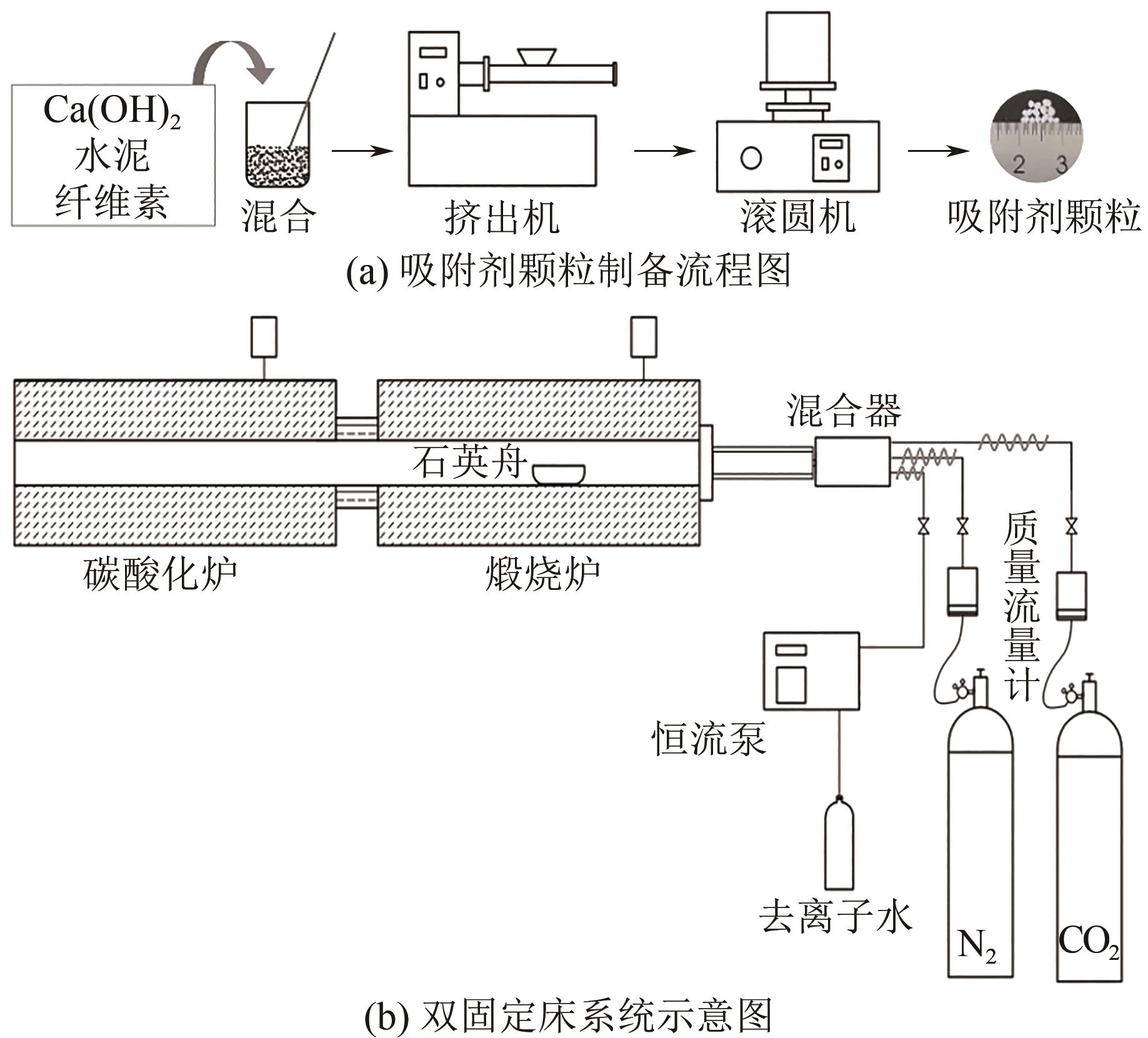
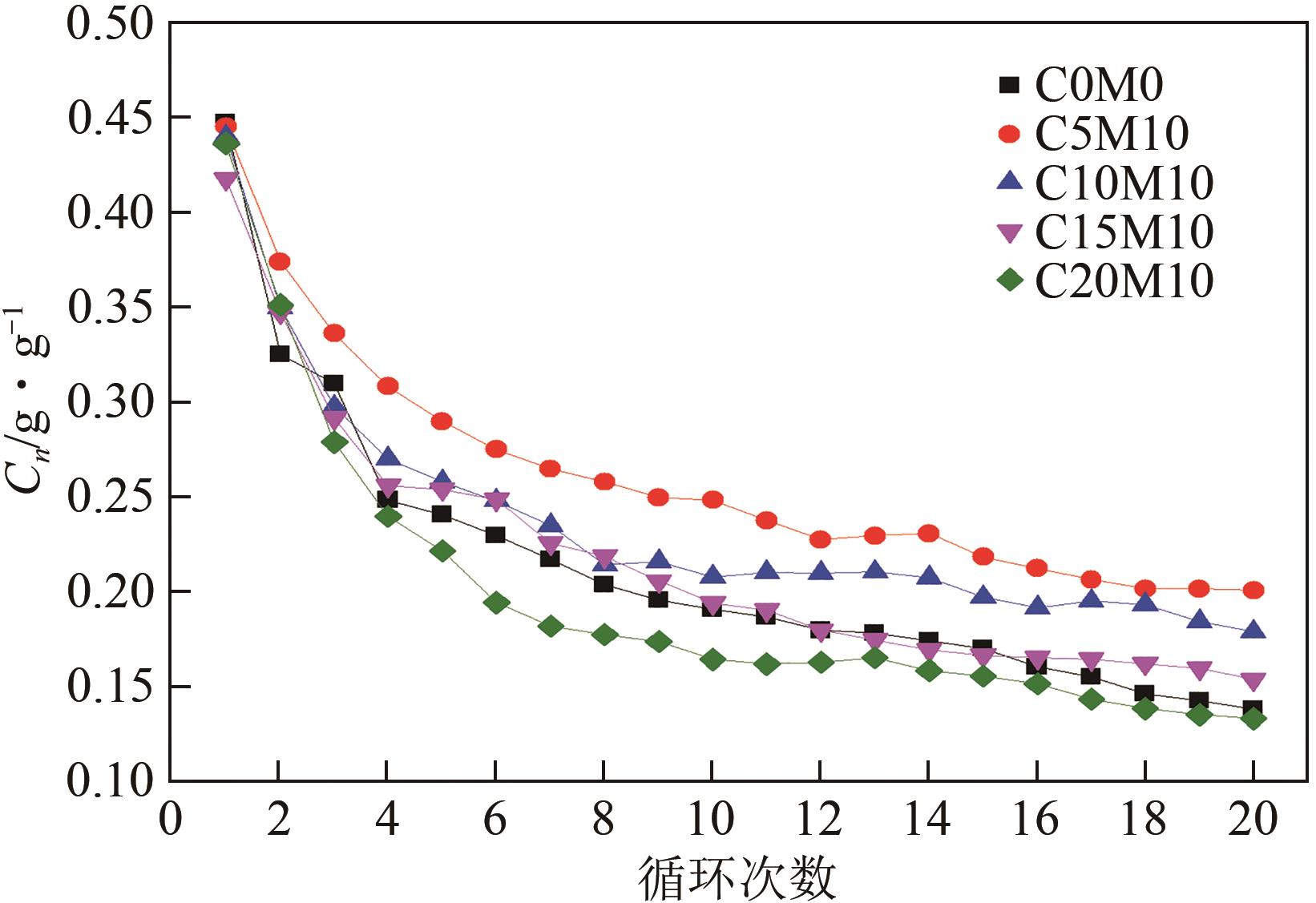
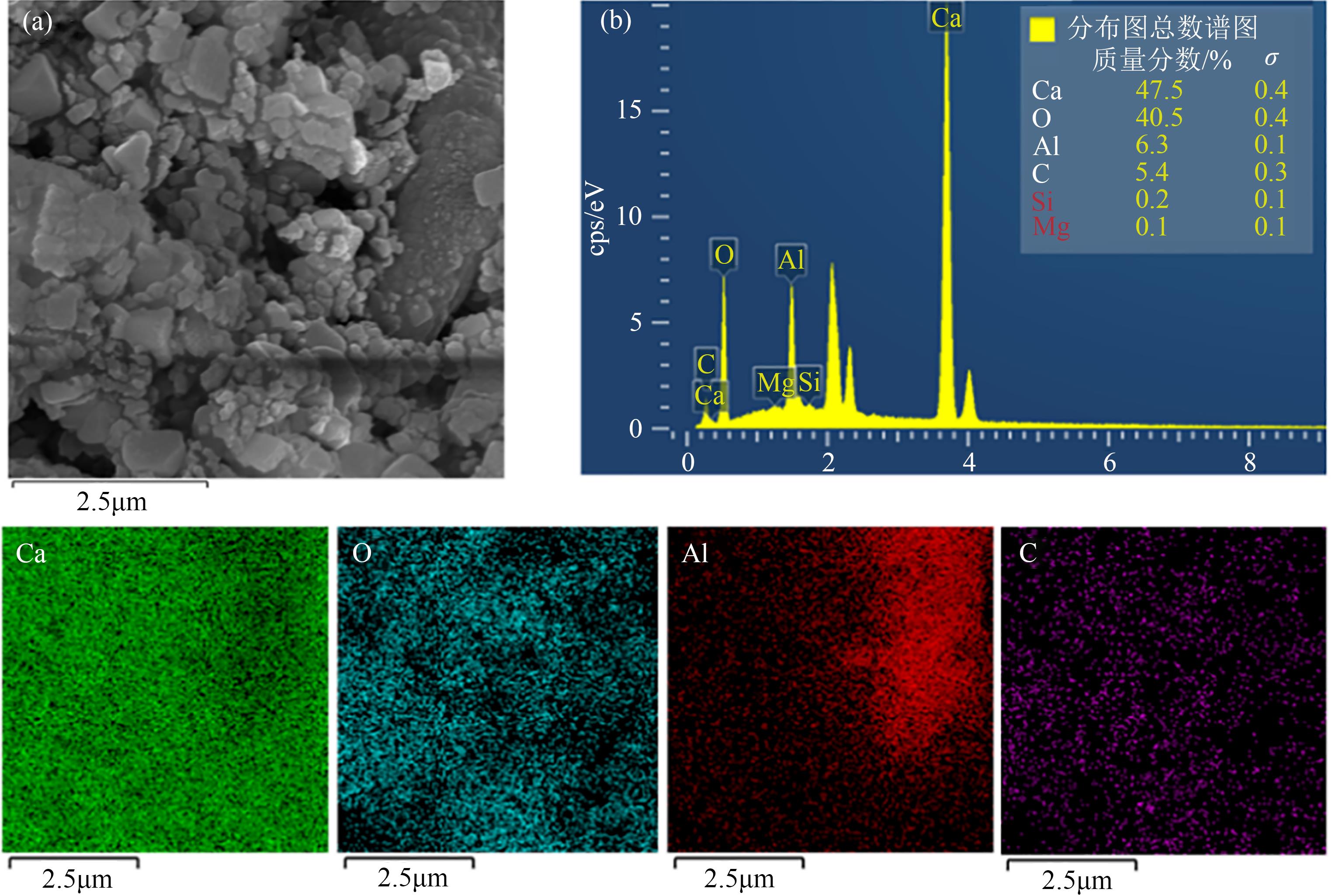
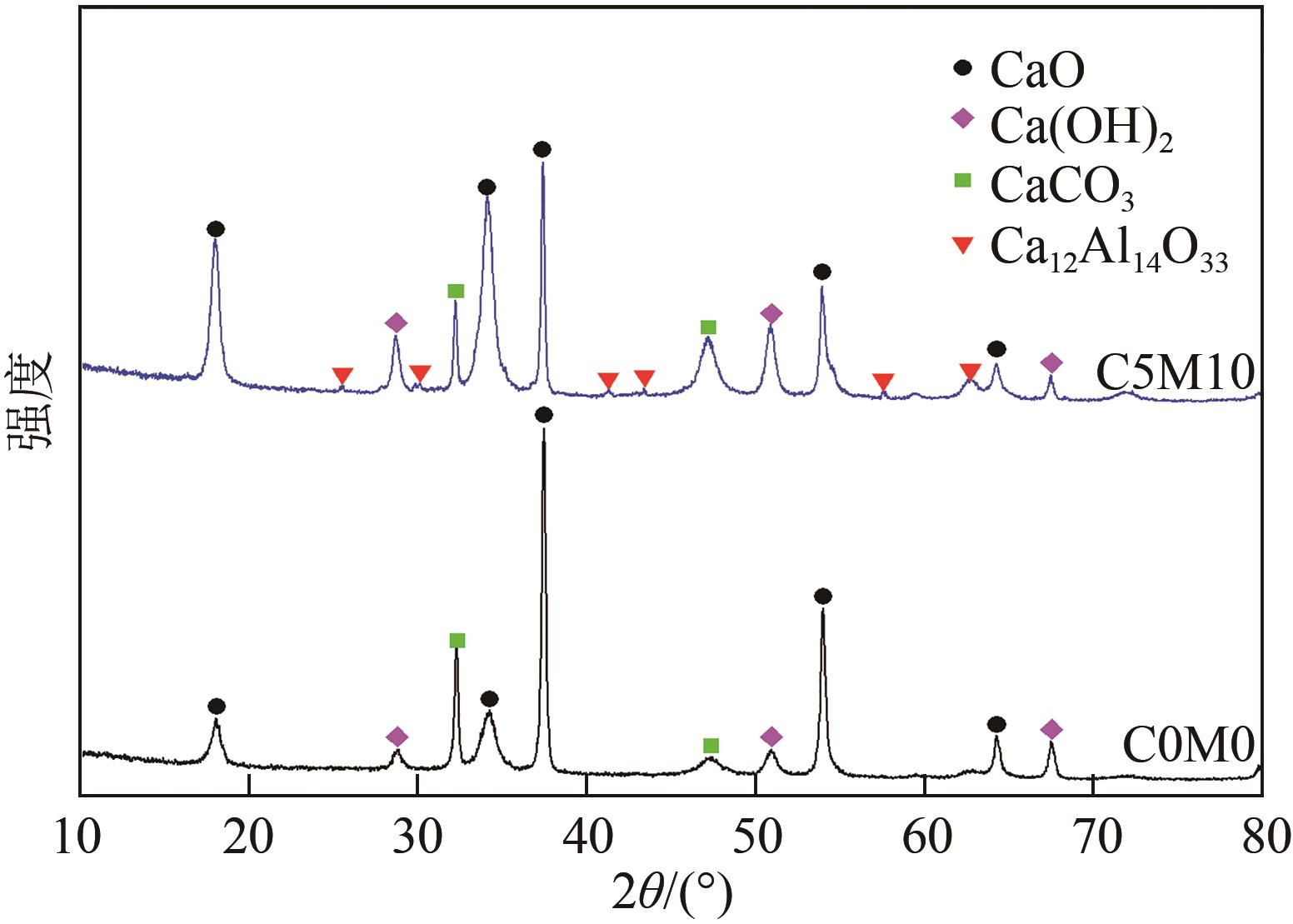
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (6): 3217-3225.DOI: 10.16085/j.issn.1000-6613.2022-1478
• Resources and environmental engineering • Previous Articles Next Articles
Enhanced CO2 capture performance and strength of cellulose-templated CaO-based pellets with steam reactivation
WANG Jiuheng1( ), RONG Nai1,2(
), RONG Nai1,2( ), LIU Kaiwei3, HAN Long4, SHUI Taotao1, WU Yan1, MU Zhengyong1, LIAO Xuqing1, MENG Wenjia1
), LIU Kaiwei3, HAN Long4, SHUI Taotao1, WU Yan1, MU Zhengyong1, LIAO Xuqing1, MENG Wenjia1
