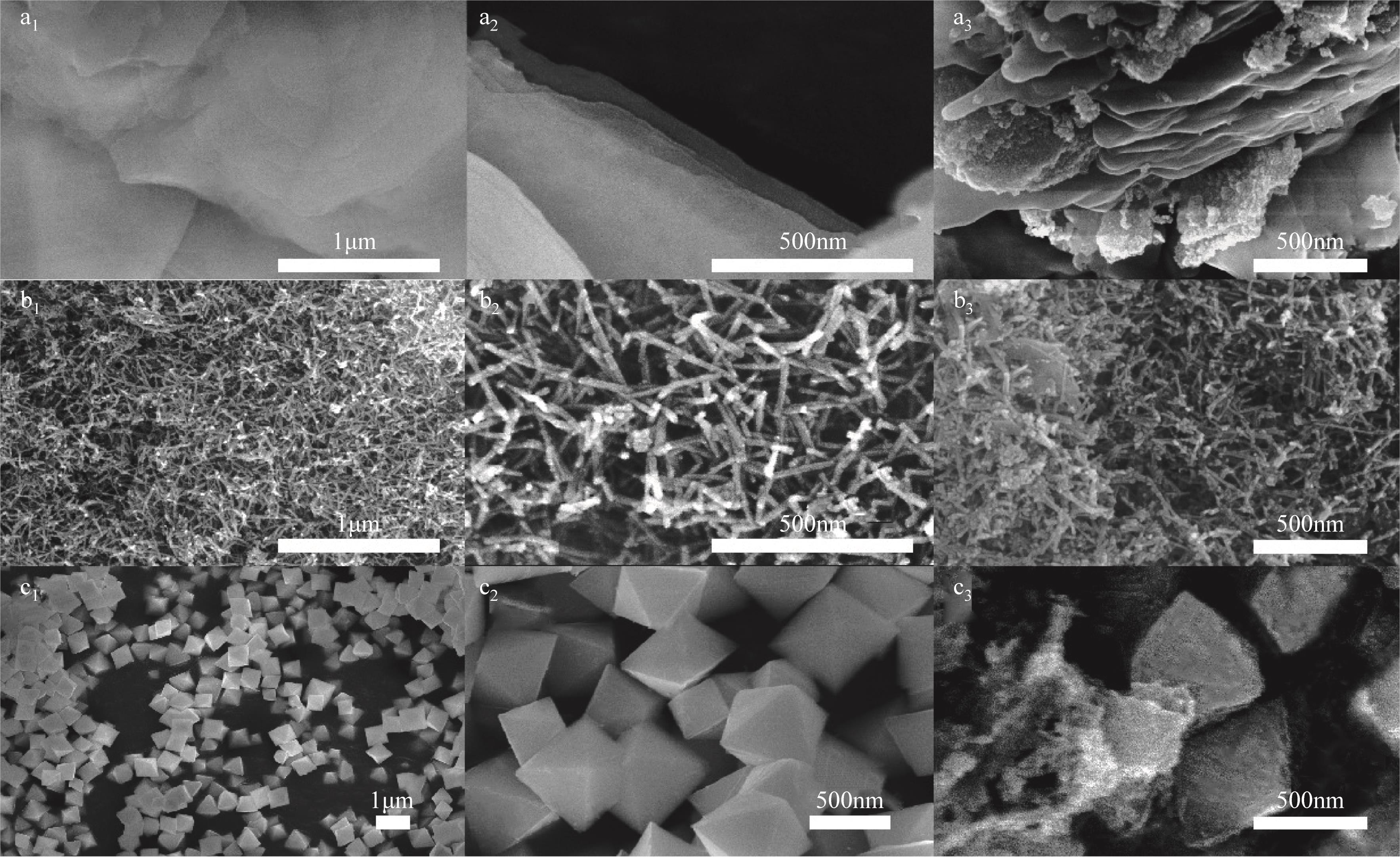
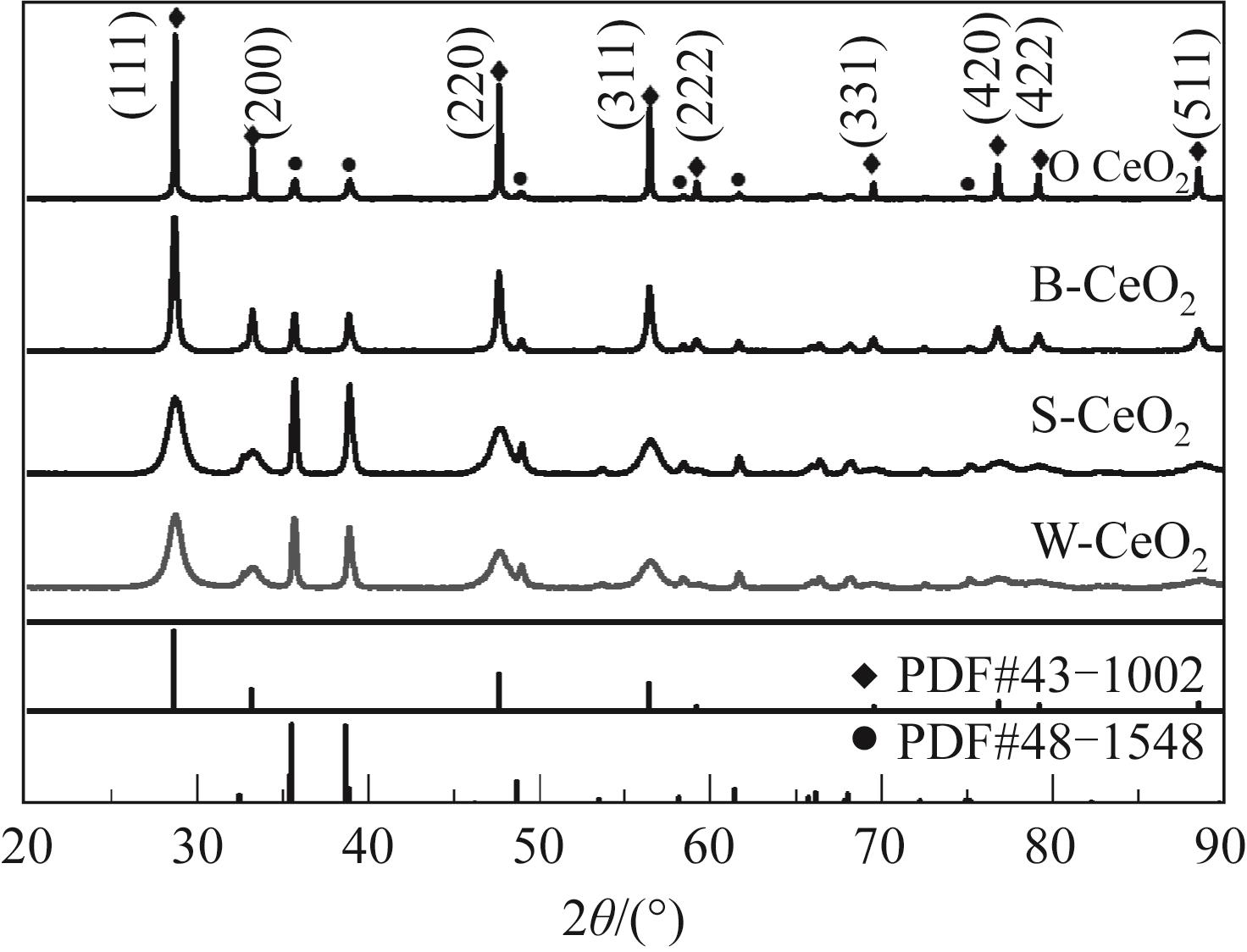
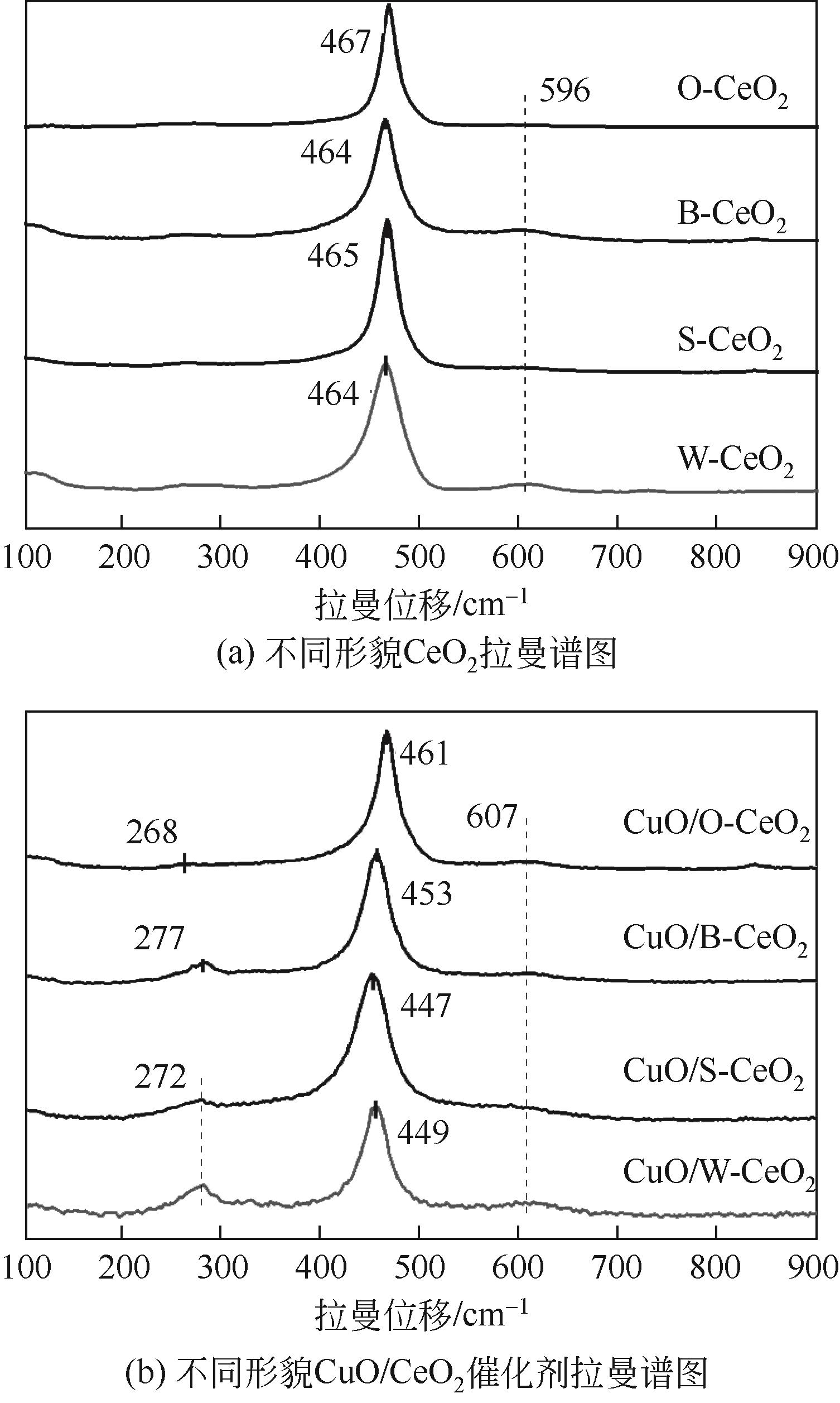
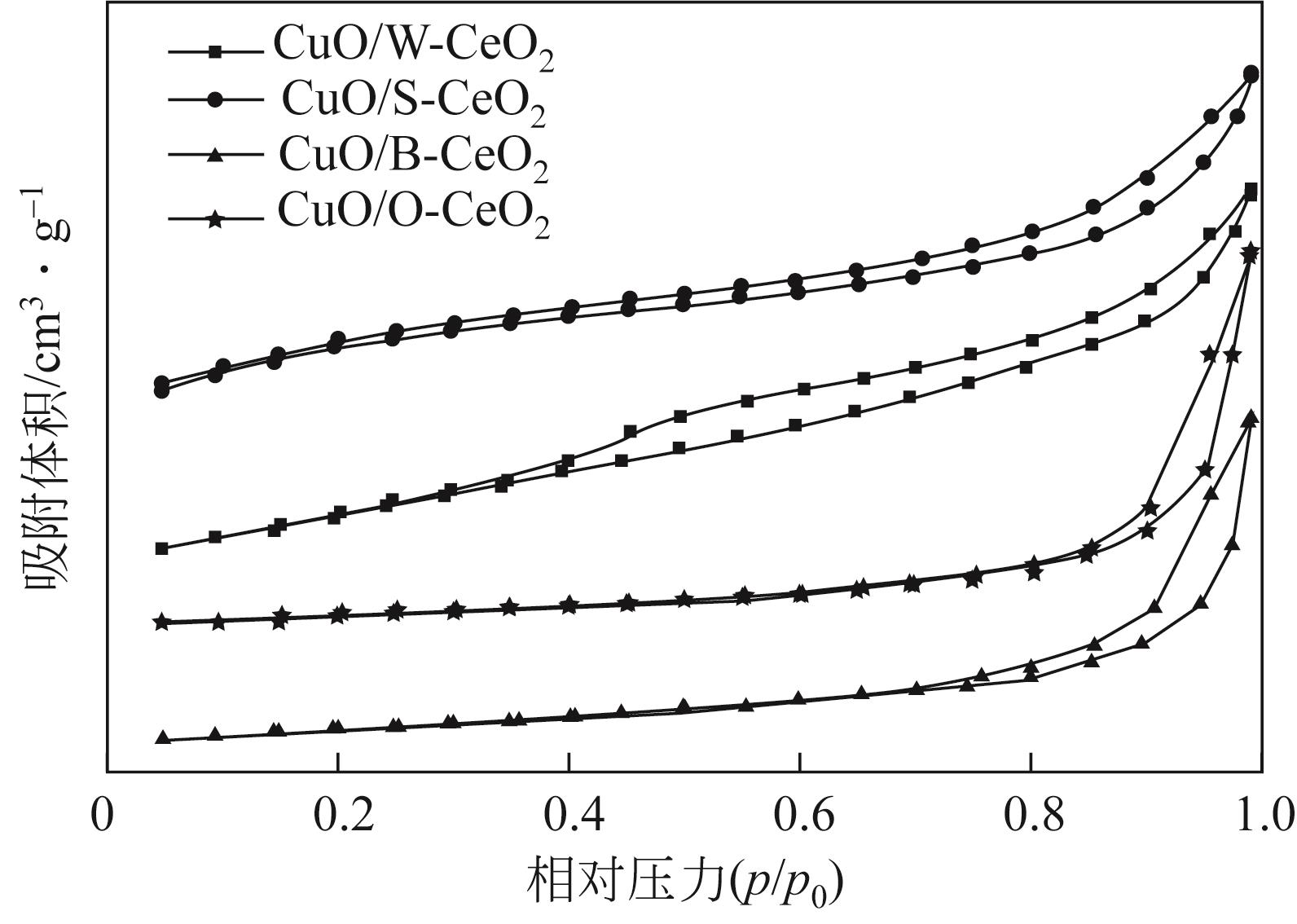
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (8): 4213-4223.DOI: 10.16085/j.issn.1000-6613.2021-1977
• Industrial catalysis • Previous Articles Next Articles
Effect of CeO2 morphology on the performance of CuO/CeO2 catalyst for CO2 hydrogenation to methanol
ZHANG Jiaqi1,2( ), LIN Lina1,2, GAO Wengui1,2,3(
), LIN Lina1,2, GAO Wengui1,2,3( ), ZHU Xing2,3
), ZHU Xing2,3
- 1.Faculty of Metallurgy and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, Yunnan, China
2.State Key Laboratory for Clean Utilization of Complex Nonferrous Metal Resources, Kunming University of Science and Technology, Kunming 650093, Yunnan, China
3.Engineering Research Center of Ministry of Education for Energy Conservation and Emission Reduction in Metallurgy, Kunming 650093, Yunnan, China
-
Received:2021-09-06Revised:2021-11-18Online:2022-08-22Published:2022-08-25 -
Contact:GAO Wengui
CeO2的形貌对CuO/CeO2催化剂CO2加氢制甲醇性能的影响
张嘉琪1,2( ), 林丽娜1,2, 高文桂1,2,3(
), 林丽娜1,2, 高文桂1,2,3( ), 祝星2,3
), 祝星2,3
- 1.昆明理工大学冶金与能源工程学院,云南 昆明 650093
2.昆明理工大学省部共建复杂有色金属资源清洁利用国家重点实验室,云南 昆明 650093
3.冶金节能减排教育部工程研究中心,云南 昆明 650093
-
通讯作者:高文桂 -
作者简介:张嘉琪(1997—),女,硕士研究生,研究方向为能源催化。E-mail:382732828@qq.com 。 -
基金资助:省部共建复杂有色金属资源清洁利用国家重点实验室自主课题(CNMRCUTS2009);国家重点研发计划(2018YFB0605402-02)
CLC Number:
Cite this article
ZHANG Jiaqi, LIN Lina, GAO Wengui, ZHU Xing. Effect of CeO2 morphology on the performance of CuO/CeO2 catalyst for CO2 hydrogenation to methanol[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4213-4223.
张嘉琪, 林丽娜, 高文桂, 祝星. CeO2的形貌对CuO/CeO2催化剂CO2加氢制甲醇性能的影响[J]. 化工进展, 2022, 41(8): 4213-4223.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-1977
| CeO2载体 | SBET/m2·g-1 | ID/IF2g | CuO/CeO2催化剂 | SBET/m2·g-1 | ID/IF2g |
|---|---|---|---|---|---|
| W-CeO2 | 66.9 | 0.035 | CuO/W-CeO2 | 76.4 | 0.126 |
| S-CeO2 | 79.2 | 0.056 | CuO/S-CeO2 | 88.8 | 0.284 |
| B-CeO2 | 114.1 | 0.048 | CuO/B-CeO2 | 122.2 | 0.154 |
| O-CeO2 | 35.8 | 0.026 | CuO/O-CeO2 | 43.5 | 0.067 |
| CeO2载体 | SBET/m2·g-1 | ID/IF2g | CuO/CeO2催化剂 | SBET/m2·g-1 | ID/IF2g |
