Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (9): 4904-4917.DOI: 10.16085/j.issn.1000-6613.2021-0594
Previous Articles Next Articles
Research progress on oxygen electrode materials for reversible solid oxide fuel cells
YANG Xiaoxing( ), MIAO He, YUAN Jinliang(
), MIAO He, YUAN Jinliang( )
)
- Faculty of Maritime and Transportation, Ningbo University, Ningbo 315222, Zhejiang, China
-
Received:2021-03-23Revised:2021-04-12Online:2021-09-13Published:2021-09-05 -
Contact:YUAN Jinliang
可逆固体氧化物燃料电池氧电极材料的研究进展
- 宁波大学海运学院,浙江 宁波 315222
-
通讯作者:袁金良 -
作者简介:杨晓幸(1997—),男,硕士研究生。E-mail:15578346001@163.com 。 -
基金资助:国家重点研发计划(2018YFB1502204);国家自然科学基金(51779025);宁波市科技创新2025重大专项(2020Z107)
CLC Number:
Cite this article
YANG Xiaoxing, MIAO He, YUAN Jinliang. Research progress on oxygen electrode materials for reversible solid oxide fuel cells[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 4904-4917.
杨晓幸, 苗鹤, 袁金良. 可逆固体氧化物燃料电池氧电极材料的研究进展[J]. 化工进展, 2021, 40(9): 4904-4917.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-0594
| SOFC模式氧电极催化 | 对应的过程 | SOEC模式氧电极催化 | 对应的过程 |
|---|---|---|---|
| 第一步 | 第一步 | ||
| 第二步 | 第二步 | ||
| 第三步 | 第三步 | ||
| 第四步 | 第四步 | ||
| 第五步 | 2 | 第五步 | |
| 第六步 | 第六步 | ||
| 第七步 | 第七步 | ||
| 第八步 |
| SOFC模式氧电极催化 | 对应的过程 | SOEC模式氧电极催化 | 对应的过程 |
|---|---|---|---|
| 第一步 | 第一步 | ||
| 第二步 | 第二步 | ||
| 第三步 | 第三步 | ||
| 第四步 | 第四步 | ||
| 第五步 | 2 | 第五步 | |
| 第六步 | 第六步 | ||
| 第七步 | 第七步 | ||
| 第八步 |
| 电池组成 | 工况 温度/℃ | 极化电阻Rp(SOFC/SOEC) /Ω·cm-2 | SOFC 功率密度/W·cm-2 | SOEC | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|
| 氧电极 | 电解质 | 燃料极 | 电压/V | 电流密度/A·cm-2 | ||||
| LSM | BaZr0.8Y0.2O3-δ(BZY2)/BaCe0.8Y0.2O3-δ(BCY2) | Ni/BaCe0.8Y0.2O3-δ(BCY2) | 700 | — | 0.131 | 1.5 | 1.528 | [ |
| LSM/YSZ | ScSZ | Ni/YSZ | 800 | 0.27/0.21 | — | — | — | [ |
| LSM/YSZ | YSZ | LCFNb/SDC | 800 | 0.39/— | 0.212 | 2.0 | 1.48 | [ |
| LSM/GDC | YSZ | Ni/YSZ | 800 | — | — | 1.3 | 1.73 | [ |
| LSM/ScSZ | ScSZ | LSTF/CeO2 | 850 | 0.13/— | 0.511 | 2.0 | 3.56 | [ |
| PdO/ZrO2/LSM/YSZ | YSZ | Ni/YSZ | 750 | 0.407/0.18 | 1.114 | 1.5 | 0.949 | [ |
| LSSMR | ScSZ | LSSMR | 750 | 0.23/— | 0.309 | 1.5 | 0.536 | [ |
| SSC/BCZY | BCZY | Ni-BCZY | 700 | 0.37/0.15 | 0.24 | 1.5 | 0.83 | [ |
| SSC/SDC | YSZ | Ag | 800 | 0.07/— | — | — | — | [ |
| LSC/CZI | CZI | Ni/CZI | 850 | 0.38/— | — | 0.3 | — | [ |
| LSCF | SSC | Ni/YSZ | 800 | 0.14/0.21 | — | — | — | [ |
| LSCF | YSZ | Ni/YSZ | 800 | 0.224/— | 2.1 | — | — | [ |
| YSZ/LSCF | YSZ | Ni/YSZ | 850 | 0.19/— | 0.486 | — | — | [ |
| YSZ/LSCF | GDC | Ni/YSZ | 800 | 0.21/— | 0.9 | 1.3 | 1.14 | [ |
| GDC/LSCN | YSZ | Ni/YSZ | 800 | 0.1/— | 1.336 | 1.6 | 2.34 | [ |
