Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (11): 5887-5895.DOI: 10.16085/j.issn.1000-6613.2022-0037
• Industrial catalysis • Previous Articles Next Articles
Nitrogen-doped carbon-confined flower-like SnS catalyst for electrochemical reduction of CO2 to HCOOH
HUANG Xin1( ), LIU Cheng2, TANG Rujia3, HAN Xinxin3, CHEN Shixia1(
), LIU Cheng2, TANG Rujia3, HAN Xinxin3, CHEN Shixia1( ), WANG Jun1
), WANG Jun1
- 1.School of Chemistry and Chemical Engineering, Nanchang University, Nanchang 330031, Jiangxi, China
2.School of Future Technology, Nanchang University, Nanchang 330031, Jiangxi, China
3.School of Resources & Environment, Nanchang University, Nanchang 330031, Jiangxi, China
-
Received:2022-01-06Revised:2022-04-10Online:2022-11-28Published:2022-11-25 -
Contact:CHEN Shixia
氮掺杂碳限域的花状SnS催化CO2电还原制甲酸
黄鑫1( ), 刘成2, 唐如佳3, 韩欣欣3, 陈世霞1(
), 刘成2, 唐如佳3, 韩欣欣3, 陈世霞1( ), 王珺1
), 王珺1
- 1.南昌大学化学化工学院,江西 南昌 330031
2.南昌大学未来技术学院,江西 南昌 330031
3.南昌大学资源与环境学院,江西 南昌 330031
-
通讯作者:陈世霞 -
作者简介:黄鑫(1998—),女,硕士研究生,研究方向为电化学还原CO2。E-mail:1270583553@qq.com。 -
基金资助:国家自然科学基金(22008101);江西省自然科学基金(20212BAB213038);江西省研究生创新基金(YC2021-S045)
CLC Number:
Cite this article
HUANG Xin, LIU Cheng, TANG Rujia, HAN Xinxin, CHEN Shixia, WANG Jun. Nitrogen-doped carbon-confined flower-like SnS catalyst for electrochemical reduction of CO2 to HCOOH[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5887-5895.
黄鑫, 刘成, 唐如佳, 韩欣欣, 陈世霞, 王珺. 氮掺杂碳限域的花状SnS催化CO2电还原制甲酸[J]. 化工进展, 2022, 41(11): 5887-5895.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0037
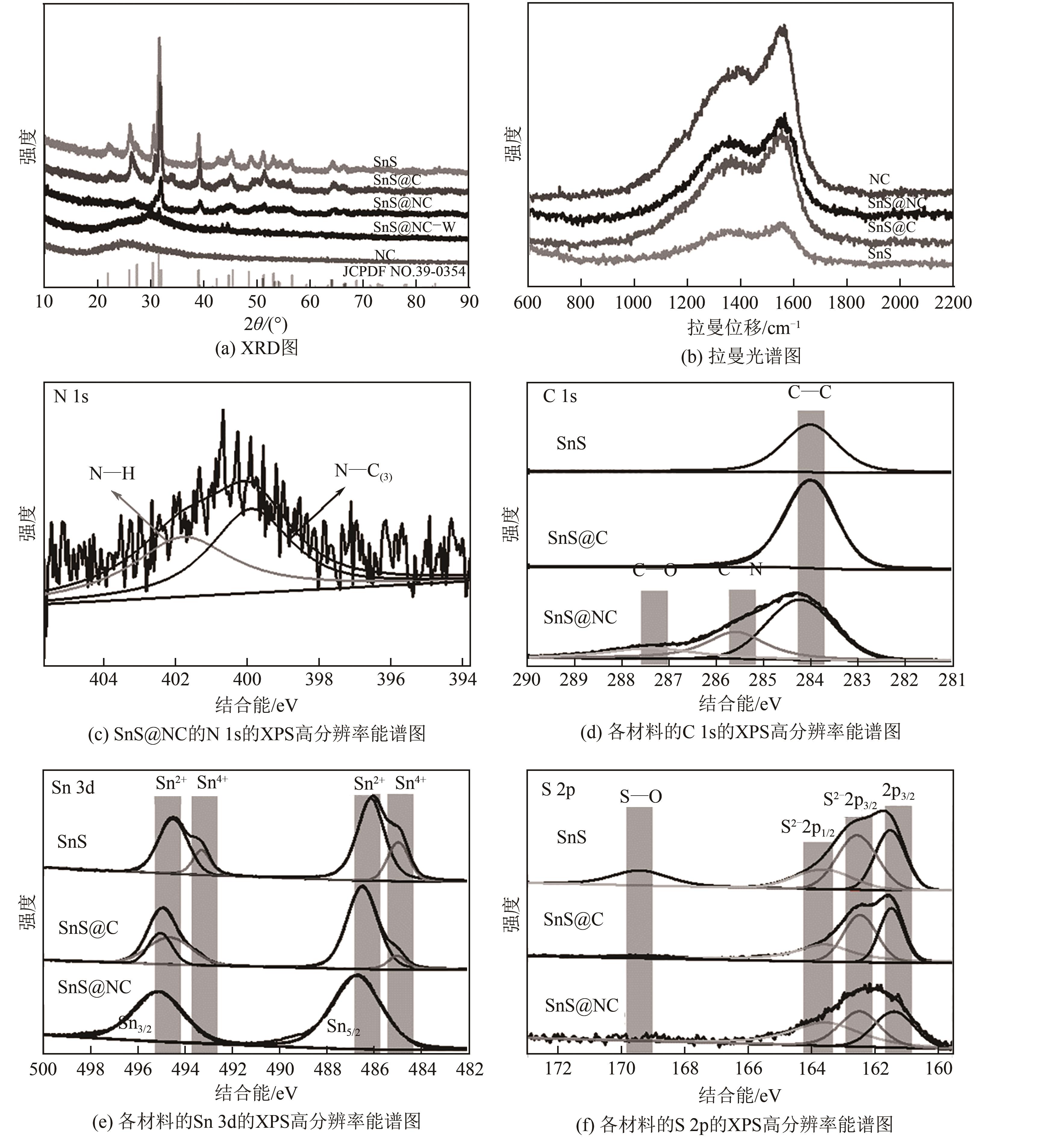
| 样品 | N/% | C/% | Sn/% | S/% |
|---|---|---|---|---|
| SnS | 2.09 | 64.22 | 18.11 | 15.15 |
| SnS@C | 2.29 | 82.40 | 7.23 | 8.08 |
| SnS@NC | 2.66 | 83.64 | 6.38 | 7.33 |
| 样品 | N/% | C/% | Sn/% | S/% |
|---|---|---|---|---|
| SnS | 2.09 | 64.22 | 18.11 | 15.15 |
| SnS@C | 2.29 | 82.40 | 7.23 | 8.08 |
| SnS@NC | 2.66 | 83.64 | 6.38 | 7.33 |
| 1 | KOTHANDARAMAN J, HELBEBRANT D J. Towards environmentally benign capture and conversion: heterogeneous metal catalyzed CO2 hydrogenation in CO2 capture solvents[J]. Green Chemistry, 2020, 22(3): 828-834. |
| 2 | BEHERA A, KAR A K, SRIVASTAVA R. Challenges and prospects in the selective photoreduction of CO2 to C1 and C2 products with nanostructured materials: a review[J]. Materials Horizons, 2022, 9: 607-639. |
| 3 | ZHANG W J, HU Y, MA L B, et al. Progress and perspective of electrocatalytic CO2 reduction for renewable carbonaceous fuels and chemicals[J]. Advanced Science, 2018, 5(1): 1700275. |
| 4 | CHOI S Y, JEONG S K, KIM H J, et al. Electrochemical reduction of carbon dioxide to formate on tin-lead alloys[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(3): 1311-1318. |
