| [1] |
中华人民共和国环境保护部. 2022年中国生态环境状况公报[M]. 北京: 中华人民共和国环境保护部, 2023.
|
|
Ministry of Ecology and Environment of the People’s Republic of China. Bulletin on China’s ecological and environmental conditions in 2022[M]. Beijing: Ministry of Ecology and Environment of the People’s Republic of China, 2023.
|
| [2] |
GUO Yingming, MA Ben, HUANG Jianxiong, et al. The simultaneous removal of bisphenol A, manganese and ammonium from groundwater by MeO x : The role of chemical catalytic oxidation for bisphenol A[J]. Water Supply, 2022, 22(2): 2106-2116.
|
| [3] |
CHEN Yiran, LIU Lu, ZHANG Lu, et al. Construction of Z-type heterojunction BiVO4/Sm/α-Fe2O3 photoanode for selective degradation: Efficient removal of bisphenol A based on multifunctional Sm-doped modification[J]. Applied Catalysis B: Environment and Energy, 2023, 333: 122775.
|
| [4] |
AHMAD Tauqir, MANZAR Mohammad Saood, GEORGIN Jordana, et al. Development of a new hyper crosslinked resin based on polyamine-isocyanurate for the efficient removal of endocrine disruptor bisphenol-A from water[J]. Journal of Water Process Engineering, 2023, 53: 103623.
|
| [5] |
LI Zixuan, YANG Ziyi, SHI Qinghong, et al. Removal of bisphenol A by integrated adsorption and biodegradation using immobilized laccase onto defective PCN-224[J]. Journal of Environmental Chemical Engineering, 2023, 11(3): 110166.
|
| [6] |
MOREIRA Carolina G, MOREIRA Mariana H, SILVA Vanessa M O C, et al. Treatment of bisphenol A (BPA) in water using UV/H2O2 and reverse osmosis (RO) membranes: Assessment of estrogenic activity and membrane adsorption[J]. Water Science and Technology, 2019, 80(11): 2169-2178.
|
| [7] |
BABAEI Hassan, GHOBADI NEJAD Zahra, YAGHMAEI Soheila, et al. Co-immobilization of multi-enzyme cascade system into the metal-organic frameworks for the removal of bisphenol A[J]. Chemical Engineering Journal, 2023, 461: 142050.
|
| [8] |
ALDHAWI Zainah A, BINSHARFAN Ibtisam I, ABDULHAMID Mahmoud A. Carboxyl-functionalized polyimides for efficient bisphenol A removal: Influence of wettability and porosity on adsorption capacity[J]. Chemosphere, 2023, 313: 137347.
|
| [9] |
郭雅婧, 越楚遥, 李金成, 等. BiOBr掺杂TiO2纳米管阵列光催化降解水中微量双酚A的性能及机理[J]. 环境工程学报, 2022, 16(9): 2817-2827.
|
|
GUO Yajing, YUE Chuyao, LI Jincheng, et al. Performance and mechanism of BiOBr modified TiO2 nanotube arrays photocatalytic degradation of trace BPA in water[J]. Chinese Journal of Environmental Engineering[J]. 2022, 16(9): 2817-2827.
|
| [10] |
KLIS Simona, BARBUSINSKI Krzysztof, THOMAS Maciej, et al. Application of potassium ferrate(Ⅵ) for oxidation of selected pollutants in aquatic environment—Short review[J]. Architecture, Civil Engineering, Environment, 2019, 12(1): 129-137.
