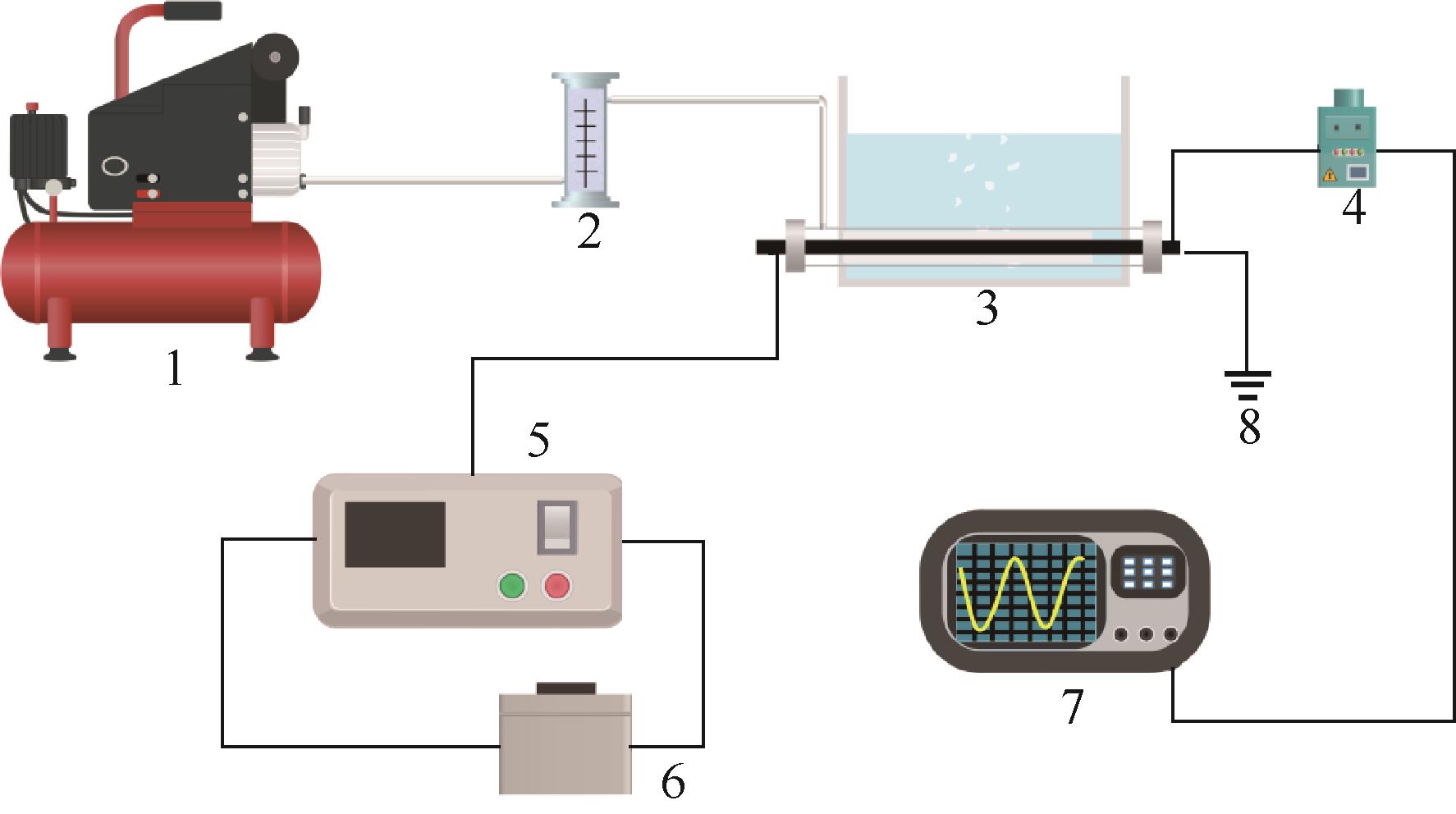
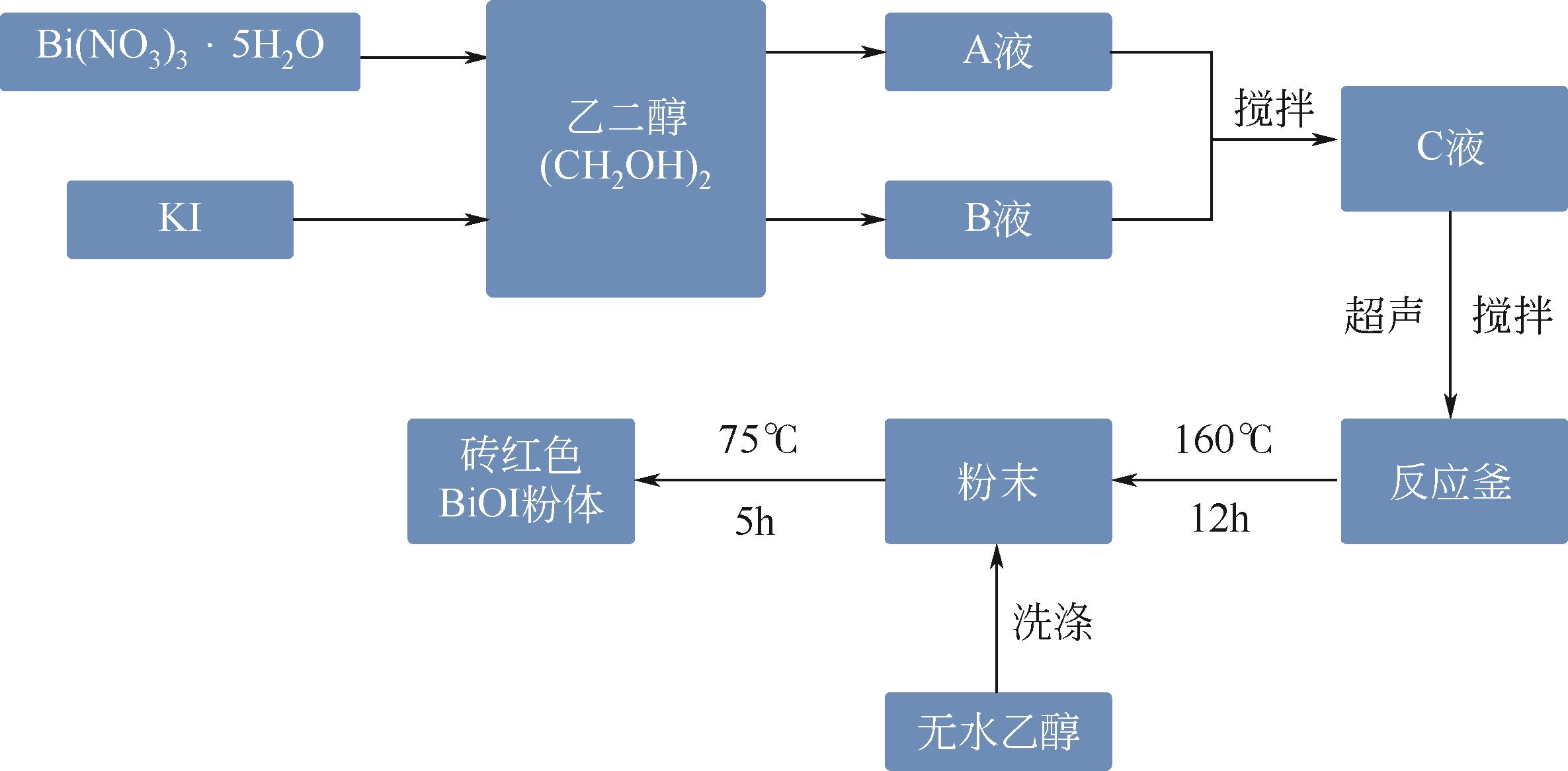
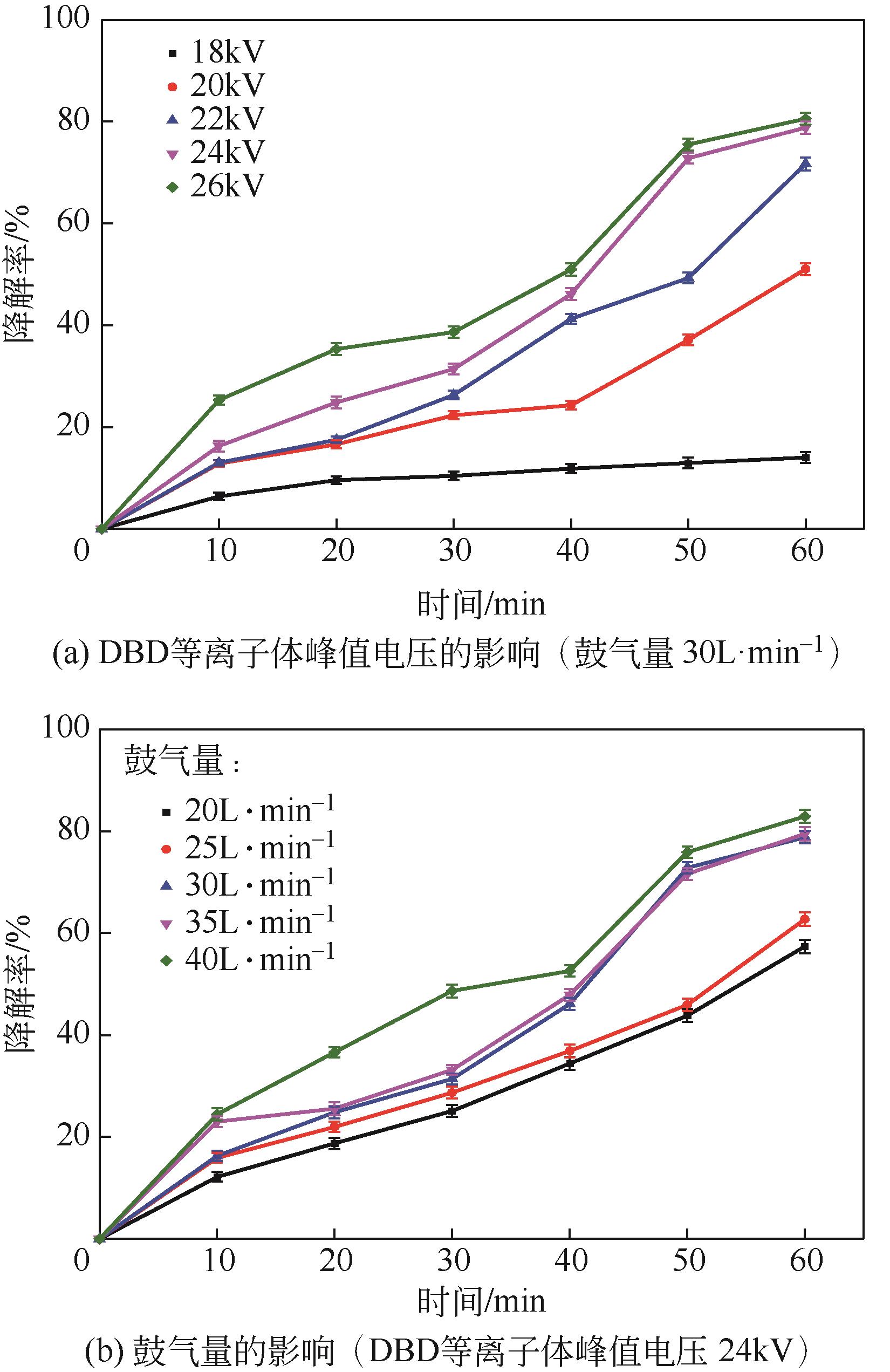
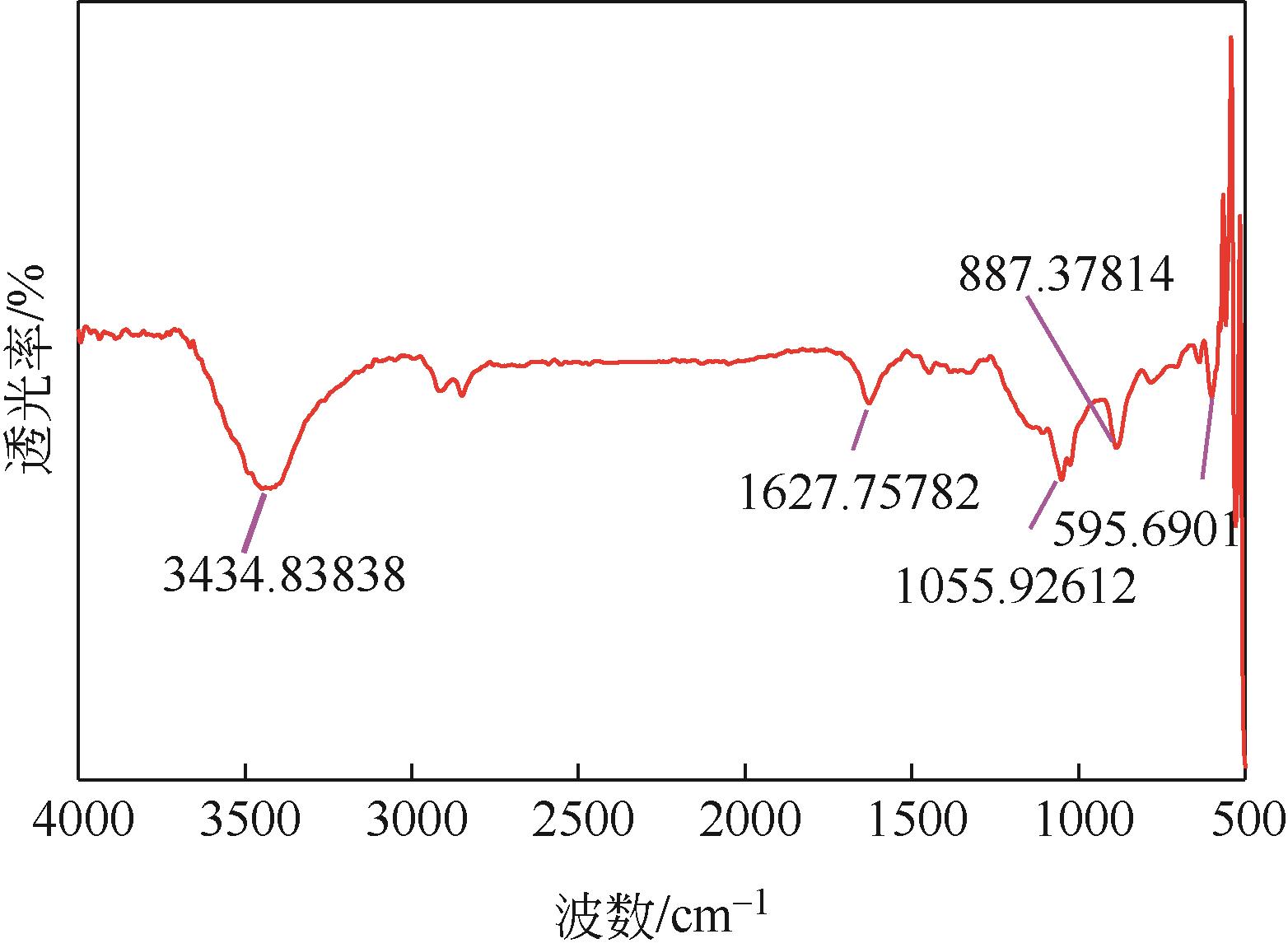
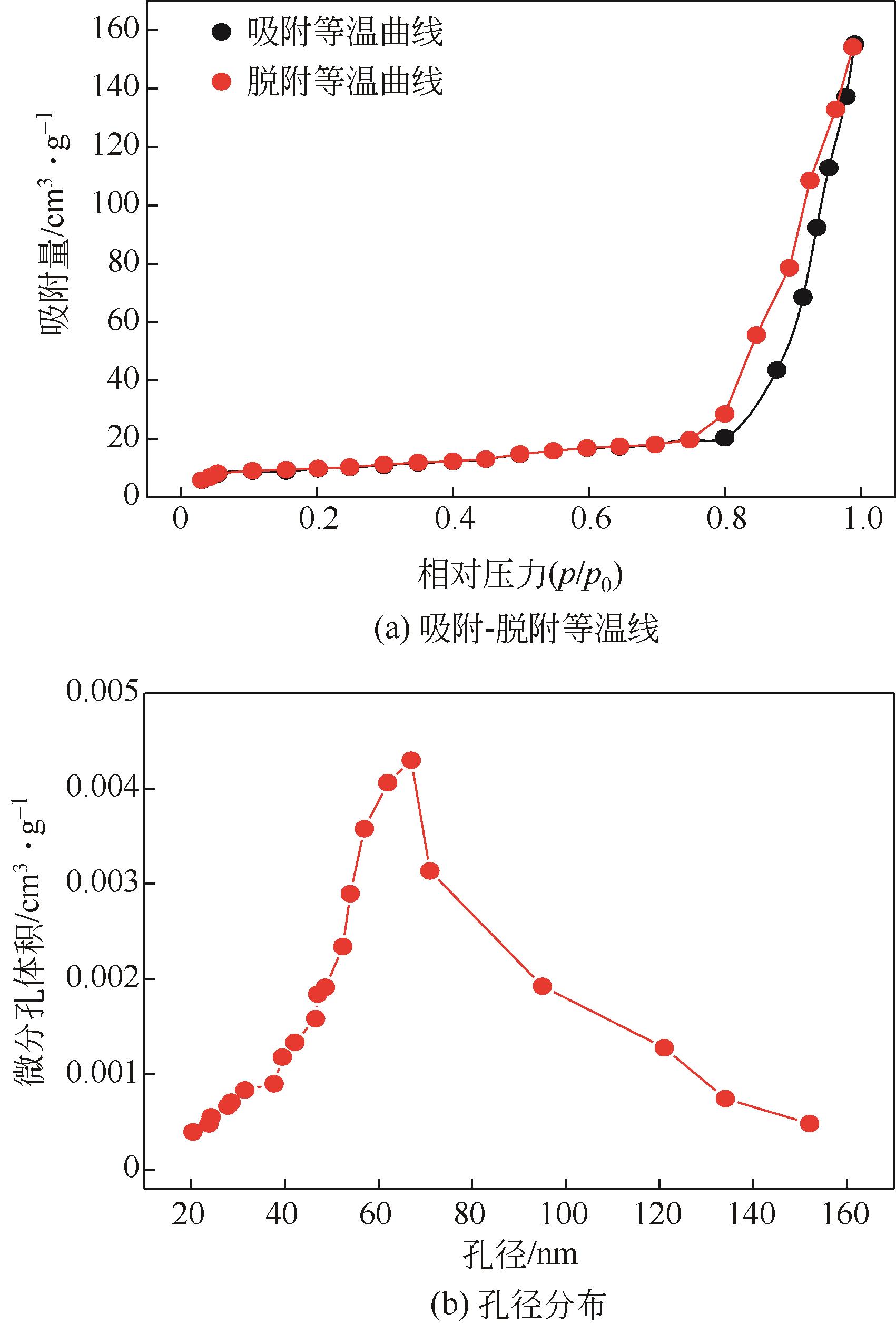
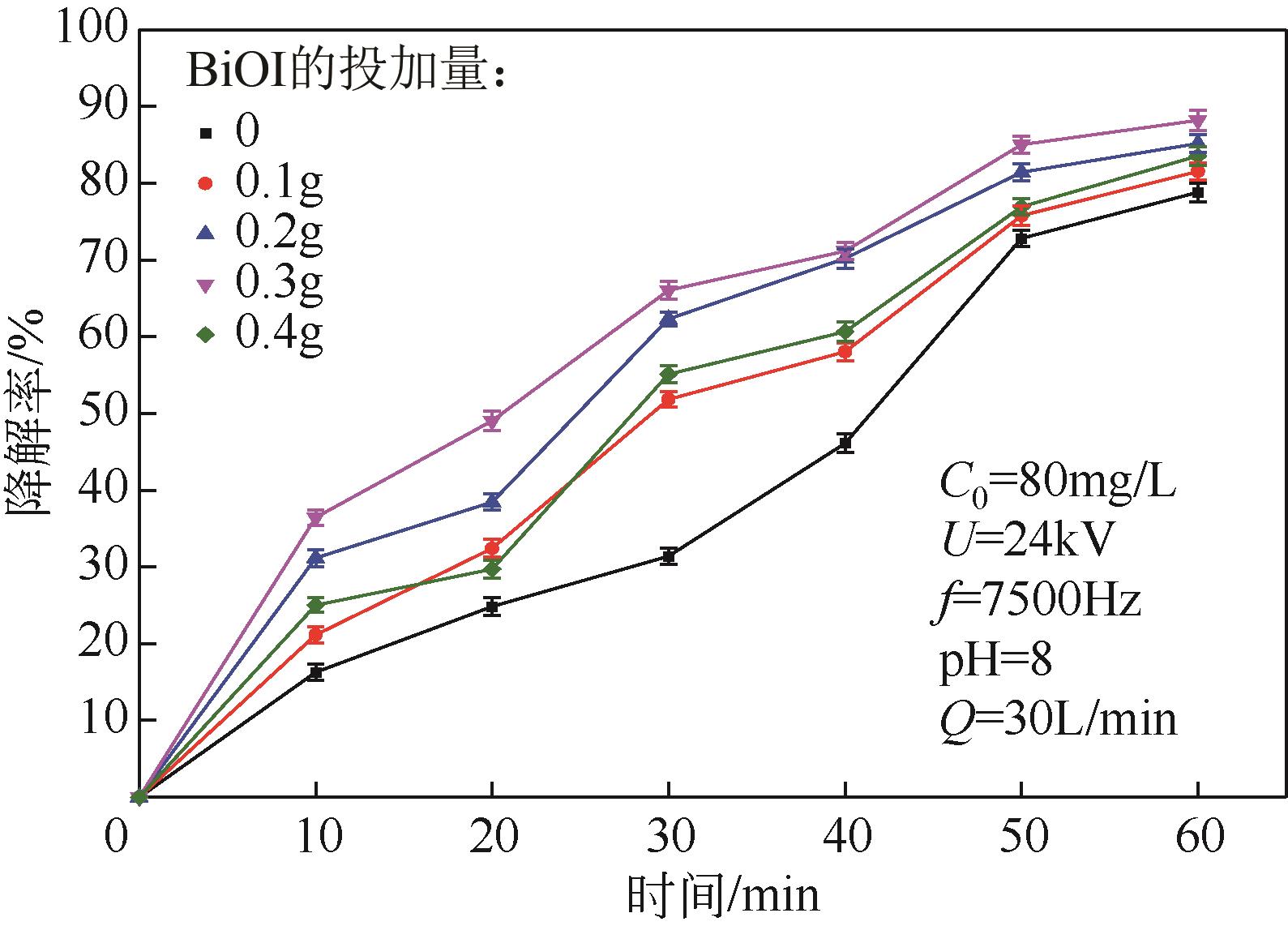
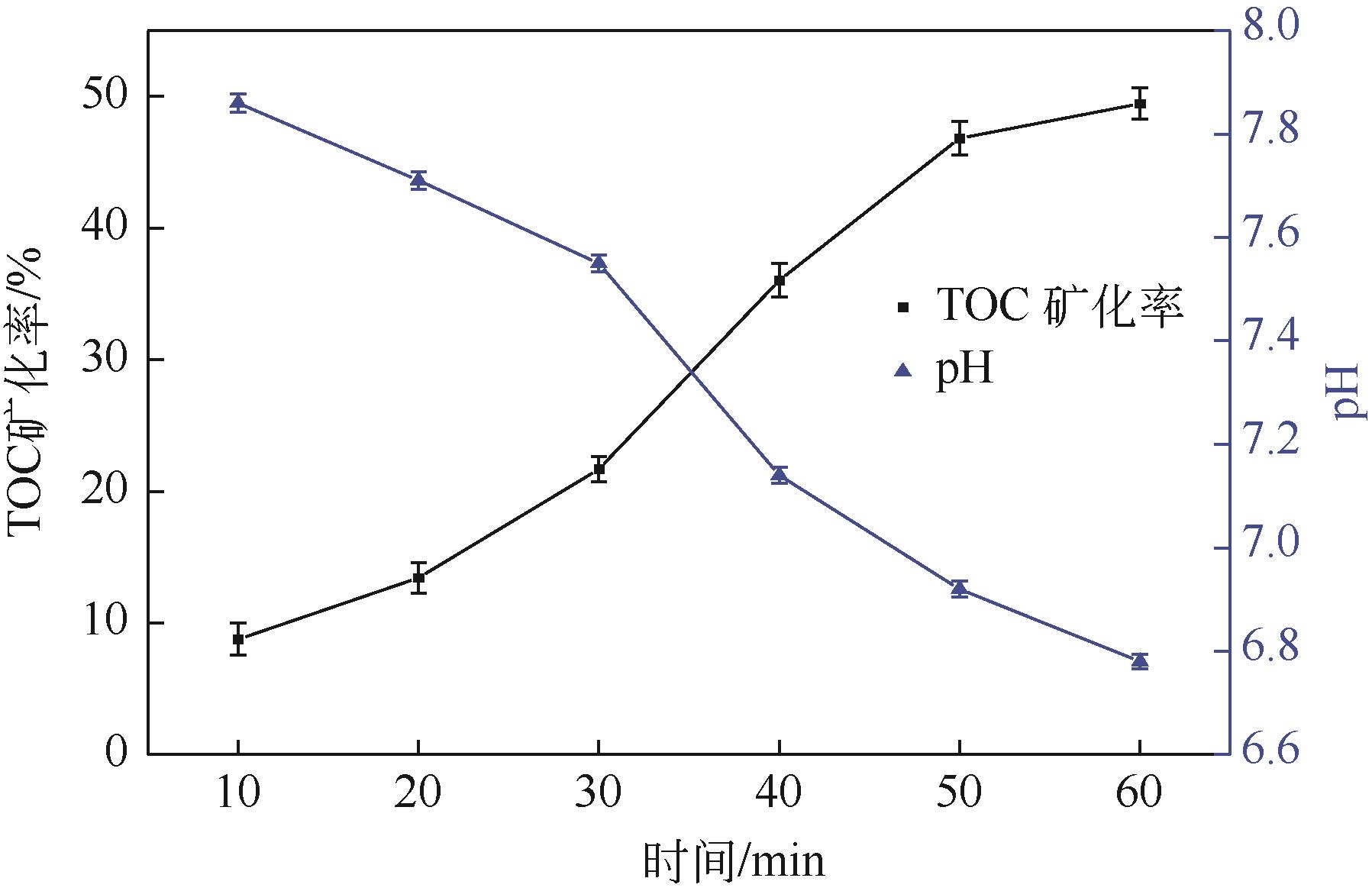
Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (3): 1565-1575.DOI: 10.16085/j.issn.1000-6613.2023-0367
• Resources and environmental engineering • Previous Articles
Performance and mechanism of the degradation of benzohydroxamic acid by DBD plasma-coupled BiOI catalytic materials
DONG Bingyan( ), LI Zhendong, WANG Peixiang, TU Wenjuan, TAN Yanwen, ZHANG Qin
), LI Zhendong, WANG Peixiang, TU Wenjuan, TAN Yanwen, ZHANG Qin
- College of Resources and Environmental Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, Jiangxi, China
-
Received:2023-03-10Revised:2023-06-28Online:2024-04-11Published:2024-03-10 -
Contact:DONG Bingyan
DBD等离子体耦合BiOI催化材料降解苯甲羟肟酸的特性与机制
- 江西理工大学资源与环境工程学院,江西 赣州 341000
-
通讯作者:董冰岩 -
作者简介:董冰岩(1974—),教授,博士生导师,研究方向为放电等离子体技术在环境保护中的应用技术。E-mail:dongbingyan1@sina.com。 -
基金资助:国家自然科学基金(51567010)
CLC Number:
Cite this article
DONG Bingyan, LI Zhendong, WANG Peixiang, TU Wenjuan, TAN Yanwen, ZHANG Qin. Performance and mechanism of the degradation of benzohydroxamic acid by DBD plasma-coupled BiOI catalytic materials[J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1565-1575.
董冰岩, 李贞栋, 王佩祥, 涂文娟, 谭艳雯, 张芹. DBD等离子体耦合BiOI催化材料降解苯甲羟肟酸的特性与机制[J]. 化工进展, 2024, 43(3): 1565-1575.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-0367
| 序号 | 产物 | 质荷比(m/z) | 结构式 |
|---|---|---|---|
| 1 | N-hydroxybenzamide (苯甲羟肟酸) | 138.055 | 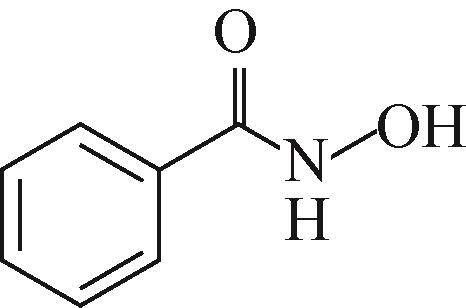 |
| 2 | benzoic acid (苯甲酸) | 123.0441 | 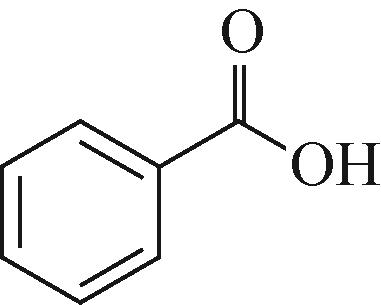 |
| 3 | 2-hydroxyacetic acid (乙醇酸) | 77.0233 | 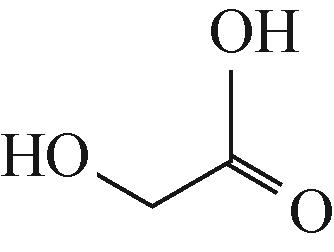 |
| 4 | but-2-ynedioic acid (丁炔二酸) | 115.0026 | 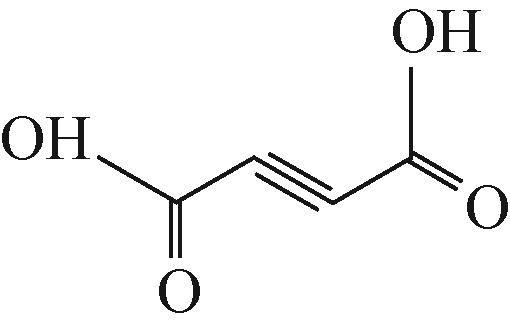 |
| 序号 | 产物 | 质荷比(m/z) | 结构式 |
|---|---|---|---|
| 1 | N-hydroxybenzamide (苯甲羟肟酸) | 138.055 |  |
| 2 | benzoic acid (苯甲酸) | 123.0441 |  |
| 3 | 2-hydroxyacetic acid (乙醇酸) | 77.0233 |  |
| 4 | but-2-ynedioic acid (丁炔二酸) | 115.0026 |  |
| 1 | YU Xinyang, ZHANG Ruirui, YANG Siyuan, et al. A novel decanedioic hydroxamic acid collector for the flotation separation of bastnäsite from calcite[J]. Minerals Engineering, 2020, 151: 106306. |
