| 1 |
CHIVERS P R A, SMITH D K. Shaping and structuring supramolecular gels[J]. Nature Reviews Materials, 2019, 4: 463-478.
|
| 2 |
裴强, 丁爱祥, 吴晋晋. 基于分子内三中心氢键的超分子组装体系及其应用[J]. 有机化学, 2021, 41: 105-125.
|
|
PEI Q, DING A X, WU J J. Supramolecular assemblies based on intramolecular three-center hydrogen bond and their applications[J]. Chinese Journal of Organic Chemistry, 2021, 41: 105-125.
|
| 3 |
裴强, 丁爱祥, 杨明丽, 等. 氢键型超分子聚合物[J]. 化工进展, 2020, 39(1): 233-249.
|
|
PEI Q, DING A X, YANG M L, et al. Hydrogen-bonding supramolecular polymers [J]. Chemical Industry and Engineering Progress, 2020, 39(1): 233-249.
|
| 4 |
YANG H K, ZHANG C, HE X N, et al. Effects of alkyl chain lengths on 12-hydroxystearic acid derivatives based supramolecular organogels[J]. Colloids and Surfaces A: physicochemical and Engineering Aspects, 2021, 616: 126319.
|
| 5 |
ZHOU Q, DONG X, ZHANG B, et al. Luminescence sensitization of terbium-loaded supramolecular gels by hydroxybenzoic acids and used for salicylates sensing[J]. Talanta, 2021, 225: 122061.
|
| 6 |
YANG Y S, YANG C, ZHANG Y P, et al. Novel coumarinbased pyrazoline derivatives organogels for Fe3+ detection and application in cell imaging[J]. Colloids and Surfaces A: physicochemical and Engineering Aspects, 2021, 624: 126798.
|
| 7 |
MARIANI G, GOUJON A, MOULIN E, et al. Integration of molecular machines into supramolecular materials: actuation between equilibrium polymers and crystal-like gels[J]. Nanoscale, 2017, 9: 18456-18466.
|
| 8 |
WU Z, SONG M, WANG J, et al. Supramolecular gel assisted synthesis of Co2P nanosheets as an effcient and stable catalyst for oxygen reduction reaction[J]. New Journal of Chemistry, 2018, 42: 8800-8804.
|
| 9 |
PATTERSON A K, SMITH D K. Two-component supramolecular hydrogel for controlled drug release[J]. Chemical Communications, 2020, 56: 11046-11049.
|
| 10 |
PEI Q, TANG Q, TAN Z L, et al. Amphiphilic oligoamides as versatile, acid-responsive gelators[J]. RSC Advances, 2017, 7: 22248-22255.
|
| 11 |
SRIDHAR S P, JOHN J, HOLMQVIST P, et al. Adsorption of anionic dyes using a poly(styrene-block-4-vinylpyridine) Block copolymer organogel[J]. Langmuir, 2021, 37: 3996-4006.
|
| 12 |
DASTIDAR P, ROY R, PARVEEN R, et al. Supramolecular synthon approach in designing molecular gels for advanced therapeutics[J]. Advances in Therapy, 2019, 2: 1800061.
|
| 13 |
裴强, 丁爱祥, 徐果. 两亲性萘双酰亚胺的合成及其凝胶性能[J]. 信阳师范学院(自然科学版), 2019, 32(2): 270-275.
|
|
PEI Q, DING A X, XU G. Synthesis and gelation properties of amphiphilic naphthalene-diimide[J]. Journal of Xinyang Normal University (Natural Science Edition ), 2019, 32(2): 270-275.
