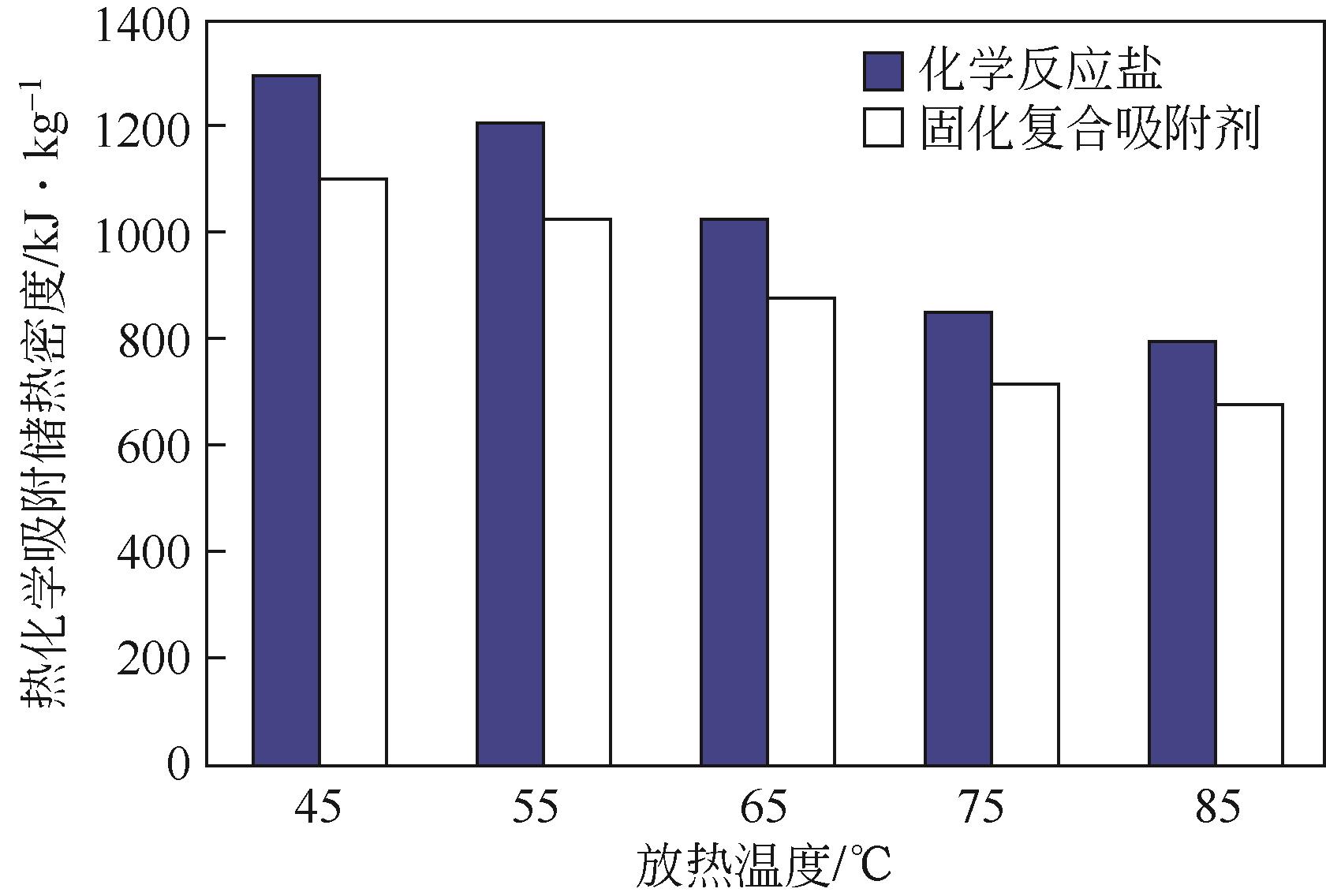
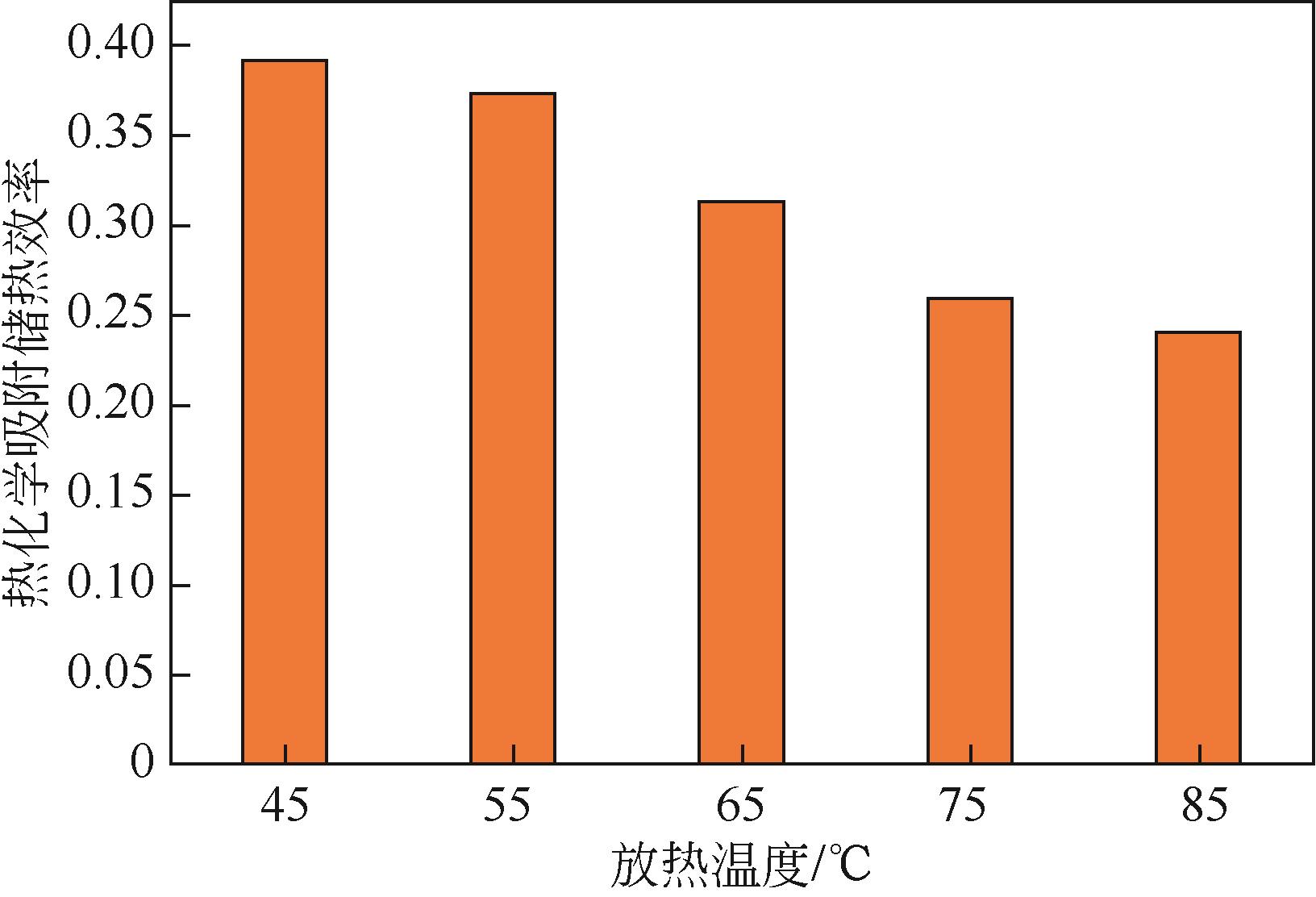
| 1 |
JUNG C W, SONG J Y, KANG Y T. Study on ammonia/water hybrid absorption/compression heat pump cycle to produce high temperature process water[J]. Energy, 2018, 145: 458-467.