| 2 | CHAI Youzheng, QIN Pufeng, ZHANG Jiachao, et al. Simultaneous removal of Fe(Ⅱ) and Mn(Ⅱ) from acid mine wastewater by electro-Fenton process[J]. Process Safety and Environmental Protection, 2020, 143: 76-90. |
| 3 | 唐清, 钟宏, 王帅, 等. 羟肟酸类化合物的合成与应用研究进展[J]. 化工进展, 2014, 33(3): 703-709. |
| TANG Qing, ZHONG Hong, WANG Shuai, et al. Research progress on the synthesis and application of hydroxamic acid compounds[J]. Chemical Industry and Engineering Progress, 2014, 33(3): 703-709. | |
| 4 | XIAO Wei, SHAO Yanhai, YU Jiayi, et al. Activation of ilmenite flotation by Al3+ in the benzohydroxamic acid (BHA) system[J]. Separation and Purification Technology, 2022, 299: 121770. |
| 5 | HAN Guihong, DU Yifan, HUANG Yanfang, et al. Efficient removal of hazardous benzohydroxamic acid (BHA) contaminants from the industrial beneficiation wastewaters by facile precipitation flotation process[J]. Separation and Purification Technology, 2021, 279: 119718. |
| 6 | PANG Wancheng, YAO Jun, KNUDSEN Tatjanašolević, et al. Degradation of three typical hydroxamic acids collectors via UVA-B activated H2O2 and persulfate: Kinetics, transformation pathway, DFT calculation and toxicity evaluation[J]. Chemical Engineering Journal, 2023, 451: 138639. |
| 7 | 张大超, 陈敏, 代振鹏, 等. 真空紫外/曝气氧化降解苯甲羟肟酸模拟废水[J]. 化工进展. 2017, 36(7): 2639-2644. |
| ZHANG Dachao, CHEN Min, DAI Zhenpeng, et al. Effect of oxidative degradation of benzoic acid simulated wastewater by VUV/air[J]. Chemical Industry and Engineering Progress, 2017, 36(7): 2639-2644. | |
| 8 | 胡纯, 王灿, 龚文琪, 等. 三种羟肟酸捕收剂的微生物降解研究[J]. 湖北农业科学, 2013, 52(11): 2505-2507. |
| HU Chun, WANG Can, GONG Wenqi, et al. Study on the biodegradation of three hydroxamic acid collectors[J]. Hubei Agricultural Sciences, 2013, 52(11): 2505-2507. | |
| 9 | 祝思频, 王春英, 王俊蔚, 等. Gd掺杂锐钛矿型TiO2光催化剂的制备及降解苯甲羟肟酸活性[J]. 硅酸盐学报, 2017, 45(10): 1495-1502. |
| ZHU Sipin, WANG Chunying, WANG Junyu, et al. Preparation and degradation activity for benzohydroxamic acid of Gd-doped anatase TiO2 photocatalysts[J]. Journal of the Chinese Ceramic Society, 2017, 45(10): 1495-1502. | |
| 10 | 章丽萍, 项俊, 严振宇, 等. O3降解水杨羟肟酸选矿废水机理研究[J]. 矿业科学学报, 2019, 4(1): 79-85. |
| ZHANG Liping, XIANG Jun, YAN Zhenyu, et al. Mechanism study on ozonization degradation of salicylhydroxamic acid in flotation wastewater[J]. Journal of Mining Science and Technology, 2019, 4(1): 79-85. | |