|
| 14 |
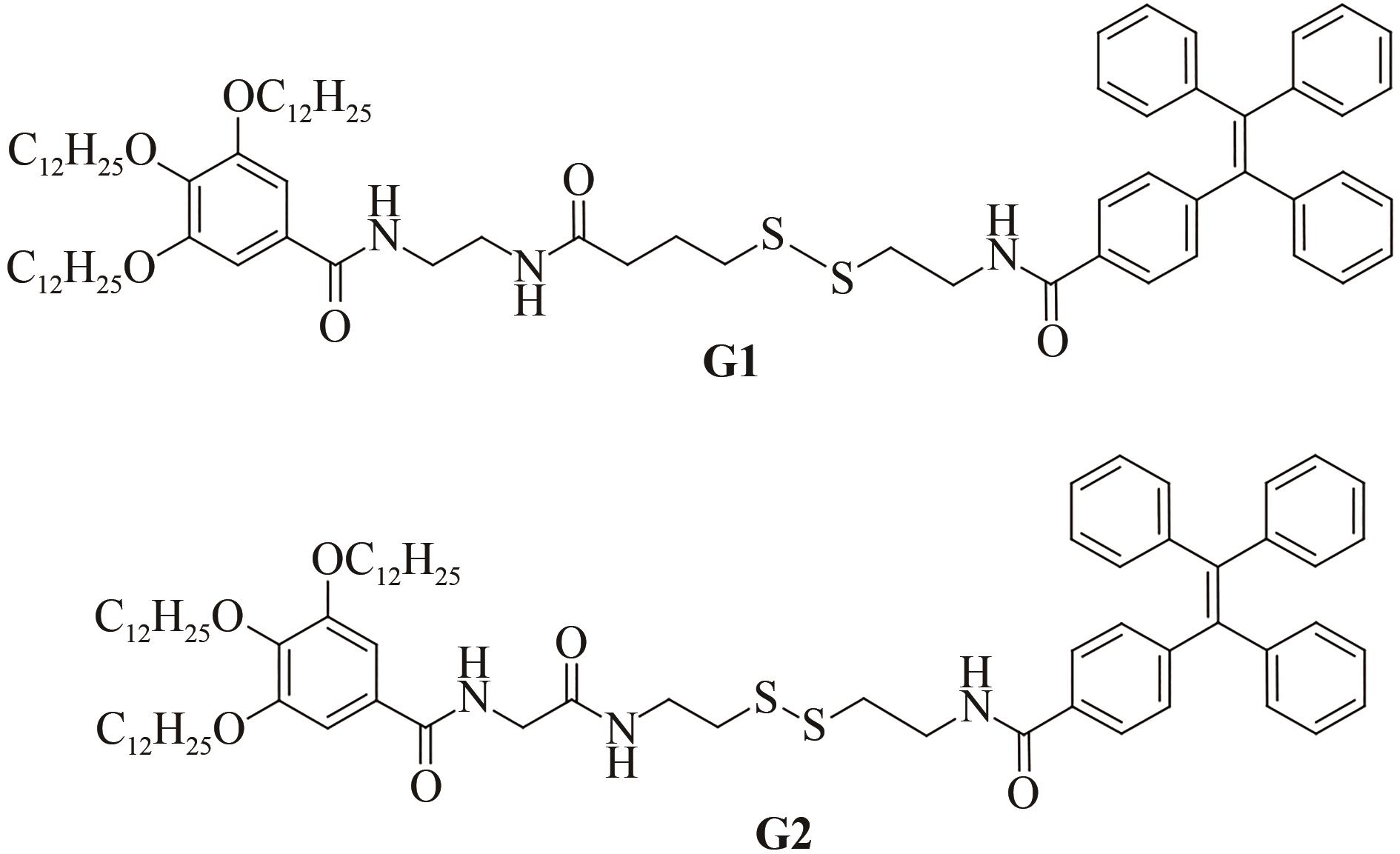
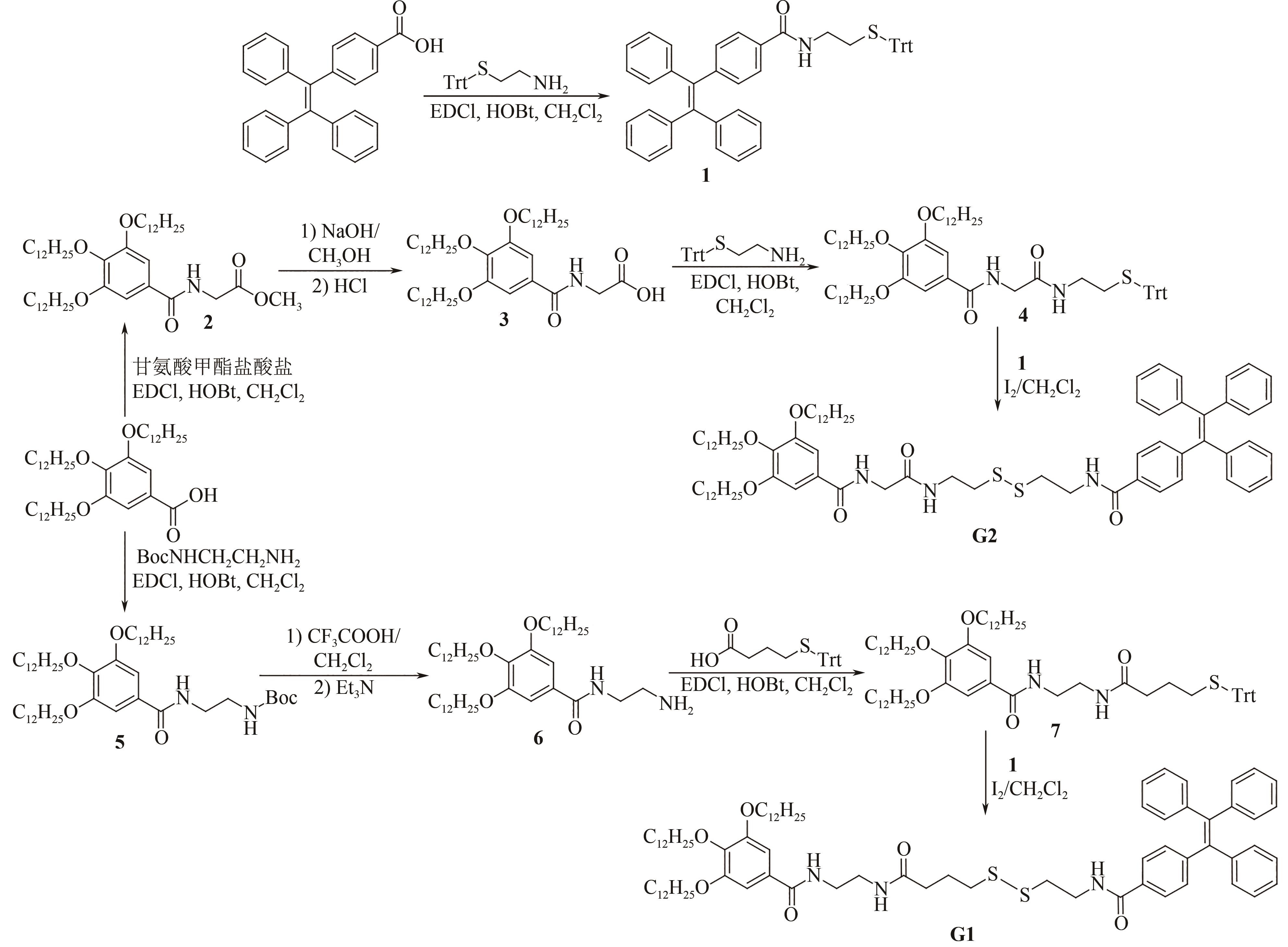