- 1.School of Environment and Energy Engineering, Anhui Jianzhu University, Hefei 230601, Anhui, China
2.Anhui Institute of Strategic Study in Carbon Emissions Peak and Carbon Neutrality in Urban-rural Development, Hefei 230601, Anhui, China
3.Anhui Province Engineering Laboratory of Advanced Building Materials, Anhui Jianzhu University, Hefei 230601, Anhui, China
4.School of Mechanical Engineering, Zhejiang University of Technology, Hangzhou 310014, Zhejiang, China
-
Received:2022-08-10Revised:2022-10-07Online:2023-06-29Published:2023-06-25 -
Contact:RONG Nai
水蒸气强化纤维素模板改性钙基吸附剂固碳性能及强度
王久衡1( ), 荣鼐1,2(
), 荣鼐1,2( ), 刘开伟3, 韩龙4, 水滔滔1, 吴岩1, 穆正勇1, 廖徐情1, 孟文佳1
), 刘开伟3, 韩龙4, 水滔滔1, 吴岩1, 穆正勇1, 廖徐情1, 孟文佳1
- 1.安徽建筑大学环境与能源工程学院,安徽 合肥 230601
2.安徽省建设领域碳达峰碳中和战略研究院,安徽 合肥 230601
3.安徽建筑大学安徽省先进建筑材料工程实验室,安徽 合肥 230601
4.浙江工业大学机械工程学院,浙江 杭州 310014
-
通讯作者:荣鼐 -
作者简介:王久衡(1997—),男,硕士研究生,研究方向为高温CO2捕集。E-mail:457356146@qq.com。 -
基金资助:国家自然科学基金(51706002);安徽省自然科学基金(1808085QE128);安徽省高校省级自然科学研究重点项目(KJ2019A0762);安徽省高校自然科学研究项目(2022AH030034)
CLC Number:
Cite this article
WANG Jiuheng, RONG Nai, LIU Kaiwei, HAN Long, SHUI Taotao, WU Yan, MU Zhengyong, LIAO Xuqing, MENG Wenjia. Enhanced CO2 capture performance and strength of cellulose-templated CaO-based pellets with steam reactivation[J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3217-3225.