|
| 2 |
杨振忠. 热能调控微纳结构材料[M]. 北京: 科学出版社, 2014.
|
|
YANG Zhenzhong. Micro/nano structural materials of thermal energy regulation [M]. Beijing: Science Press, 2014.
|
| 3 |
YAN T, WANG R Z, LI T X. Experimental investigation on thermochemical heat storage using manganese chloride/ammonia[J]. Energy, 2018, 143: 562-574.
|
| 4 |
闫霆, 王文欢, 王程遥. 化学储热技术的研究现状及进展[J]. 化工进展, 2018, 37(12): 4586-4595.
|
|
YAN Ting, WANG Wenhuan, WANG Chengyao. Research situation and progress on chemical heat storage technology[J]. Chemical Industry and Engineering Progress, 2018, 37(12): 4586-4595.
|
| 5 |
林文珠, 凌子夜, 方晓明, 等. 相变储热的传热强化技术研究进展[J]. 化工进展, 2021, 40(9): 5166-5179.
|
|
LIN Wenzhu, LING Ziye, FANG Xiaoming, et al. Research progress on heat transfer of phase change material heat storage technology[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 5166-5179.
|
| 6 |
N’TSOUKPOE K E, LIU H, LE PIERRÈS N, et al. A review on long-term sorption solar energy storage[J]. Renewable and Sustainable Energy Reviews, 2009, 13(9): 2385-2396.
|
| 7 |
YAN T, WANG R Z, LI T X, et al. A review of promising candidate reactions for chemical heat storage[J]. Renewable and Sustainable Energy Reviews, 2015, 43: 13-31.
|
| 8 |
YU N, WANG R Z, WANG L W. Sorption thermal storage for solar energy[J]. Progress in Energy and Combustion Science, 2013, 39(5): 489-514.
|
| 9 |
PATHAK A D, NEDEA S, ZONDAG H, et al. Diffusive transport of water in magnesium chloride dihydrate under various external conditions for long term heat storage: a ReaxFF-MD study[J]. European Journal of Mechanics B: Fluids, 2017, 64: 93-101.
|
| 10 |
KUZNIK F, JOHANNES K. A review on chemisorption heat storage in low-energy buildings[J]. Energy Procedia, 2014, 57: 2333-2341.
|
| 11 |
闫霆, 王文欢, 王如竹. 化学吸附储热技术的研究现状及进展[J]. 材料导报, 2018, 32(23): 4107-4115, 4124.
|
|
YAN Ting, WANG Wenhuan, WANG Ruzhu. Present status and progress of research on chemical adsorption heat storage[J]. Materials Review, 2018, 32(23): 4107-4115, 4124.
|
| 12 |
李琳, 黄宏宇, 邓立生, 等. 低品位能源化学储热材料研究进展[J]. 化工进展, 2020, 39(9): 3608-3616.
|
|
LI Lin, HUANG Hongyu, DENG Lisheng, et al. Research progress of low-grade energy chemical heat storage materials[J]. Chemical Industry and Engineering Progress, 2020, 39(9): 3608-3616.
|
| 13 |
吴韶飞, 安国亮, 田宜聪, 等. 基于双盐复合吸附剂的跨季节冷热自适应调控新策略[J]. 中国科学: 技术科学, 2022, 52(5): 755-772.
|
|
WU Shaofei, AN Guoliang, TIAN Yicong, et al. Cold-heat adaptive control for cross-season based on bi-salt composite sorbent[J]. Scientia Sinica (Technologica), 2022, 52(5): 755-772.