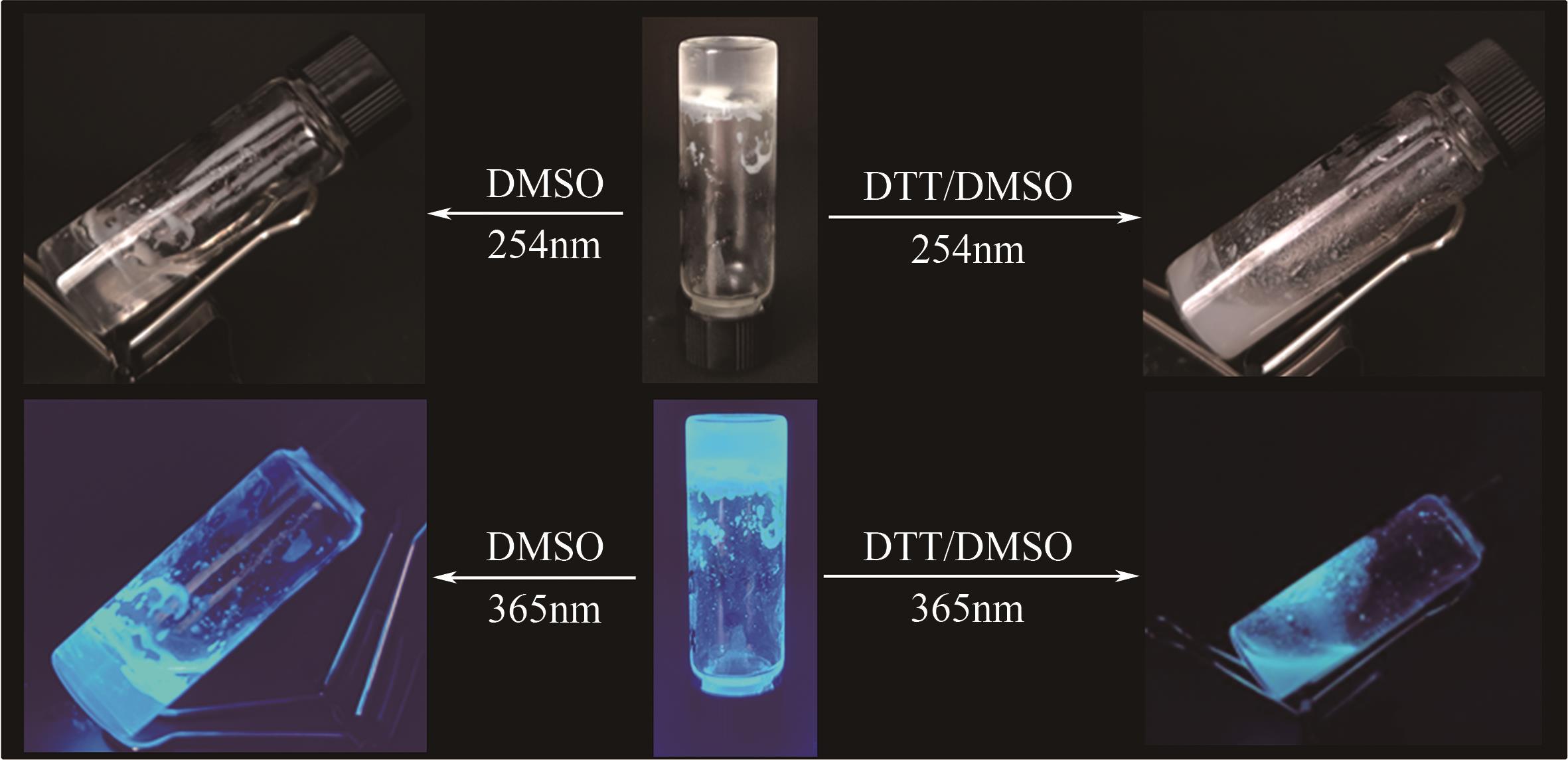
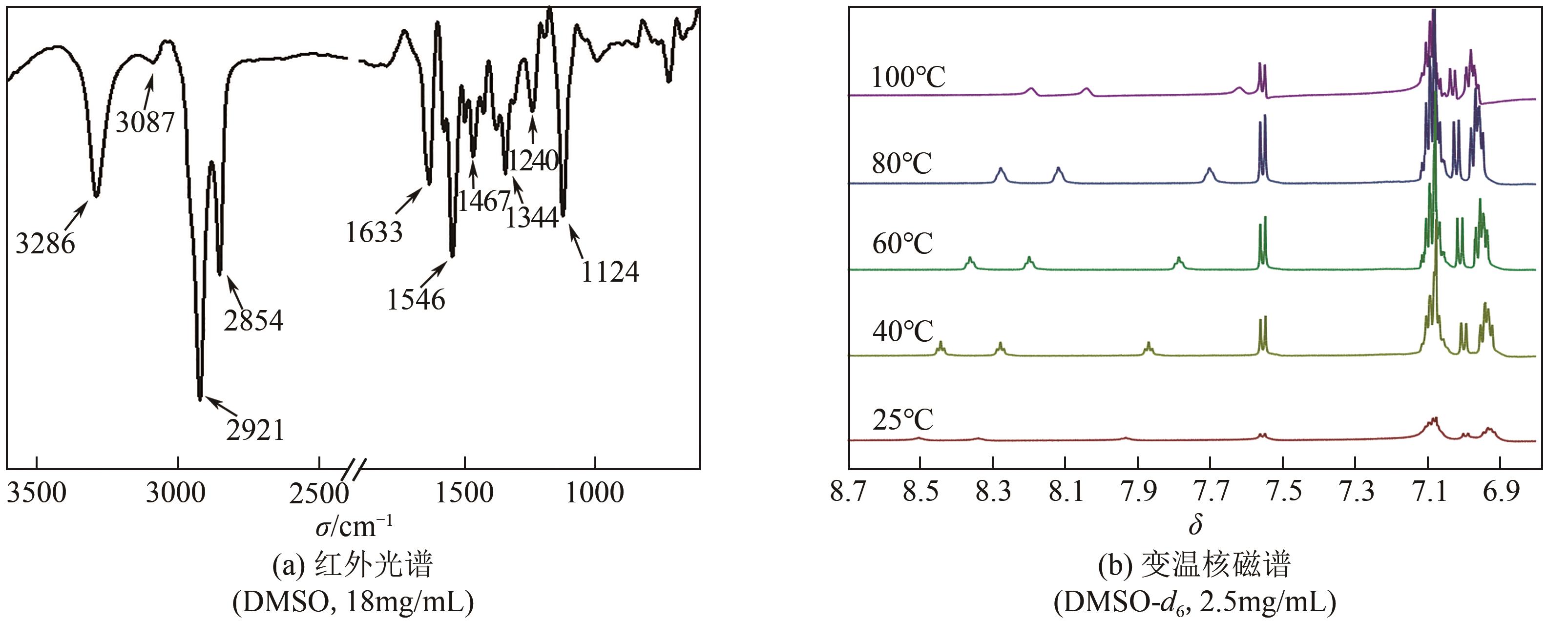
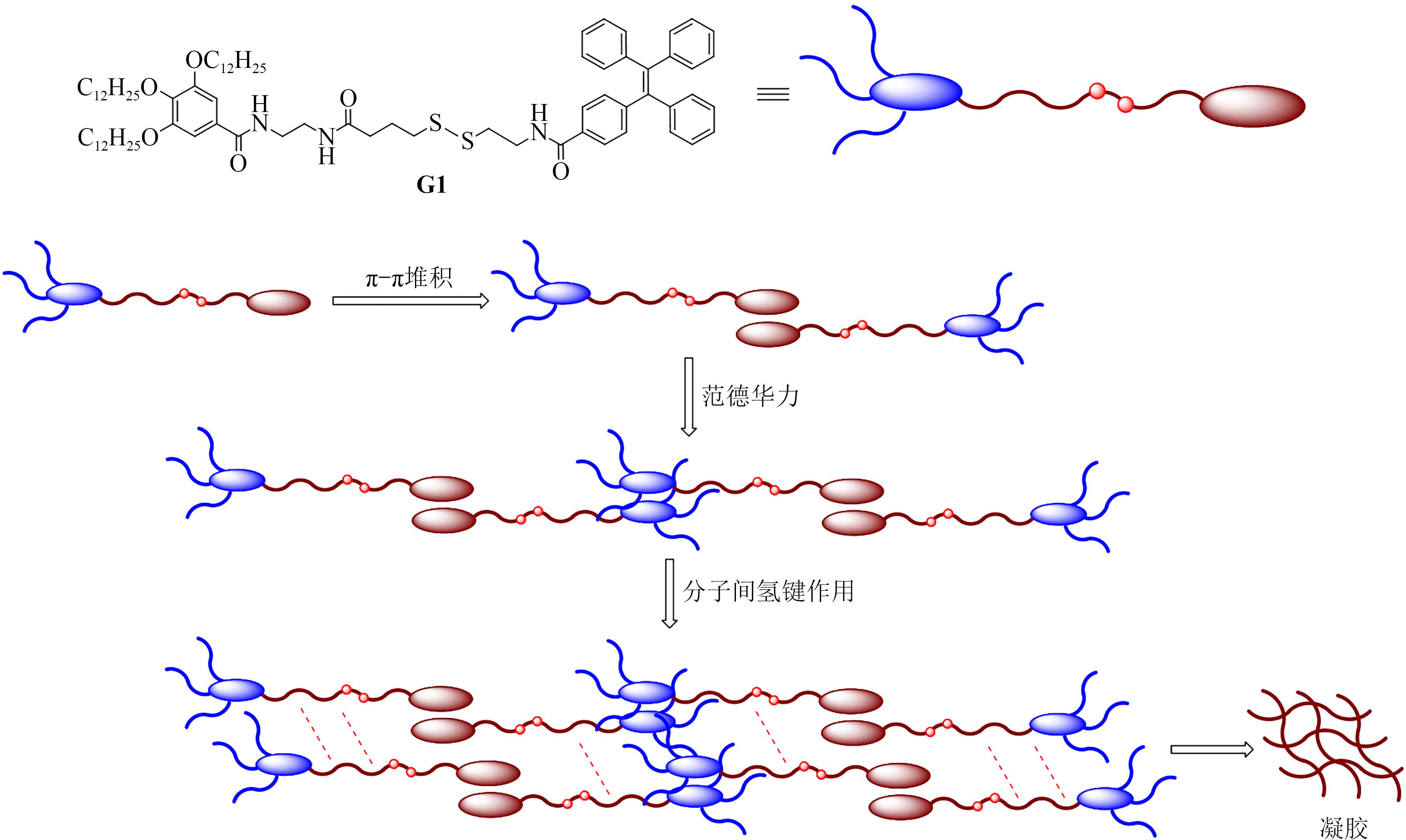
PEI Q, HAN Q Q, TANG F, et al. Gallic-acid-modified naphthalimide containing disulfide bond as reduction-responsive supramolecular organogelator[J]. ChemistrySelect, 2022, 7: e202201296.