王久衡, 荣鼐, 刘开伟, 韩龙, 水滔滔, 吴岩, 穆正勇, 廖徐情, 孟文佳. 水蒸气强化纤维素模板改性钙基吸附剂固碳性能及强度[J]. 化工进展, 2023, 42(6): 3217-3225.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-1478
| 样品 | CaO | Al2O3 | MgO | SiO2 | K2O | Fe2O3 | SrO | 其他 |
|---|---|---|---|---|---|---|---|---|
| C0M0 | 98.543 | 0.196 | 0.302 | 0.311 | 0.052 | 0.297 | 0.099 | 0.201 |
| C5M10 | 95.477 | 3.518 | 0.568 | 0.179 | 0.013 | 0.123 | 0.022 | 0.101 |
| C10M10 | 91.877 | 7.127 | 0.529 | 0.198 | 0.015 | 0.124 | 0.025 | 0.106 |
| C15M10 | 87.682 | 11.29 | 0.498 | 0.228 | 0 | 0.179 | 0.017 | 0.105 |
| C20M10 | 83.876 | 15.017 | 0.541 | 0.231 | 0.013 | 0.149 | 0.021 | 0.151 |
| 样品 | CaO | Al2O3 | MgO | SiO2 | K2O | Fe2O3 | SrO | 其他 |
|---|---|---|---|---|---|---|---|---|
| C0M0 | 98.543 | 0.196 | 0.302 | 0.311 | 0.052 | 0.297 | 0.099 | 0.201 |
| C5M10 | 95.477 | 3.518 | 0.568 | 0.179 | 0.013 | 0.123 | 0.022 | 0.101 |
| C10M10 | 91.877 | 7.127 | 0.529 | 0.198 | 0.015 | 0.124 | 0.025 | 0.106 |
| C15M10 | 87.682 | 11.29 | 0.498 | 0.228 | 0 | 0.179 | 0.017 | 0.105 |
| C20M10 | 83.876 | 15.017 | 0.541 | 0.231 | 0.013 | 0.149 | 0.021 | 0.151 |
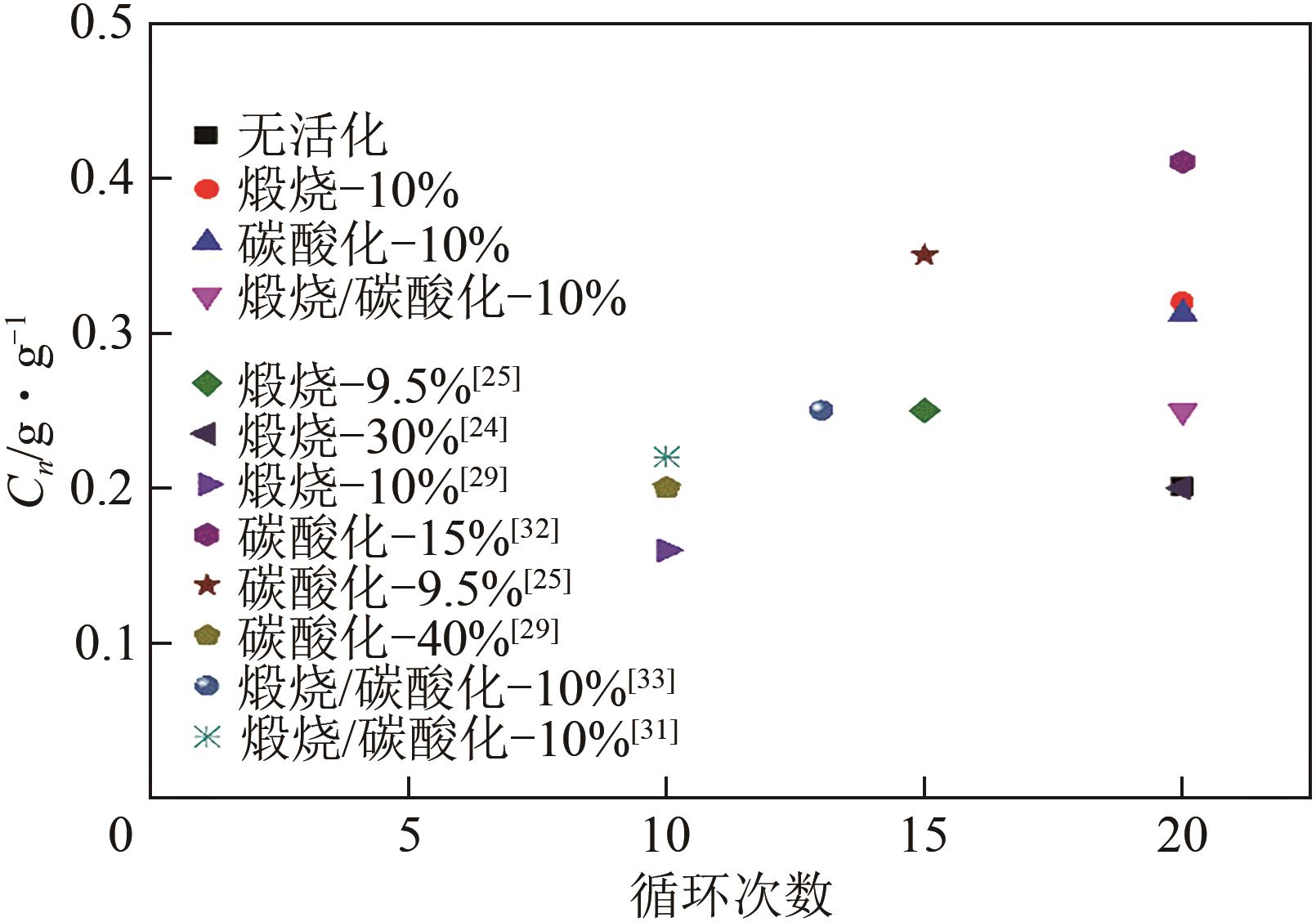
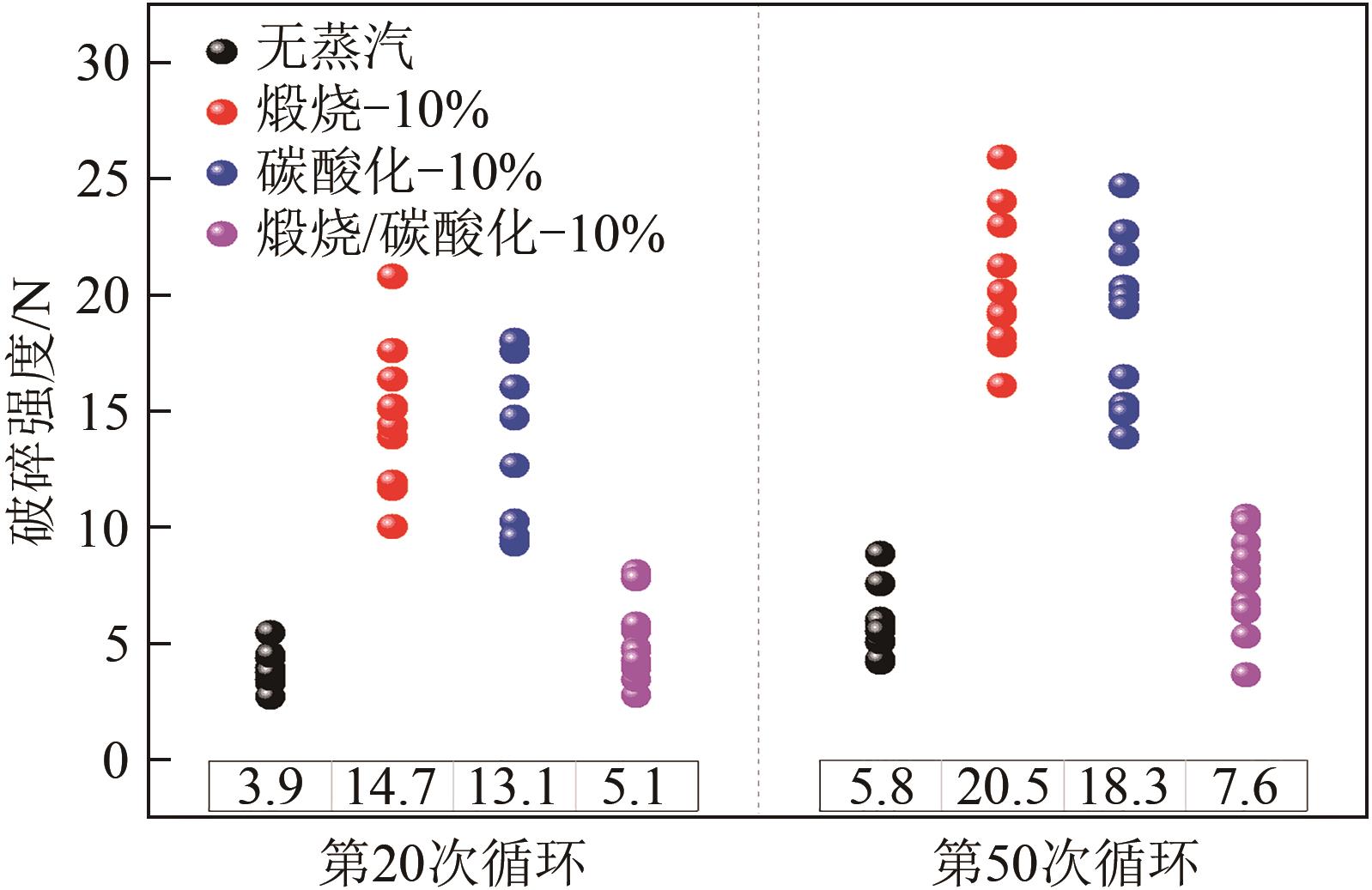
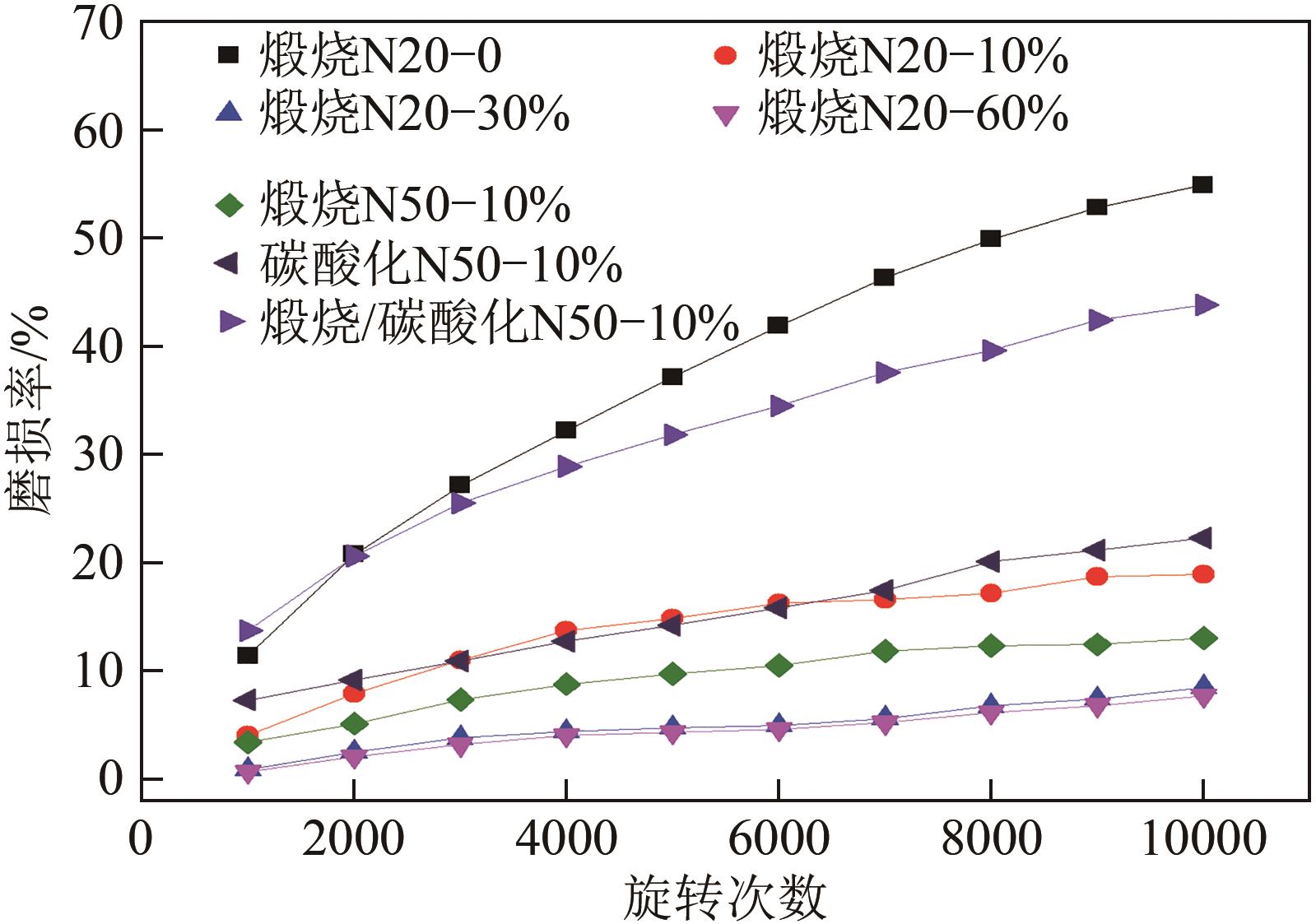
| 蒸汽 体积分数/% | C20/g·g-1 | 提升幅度 (与C0M0对比)/% | 提升幅度 (与未活化C5M10对比)/% |
|---|---|---|---|
| 0 | 0.20 | 45 | 0 |
| 10 | 0.32 | 132 | 60 |
| 30 | 0.245 | 77.5 | 22.5 |
| 60 | 0.22 | 59.0 | 10 |
| 蒸汽 体积分数/% | C20/g·g-1 | 提升幅度 (与C0M0对比)/% | 提升幅度 (与未活化C5M10对比)/% |
|---|---|---|---|
| 0 | 0.20 | 45 | 0 |
| 10 | 0.32 | 132 | 60 |
| 30 | 0.245 | 77.5 | 22.5 |
| 60 | 0.22 | 59.0 | 10 |
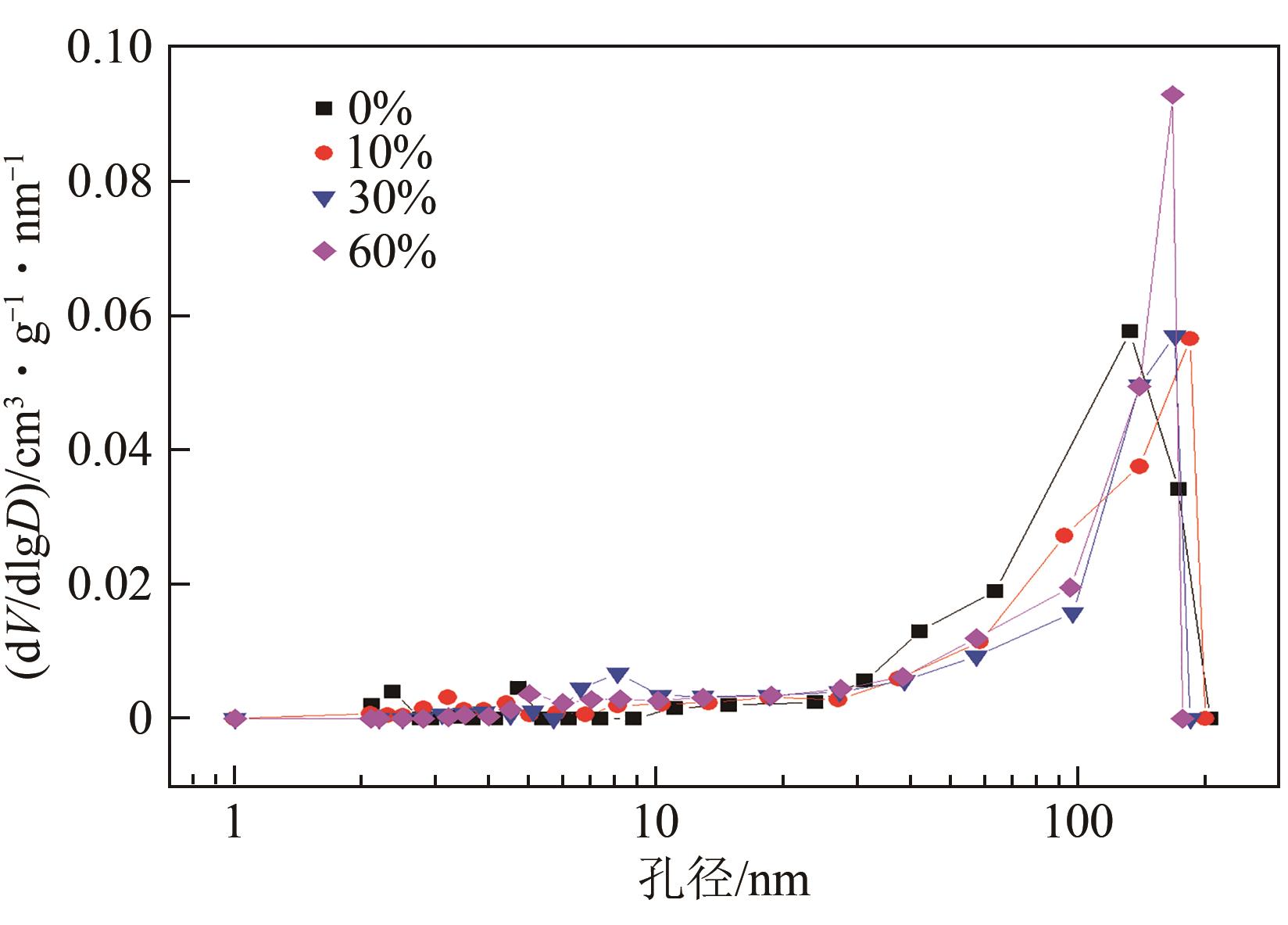
| 蒸汽体积分数/% | 比表面积/m2·g-1 | 比孔容/cm3·g-1 |
|---|---|---|
| 0 | 3.242 | 0.0322 |
| 10 | 3.124 | 0.0216 |
| 30 | 2.602 | 0.0310 |
| 60 | 2.705 | 0.0231 |
| 蒸汽体积分数/% | 比表面积/m2·g-1 | 比孔容/cm3·g-1 |
|---|---|---|
| 0 | 3.242 | 0.0322 |
| 10 | 3.124 | 0.0216 |
| 30 | 2.602 | 0.0310 |
| 60 | 2.705 | 0.0231 |
| 1 | IPCC. Climate change 2021: the physical science basis[M]. Cambridge: Cambridge University Press, 2021. |
| 2 | ROGELJ J, DEN E M, HÖHNE N, et al. Paris Agreement climate proposals need a boost to keep warming well below 2℃[J]. Nature, 2016, 534(7609): 631-639. |
| 3 | LEONZIO G, FOSCOLO P U, ZONDERVAN E, et al. Scenario analysis of carbon capture, utilization (particularly producing methane and methanol), and storage (CCUS) systems[J]. Industrial & Engineering Chemistry Research, 2020, 59(15): 6961-6976. |