|
| [11] |
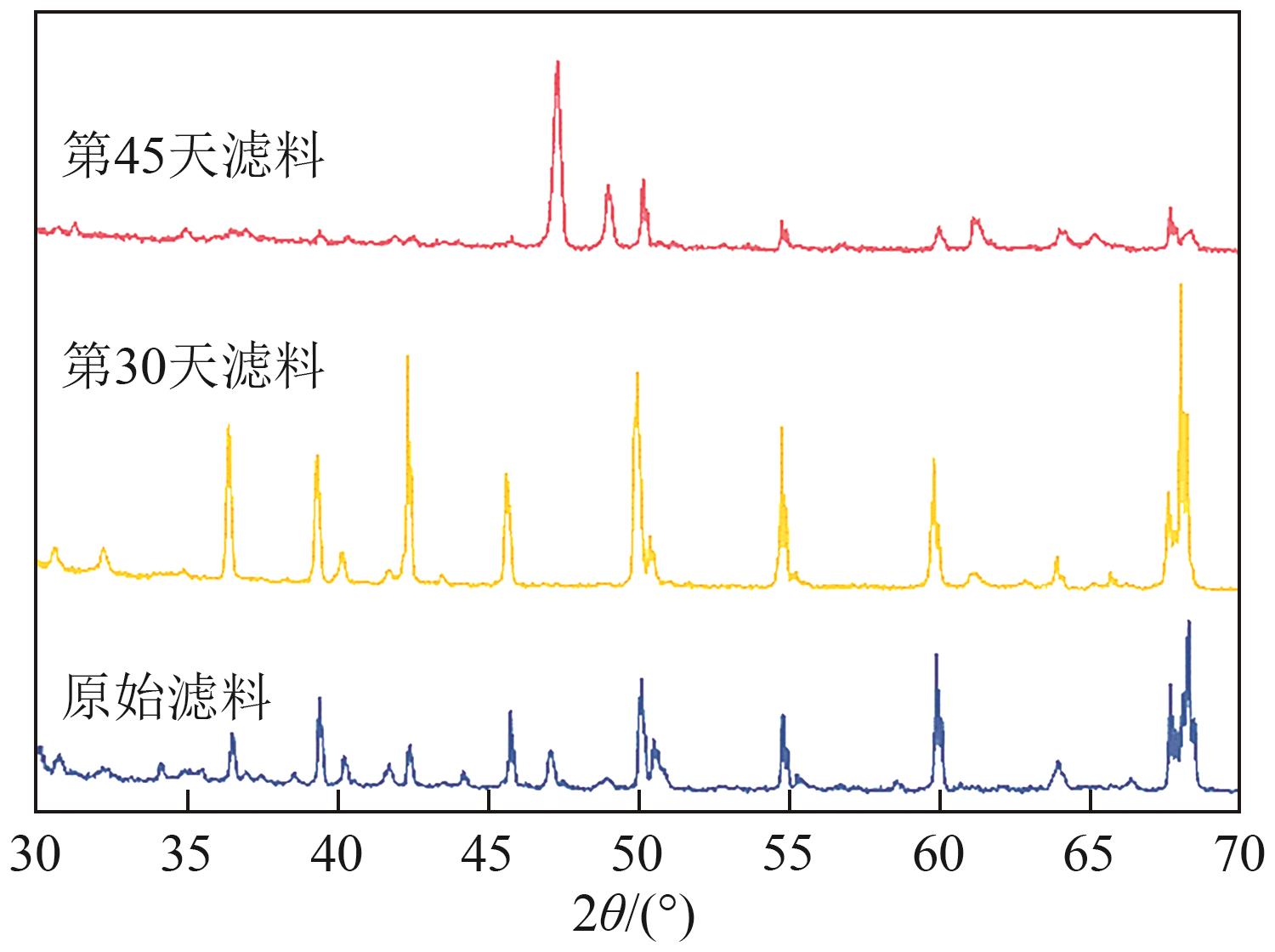
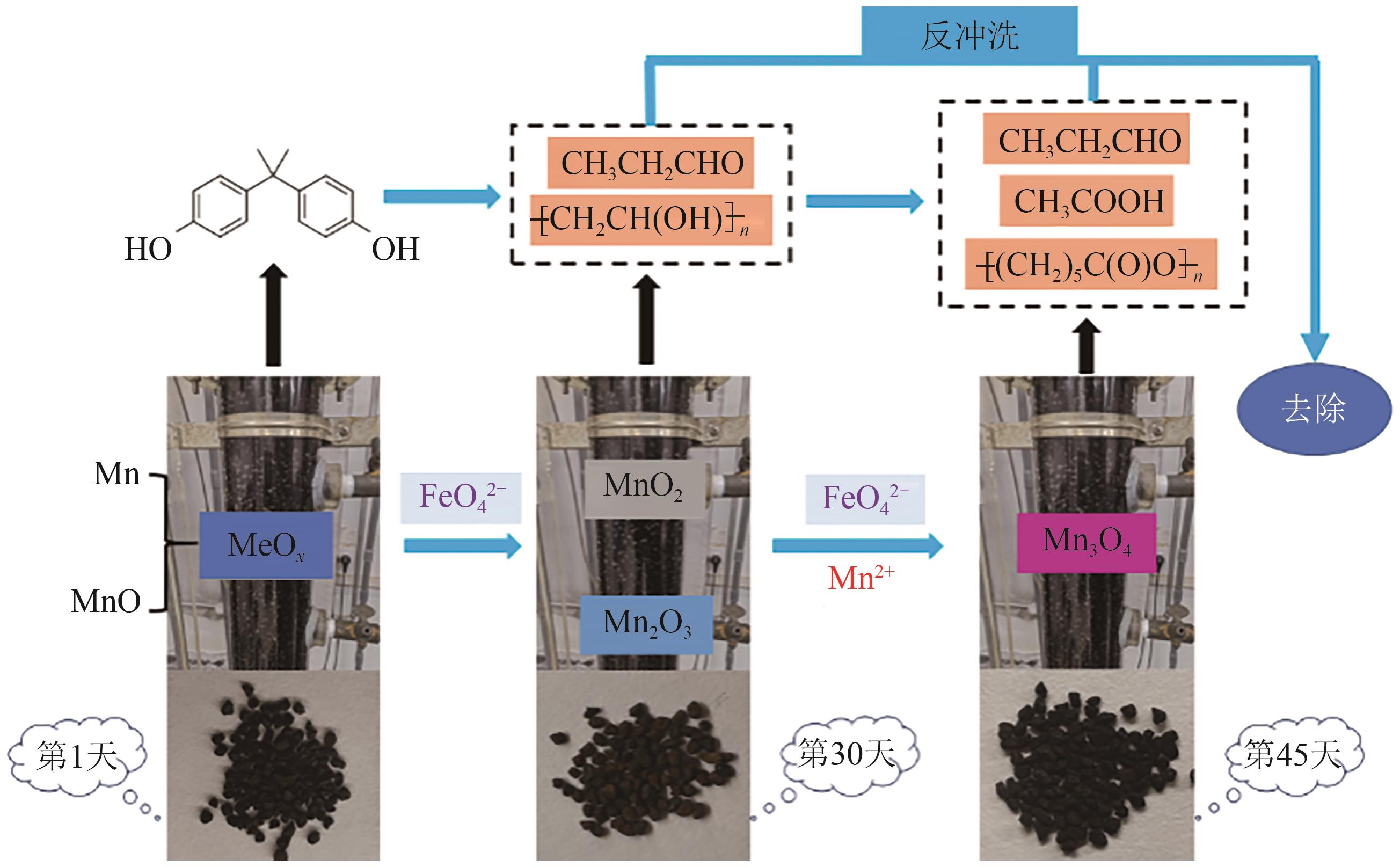
GUO Yingming, MA Ben, YUAN Shengchen, et al. Simultaneous removal of CODMn and ammonium from water by potassium ferrate-enhanced iron-manganese co-oxide film[J]. Water, 2022, 14(17): 2651.
|
| [12] |
郭英明, 张宇宏, 麻奔, 等. 高铁酸钾强化铁锰氧化膜过滤去除水中有机物[J]. 化工进展, 2022, 41(11): 6130-6138.
|
|
GUO Yingming, ZHANG Yuhong, MA Ben, et al. Removal of CODMn in water by potassium ferrate enhanced iron-manganeseoxide film filtration and its influencing factors[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 6130-6138.
|
| [13] |
ZHAO Junfeng, WANG Qun, FU Yongsheng, et al. Kinetics and mechanism of diclofenac removal using ferrate(Ⅵ): Roles of Fe3+, Fe2+, and Mn2+ [J]. Environmental Science and Pollution Research International, 2018, 25(23): 22998-23008.
|
| [14] |
GOODWILL Joseph E, Xuyen MAI, JIANG Yanjun, et al. Oxidation of manganese(Ⅱ) with ferrate: Stoichiometry, kinetics, products and impact of organic carbon[J]. Chemosphere, 2016, 159: 457-464.