| LSC/YSZ | YSZ | Ni/YSZ | 800 | 0.067/— | 1.56 | — | — | [ |
| LGFC | GDC | LGFC | 800 | 0.07/— | — | — | — | [ |
| LSTF/CeO2 | ScSZ | LSTF/CeO2 | 850 | 0.203/— | 0.357 | 2.0 | 1.9 | [ |
| SFCM | YSZ | Pt | 700 | 0.2/— | — | — | — | [ |
| LSCF/GDC | YSZ | Ni/YSZ | 800 | — | 0.695 | 1.3 | 0.845 | [ |
| LSFM | LSGM | LSFM | 850 | 0.08/— | 0.64 | 1.3 | 1 | [ |
| LSFN/GDC | YSZ | Ni/YSZ | 850 | 0.142/— | 0.961 | 1.3 | 1.09 | [ |
| LSF/GDC | YSZ | NiO/YSZ | 800 | — | 1.36 | 1.3 | 1.52 | [ |
| PSF | LSGM | RP/O-PSF | 800 | 0.152/0.126 | 0.591 | 1.6 | 1.388 | [ |
| SFM/GDC | YSZ | SFM-GDC | 750 | 0.53/— | 1.913 | 1.3 | 0.2017 | [ |
| SFM/YSZ | YSZ | Ni/YSZ | 750 | — | 0.256 | 1.5 | 0.618 | [ |
| PBSCF | YSZ | Ni/YSZ | 800 | 0.314/0.176 | 0.986 | 1.3 | 1.3 | [ |
| NBCCF/GDC | YSZ | Ni/YSZ | 800 | —/0.079 | 0.941 | 1.3 | 0.92 | [ |
| SSC/LSGM | LSGM | Ni/SDC | 600 | —/0.231 | 1.817 | 1.3 | 2.472 | [ |
| LSN/ BCZY7 | BCZY7 | Ni/BCZY7 | 700 | —/0.15 | 0.46 | 1.3 | 1.41 | [ |
| CC/ BCZY | BCZY | Ni/BCZY | 700 | —/0.19 | 0.29 | 1.36 | 0.58 | [ |
| PNO/CGO | YSZ | Ni/YSZ | 800 | — | — | 1.2 | 0.98 | [ |
| 电池组成 | 工况 温度/℃ | 极化电阻Rp(SOFC/SOEC) /Ω·cm-2 | SOFC 功率密度/W·cm-2 | SOEC | 参考文献 | |||
|---|---|---|---|---|---|---|---|---|
| 氧电极 | 电解质 | 燃料极 | 电压/V | 电流密度/A·cm-2 | ||||
| LSM | BaZr0.8Y0.2O3-δ(BZY2)/BaCe0.8Y0.2O3-δ(BCY2) | Ni/BaCe0.8Y0.2O3-δ(BCY2) | 700 | — | 0.131 | 1.5 | 1.528 | [ |
| LSM/YSZ | ScSZ | Ni/YSZ | 800 | 0.27/0.21 | — | — | — | [ |
| LSM/YSZ | YSZ | LCFNb/SDC | 800 | 0.39/— | 0.212 | 2.0 | 1.48 | [ |
| LSM/GDC | YSZ | Ni/YSZ | 800 | — | — | 1.3 | 1.73 | [ |
| LSM/ScSZ | ScSZ | LSTF/CeO2 | 850 | 0.13/— | 0.511 | 2.0 | 3.56 | [ |
| PdO/ZrO2/LSM/YSZ | YSZ | Ni/YSZ | 750 | 0.407/0.18 | 1.114 | 1.5 | 0.949 | [ |
| LSSMR | ScSZ | LSSMR | 750 | 0.23/— | 0.309 | 1.5 | 0.536 | [ |
| SSC/BCZY | BCZY | Ni-BCZY | 700 | 0.37/0.15 | 0.24 | 1.5 | 0.83 | [ |
| SSC/SDC | YSZ | Ag | 800 | 0.07/— | — | — | — | [ |
| LSC/CZI | CZI | Ni/CZI | 850 | 0.38/— | — | 0.3 | — | [ |
| LSCF | SSC | Ni/YSZ | 800 | 0.14/0.21 | — | — | — | [ |
| LSCF | YSZ | Ni/YSZ | 800 | 0.224/— | 2.1 | — | — | [ |
| YSZ/LSCF | YSZ | Ni/YSZ | 850 | 0.19/— | 0.486 | — | — | [ |
| YSZ/LSCF | GDC | Ni/YSZ | 800 | 0.21/— | 0.9 | 1.3 | 1.14 | [ |
| GDC/LSCN | YSZ | Ni/YSZ | 800 | 0.1/— | 1.336 | 1.6 | 2.34 | [ |
| LSC/YSZ | YSZ | Ni/YSZ | 800 | 0.067/— | 1.56 | — | — | [ |
| LGFC | GDC | LGFC | 800 | 0.07/— | — | — | — | [ |
| LSTF/CeO2 | ScSZ | LSTF/CeO2 | 850 | 0.203/— | 0.357 | 2.0 | 1.9 | [ |
| SFCM | YSZ | Pt | 700 | 0.2/— | — | — | — | [ |
| LSCF/GDC | YSZ | Ni/YSZ | 800 | — | 0.695 | 1.3 | 0.845 | [ |