| 11 | NIPPATLAPALLI Narasamma, RAMAKRISHNAN Kamaraj, PHILIP Ligy. Enhanced degradation of complex organic compounds in wastewater using different novel continuous flow non-Thermal pulsed corona plasma discharge reactors[J]. Environmental Research, 2022, 203: 111807. |
| 12 | 董冰岩, 李贞栋, 王佩祥,等. 脉冲气液两相放电等离子体耦合Fe改性的TiO2催化剂降解废水中的4-氯酚[J]. 化工进展, 2021, 40(12): 6721-6728. |
| DONG Bingyan, LI Zhendong, WANG Peixiang, et al. 4-Chlorophenol containing wastewater joint treated by pulsed discharge plasma in gas-liquid two phase and Fe-modified TiO2 catalyst[J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6721-6728. | |
| 13 | GAO Xiaoting, HUANG Keliang, ZHANG Ai, et al. Simultaneous degradation of glucocorticoids and sterilization using bubbling corona discharge plasma based systems: A promising terminal water treatment facility for hospital wastewater[J]. Chemical Engineering Journal, 2022, 430: 132845. |
| 14 | FANG Cao, WANG Shenhao, SHAO Changsheng, et al. Study of detoxification of methyl parathion by dielectric barrier discharge (DBD) non-thermal plasma at gas-liquid interface:Mechanism and bio-toxicity evaluation[J]. Chemosphere, 2022, 307: 135620. |
| 15 | 董博, 王保伟, 迟春梅, 等. 添加剂对等离子体降解亚甲基蓝的影响[J]. 化工进展, 2017, 36(2): 705-711. |
| DONG Bo, WANG Baowei, CHI Chunmei, et al. The effects of additives on the degradation of methylene blue using plasma[J]. Chemical Industry and Engineering Progress, 2017, 36(2): 705-711. | |
| 16 | WANG Xiaoping, HUANG Qiulin, DING Sheguang, et al. Micro hollow cathode excited dielectric barrier discharge (DBD) plasma bubble and the application in organic wastewater treatment[J]. Separation and Purification Technology, 2020, 240: 116659. |
| 17 | REN Jingyu, JIANG Nan, SHANG Kefeng, et al. Synergistic degradation of trans-ferulic acid by water falling film DBD plasma coupled with cobalt oxyhydroxide: performance and mechanisms[J]. Chemical Engineering Journal, 2019, 372: 321-331. |
| 18 | 王思怡, 李月慧, 葛玉洁, 等. NTP-DBD气化城市污泥及其模型化合物:气氛对产物分布及特性的影响[J]. 化工进展, 2022, 41(4): 2150-2160. |
| WANG Siyi, LI Yuehui, GE Yujie, et al. Gasification of sewage sludge and its model compounds with NTP-DBD: Effect of atmosphere on product distribution and properties[J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2150-2160. | |
| 19 | WU Jiali, XIONG Qing, LIANG Jialiang, et al. Degradation of benzotriazole by DBD plasma and peroxymonosulfate: mechanism, degradation pathway and potential toxicity[J]. Chemical Engineering Journal, 2020, 384: 123300. |