|
| 14 |
朱芳啟, 江龙, 王丽伟, 等. MnCl2-CaCl2-NH3再吸附温度提升系统储能特性[J]. 化工学报, 2016, 67(4): 1453-1458.
|
|
ZHU Fangqi, JIANG Long, WANG Liwei, et al. Energy storage properties of MnCl2-CaCl2-NH3 resorption temperature-lifting system[J]. CIESC Journal, 2016, 67(4): 1453-1458.
|
| 15 |
江龙, 王丽伟, 王如竹, 等. MnCl2/CaCl2/NH3再吸附工质对性能研究[J]. 工程热物理学报, 2015, 36(4): 720-723.
|
|
JIANG Long, WANG Liwei, WANG Ruzhu, et al. Experimental study on the performance of MnCl2/CaCl2/NH3 working pairs for resorption refrigeration[J]. Journal of Engineering Thermophysics, 2015, 36(4): 720-723.
|
| 16 |
周志松, 江龙, 王丽伟, 等. 非平衡条件下氯化锰-氨的吸附/解吸特性分析[J]. 上海交通大学学报, 2016, 50(4): 583-587, 594.
|
|
ZHOU Zhisong, JIANG Long, WANG Liwei, et al. Adsorption/desorption non-equilibrium characteristics of composite MnCl2-NH3 working pair[J]. Journal of Shanghai Jiao Tong University, 2016, 50(4): 583-587, 594.
|
| 17 |
LI T X, WANG R Z, YAN T. Solid-gas thermochemical sorption thermal battery for solar cooling and heating energy storage and heat transformer[J]. Energy, 2015, 84: 745-758.
|
| 18 |
YAN T, KUAI Z H, WU S F. Multi-mode solid-gas thermochemical resorption heat transformer using NiCl2-SrCl2/NH3 [J]. Applied Thermal Engineering, 2020, 167: 114800.
|
| 19 |
JIANG L, ROSKILLY A P, WANG R Z, et al. Analysis on innovative modular sorption and resorption thermal cell for cold and heat cogeneration[J]. Applied Energy, 2017, 204: 767-779.
|
| 20 |
LI T X, WANG R Z, KIPLAGAT J K. A target-oriented solid-gas thermochemical sorption heat transformer for integrated energy storage and energy upgrade[J]. AIChE Journal, 2013, 59(4): 1334-1347.
|
| 21 |
WANG R Z, WANG L W, WU J Y. Adsorption refrigeration technology[M]. New York: Wiley, 2014.
|
| 22 |
MAURAN S, PRADES P, L'HARIDON F. Heat and mass transfer in consolidated reacting beds for thermochemical systems[J]. Heat Recovery Systems and CHP, 1993, 13(4): 315-319.
|
| 23 |
闫霆. 中低温热化学吸附储热机理及实验研究[D]. 上海: 上海交通大学, 2016.
|
|
YAN Ting. Theoretical and experimental research of thermochemical sorption heat storage at middle-low temperature[D]. Shanghai: Shanghai Jiaotong University, 2016.
|
| 24 |
LI T X, WANG R Z, YAN T, et al. Integrated energy storage and energy upgrade, combined cooling and heating supply, and waste heat recovery with solid-gas thermochemical sorption heat transformer[J]. International Journal of Heat and Mass Transfer, 2014, 76: 237-246.
|
| 25 |
JIANG L, ZHU F Q, WANG L W, et al. Experimental investigation on a MnCl2-CaCl2-NH3 thermal energy storage system[J]. Renewable Energy, 2016, 91: 130-136.
|
| 26 |
叶大伦, 胡建华. 实用无机物热力学数据手册[M]. 2版. 北京: 冶金工业出版社, 2002.
|
|
YE Dalun, HU Jianhua. Practical inorganic thermodynamic data manual[M]. Beijing: Metallurgical Industry Press, 2002.
|
 ), YAN Ting(
), YAN Ting( ), HUO Yingjie
), HUO Yingjie