| LSFM | LSGM | LSFM | 850 | 0.08/— | 0.64 | 1.3 | 1 | [ |
| LSFN/GDC | YSZ | Ni/YSZ | 850 | 0.142/— | 0.961 | 1.3 | 1.09 | [ |
| LSF/GDC | YSZ | NiO/YSZ | 800 | — | 1.36 | 1.3 | 1.52 | [ |
| PSF | LSGM | RP/O-PSF | 800 | 0.152/0.126 | 0.591 | 1.6 | 1.388 | [ |
| SFM/GDC | YSZ | SFM-GDC | 750 | 0.53/— | 1.913 | 1.3 | 0.2017 | [ |
| SFM/YSZ | YSZ | Ni/YSZ | 750 | — | 0.256 | 1.5 | 0.618 | [ |
| PBSCF | YSZ | Ni/YSZ | 800 | 0.314/0.176 | 0.986 | 1.3 | 1.3 | [ |
| NBCCF/GDC | YSZ | Ni/YSZ | 800 | —/0.079 | 0.941 | 1.3 | 0.92 | [ |
| SSC/LSGM | LSGM | Ni/SDC | 600 | —/0.231 | 1.817 | 1.3 | 2.472 | [ |
| LSN/ BCZY7 | BCZY7 | Ni/BCZY7 | 700 | —/0.15 | 0.46 | 1.3 | 1.41 | [ |
| CC/ BCZY | BCZY | Ni/BCZY | 700 | —/0.19 | 0.29 | 1.36 | 0.58 | [ |
| PNO/CGO | YSZ | Ni/YSZ | 800 | — | — | 1.2 | 0.98 | [ |
| 1 | PANWAR N L, KAUSHIK S C, KOTHARI S. Role of renewable energy sources in environmental protection: a review[J]. Renewable and Sustainable Energy Reviews, 2011, 15(3): 1513-1524. |
| 2 | MOMIRLAN M, VEZIROGLU T N. Current status of hydrogen energy[J]. Renewable and Sustainable Energy Reviews, 2002, 6(1/2): 141-179. |
| 3 | AKIKUR R K, SAIDUR R, PING H W, et al. Comparative study of stand-alone and hybrid solar energy systems suitable for off-grid rural electrification: a review[J]. Renewable and Sustainable Energy Reviews, 2013, 27: 738-752. |
| 4 | HOTZA D, DINIZ DA COSTA J C. Fuel cells development and hydrogen production from renewable resources in Brazil[J]. International Journal of Hydrogen Energy, 2008, 33(19): 4915-4935. |
| 5 | AKIKUR R K, SAIDUR R, PING H W, et al. Performance analysis of a co-generation system using solar energy and SOFC technology[J]. Energy Conversion and Management, 2014, 79: 415-430. |
| 6 | ZHANG Z H, ZHOU J, ZONG Z, et al. Development and modelling of a novel electricity-hydrogen energy system based on reversible solid oxide cells and power to gas technology[J]. International Journal of Hydrogen Energy, 2019, 44(52): 28305-28315. |
| 7 | DI GIORGIO P, DESIDERI U. Potential of reversible solid oxide cells as electricity storage system[J]. Energies, 2016, 9(8): 662. |
| 8 | WENDEL C H, BRAUN R J. Design and techno-economic analysis of high efficiency reversible solid oxide cell systems for distributed energy storage[J]. Applied Energy, 2016, 172: 118-131. |
| 9 | BAEK S W, BAE J, YOO Y S. Cathode reaction mechanism of porous-structured Sm0.5Sr0.5CoO3-δ and Sm0.5Sr0.5CoO3-δ/Sm0.2Ce0.8O1.9 for solid oxide fuel cells[J]. Journal of Power Sources, 2009, 193(2): 431-440. |