| 20 | RAHIMPOUR Mohammad, TAGHVAEI Hamed, RAHIMPOUR Mohammad Reza. Degradation of crystal violet in water solution using post discharge DBD plasma treatment: Factorial design experiment and modeling[J]. Chemosphere, 2019, 232: 213-223. |
| 21 | 刘丹, 张连成, 黄逸凡, 等. 双杆介质阻挡放电降解酸性红73废水[J]. 化工进展, 2018, 37(9): 3640-3648. |
| LIU Dan, ZHANG Liancheng, HUANG Yifan, et al. Degradation of Brilliant Crocein wastewater by double-rod dielectric barrier discharge[J]. Chemical Industry and Engineering Progress, 2018, 37(9): 3640-3648. | |
| 22 | LI Zhen, WANG Yawen, GUO He, et al. Insights into water film DBD plasma driven by pulse power for ibuprofen elimination in water: Performance, mechanism and degradation route[J]. Separation and Purification Technology, 2021, 277: 119415. |
| 23 | HUANG Qing, FANG Cao. Degradation of 3,3’,4,4’-tetrachlorobiphenyl (PCB77) by dielectric barrier discharge (DBD) non-thermal plasma: Degradation mechanism and toxicity evaluation[J]. Science of the Total Environment, 2020, 739: 139926. |
| 24 | KHAN Muhammad Saiful Islam, KIM Yun Ji. Dielectric barrier discharge (DBD) plasma induced flavonoid degradation kinetics and mechanism in water[J]. LWT, 2020, 118: 108777. |
| 25 | ZHANG Huihui, XIAO Sisi, DU Yansheng, et al. Catalysis of MnO2-cellulose acetate composite films in DBD plasma system and sulfamethoxazole degradation by the synergistic effect[J]. Separation and Purification Technology, 2022, 298: 121608. |
| 26 | DENG Ruoyu, HE Qiang, YANG Dongxu, et al. Enhanced synergistic performance of nano-Fe0-CeO2 composites for the degradation of diclofenac in DBD plasma[J]. Chemical Engineering Journal, 2021, 406: 126884. |
| 27 | SHANG Kefeng, LI Wenfeng, WANG Xiaojing, et al. Degradation of p-nitrophenol by DBD plasma/Fe2+/persulfate oxidation process[J]. Separation and Purification Technology, 2019, 218: 106-112. |
| 28 | PENG Haiyang, DUAN Lijuan, XIE Wuming, et al. Enhanced removal of Cr(Ⅵ) from wastewater by dielectric barrier discharge plasma coupled with TiO2/rGO nanocomposites: Catalytic performance and reduction mechanism[J]. Separation and Purification Technology, 2022, 298: 121609. |
| 29 | LU Wei, SANG Wenjiao, JIA Danni, et al. Improvement of degradation of Orange G in aqueous solution by Fe2+ added in dielectric barrier discharge plasma system[J]. Journal of Water Process Engineering, 2022, 47: 102707. |