| 5 | CHANG J F, FENG L G, LIU C P, et al. An effective Pd-Ni2P/C anode catalyst for direct formic acid fuel cells[J]. Angewandte Chemie, 2014, 126(1): 122-130. |
| 6 | ALVAREZ-GUERRA M, QUINTANILLA S, IRABIEN A. Conversion of carbon dioxide into formate using a continuous electrochemical reduction process in a lead cathode[J]. Chemical Engineering Journal, 2012, 207/208: 278-284. |
| 7 | ZHOU B W, KONG X H, VANKA S, et al. A GaN: Sn nanoarchitecture integrated on a silicon platform for converting CO2 to HCOOH by photoelectrocatalysis[J]. Energy & Environmental Science, 2019, 12(9): 2842-2848. |
| 8 | XING Y L, KONG X D, GUO X, et al. Bi@Sn core–shell structure with compressive strain boosts the electroreduction of CO2 into formic acid[J]. Advanced Science, 2020, 7(22): 1902989. |
| 9 | HUANG J J, CHEN S X, YANG F Q, et al. Nickel nanoparticles with narrow size distribution confined in nitrogen-doped carbon for efficient reduction of CO2 to CO[J]. Catalysis Letters, 2022, 152(2): 600-609. |
| 10 | BOK J, LEE S Y, LEE B H, et al. Designing atomically dispersed Au on tensile-strained Pd for efficient CO2 electroreduction to formate[J]. Journal of the American Chemical Society, 2021, 143(14): 5386-5395. |
| 11 | ZOUAOUI N, OSSONON B D, FAN M Y, et al. Electroreduction of CO2 to formate on amine modified Pb electrodes[J]. Journal of Materials Chemistry A, 2019, 7(18): 11272-11281. |
| 12 | PENG L W, WANG Y X, MASOOD I, et al. Self-growing Cu/Sn bimetallic electrocatalysts on nitrogen-doped porous carbon cloth with 3D-hierarchical honeycomb structure for highly active carbon dioxide reduction[J]. Applied Catalysis B: Environmental, 2020, 264: 118447. |
| 13 | ZHANG A, LIANG Y X, LI H P, et al. Harmonizing the electronic structures of the adsorbate and catalysts for efficient CO2 reduction[J]. Nano Letters, 2019, 19(9): 6547-6553. |
| 14 | LI J C, KUANG Y, MENG Y T, et al. Electroreduction of CO2 to formate on a copper-based electrocatalyst at high pressures with high energy conversion efficiency[J]. Journal of the American Chemical Society, 2020, 142: 7276-7282. |
| 15 | KIM M K, LEE H, WON J H, et al. Design of less than 1nm scale spaces on SnO2 nanoparticles for high-performance electrochemical CO2 reduction[J]. Advanced Functional Materials, 2022, 32: 2107349. |
| 16 | YANG Z J, YANG C Y, HAN J Y, et al. Boosting electrochemical CO2 reduction to formate using SnO2/graphene oxide with amide linkages[J]. Journal of Materials Chemistry A, 2021, 9: 19681-19686. |