| 10 | HE F, SONG D, PENG R R, et al. Electrode performance and analysis of reversible solid oxide fuel cells with proton conducting electrolyte of BaCe0.5Zr0.3Y0.2O3-δ[J]. Journal of Power Sources, 2010, 195(11): 3359-3364. |
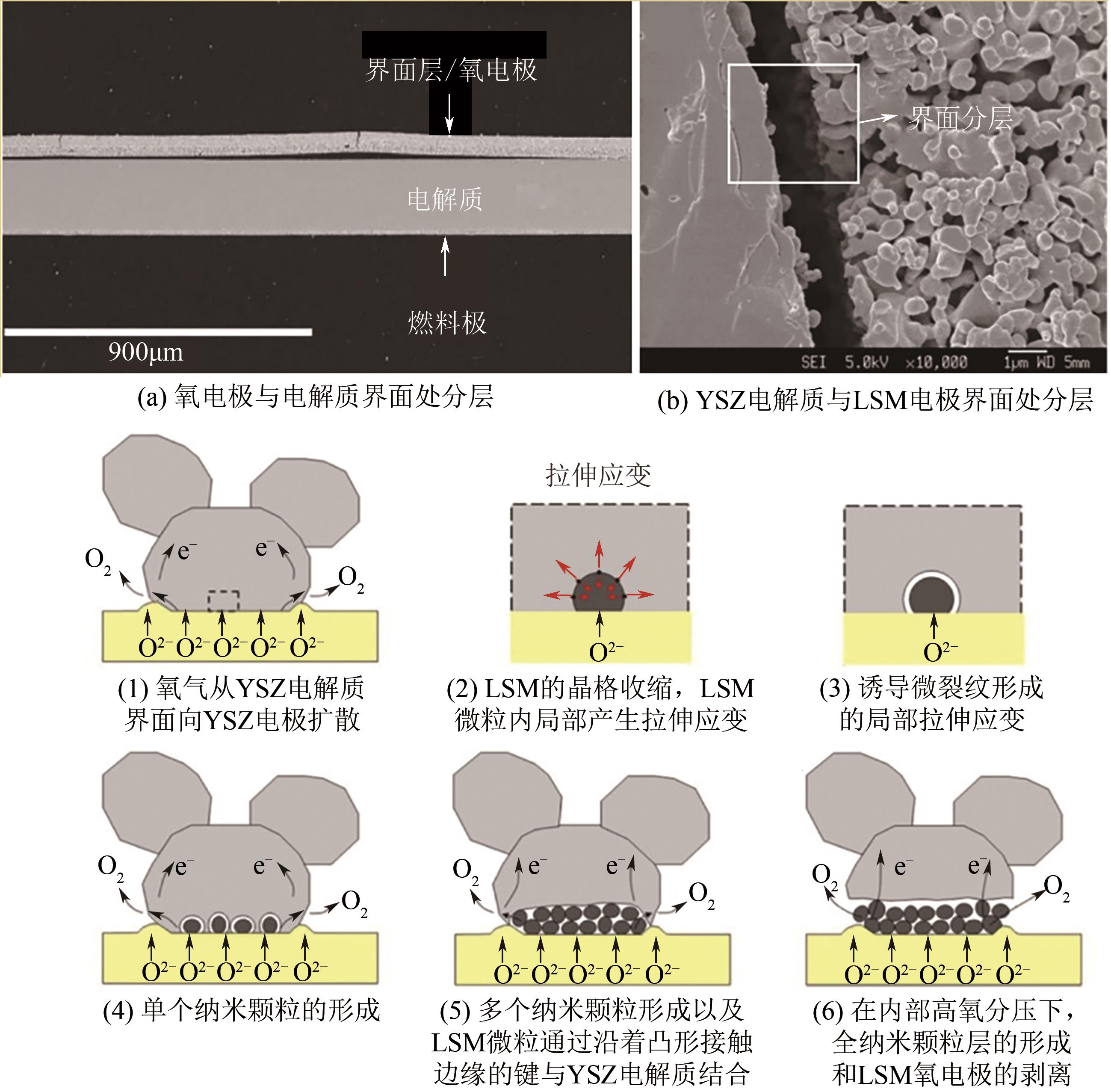
| 11 | VIRKAR A V. Mechanism of oxygen electrode delamination in solid oxide electrolyzer cells[J]. International Journal of Hydrogen Energy, 2010, 35(18): 9527-9543. |
| 12 | CHEN K F, JIANG S P. Failure mechanism of (La, Sr)MnO3 oxygen electrodes of solid oxide electrolysis cells[J]. International Journal of Hydrogen Energy, 2011, 36(17): 10541-10549. |
| 13 | MOÇOTEGUY P, BRISSE A. A review and comprehensive analysis of degradation mechanisms of solid oxide electrolysis cells[J]. International Journal of Hydrogen Energy, 2013, 38(36): 15887-15902. |
| 14 | BLUMENTHAL R N, SHARMA R K. Electronic conductivity in nonstoichiometric cerium dioxide[J]. Journal of Solid State Chemistry, 1975, 13(4): 360-364. |
| 15 | LAGUNA-BERCERO M A, KILNER J A, SKINNER S J. Development of oxygen electrodes for reversible solid oxide fuel cells with scandia stabilized zirconia electrolytes[J]. Solid State Ionics, 2011, 192(1): 501-504. |
| 16 | LAGUNA-BERCERO M A. Recent advances in high temperature electrolysis using solid oxide fuel cells: a review[J]. Journal of Power Sources, 2012, 203: 4-16. |
| 17 | B-K PARK, ZHANG Q, VOORHEES P W, et al. Conditions for stable operation of solid oxide electrolysis cells: oxygen electrode effects[J]. Energy & Environmental Science, 2019, 12(10): 3053–3062. |
| 18 | KHAN M S, XU X, KNIBBE R, et al. Air electrodes and related degradation mechanisms in solid oxide electrolysis and reversible solid oxide cells[J]. Renewable and Sustainable Energy Reviews, 2021, 143: 110918. |
| 19 | ZHANG Y, CHEN B, GUAN D, et al. Thermal-expansion offset for high-performance fuel cell cathodes[J]. Nature, 2021, 591(7849): 246-251. |
| 20 | LU F, YANG M J, SHI Y J, et al. Application of a negative thermal expansion oxide in SOFC cathode[J]. Ceramics International, 2021, 47(1): 1095-1100. |
| 21 | WEST A R. Inorganic functional materials: optimization of properties by structural and compositional control[J]. Chemical Record (New York, N Y), 2006, 6(4): 206-216. |
| 22 | MOGENSEN M, LYBYE D, BONANOS N, et al. Factors controlling the oxide ion conductivity of fluorite and perovskite structured oxides[J]. Solid State Ionics, 2004, 174(1/2/3/4): 279-286. |
| 23 | AFROZE S, KARIM A, CHEOK Q, et al. Latest development of double perovskite electrode materials for solid oxide fuel cells: a review[J]. Frontiers in Energy, 2019, 13(4): 770-797. |
