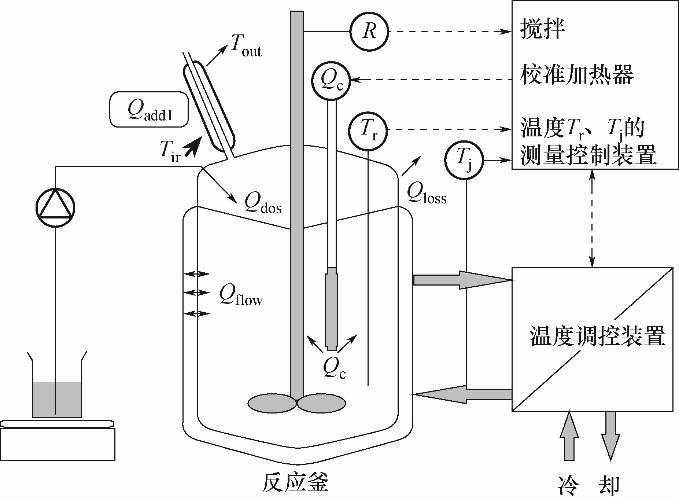
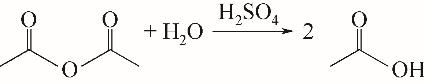| 1 |
牟善军. 化工过程安全管理与技术: 预防重特大事故的系统方法[M]. 北京: 中国石化出版社, 2018.
|
|
MU Shanjun. Chemical process safety management and technology[M]. Beijing: China Petrochemical Press, 2018.
|
| 2 |
国家安全生产监督管理总局.安全监管总局关于加强化工过程安全管理的指导意见[EB/OL]. [2013-07-23]. .
|
|
State Administration of Work Safety. Guidelines of State Administration of Work Safety on strengthening chemical process safety management[EB/OL]. [2013-07-23]. .
|
| 3 |
国务院安全生产委员会.全国安全生产专项整治三年行动计划[EB/OL]. [2020-04-01]. .
|
|
Work Safety Committee of the State Council. Three-year action plan for the National Work Safety Campaign [EB/OL]. [2020-04-01]. .
|
| 4 |
国务院安全生产委员会.全国危险化学品安全风险集中治理方案[EB/OL]. [2021-12-31]. .
|
|
Work Safety Committee of the State Council. National Plan for centralized control of safety risks of Hazardous chemicals [EB/OL]. [2021-12-31]. .
|
| 5 |
应急管理部.关于印发《危险化学品生产建设项目安全风险防控指南(试行)》的通知[EB/OL]. [2022-06-10]. .
|
|
Ministry of Emergency Management of the People’s Republic of China. Notice on the issuance of the Guidelines for the Prevention and Control of Safety Risks in Production and Construction Projects of Hazardous Chemicals (Trial) [EB/OL]. [2022-06-10]. .
|
| 6 |
国家安全生产监督管理总局.关于加强精细化工反应安全风险评估工作的指导意见[EB/OL]. [2017-01-05]. .
|
|
State Administration of Work Safety. Guidelines on strengthening the safety risk assessment of fine chemical reactions [EB/OL]. [2017-01-05]. .
|
| 7 |
中华人民共和国国家市场监督管理总局, 中国国家标准化管理委员会. 检测和校准实验室能力的通用要求: [S]. 北京: 中国标准出版社, 2019.
|
|
State Administration for Market Regulation, Standardization Administration of the People's Republic of China. General requirements for the competence of testing and calibration laboratories: [S]. Beijing: Standards Press of China, 2019.
|
| 8 |
刘纯, 潘旭海, 陈发明, 等. 反应量热仪RC1在化工热危险性分析中的应用[J]. 工业安全与环保, 2011, 37(5): 26-27, 32.
|
|
LIU Chun, PAN Xuhai, CHEN Faming, et al. Application of reaction calorimetry in the thermal hazard analysis of chemical process[J]. Industrial Safety and Environmental Protection, 2011, 37(5): 26-27, 32.
|
| 9 |
WANG Jingyao, HUANG Yanyan, WILHITE B, et al. Toward the identification of intensified reaction conditions using response surface methodology: A case study on 3-methylpyridine N-oxide synthesis[J]. Industrial & Engineering Chemistry Research, 2018, 58(15): 6093–6104.
|
| 10 |
陈利平, 蔡刘霖, 彭金华, 等. 硝酸甲胺反应过程的安全性研究[J]. 中国安全科学学报, 2006, 16(12): 97-102, 171.
|
|
CHEN Liping, CAI Liulin, PENG Jinhua, et al. Study on the safety synthesis of methylamine nitrate[J]. China Safety Science Journal (CSSJ), 2006, 16(12): 97-102, 171.
|
| 11 |
FERGUSON H, FRURIP D, PASTOR A, et al. A review of analytical applications of calorimetry[J]. Thermochimica Acta, 2000, 363: 1-21.
|
| 12 |
中国化工协会, 化学反应量热试验规程: T/CIE [S]. 北京: 中国标准出版社, 2020
|
|
China Chemical Industry Association, Calorimetric test code for chemical reactions: T/CIE [S]. Beijing: Standards Press of China, 2020.
|
| 13 |
Yih Shing DUH, HSU Chang Chia, Chenshan KAO, et al. Applications of reaction calorimetry in reaction kinetics and thermal hazard evaluation[J]. Thermochimica Acta, 1996, 285(1): 67-79.
|
| 14 |
国家质量监督检验检疫总局, 中国国家标准化管理委员会. 合格评定 化学分析方法确认和验证指南: [S]. 北京: 中国标准出版社, 2017.
|
|
General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration of the People's Republic of China. Comformity assessment—Guidance on validation and verification of chemical analytical methods: [S]. Beijing: Standards Press of China, 2017.
|
| 15 |
中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 数据的统计处理和解释 正态样本离群值的判断和处理: [S]. 北京: 中国标准出版社, 2008
|
|
General Administration of Quality Supervision, Inspection and Quarantine of P. R.C., Standardization Administration of P.R.C. Statistical interpretation of data—Detection and treatment of outliers in the normal sample: [S]. Beijing: Standards Press of China, 2008.
|
| 16 |
RC 1 e反应量热仪操作说明手册[Z]. 2021.
|
|
RC 1 e Operation instruction manual of reaction calorimeter[Z]. 2021.
|
| 17 |
刘光启, 马连湘, 项曙光. 化学化工物性数据手册——无机卷[M]. 2版. 北京: 化学工业出版社, 2013.
|
|
LIU Guangqi, MA Lianxiang, XIANG Shuguang. Chemical Chemical Physical Properties Data Manual (Updated edition of Inorganic Volume)[M]. Beijing: Chemical Industry Press, 2013.
|
 ), CHEN Yanqing1, KONG Xiangbei2, JIN Manping3, LIU Fufang4(
), CHEN Yanqing1, KONG Xiangbei2, JIN Manping3, LIU Fufang4( )
)
 ), 陈延青1, 孔祥北2, 金满平3, 刘付芳4(
), 陈延青1, 孔祥北2, 金满平3, 刘付芳4( )
)