|---|---|---|---|---|---|
| W-CeO2 | 66.9 | 0.035 | CuO/W-CeO2 | 76.4 | 0.126 |
| S-CeO2 | 79.2 | 0.056 | CuO/S-CeO2 | 88.8 | 0.284 |
| B-CeO2 | 114.1 | 0.048 | CuO/B-CeO2 | 122.2 | 0.154 |
| O-CeO2 | 35.8 | 0.026 | CuO/O-CeO2 | 43.5 | 0.067 |
| 催化剂 | α | β |  | |||
|---|---|---|---|---|---|---|
| 面积/mV·℃ | Tmax/℃ | 面积/mV·℃ | Tmax/℃ | |||
| CuO/W-CeO2 | 5200 | 323 | 16820 | 390 | 0.236 | |
| CuO/S-CeO2 | 3200 | 311 | 10256 | 358 | 0.238 | |
| CuO/B-CeO2 | 5395 | 428 | 18202 | 470 | 0.229 | |
| CuO/O-CeO2 | 3143 | 312 | 11824 | 372 | 0.210 | |
| 催化剂 | α | β |  | |||
|---|---|---|---|---|---|---|
| 面积/mV·℃ | Tmax/℃ | 面积/mV·℃ | Tmax/℃ | |||
| CuO/W-CeO2 | 5200 | 323 | 16820 | 390 | 0.236 | |
| CuO/S-CeO2 | 3200 | 311 | 10256 | 358 | 0.238 | |
| CuO/B-CeO2 | 5395 | 428 | 18202 | 470 | 0.229 | |
| CuO/O-CeO2 | 3143 | 312 | 11824 | 372 | 0.210 | |
| 催化剂 | 弱碱性位点 | 中碱性位点 | 强碱性位点 | 总位点 |
|---|---|---|---|---|
| CuO/W-CeO2 | 1220 | 902 | 30 | 2152 |
| CuO/S-CeO2 | 1126 | 1835 | 35 | 2996 |
| CuO/B-CeO2 | 250 | 986 | 11 | 1247 |
| CuO/O-CeO2 | 55 | 579 | 24 | 658 |
| 催化剂 | 弱碱性位点 | 中碱性位点 | 强碱性位点 | 总位点 |
|---|---|---|---|---|
| CuO/W-CeO2 | 1220 | 902 | 30 | 2152 |
| CuO/S-CeO2 | 1126 | 1835 | 35 | 2996 |
| CuO/B-CeO2 | 250 | 986 | 11 | 1247 |
| CuO/O-CeO2 | 55 | 579 | 24 | 658 |
| 催化剂 | SCO/% | DCu/% | |||
|---|---|---|---|---|---|
| CuO/W-CeO2 | 5.47 | 99.04 | 0.96 | 5.42 | 15.8 |
| CuO/S-CeO2 | 8.56 | 96.30 | 3.70 | 8.24 | 19.2 |
| CuO/B-CeO2 | 6.01 | 98.09 | 1.91 | 5.90 | 17.4 |
| CuO/O-CeO2 | 3.97 | 99.99 | 0.01 | 3.97 | 11.9 |
| 催化剂 | SCO/% | DCu/% | |||
|---|---|---|---|---|---|
| CuO/W-CeO2 | 5.47 | 99.04 | 0.96 | 5.42 | 15.8 |
| CuO/S-CeO2 | 8.56 | 96.30 | 3.70 | 8.24 | 19.2 |
| CuO/B-CeO2 | 6.01 | 98.09 | 1.91 | 5.90 | 17.4 |
| CuO/O-CeO2 | 3.97 | 99.99 | 0.01 | 3.97 | 11.9 |
| 1 | KOPP R E, KEMP A C, BITTERMANN K, et al. Temperature-driven global sea-level variability in the Common Era[J]. PNAS, 2016, 113(11): E1434-E1441. |
| 2 | KEMP A C, HORTON B P, DONNELLY J P, et al. Climate related sea-level variations over the past two millennia[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(27): 11017-11022. |
| 3 | 郝静, 宛霞. 过去五年史上最热 《2019年全球气候状况声明》发布[J]. 科学大观园, 2020(8): 18-19. |