| 24 | YIN W-J, WENG B, GE J, et al. Oxide perovskites, double perovskites and derivatives for electrocatalysis, photocatalysis, and photovoltaics[J]. Energy & Environmental Science, 2019, 12(2): 442–462. |
| 25 | VASALA S, KARPPINEN M. A2B'B″O6 perovskites: a review[J]. Progress in Solid State Chemistry, 2015, 43(1/2): 1-36. |
| 26 | XU X M, ZHONG Y J, SHAO Z P. Double perovskites in catalysis, electrocatalysis, and photo(electro)catalysis[J]. Trends in Chemistry, 2019, 1(4): 410-424. |
| 27 | SENGODAN S, CHOI S, JUN A, et al. Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells[J]. Nature Materials, 2015, 14(2): 205-209. |
| 28 | GEIGER S, KASIAN O, LEDENDECKER M, et al. The stability number as a metric for electrocatalyst stability benchmarking[J]. Nature Catalysis, 2018, 1(7): 508-515. |
| 29 | GRIMAUD A, MAY K J, CARLTON C E, et al. Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution[J]. Nature Communications, 2013, 4: 2439. |
| 30 | ZHOU L, XU Y F, CHEN B X, et al. Synthesis and photocatalytic application of stable lead-free Cs2AgBiBr6 perovskite nanocrystals[J]. Small, 2018, 14(11): 1703762. |
| 31 | TARRAGÓ D P, MORENO B, CHINARRO E, et al. Deposition of nanostructured LSM perovskite thin film on dense YSZ substrate by airbrushed solution combustion (ASC) for application in SOFC cathodes[J]. International Journal of Hydrogen Energy, 2020, 45(20): 11749-11760. |
| 32 | MEN H J, TIAN N, QU Y M, et al. Improved performance of a lanthanum strontium manganite-based oxygen electrode for an intermediate-temperature solid oxide electrolysis cell realized via ionic conduction enhancement[J]. Ceramics International, 2019, 45(6): 7945-7949. |
| 33 | MOHAMMAD S F, AHMAD S, RAHMAN H A, et al. Effect of SSC loading on the microstructural stability SSC-SDCC composite cathode as new potential SOFC[J]. International Journal of Integrated Engineering, 2019, 11(7): 162–168. |
| 34 | SINDIRAC C. Improvement of IT-SOFC by tailoring the microstructure of ISCF cathode and gdc electrolyte[EB/OL]. 2019. |
| 35 | AHMAD S, MOHAMMAD S F, RAHMAN H A, et al. Comparative characterization on structural properties of calcined Sm0.5Sr0.5CoO3–δ - Sm0.2Ce0.8O1.9 carbonate as potential composite cathode for SOFC[J]. IOP Conference Series: Materials Science and Engineering, 2019, 494: 012003. |
| 36 | MAIDE M, PAISTE P, MÖLLER P, et al. Influence of A- and B-site modifications of (La1-xSrx)yCr0.5-zMn0.5-wNiz+wO3-δ on electrochemical impedance characteristics of reversible solid oxide cell[J]. Journal of the Electrochemical Society, 2019, 166(15): F1148-F1156. |