| 4 | DUNSTAN M T, DONAT F, BORK A H, et al. CO2 capture at medium to high temperature using solid oxide-based sorbents: fundamental aspects, mechanistic insights, and recent advances[J]. Chemical Reviews, 2021, 121(20): 12681-12745. |
| 5 | SUN H, WU C, SHEN B, et al. Progress in the development and application of CaO-based adsorbents for CO2 capture: a review[J]. Materials Today Sustainability, 2018, 1/2: 1-27. |
| 6 | CHEN J, DUAN L B, SUN Z K. Review on the development of sorbents for calcium looping[J]. Energy & Fuels, 2020, 34(7): 7806-7836. |
| 7 | WANG K, GU F, CLOUGH P T, et al. CO2 capture performance of gluconic acid modified limestone-dolomite mixtures under realistic conditions[J]. Energy & Fuels, 2019, 33(8): 7550-7560. |
| 8 | HU Y C, LIU W Q, SUN J, et al. Structurally improved CaO-based sorbent by organic acids for high temperature CO2 capture[J]. Fuel, 2016, 167: 17-24. |
| 9 | 王保文, 姜涛, 许炳辉, 等. 废弃蛋壳源高性能钙基吸收剂的制备及其碳捕集性能[J]. 化工进展, 2022, 41(3): 1289-1297. |
| WANG Baowen, JIANG Tao, XU Binghui, et al. Preparation of the calcium based adsorbent derived from egg shell waste and its CO2 capture performance in the calcium looping[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1289-1297. | |
| 10 | GUO H X, KOU X C, ZHAO Y J, et al. Effect of synergistic interaction between Ce and Mn on the CO2 capture of calcium-based sorbent: textural properties, electron donation, and oxygen vacancy[J]. Chemical Engineering Journal, 2018, 334: 237-246. |
| 11 | 郭红霞, 南雁, 寇晓晨, 等. 钙基CO2吸附剂的惰性掺杂和形貌调控研究进展[J]. 化工进展, 2019, 38(1): 457-466. |
| GUO Hongxia, Yan NAN, KOU Xiaochen, et al. Research on doping modification and morphology control of calcium-based CO2 sorbents[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 457-466. | |
| 12 | CHEN Y N, LONG Y, SUN J, et al. Core-in-shell, cellulose-templated CaO-based sorbent pellets for CO2 capture at elevated temperatures[J]. Energy & Fuels, 2021, 35(16): 13215-13223. |
| 13 | SUN J, LIU W Q, HU Y C, et al. Structurally improved, core-in-shell, CaO-based sorbent pellets for CO2 capture[J]. Energy & Fuels, 2015, 29(10): 6636-6644. |
| 14 | SEDGHKERDAR M H, MAHINPEY N, SOLEIMANISALIM A H, et al. Core-shell structured CaO-based pellets protected by mesoporous ceramics shells for high-temperature CO2 capture[J]. The Canadian Journal of Chemical Engineering, 2016, 94(11): 2038-2044. |
| 15 | 迟长云, 李英杰. 钙基碳载体造粒的捕集CO2特性及力学性能[J]. 化工进展, 2018, 37(12): 4908-4916. |