| HAO Jing, WAN Xia. Hottest on record for the past five years “State of the Global Climate 2019 Statement” released[J]. Grand Garden of Science, 2020(8): 18-19. | |
| 4 | 惠婕. 应对气候变化,拯救我们的星球[J]. 世界环境, 2021(1): 14-15. |
| HUI Jie. Address climate change and save our planet[J]. World Environment, 2021(1): 14-15. | |
| 5 | 赵信国, 刘广绪. 海洋酸化对海洋无脊椎动物的影响研究进展[J]. 生态学报, 2015, 35(7): 2388-2398. |
| ZHAO Xinguo, LIU Guangxu. Advances in the effects of ocean acidification on marine invertebrates[J]. Acta Ecologica Sinica, 2015, 35(7): 2388-2398. | |
| 6 | CHU S, MAJUMDAR A. Opportunities and challenges for a sustainable energy future[J]. Nature, 2012, 488 (7411): 294-303. |
| 7 | 王克, 刘芳名, 尹明健, 等. 1.5℃温升目标下中国碳排放路径研究[J]. 气候变化研究进展, 2021, 17(1): 7-17. |
| WANG Ke, LIU Fangming, YIN Mingjian, et al. Research on China’s carbon emissions pathway under the 1.5℃ target[J]. Climate Change Research, 2021, 17(1): 7-17. | |
| 8 | RAMACHANDRIYA K D, KUNDIYANA D K, WILKINS M R, et al. Carbon dioxide conversion to fuels and chemicals using a hybrid green process[J]. Applied Energy, 2013, 112: 289-299. |
| 9 | 徐敏杰, 朱明辉, 陈天元, 等. CO2高值化利用:CO2加氢制甲醇催化剂研究进展[J]. 化工进展, 2021, 40(2): 565-576. |
| XU Minjie, ZHU Minghui, CHEN Tianyuan, et al. High value utilization of CO2: research progress of catalyst for hydrogenation of CO2 to methanol[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 565-576. | |
| 10 | ACRES Gary. Beyond oil and gas: the methanol econnmy[J].Chemistry and Industry, 2006(14): 26-27. |
| 11 | AWAD O I, MAMAT R, IBRAHIM T K, et al. Overview of the oxygenated fuels in spark ignition engine: environmental and performance[J]. Renewable and Sustainable Energy Reviews, 2018, 91: 394-408. |
| 12 | ALI K A, ABDULLAH A Z, MOHAMED A R. Recent development in catalytic technologies for methanol synthesis from renewable sources: a critical review[J]. Renewable and Sustainable Energy Reviews, 2015, 44: 508-518. |
| 13 | KOTHANDARAMAN J, KAR S, GOEPPERT A, et al. Advances in homogeneous catalysis for low temperature methanol reforming in the context of the methanol economy[J]. Topics in Catalysis, 2018, 61(7/8): 542-559. |