|
| 15 |
PEI Q, HAN Q Q, TANG F, et al. In situ synthesis of reduction-responsive organogelators via oxidative coupling of tritylthio-terminated gallic acid derivatives[J]. Colloids and Surfaces A: physicochemical and Engineering Aspects, 2022, 641: 128602.
|
| 16 |
DING A X, TANG Q, GAO Y G, et al. [12]aneN3 modified tetraphenyl ethene molecules as high performance sensing, condensing, and delivering agents toward DNAs[J]. ACS Applied Materials & Interfaces, 2016, 8: 14367-14378.
|
| 17 |
DING A X, HAO H J, GAO Y G, et al. D-A-D type chromophores with aggregation-induced emission and two-photon absorption: synthesis, optical characteristics and cell imaging [J]. Journal of Materials Chemistry C, 2016, 4(23): 5379-5389.
|
| 18 |
YANG Q L, BAI L, ZHANG Y Q, et al. Dynamic covalent diblock copolymers: instructed coupling, micellation and redox responsiveness[J]. Macromolecules, 2014, 47(21): 7431-7442.
|
| 19 |
JADHAV S R, VEMULA P K, KUMAR R, et al. Sugar-derived phaseselective molecular gelators as model solidifers for oil spills[J]. Angewandte Chemie International Edition, 2010, 49: 7695-7698.
|
| 20 |
SONG N, LOU X Y, HOU W, et al. Pillararene-based fluorescent supramolecular systems: the key role of chain length in gelation[J]. Macromolecular Rapid Communications, 2018, 39: 1800593.
|
| 21 |
YU G C, YAN X Z, HAN C Y, et al. Characterization of supramolecular gels[J]. Chemical Society Reviews, 2013, 42(16): 6697-6722.
|
| 22 |
CAO X H, DING Q Q, LI Y R, et al. Continuous multi-channel sensing of volatile acid and organic amine gases using a fluorescent self-assembly system[J]. Journal of Materials Chemistry C, 2019, 7(1): 133-142.
|
| 23 |
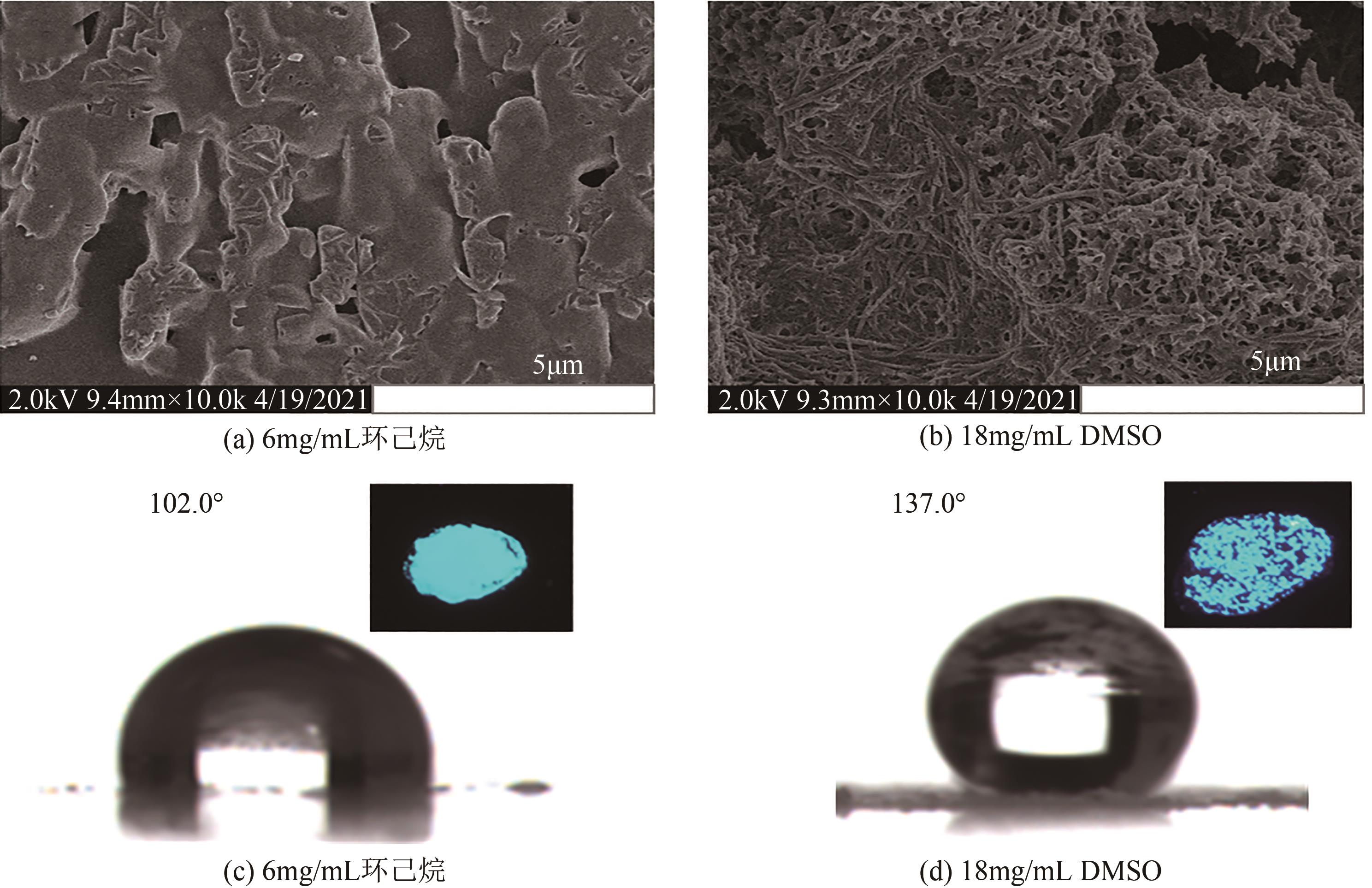