| 17 | ZHANG B H, CHEN S, WULAN B, et al. Surface modification of SnO2 nanosheets via ultrathin N-doped carbon layers for improving CO2 electrocatalytic reduction[J]. Chemical Engineering Journal, 2021, 421: 130003. |
| 18 | WANG Q N, WU Y L, ZHU C Q, et al. Sn nanoparticles deposited onto a gas diffusion layer via impregnation-electroreduction for enhanced CO2 electroreduction to formate[J]. Electrochimica Acta, 2021, 369: 137662. |
| 19 | LI Q Q, ZHANG Y X, ZHANG X R, et al. Novel Bi, BiSn, Bi2Sn, Bi3Sn, and Bi4Sn catalysts for efficient electroreduction of CO2 to formic acid[J]. Industrial & Engineering Chemistry Research, 2019, 59(15): 6806-6814. |
| 20 | CHEN H L, CHEN J X, SI J C, et al. Ultrathin tin monosulfide nanosheets with the exposed (001) plane for efficient electrocatalytic conversion of CO2 into formate[J]. Chemical Science, 2020, 11(15): 3952-3958. |
| 21 | VANDAELE K, MOT B T, PUPO M, et al. Sn-based electrocatalyst stability: a crucial piece to the puzzle for the electrochemical CO2 reduction toward formic acid[J]. ACS Energy Letter, 2021, 6: 4317-4327. |
| 22 | MELCHIONNA M, FORNASIERO P, PRATO M, et al. Electrocatalytic CO2 reduction: role of the cross-talk at nano-carbon interfaces[J]. Energy & Environmental Science, 2021, 14(11): 5816-5833. |
| 23 | LI Z J, CAO A, ZHENG Q, et al. Elucidation of the synergistic effect of dopants and vacancies on promoted selectivity for CO2 electroreduction to formate[J]. Advanced Materials, 2020, 33(2): 2005113. |
| 24 | YE G Y, LIU S Q, HUANG K, et al. Domain-confined etching strategy to regulate defective sites in carbon for high-efficiency electrocatalytic oxygen reduction[J]. Advanced Functional Materials, 2022: 2111396. |
| 25 | LU Q, CHEN C, DI Q, et al. Dual role of pyridinic-N doping in carbon-coated Ni nanoparticles for highly efficient electrochemical CO2 reduction to CO over a wide potential range[J]. ACS Catalysis, 2022, 12(2): 1364-1374. |
| 26 | YU A, MA G M, ZHU L T, et al. Conversion of CO2 to defective porous carbons in one electro-redox cycle for boosting electrocatalytic H2O2 production[J]. Applied Catalysis B: Environmental, 2022, 307: 121161. |
| 27 | CHI SHAOYI, CHEN Q, ZHAO S S, et al. Three-dimensional porphyrinic covalent organic frameworks for highly efficient electroreduction of carbon dioxide[J]. Journal of Materials Chemistry A, 2022, 10(9): 4653-4659. |
| 28 | LIU S, YANG H B, HUANG X, et al. Identifying active sites of nitrogen-doped carbon materials for the CO2 reduction reaction[J]. Advanced Functional Materials, 2018, 28(21): 1800499. |