| 14 | 贾晨喜, 邵敬爱, 白小薇, 等. 二氧化碳加氢制甲醇铜基催化剂性能的研究进展[J]. 化工进展, 2020, 39(9): 3658-3668. |
| JIA Chenxi, SHAO Jingai, BAI Xiaowei, et al. Review on Cu-based catalysts for CO2 hydrogenation to methanol[J]. Chemical Industry and Engineering Progress, 2020, 39(9): 3658-3668. | |
| 15 | HAYWARD J S, SMITH P J, KONDRAT S A, et al. The effects of secondary oxides on copper-based catalysts for green methanol synthesis[J]. ChemCatChem, 2017, 9(9): 1655-1662. |
| 16 | TURSUNOV O, KUSTOV L, TILYABAEV Z. Methanol synthesis from the catalytic hydrogenation of CO2 over CuO-ZnO supported on aluminum and silicon oxides[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 78: 416-422. |
| 17 | HU Nian, LI Xiaoyun, LIU Siming, et al. Enhanced stability of highly-dispersed copper catalyst supported by hierarchically porous carbon for long term selective hydrogenation[J]. Chinese Journal of Catalysis, 2020, 41(7): 1081-1090. |
| 18 | YANG Bin, DENG Wei, GUO Limin, et al. Copper-ceria solid solution with improved catalytic activity for hydrogenation of CO2 to CH3OH[J]. Chinese Journal of Catalysis, 2020, 41(9): 1348-1359. |
| 19 | RAUDASKOSKI R, NIEMELÄ M V, KEISKI R L. The effect of ageing time on co-precipitated Cu/ZnO/ZrO2 catalysts used in methanol synthesis from CO2 and H2 [J]. Topics in Catalysis, 2007, 45(1/2/3/4): 57-60. |
| 20 | GRACIANI J, MUDIYANSELAGE K, XU Fang, et al. Catalysis. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO₂[J]. Science, 2014, 345(6196): 546-550. |
| 21 | OUYANG Bi, TAN Weiling, LIU Bing. Morphology effect of nanostructure ceria on the Cu/CeO2 catalysts for synthesis of methanol from CO2 hydrogenation[J]. Catalysis Communications, 2017, 95: 36-39. |
| 22 | KASPAR J. Catalysis by ceria and related materials[M]. Italy: IMPERIAL, 2015: 221-252. |
| 23 | BADWAL S P S, FINI D, CIACCHI F T, et al. Structural and microstructural stability of ceria-gadolinia electrolyte exposed to reducing environments of high temperature fuel cells[J]. Journal of Materials Chemistry A, 2013, 1(36): 10768. |
| 24 | ZHANG Y W, SI R, LIAO C S, et al. Facile alcohothermal synthesis, size-dependent ultraviolet absorption, and enhanced CO conversion activity of ceria nanocrystals[J]. The Journal of Physical Chemistry B, 2003, 107(37): 10159-10167. |