|
| [15] |
MONFORT Olivier, USMAN Muhammad, SOUTREL Isabelle, et al. Ferrate(Ⅳ) based chemical oxidation for the remediation of aged PCB contaminated soil: Comparison with conventional oxidants and study of limiting factors[J]. Chemical Engineering Journal, 2019, 355: 109-117.
|
| [16] |
BARBER M, CONNOR J A, GUEST M F, et al. Bonding in some donor-acceptor complexes involving boron trifluoride. Study by means of ESCA and molecular orbital calculations[J]. J Chem Soc, Faraday Trans 2, 1973, 69: 551-558.
|
| [17] |
SCHULZ Kirk H, David F COX. Surface reactions of acrolein and propionaldehyde on cuprous oxide(100): Nonselective oxidation and enolate-mediated side reactions to C3 products[J]. The Journal of Physical Chemistry, 1993, 97(14): 3555-3564.
|
| [18] |
GELIUS U, P-F HEDÉN, HEDMAN J, et al. Molecular spectroscopy by means of ESCA Ⅲ. Carbon compounds[J]. Physica Scripta, 1970, 2(1/2): 70-80.
|
 ), GUO Yingming(
), GUO Yingming( ), CAO Yuanyuan, ZHANG Yuhong, ZHANG Zhekai
), CAO Yuanyuan, ZHANG Yuhong, ZHANG Zhekai