| CHI Changyun, LI Yingjie. CO2 capture performance and mechanical properties of granulated calcium-based sorbent[J]. Chemical Industry and Engineering Progress, 2018, 37(12): 4908-4916. | |
| 16 | 梁成. 生物质模板改性钙基吸收剂颗粒循环脱碳性能研究[D]. 南京: 南京师范大学, 2019. |
| LIANG Cheng. Study on cyclic CO2 capture performance of biomass-templated CaO-based sorbent pellets[D]. Nanjing: Nanjing Normal University, 2019. | |
| 17 | ZHOU Y, CHEN Y N, LI W L, et al. High-temperature CO2 uptake and mechanical strength enhancement of the calcium aluminate cement-bound carbide slag pellets[J]. Energy & Fuels, 2021, 35(9): 8117-8125. |
| 18 | SUN J, WANG W Y, YANG Y D, et al. Reactivation mode investigation of spent CaO-based sorbent subjected to CO2 looping cycles or sulfation[J]. Fuel, 2020, 266: 117056. |
| 19 | RONG N, WU Y, WANG J H, et al. Steam reactivation of biotemplated CaO-based pellets for cyclic CO2 capture[J]. Energy & Fuels, 2021, 35(7): 6056-6067. |
| 20 | 余志健, 段伦博, 苏成林, 等. 蒸汽活化水泥支撑钙基吸收剂活性及强度特性[J]. 化工学报, 2017, 68(4): 1637-1645. |
| YU Zhijian, DUAN Luobo, SU Chenglin, et al. Reactivity and strength of cement-supported calcium sorbent reactivated by steam for CO2 capture[J]. CIESC Journal, 2017, 68(4): 1637-1645. | |
| 21 | GUO H X, YAN S L, ZHAO Y J, et al. Influence of water vapor on cyclic CO2 capture performance in both carbonation and decarbonation stages for Ca-Al mixed oxide[J]. Chemical Engineering Journal, 2019, 359: 542-551. |
| 22 | CHEN L Y, QIAN N. The effects of water vapor and coal ash on the carbonation behavior of CaO-sorbent supported by γ-Al2O3 for CO2 capture[J]. Fuel Processing Technology, 2018, 177: 200-209. |
| 23 | HE Z R, LI Y J, MA X T, et al. Influence of steam in carbonation stage on CO2 capture by Ca-based industrial waste during calcium looping cycles[J]. International Journal of Hydrogen Energy, 2016, 41(7): 4296-4304. |
| 24 | CHOU Y C, CHENG J Y, LIU W H, et al. Effects of steam addition during calcination on carbonation behavior in a calcination/carbonation loop[J]. Chemical Engineering & Technology, 2018, 41(10): 1921-1927. |
| 25 | SHOKROLLAHI Y M, RADFARNIA H R, ILIUTA M C. Influence of steam addition during carbonation or calcination on the CO2 capture performance of Ca9Al6O18 CaO sorbent[J]. Journal of Natural Gas Science and Engineering, 2016, 36: 1062-1069. |
| 26 | SILAKHORI M, JAFARIAN M, CHINNICI A, et al. Effects of steam on the kinetics of calcium carbonate calcination[J]. Chemical Engineering Science, 2021, 246: 116987. |