| 30 | HOJAMBERDIEV Mirabbos, KADIROVA Zukhra C, MAKINOSE Yuki, et al. Influence of BiOI content on the photocatalytic activity of Bi2WO6/BiOI/allophane composites and molecular modeling studies of acetaldehyde adsorption[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 81: 258-264. |
| 31 | 张进, 徐丹, 冒彩云. 碘氧化铋的制备及可见光催化性能研究[J]. 化工新型材料, 2018, 46(5): 192-194. |
| ZHANG Jin, XU Dan, MAO Caiyun. Synthesis and photocatalytic application of visible-light-driven BiOI photocatalyst[J]. New Chemical Materials, 2018, 46(5):192-194. | |
| 32 | ZHU Jiaqian, LI Jiaying, LI Yuying, et al. Adsorption of phosphate and photodegradation of cationic dyes with BiOI in phosphate-cationic dye binary system[J]. Separation and Purification Technology, 2019, 223: 196-202. |
| 33 | LONG Yang, WANG Yi, ZHANG Dun, et al. Facile synthesis of BiOI in hierarchical nanostructure preparation and its photocatalytic application to organic dye removal and biocidal effect of bacteria[J]. Journal of Colloid and Interface Science, 2016, 481: 47-56. |
| 34 | DIEZ Laurent, LIVERTOUX Marie Hélène, STARK Avishay Abraham, et al. High-performance liquid chromatographic assay of hydroxyl free radical using salicylic acid hydroxylation during in vitro experiments involving thiols[J]. Journal of Chromatography B: Biomedical Sciences and Applications, 2001, 763(1): 185-193. |
| 35 | SHEN Tianyao, WANG Xiaojing, XU Peng, et al. Effect of dielectric barrier discharge plasma on persulfate activation for rapid degradation of atrazine: Optimization, mechanism and energy consumption[J]. Environmental Research, 2022, 212: 113287. |
| 36 | FANG Cao, SHAO Changsheng, WANG Shenhao, et al. Simultaneous removal of levofloxacin and sulfadiazine in water by dielectric barrier discharge (DBD) plasma: Enhanced performance and degradation mechanism[J]. Process Safety and Environmental Protection, 2023, 171: 459-469. |
| 37 | DONG Shanshan, FAN Liumin, MA Yunfang, et al. Inactivation of polyphenol oxidase by dielectric barrier discharge (DBD) plasma: Kinetics and mechanisms[J]. LWT, 2021, 145: 111322. |
| 38 | GUO He, YANG Hao, HUANG Jingwen, et al. Theoretical and experimental insight into plasma-catalytic degradation of aqueous p-nitrophenol with graphene-ZnO nanoparticles[J]. Separation and Purification Technology, 2022, 295: 121362. |
| 39 | 姬磊, 于瑞敏, 王浩人, 等. BiOCl/NaBiO3复合材料的原位合成及光催化性能[J]. 高等学校化学学报, 2015, 36(3): 551-558. |