| 25 | ZHANG Jingcai, YANG Hongxiao, WANG Shuping, et al. Mesoporous CeO2 nanoparticles assembled by hollow nanostructures: formation mechanism and enhanced catalytic properties[J]. CrystEngComm, 2014, 16(37): 8777-8785. |
| 26 | CHEN Shilong, XIONG Feng, HUANG Weixin. Surface chemistry and catalysis of oxide model catalysts from single crystals to nanocrystals[J]. Surface Science Reports, 2019, 74(4): 100471. |
| 27 | POLO-GARZON F, BAO Zhenghong, ZHANG Xuanyu, et al. Surface reconstructions of metal oxides and the consequences on catalytic chemistry[J]. ACS Catalysis, 2019, 9(6): 5692-5707. |
| 28 | HUANG Weixin, GAO Yuxian. ChemInform abstract: morphology-dependent surface chemistry and catalysis of CeO2 nanocrystals[J]. Catalysis Science & Technology, 2014, 4(11): 3772-3784. |
| 29 | On the question of speed of growth and dissolution of crystal surfaces[J]. Zeitschrift Für Kristallographie-Crystalline Materials, 1901, 34(1/2/3/4/5/6): 449-530. |
| 30 | ZHANG Dengsong, DU Xianjun, SHI Liyi, et al. Shape-controlled synthesis and catalytic application of ceria nanomaterials[J]. Dalton Transactions, 2012,41(48): 14455-14475. |
| 31 | 位忠斌, 崔育倩, 郭培志, 等. 二氧化铈八面体的水热合成与表征[J]. 无机化学学报, 2011, 27(7): 1399-1404. |
| WEI Zhongbin, CUI Yuqian, GUO Peizhi, et al. Hydrothermal synthesis and characterization of ceria octahedrons[J]. Chinese Journal of Inorganic Chemistry, 2011, 27(7): 1399-1404. | |
| 32 | TANA, ZHANG Milin, LI Juan, et al. Morphology-dependent redox and catalytic properties of CeO2 nanostructures: Nanowires, nanorods and nanoparticles[J]. Catalysis Today, 2009, 148(1/2): 179-183. |
| 33 | WANG Xue, JIANG Zhiyuan, ZHENG Binjie, et al. Synthesis and shape-dependent catalytic properties of CeO2 nanocubes and truncated octahedra[J]. CrystEngComm, 2012, 14(22): 7579. |
| 34 | SENANAYAKE S D, RAMÍREZ P J, WALUYO I, et al. Hydrogenation of CO2 to methanol on CeO x /Cu(111) and ZnO/Cu(111) catalysts: role of the metal-oxide interface and importance of Ce3+ sites[J]. The Journal of Physical Chemistry C, 2016, 120(3): 1778-1784. |