| 27 | HAN L, LIU Q, ZHANG Y, et al. A novel hybrid iron-calcium catalyst/absorbent for enhanced hydrogen production via catalytic tar reforming with in-situ CO2 capture[J]. International Journal of Hydrogen Energy, 2020, 45(18): 10709-10723. |
| 28 | 马晓彤. 高效钙铝碳载体循环捕集CO2性能及其反应机理的密度泛函理论研究[D]. 济南:山东大学, 2019. |
| MA Xiaotong. Research on cyclic CO2 capture performance of Ca/Al sorbents and reaction mechanism by experiment and DTF caclculations[D]. Jinan: Shandong University, 2019. | |
| 29 | DONG J, TANG Y J, NZIHOU A, et al. Effect of steam addition during carbonation, calcination or hydration on re-activation of CaO sorbent for CO2 capture[J]. Journal of CO2 Utilization, 2020, 39: 101167. |
| 30 | PHALAK N, DESHPANDE N, FAN L S. Investigation of high-temperature steam hydration of naturally derived calcium oxide for improved carbon dioxide capture capacity over multiple cycles[J]. Energy & Fuels, 2012, 26(6): 3903-3909. |
| 31 | DONAT F, FLORIN N H, ANTHONY E J, et al. Influence of high-temperature steam on the reactivity of CaO sorbent for CO2 capture[J]. Environmental Science & Technollogy, 2012, 46(2): 1262-1269. |
| 32 | RIDHA F N, WU Y, MANOVIC V, et al. Enhanced CO2 capture by biomass-templated Ca(OH)2-based pellets[J]. Chemical Engineering Journal, 2015, 274: 69-75. |
| 33 | GONZÁLEZ B, BLAMEY J, AL-JEBOORI M J, et al. Additive effects of steam addition and HBr doping for CaO-based sorbents for CO2 capture[J]. Chemical Engineering and Processing: Process Intensification, 2016, 103: 21-26. |
| 34 | XU Y Q, DING H R, LUO C, et al. Effect of lignin, cellulose and hemicellulose on calcium looping behavior of CaO-based sorbents derived from extrusion-spherization method[J]. Chemical Engineering Journal, 2018, 334: 2520-2529. |
| 35 | CHAMPAGNE S, LU D Y, SYMONDS R T, et al. The effect of steam addition to the calciner in a calcium looping pilot plant[J]. Powder Technology, 2016, 290: 114-123. |
| [1] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [2] | DAI Huantao, CAO Lingyu, YOU Xinxiu, XU Haoliang, WANG Tao, XIANG Wei, ZHANG Xueyang. Adsorption properties of CO2 on pomelo peel biochar impregnated by lignin [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 356-363. |
| [3] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [4] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [5] | BAI Yadi, DENG Shuai, ZHAO Ruikai, ZHAO Li, YANG Yingxia. Exploration on standardized test scheme and experimental performance of temperature swing adsorption carbon capture unit [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3834-3846. |