| 29 | SI Z, LYU Z Z, LU L H, et al. Nitrogen-doped graphene chainmail wrapped IrCo alloy particles on nitrogen-doped graphene nanosheet for highly active and stable full water splitting[J]. ChemCatChem, 2019, 11: 5457-6465. |
| 30 | CHEN J Y, WANG T T, WANG X Y, et al. Promoting electrochemical CO2 reduction via boosting activation of adsorbed intermediates on iron single-atom catalyst[J]. Advanced Functional Materials: 2022: 2110174. |
| 31 | CHEN J Y, LI Z G, WANG X Y, et al. Promoting CO2 electroreduction kinetics on atomically dispersed monovalent ZnI sites by rationally engineering proton-feeding centers[J]. Angewandte Chemie International Edition, 2022, 134 (7): e202111683. |
| 32 | ZHANG Y K, WANG X Y, ZHENG S X, et al. Hierarchical cross-linked carbon aerogels with transition metal-nitrogen sites for highly efficient industrial-level CO2 electroreduction[J]. Advanced Functional Materials, 2021, 31(45): 2104377. |
| 33 | WANG X Y, SANG X H, DONG C L, et al. Proton capture strategy for enhancing electrochemical CO2 reduction on atomically dispersed metal-nitrogen active sites[J]. Angewandte Chemie International Edition, 2021, 60(21): 11959-11965. |
| 34 | WANG X Y, FENG X H, LU W C, et al. A new strategy for accelerating dynamic proton transfer of electrochemical CO2 reduction at high current densities[J]. Advanced Functional Materials, 2021, 31(50): 2104243. |
| 35 | FAN Q K, ZHANG X, GE X H, et al. Manipulating Cu nanoparticle surface oxidation states tunes catalytic selectivity toward CH4 or C2+ products in CO2 electroreduction[J]. Advanced Energy Materials, 2021, 11(36): 2101424. |
| 36 | CHEN S X, LI Y W, BU Z G, et al. Boosting CO2-to-CO conversion on a robust single-atom copper decorated carbon catalyst by enhancing intermediate binding strength[J]. Journal of Materials Chemistry A, 2021, 9(3): 1705-1712. |
| 37 | HOU Y, LIANG Y L, SHI P C, et al. Atomically dispersed Ni species on N-doped carbon nanotubes for electroreduction of CO2 with nearly 100% CO selectivity[J]. Applied Catalysis B: Environmental, 2020, 271: 118929. |
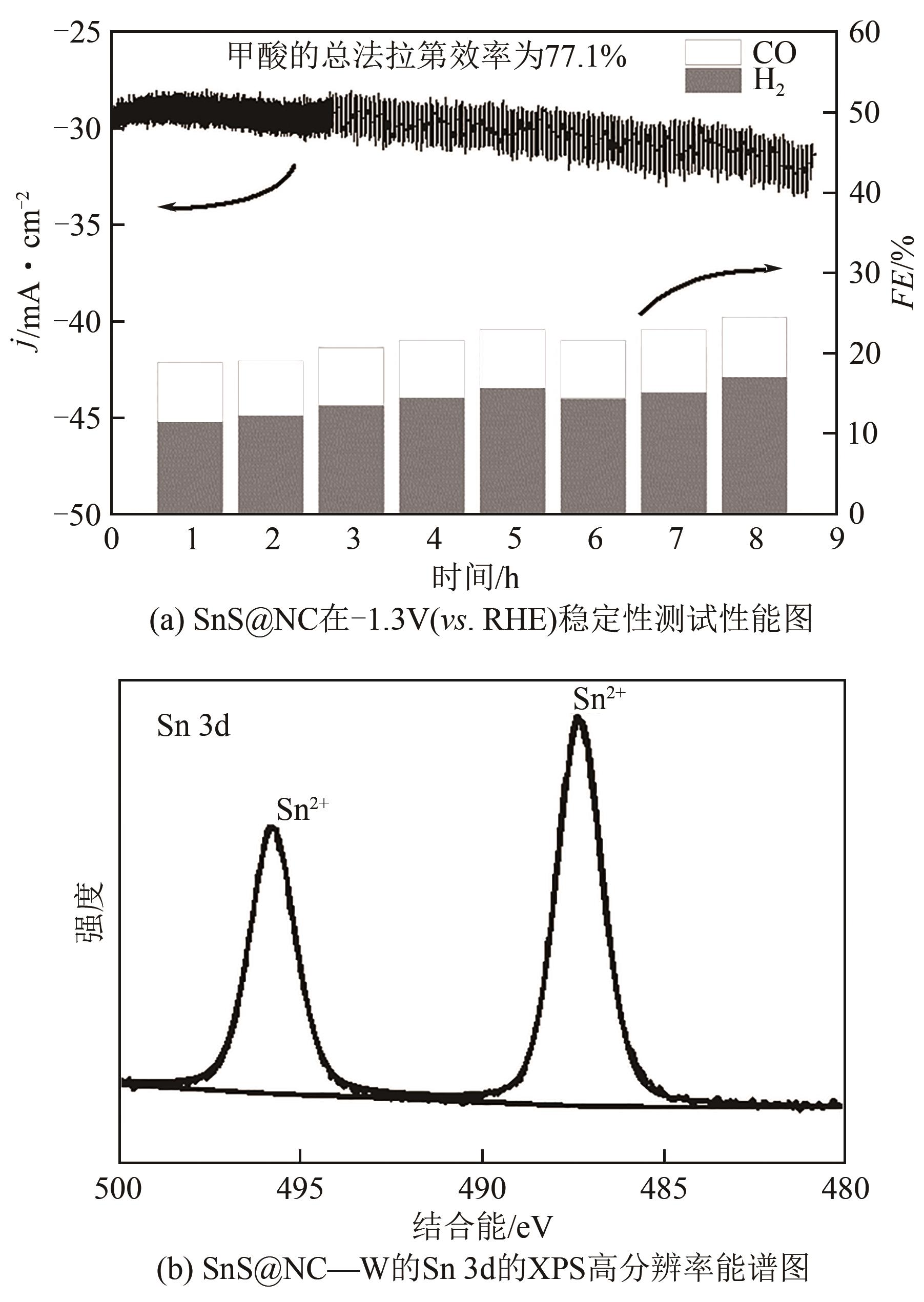
| 38 | CHEN Z P, ZHANG X X, JIAO M Y, et al. Engineering electronic structure of stannous sulfide by amino‐functionalized carbon: toward efficient electrocatalytic reduction of CO2 to formate[J]. Advanced Energy Materials, 2020, 10(8): 1903664. |
| 39 | VELASCO-VELEZ J J, JONES T, GAO D F, et al. The role of the copper oxidation state in the electrocatalytic reduction of CO2 into valuable hydrocarbons[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(1): 1485-1492. |