| 35 | GAO Yuxian, ZHANG Zhenhua, LI Zhaorui, et al. Understanding morphology-dependent CuO x -CeO2 interactions from the very beginning[J]. Chinese Journal of Catalysis, 2020, 41(6): 1006-1016. |
| 36 | HUANG Weixin. Oxide nanocrystal model catalysts[J]. Accounts of Chemical Research, 2016, 49(3): 520-527. |
| 37 | LUO Mengfei, SONG Yupeng, LU Jiqing, et al. Identification of CuO species in high surface area CuO-CeO2 catalysts and their catalytic activities for CO oxidation[J]. The Journal of Physical Chemistry C, 2007, 111(34): 12686-12692. |
| 38 | NOLAN M, GRIGOLEIT S, SAYLE D C, et al. Density functional theory studies of the structure and electronic structure of pure and defective low index surfaces of ceria[J]. Surface Science, 2005, 576(1/2/3): 217-229. |
| 39 | GAO Yuxian, WANG Wendong, CHANG Sujie, et al. Morphology effect of CeO2 support in the preparation, metal-support interaction, and catalytic performance of Pt/CeO2 catalysts[J]. ChemCatChem, 2013, 5(12): 3610-3620. |
| 40 | GRACIANI J, VIDAL A B, RODRIGUEZ J A, et al. Unraveling the nature of the oxide-metal interaction in ceria-based noble metal inverse catalysts[J]. The Journal of Physical Chemistry C, 2014, 118(46): 26931-26938. |
| 41 | CÁMARA A L, BERA P, CONESA J C, et al. Characterization of active sites/entities and redox/catalytic correlations in copper-ceria-based catalysts for preferential oxidation of CO in H2-rich streams[J]. Catalysts, 2013, 3(2): 378-400. |
| 42 | LI L, ZHAN Y Y, CHEN C Q, et al. Effect of CeO2 support prepared with different methods on the activity and stability of CuO/CeO2 catalysts for the water-gas shift reaction[J]. Journal of Physical Chemistry, 2009, 25(7): 1397-1404. |
| 43 | KOSTIĆ R, AŠKRABIĆ S, DOHČEVIĆ-MITROVIĆ Z, et al. Low-frequency Raman scattering from CeO2 nanoparticles[J]. Applied Physics A, 2008, 90(4): 679-683. |
| 44 | HE Chi, YU Yanke, YUE Lin, et al. Low-temperature removal of toluene and propanal over highly active mesoporous CuCeO x catalysts synthesized via a simple self-precipitation protocol[J]. Applied Catalysis B: Environmental, 2014, 147: 156-166. |
| 45 | WU Xinping, GONG Xueqing. Clustering of oxygen vacancies at CeO2(111): critical role of hydroxyls[J]. Physical Review Letters, 2016, 116(8): 086102. |