| [6] | LU Shijian, ZHANG Yuanyuan, WU Wenhua, YANG Fei, LIU Ling, KANG Guojun, LI Qingfang, CHEN Hongfu, WANG Ning, WANG Feng, ZHANG Juanjuan. Health risk assessment of nitrosamine pollutant diffusion in a million ton CO2 capture project [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3209-3216. |
| [7] | GU Shiya, DONG Yachao, LIU Linlin, ZHANG Lei, ZHUANG Yu, DU Jian. Design and optimization of pipeline system for carbon capture considering intermediate nodes [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2799-2808. |
| [8] | SANG Wei, TANG Jianfeng, HUA Yihuai, CHEN Jie, SUN Peiyuan, XU Yifei. Effects of physical solvent and amine properties on the performance of biphasic solvent [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2151-2159. |
| [9] | SHANG Yu, XIAO Man, CUI Qiufang, TU Te, YAN Shuiping. Recovery characteristics of PVDF/BN-OH flat composite membrane for waste heat of hot stripped gas in CO2 capture process [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1618-1628. |
| [10] | KONG Xiangru, ZHANG Xiaoyang, SUN Pengxiang, CUI Lin, DONG Yong. Research progress of solid porous materials for direct CO2 capture from air [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1471-1483. |
| [11] | WANG Lu, ZHANG Lei, DU Jian. High-throughput screening of zeolite materials for CO2/N2 selective adsorption separation by machine learning [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 148-158. |
| [12] | LU Shijian, LIU Miaomiao, LIU Ling, KANG Guojun, MAO Songbai, WANG Feng, ZHANG Juanjuan, GONG Yuping. Progress and future development trend of amine method of CO2 capture technology from flue gas [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 435-444. |
| [13] | SHEN Tianxu, SHEN Laihong. Investigation of multi-fuel chemical looping combustion in a 3kW interconnected fluidized bed reactors [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 138-147. |
| [14] | MENG Lingding, MAO Menglei, LIAO Qiyong, MENG Zihui, LIU Wenfang. Recent advance in stability of carbonic anhydrase and formate dehydrogenase [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 436-447. |
| [15] | FANG Longlong, ZHENG Wenji, NING Mengjia, ZHANG Mingyang, YANG Yuqing, DAI Yan, HE Gaohong. Enhanced CO2 separation of mixed matrix membranes by functionalized Zr-MOF [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4954-4962. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||