| 40 | LIU S B, TAO H B, ZENG L, et al. Shape-dependent electrocatalytic reduction of CO2 to CO on triangular silver nanoplates[J]. Journal of the American Chemical Society, 2017, 139: 2160-2163. |
| 41 | YANG F, WANG J, LIU L, et al. Synthesis of porous carbons with high N-content from shrimp shells for efficient CO2-capture and gas separation[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 15550-15559. |
| 42 | LI F W, CHEN L, XUE M Q, et al. Towards a better Sn: efficient electrocatalytic reduction of CO2 to formate by Sn/SnS2 derived from SnS2 nanosheets[J]. Nano Energy, 2017, 31: 270-277. |
| 43 | RABIEE H, ZHANG X Q, GE L, et al. Tuning the product selectivity of the Cu hollow fiber gas diffusion electrode for efficient CO2 reduction to formate by controlled surface Sn electrodeposition[J]. ACS Applied Materials & Interfaces, 2020, 12(19): 21670-21681. |
| 44 | KUANG Z Y, ZHAO W N, PENG C, et al. Hierarchically porous SnO2 coupled organic carbon for CO2 electroreduction[J]. ChemSusChem, 2020, 13(22): 5896-5900. |
| 45 | GENG W H, CHEN W, LI G H, et al. Induced CO2 electroreduction to formic acid on metal-organic frameworks via node doping[J]. ChemSusChem, 2020, 13(16): 4035-4040. |
| 46 | YE K, CAO A, SHAO J Q, et al. Synergy effects on Sn-Cu alloy catalyst for efficient CO2 electroreduction to formate with high mass activity[J]. Science Bulletin, 2020, 65(19): 711-719. |
| 47 | LIUS Y, PAG F J, ZHANG Q Y, et al. Stable nanoporous Sn/SnO2 composites for efficient electroreduction of CO2 to formate over wide potential range[J]. Applied Materials Today, 2018, 13: 135-143. |
| 48 | DAIYAN R, LU X Y, NG Y H, et al. Surface engineered tin foil for electrocatalytic reduction of carbon dioxide to formate[J]. Catalysis Science &Technology, 2017, 7(12): 2542-2550. |
| 49 | ZHANG Y, ZHANG X L, BOND A M, et al. Identification of a new substrate effect that enhances the electrocatalytic activity of dendritic tin in CO2 reduction[J]. Physical Chemistry Chemical Physics, 2018, 20(8): 5936-5941. |
| 50 | KUMAR B, ATLAY, BRIAN J P, et al. Reduced SnO2 porous nanowires with a high density of grain boundaries as catalysts for efficient electrochemical CO2-into-HCOOH conversion[J]. Angewandte Chemie International Edition, 2017, 56(13): 3645-3649. |
| 51 | DONG W J, YOO C J, LEE J L. Monolithic nanoporous In-Sn aloy for electrochemical reduction of carbon doxide[J]. ACS Applied Materials & Interfaces, 2017, 9(50): 43575-43582. |
| 52 | HE J, YANG C, YANG X, et al. Hydrophobic electrocatalyst for the enhanced activity of oxygen reduction reaction through controllable liquid/gas/solid interface[J]. Applied Surface Science, 2020, 532: 147357. |