| 37 | MOGENSEN M B. Materials for reversible solid oxide cells[J]. Current Opinion in Electrochemistry, 2020, 21: 265-273. |
| 38 | KIM Y D, YANG J Y, SAQIB M, et al. Cobalt-free perovskite Ba1-xNdxFeO3-δ air electrode materials for reversible solid oxide cells[J]. Ceramics International, 2021, 47(6): 7985-7993. |
| 39 | BABINDAMANA D, JIA C, HAN M F. Study of a promising co-doped double perovskite cathode material for IT-SOFCs[J]. ECS Transactions, 2019, 91(1): 1437-1443. |
| 40 | LIN T N, KUO H Y, YEH C Y, et al. Performance and durability evaluation for anode-supported solid oxide fuel cell with Ce0.8Sm0.2O2–δ-SmBa0.5Sr0.5Co2O5+δ composite cathodes[J]. ECS Transactions, 2019, 91(1): 1239-1246. |
| 41 | LI P Z, YANG W, TIAN C J, et al. Electrochemical performance of La2NiO4+δ-Ce0.55La0.45O2-δ as a promising bifunctional oxygen electrode for reversible solid oxide cells[J]. Journal of Advanced Ceramics, 2021, 10(2): 328-337. |
| 42 | NAJJAR H, BATIS H. Development of Mn-based perovskite materials: chemical structure and applications[J]. Catalysis Reviews, 2016, 58(3): 371-438. |
| 43 | JENSEN S H, LARSEN P H, MOGENSEN M. Hydrogen and synthetic fuel production from renewable energy sources[J]. International Journal of Hydrogen Energy, 2007, 32(15): 3253-3257. |
| 44 | NOH T, RYU J, KIM J, et al. Structural and impedance analysis of copper doped LSM cathode for IT-SOFCs[J]. Journal of Alloys and Compounds, 2013, 557: 196-201. |
| 45 | JIANG S P. A review of wet impregnation—An alternative method for the fabrication of high performance and nano-structured electrodes of solid oxide fuel cells[J]. Materials Science and Engineering A, 2006, 418(1/2): 199-210. |
| 46 | 王莹莹, 罗凌虹, 徐序, 等. LSM/SUS430复合连接体材料的溶胶-凝胶法制备及性能研究[J]. 中国陶瓷, 2013, 49(7): 38-40. |
| WANG Yingying, LUO Linghong, XU Xu, et al. Properties studies on sol-gel derived LSM coating of SOFC metallic interconnect[J]. China Ceramics, 2013, 49(7): 38-40. | |
| 47 | WEN Y B, YANG S J, GU S, et al. Fabrication and characterization of a double-layer electrolyte membrane for BaCeO3-based reversible solid oxide cells (RSOCs)[J]. Solid State Ionics, 2017, 308: 167-172. |
| 48 | KIM J D, KIM G D, MOON J W, et al. Characterization of LSM-YSZ composite electrode by AC impedance spectroscopy[J]. Solid State Ionics, 2001, 143(3/4): 379-389. |
| 49 | SU Q, YOON D, SISMAN Z, et al. Vertically aligned nanocomposite La0.8Sr0.2MnO3-δ/Zr0.92Y0.08O1.96 thin films as electrode/electrolyte interfacial layer for solid oxide reversible fuel cells[J]. International Journal of Hydrogen Energy, 2013, 38(36): 16320-16327. |