| JI Lei, YU Ruimin, WANG Haoren, et al. In-situ synthesis of BiOCl/NaBiO3 composites and their photocatalytic activities[J]. Chemical Journal of Chinese Universities, 2015, 36(3): 551-558. | |
| 40 | SUN Xiaoming, LU Jia, WU Jiang, et al. Enhancing photocatalytic activity on gas-phase heavy metal oxidation with self-assembled BiOI/BiOCl microflowers[J]. Journal of Colloid and Interface Science, 2019, 546: 32-42. |
| 41 | 卞俊杰, 王万圆, 满恒孝, 等. BiOX(Cl, Br, I)/Bi2WO6异质结型复合光催化剂用于高浓度氮氧化物的脱除[J]. 化工进展, 2021, 40(11): 6094-6101. |
| BIAN Junjie, WANG Wanyuan, MAN Hengxiao, et al. BiOX(CI,Br,I)/Bi2WO6 heterojunction composites as photocatalysts for high concentration NO removal[J]. Chemical Industry and Engineering Progress, 2021, 40(11): 6094-6101. | |
| 42 | NIU Jinfen, DAI Peixuan, WANG Kai, et al. Microwave-assisted synthesis of high efficient α-Fe2O3/BiOI composites and its performance in photocatalytic degardation of organic pollutants[J]. Advanced Powder Technology, 2020, 31(6): 2327-2336. |
| 43 | ARUMUGAM Malathi, YU Yiseul, JUNG Hyeon Jin, et al. Solvent-mediated synthesis of BiOI with a tunable surface structure for effective visible light active photocatalytic removal of Cr(Ⅵ) from wastewater[J]. Environmental Research, 2021, 197: 111080. |
| 44 | VAHABIRAD Samira, Alireza NEZAMZADEH-EJHIEH. Co-precipitation synthesis of BiOI/(BiO)2CO3: Brief characterization and the kinetic study in the photodegradation and mineralization of sulfasalazine[J]. Journal of Solid State Chemistry, 2022, 310: 123018. |
| 45 | LI Jianhui, YANG Chunmei, ZHANG Shen, et al. Synthesis and property analysis of high magnetic and stable ternary composite Fe3O4/BiOBr/BiOI[J]. Chemical Physics Letters, 2021, 785: 139159. |
| 46 | WANG Xiaobing, WANG Lu, LIU Kai, et al. Study on degradation of wastewater containing PAM catalyzed by nonequilibrium plasma and γ-Al2O3 [J]. Journal of Water Process Engineering, 2022, 49: 103065. |
| 47 | FENG Jingwei, ZHENG Zheng, SUN Yabing, et al. Degradation of diuron in aqueous solution by dielectric barrier discharge[J]. Journal of Hazardous Materials, 2008, 154(1/2/3): 1081-1089. |
| 48 | 谢瑞, 陈超, 李武华, 等. 气液相等离子体放电水处理反应器及苯酚降解分析[J]. 高电压技术, 2010, 36(11): 2791-2796. |
| XIE Rui, CHEN Chao, LI Wuhua, et al. Analysis on gas-liquid hybrid plasma discharge reactor for wastewater treatment and phenol degradation[J]. High Voltage Engineering, 2010, 36(11): 2791-2796. | |
| 49 | CHEN Yongyang, SUN Xiaomin, ZHENG Lijiao, et al. Synergistic catalysis induced by a multi-component system constructed by DBD plasma combined with α-Fe2O3/FeVO4/HCP and peroxymonosulfate for gatifloxacin removal[J]. Chemosphere, 2023, 332: 138838. |
| 50 | DONG Senlin, WANG Yifan, YANG Jie, et al. Performance and mechanism analysis of degradation of toluene by DBD plasma-catalytic method with MnO x /Al2O3 catalyst[J]. Fuel, 2022, 319: 123721. |
| 51 | AGGELOPOULOS C A, MEROPOULIS S, HATZISYMEON M, et al. Degradation of antibiotic enrofloxacin in water by gas-liquid nsp-DBD plasma: Parametric analysis, effect of H2O2 and CaO2 additives and exploration of degradation mechanisms[J]. Chemical Engineering Journal, 2020, 398: 125622. |
| 52 | DONG Bingyan, WANG Peixiang, LI Zhendong, et al. Degrading hazardous benzohydroxamic acid in the industrial beneficiation wastewater by dielectric barrier discharge reactor[J]. Separation and Purification Technology, 2022, 299: 121644. |
| 53 | LI Mengke, HE Zhiguo, ZHONG Hui, et al. A novel multi-components hierarchical porous composite prepared from solid wastes for benzohydroxamic acid degradation[J]. Journal of Colloid and Interface Science, 2023, 630: 714-726. |
| [1] | LIU Fangwang, HAN Yi, ZHANG Jiajia, BU Honghong, WANG Xingpeng, YU Chuanfeng, LIU Mengshuai. Research advance of heterogeneous catalytic system for the coupling between CO2 and epoxide into propylene carbonate [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1252-1265. |
| [2] |
ZHANG Pengfei, YAN Zhangyan, REN Liang, ZHAGN Kui, LIANG Jialin, ZHAO Guangle, ZHANG Fanbin, HU Zhihai.
Research progress in the catalytic hydrodealkylation of C |
| [3] | GU Xingpeng, MA Hongqin, LIU Jiahao. Modification of Rainey nickel with phosphorus quantum dots and its catalytic hydrodesulfurization performances [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1293-1301. |
| [4] | ZHANG Shuming, LIU Huazhang. Optimization of Fe1-x O ammonia synthesis catalyst by BP neural network model [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1302-1308. |
| [5] | LI Kairui, GAO Zhaohua, LIU Tiantian, LI Jing, WEI Haisheng. Tuning the catalytic performance of Rh/FePO4 catalyst by reduction temperature for quinoline selective hydrogenation [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1342-1349. |