CAO X H, LI Y R, ZHANG X Y, et al. Surface wettability and emission behavior tuned via solvent in a supramolecular self-assembly system based on a naphthalene diimides derivative[J]. Applied Surface Science, 2020, 501: 144256.
|
| 24 |
ROY S, KAR B, DAS S, et al. Effect of hydrogen bonding and hydrophobicity on gel emulsions by benzenesulphonamide moiety-based amphiphiles: entrapment and release of vitamin B12 [J]. Chemical Papers, 2020, 74: 2635-2652.
|
| 25 |
NANDI M, MAITI B, BANERJEE S, et al. Hydrogen bonding driven self-assembly of side-chain amino acid and fatty acid appended poly(methacrylate)s: gelation and application in oil spill recovery[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2019, 57: 511-521.
|
| 26 |
WEI J, CHAI Q, HE L, et al. An anthracene-based organogel with colorimetric fluoride-responsive and fluorescence-enhanced properties[J]. Tetrahedron, 2016, 72: 3073-3076.
|
| 27 |
WANG H, HAN Y, YUAN W, et al. Self-assembly of azobenzene derivatives into organogels and photoresponsive liquid crystals[J]. Chemistry-An Asian Journal, 2018,13: 1173-1179.
|
| 28 |
SIMALOU O, XUE P, LU R. A potent triphenylbenzene-based H-bonding donor to assist formation of two-component organogels with stilbazoles[J]. Tetrahedron Letters, 2010, 51: 3685-3690.
|
| 29 |
KE C, ZHANG C, WU X, et al. Highly transparent and robust superhydrophobic coatings fabricated via a facile sol-gel process[J]. Thin Solid Films, 2021, 723: 138583.
|
| 30 |
CAO X H, ZHAO N, GAO A P, et al. Bis-naphthalimides self-assembly organogel formation and application in detection of p-phenylenediamine[J]. Materials Science and Engineering C, 2017, 70: 216-222.
|
 ), HU Wenjing1, NIE Lizhu1, SHI Yanan1, DING Aixiang2
), HU Wenjing1, NIE Lizhu1, SHI Yanan1, DING Aixiang2