| 46 | MCBRIDE J R, HASS K C, POINDEXTER B D, et al. Raman and X-ray studies of Ce1- x RE x O2- y, where RE=La, Pr, Nd, Eu, Gd, and Tb[J]. Journal of Applied Physics, 1994, 76(4): 2435-2441. |
| 47 | WEBER W H, HASS K C, MCBRIDE J R. Raman study of CeO2: second-order scattering, lattice dynamics, and particle-size effects[J]. Physical Review B, Condensed Matter, 1993, 48(1): 178-185. |
| 48 | 王禹皓. Cu-ZnO-ZrO2界面相互作用及其催化CO2加氢选择性合成甲醇的研究[D]. 昆明: 昆明理工大学, 2018. |
| WANG Yuhao. Cu-ZnO-ZrO2 interfacial interaction and its catalytic hydrogenation of CO2 for selective synthesis of methanol[D]. Kunming: Kunming University of Science and Technology, 2018. | |
| 49 | WANG Xianqin, RODRIGUEZ J A, HANSON J C, et al. In situ studies of the active sites for the water gas shift reaction over Cu-CeO2 catalysts: complex interaction between metallic copper and oxygen vacancies of ceria[J]. The Journal of Physical Chemistry B, 2006, 110(1): 428-434. |
| 50 | YANG Zhijie, HAN Dongqing, MA Donglin, et al. Fabrication of monodisperse CeO2 hollow spheres assembled by nano-octahedra[J]. Crystal Growth & Design, 2010, 10(1): 291-295. |
| 51 | PALOMINO R M, RAMÍREZ P J, LIU Zongyuan, et al. Hydrogenation of CO2 on ZnO/Cu(100) and ZnO/Cu(111) catalysts: role of copper structure and metal-oxide interface in methanol synthesis[J]. The Journal of Physical Chemistry B, 2018, 122(2): 794-800. |
| 52 | SKORODUMOVA N V, BAUDIN M, HERMANSSON K. Surface properties of CeO2 from first principles[J]. Physical Review B, 2004, 69(7): 075401. |
| 53 | SI Rui, RAITANO J, YI Nan, et al. Structure sensitivity of the low-temperature water-gas shift reaction on Cu-CeO2 catalysts[J]. Catalysis Today, 2012, 180(1): 68-80. |
| 54 | KAMMERT J, MOON J, WU Zili. A review of the interactions between ceria and H2 and the applications to selective hydrogenation of alkynes[J]. Chinese Journal of Catalysis, 2020, 41(6): 901-914. |
| 55 | MARTÍNEZ-ARIAS A, GAMARRA D, FERNÁNDEZ-GARCÍA M, et al. Comparative study on redox properties of nanosized CeO2 and CuO/CeO2 under CO/O2 [J]. Journal of Catalysis, 2006, 240(1): 1-7. |