| [1] | YANG Hanyue, KONG Lingzhen, CHEN Jiaqing, SUN Huan, SONG Jiakai, WANG Sicheng, KONG Biao. Decarbonization performance of downflow tubular gas-liquid contactor of microbubble-type [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 197-204. |
| [2] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [3] | ZHENG Qian, GUAN Xiushuai, JIN Shanbiao, ZHANG Changming, ZHANG Xiaochao. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. |
| [4] | HU Xi, WANG Mingshan, LI Enzhi, HUANG Siming, CHEN Junchen, GUO Bingshu, YU Bo, MA Zhiyuan, LI Xing. Research progress on preparation and sodium storage properties of tungsten disulfide composites [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 344-355. |
| [5] | ZHANG Jie, BAI Zhongbo, FENG Baoxin, PENG Xiaolin, REN Weiwei, ZHANG Jingli, LIU Eryong. Effect of PEG and its compound additives on post-treatment of electrolytic copper foils [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 374-381. |
| [6] | ZHAO Wei, ZHAO Deyin, LI Shihan, LIU Hongda, SUN Jin, GUO Yanqiu. Synthesis and application of triazine drag reducing agent for nature gas pipeline [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 391-399. |
| [7] | ZHANG Jie, WANG Fangfang, XIA Zhonglin, ZHAO Guangjin, MA Shuangchen. Current SF6 emission, emission reduction and future prospects under “carbon peaking and carbon neutrality” [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 447-460. |
| [8] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [9] | ZHAO Jingchao, TAN Ming. Effect of surfactants on the reduction of industrial saline wastewater by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 529-535. |
| [10] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [11] | SUN Yuyu, CAI Xinlei, TANG Jihai, HUANG Jingjing, HUANG Yiping, LIU Jie. Optimization and energy-saving of a reactive distillation process for the synthesis of methyl methacrylate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 56-63. |
| [12] | SHU Bin, CHEN Jianhong, XIONG Jian, WU Qirong, YU Jiangtao, YANG Ping. Necessity analysis of promoting the development of green methanol under the goal of carbon neutrality [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4471-4478. |
| [13] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [14] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [15] | ZHU Chuanqiang, RU Jinbo, SUN Tingting, XIE Xingwang, LI Changming, GAO Shiqiu. Characteristics of selective non-catalytic reduction of NO x with solid polymer denitration agent [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4939-4946. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||