| 50 | JUNG G B, CHANG C T, YEH C C, et al. Study of reversible solid oxide fuel cell with different oxygen electrode materials[J]. International Journal of Hydrogen Energy, 2016, 41(46): 21802-21811. |
| 51 | LIU L M, ZHOU X L, WANG Y, et al. Study of high active and redox-stable La0.9Ca0.1Fe0.9Nb0.1O3-δ/Sm0.1Ce0.9O2-δ composite ceramic electrode for solid oxide reversible cells[J]. Electrochimica Acta, 2017, 236: 371-377. |
| 52 | SHIMADA H, FUJIMAKI Y, FUJISHIRO Y. Highly active and durable La0.4Sr0.6MnO3-δ and Ce0.8Gd0.2O1.9 nanocomposite electrode for high-temperature reversible solid oxide electrochemical cells[J]. Ceramics International, 2020, 46(11): 19617-19623. |
| 53 | XU J, ZHOU X L, PAN L, et al. Oxide composite of La0.3Sr0.7Ti0.3Fe0.7O3-δ and CeO2 as an active fuel electrode for reversible solid oxide cells[J]. Journal of Power Sources, 2017, 371: 1-9. |
| 54 | TAN Y, GAO S, XIONG C Y, et al. Nano-structured LSM-YSZ refined with PdO/ZrO2 oxygen electrode for intermediate temperature reversible solid oxide cells[J]. International Journal of Hydrogen Energy, 2020, 45(38): 19823-19830. |
| 55 | TAN Y, WANG A, JIA L C, et al. High-performance oxygen electrode for reversible solid oxide cells with power generation and hydrogen production at intermediate temperature[J]. International Journal of Hydrogen Energy, 2017, 42(7): 4456-4464. |
| 56 | WEI T, JIA L C, ZHENG H Y, et al. LaMnO3-based perovskite with in situ exsolved Ni nanoparticles: a highly active, performance stable and coking resistant catalyst for CO2 dry reforming of CH4[J]. Applied Catalysis A: General, 2018, 564: 199-207. |
| 57 | CHOI M B, SINGH B, WACHSMAN E D, et al. Performance of La0.1Sr0.9Co0.8Fe0.2O3-δ and La0.1Sr0.9Co0.8Fe0.2O3-δ-Ce0.9Gd0.1O2 oxygen electrodes with Ce0.9Gd0.1O2 barrier layer in reversible solid oxide fuel cells[J]. Journal of Power Sources, 2013, 239: 361-373. |
| 58 | CHRZAN A, OVTAR S, JASINSKI P, et al. High performance LaNi1-xCoxO3-δ (x=0.4 to 0.7) infiltrated oxygen electrodes for reversible solid oxide cells[J]. Journal of Power Sources, 2017, 353: 67-76. |
| 59 | SHAHROKHI S, BABAEI A, ZAMANI C. Reversible operation of La0.8Sr0.2MnO3 oxygen electrode infiltrated with Ruddlesden-Popper and perovskite lanthanum nickel cobaltite[J]. International Journal of Hydrogen Energy, 2018, 43(52): 23091-23100. |
| 60 | ZHOU J, WANG N, CUI J J, et al. Structural and electrochemical properties of B-site Ru-doped (La0.8Sr0.2)0.9Sc0.2Mn0.8O3-δ as symmetrical electrodes for reversible solid oxide cells[J]. Journal of Alloys and Compounds, 2019, 792: 1132-1140. |
| 61 | PENA M A, FIERRO J L G. