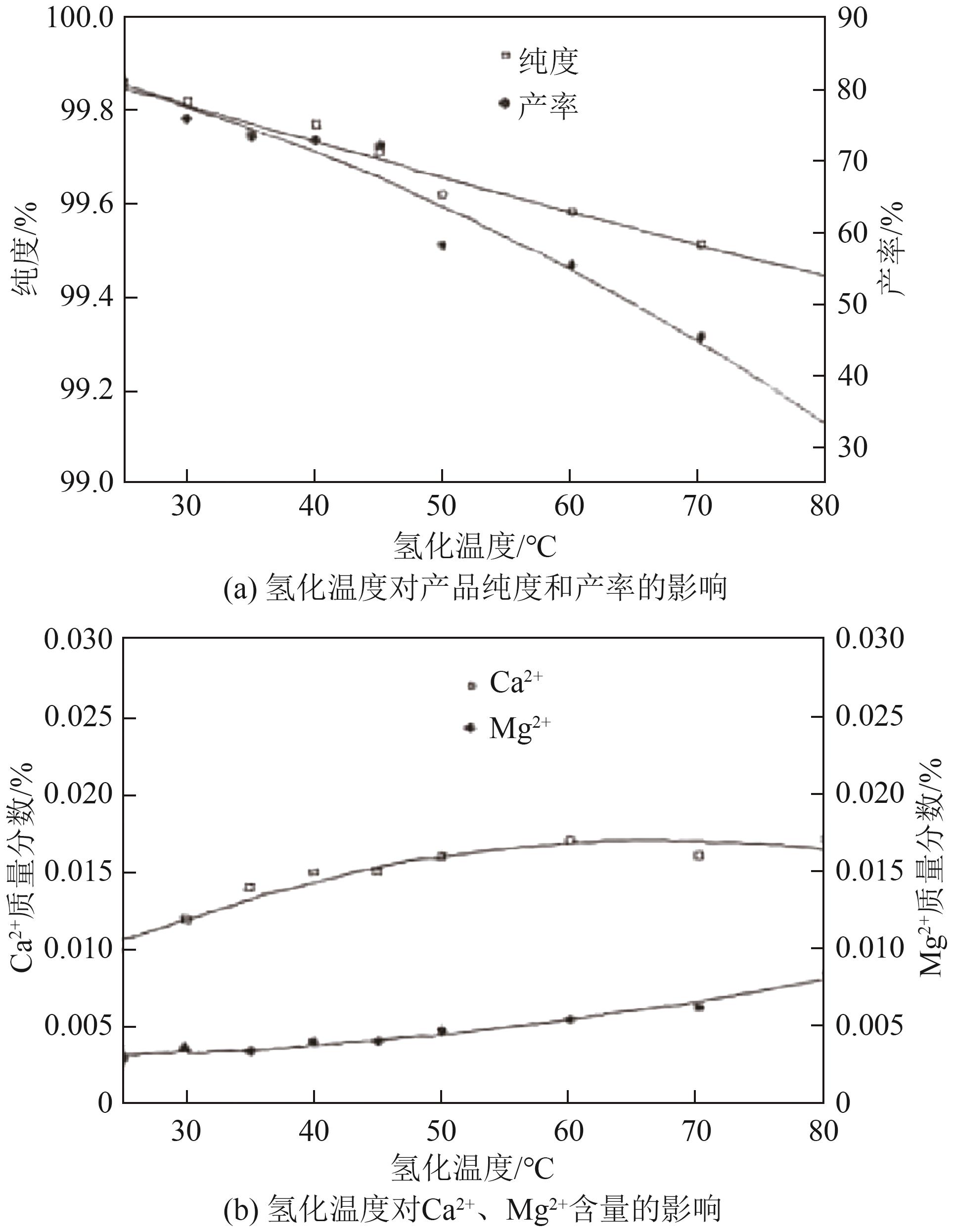
| [46] | ZHAO Quanfeng, WU Jian, YAO Yaochun. Effect of carbonation temperature on the purification of lithium carbonate[J]. Advanced Materials Research, 2013, 846/847: 1911-1914. |
| [64] | 李芯, 袁波, 易美桂. 水热法脱除碳酸锂中微量硫杂质的研究[J]. 无机盐工业, 2019, 51(11): 28-30, 53. |
| LI Xin, YUAN Bo, YI Meigui. Study on removal of trace sulfur impurities from lithium carbonate by hydrothermal method[J]. Inorganic Chemicals Industry, 2019, 51(11): 28-30, 53. |
| [1] | ZHANG Yuxin, DENG Ziyang, WANG Can, ZENG Dan. Recent advances of extraction technology and application of fucoxanthin [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 422-433. |
| [2] | LIU Ying, BAO Cheng, ZHANG Xinxin. Modified copper-carrying activated carbon for hydrogen purification [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 413-421. |
| [3] | ZOU Xianzhi, LIAO Yalong, YANG Shuangyu. Research progress on purification and impurity removal in copper electrolyte [J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 492-503. |
| [4] | LIU Jianhua, YUAN Zhenjun, CHANG Xin, ZHAO Xizhe, WAN Ye, YU Xuegong, YANG Deren. Application and technological progress of silicon-based precursors in advanced integrated circuit manufacturing [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5255-5264. |
| [5] | WANG Xiaoguang, DONG Qing, LANG Wenli, HONG Xiangxin, HUANG Zhenxiang, TAN Fengyu, LEI Yizhu, YU Ziyi. Progress on emission reduction and resource utilization of ultra-low concentration methane [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5363-5376. |
| [6] | XU Ruting, ZHAO Jian, SUN Kang, LU Xincheng, JIANG Jianchun, SU Zhonggao, LIU Junli, CHEN Zibiao, SU Zihan. Modification of activated carbon and its purification performance for simulated waste lubricating oil [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 4022-4031. |
| [7] | FU Yu, LI Xiaoyu, WU Yue, TAO Chunhui, DUAN Ran, ZHANG Wenxiang, MA Heping. Removal of trace NF3 impurities from electronic grade CF4 by adsorption [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3570-3578. |
| [8] | DING Wei, BAO Shenxu, XIN Chunfu, WANG Zhanhao, ZHANG Hongwei, KUANG Buxiao. Research progress on preparation and purification technology of wet-process phosphoric acid from middle-low-grade phosphorus ore [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2274-2284. |
| [9] | CAI Ruiyun, JIAO Rui, SUN Hanxue, LI An. Design, preparation and application of asymmetrically wettable Janus organic porous membrane [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2057-2067. |
| [10] | JIANG Yang, CHEN Wei, ZHOU Kanggen, LI Hongbo, LAI Qizhou, NONG Runqiao, PENG Changhong. Optimization and pilot testing of Al/Fe removal processes for acid leachate from spent ternary lithium-ion battery powders [J]. Chemical Industry and Engineering Progress, 2025, 44(10): 6016-6022. |
| [11] | LIU Chuanlei, CHEN Yuxiang, GUO Guanchu, ZHAO Qiyue, JIANG Hao, SUN Hui, SHEN Benxian. Designing novel alkoxypropylamine solvents for removing mercaptans from high-acidity natural gas [J]. Chemical Industry and Engineering Progress, 2025, 44(1): 184-191. |
| [12] | ZHANG Lei, DU Hongying, FENG Wenhao, GUO Junkang. Optimization of interfacial solar photothermal evaporation system based on two-dimensional photothermal materials [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4571-4586. |
| [13] | CHENG Chunhui, MING Shujun, PANG Lei, TIAN Shidong, LI Kelun, LI Tao. Developments in solid porous materials for methane enrichment in coalbed gas [J]. Chemical Industry and Engineering Progress, 2024, 43(11): 6215-6232. |
| [14] | SHANG Gaoyuan, YU Jinpeng, CUI Kai, GUO Kun. Impact of cathode potentials on methane production from high-concentration potato starch wastewater in electro-fermentation systems [J]. Chemical Industry and Engineering Progress, 2024, 43(11): 6533-6542. |
| [15] | SU Shikun, LIU Tang, JIN Ye, ZHENG Jinyu. Advances of adsorption materials for hydrogen purification [J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5612-5632. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||