| 56 | ZABILSKIY M, DJINOVIĆ P, TCHERNYCHOVA E, et al. Nanoshaped CuO/CeO2 materials: effect of the exposed ceria surfaces on catalytic activity in N2O decomposition reaction[J]. ACS Catalysis, 2015, 5(9): 5357-5365. |
| 57 | MENON U, POELMAN H, BLIZNUK V, et al. Nature of the active sites for the total oxidation of toluene by CuOCeO2/Al2O3 [J]. Journal of Catalysis, 2012, 295: 91-103. |
| 58 | WANG Shuxian, GUO Ruitang, PAN Weiguo, et al. The deactivation of Ce/TiO2 catalyst for NH3-SCR reaction by alkali metals: TPD and DRIFT studies[J]. Catalysis Communications, 2017, 89: 143-147. |
| 59 | GIORDANO F, TROVARELLI A, DE LEITENBURG C, et al. Some insight into the effects of oxygen diffusion in the reduction kinetics of ceria[J]. Industrial & Engineering Chemistry Research, 2001, 40(22): 4828-4835. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | ZHENG Qian, GUAN Xiushuai, JIN Shanbiao, ZHANG Changming, ZHANG Xiaochao. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. |
| [6] | XU Jiaheng, LI Yongsheng, LUO Chunhuan, SU Qingquan. Optimization of methanol steam reforming process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 41-46. |
| [7] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [8] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [9] | SUN Yuyu, CAI Xinlei, TANG Jihai, HUANG Jingjing, HUANG Yiping, LIU Jie. Optimization and energy-saving of a reactive distillation process for the synthesis of methyl methacrylate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 56-63. |
| [10] | YANG Hanyue, KONG Lingzhen, CHEN Jiaqing, SUN Huan, SONG Jiakai, WANG Sicheng, KONG Biao. Decarbonization performance of downflow tubular gas-liquid contactor of microbubble-type [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 197-204. |
| [11] | SHU Bin, CHEN Jianhong, XIONG Jian, WU Qirong, YU Jiangtao, YANG Ping. Necessity analysis of promoting the development of green methanol under the goal of carbon neutrality [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4471-4478. |
| [12] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [13] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [14] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [15] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||