| [6] | LIU Bin, WANG Yongjun, LYU Wangyang, CHEN Wenxing. Preparation and application of high stability titanium polyester catalyst TiOC@SiO2 [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1395-1402. |
| [7] | WANG Xiong, KANG Wenqian, REN Yue, QIAO Tongsen, ZHANG Peng, HUANG Anping, LI Guangquan. Pilot scale production of porous organic polymers and their application in polyolefin catalysts [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1412-1417. |
| [8] | YAN Shoucheng, ZHANG Huihua, XU Qianqian, WANG Yukun. Application of graphene composite supported catalyst in the removal of NO from diesel exhaust [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1456-1465. |
| [9] | CHEN Xiaozhen, LIU Li, YANG Chengmin, ZHENG Bumei, YIN Xiaoying, SUN Jin, YAO Yunhai, DUAN Weiyu. Research progress of alumina-supported hydrodesulfurization catalyst [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 948-961. |
| [10] | DING Kang, HE Junqiao, CHEN Yuanjie, YANG Xiazhen, LIU Huazhang, HUO Chao. Effect of hydrochloric acid treatment on catalytic performance of Ru/Ba-MgO catalyst template cotton fiber [J]. Chemical Industry and Engineering Progress, 2024, 43(2): 962-970. |
| [11] | WANG Darui, SUN Hongmin, WANG Yiyan, TANG Zhimou, LI Rui, FAN Xueyan, YANG Weimin. Recent progress in zeolite for efficient catalytic reaction process [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 1-18. |
| [12] | LUO Fen, YANG Xiaoqi, DUAN Fanglin, LI Xiaojiang, WU Liang, XU Tongwen. Recent advances in the bipolar membrane and its applications [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 145-163. |
| [13] | GAI Hongwei, ZHANG Chenjun, QU Jingying, SUN Huailu, TUO Yongxiao, WANG Bin, JIN Xu, ZHANG Xi, FENG Xiang, CHEN De. Research progress on catalytic dehydrogenation process intensification for liquid organic hydride carrier hydrogen storage [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 164-185. |
| [14] | ZHANG Jiahao, LI Yingying, XU Yanlin, YIN Jiabin, ZHANG Jisong. Research advancement of continuous reductive amination in microreactors [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 186-197. |
| [15] | HENG Linyu, DENG Zhuoran, CHENG Daojian, WEI Bin, ZHAO Liqiang. Progress of high-throughput synthesis device for process reinforcement of metal catalyst preparation [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 246-259. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||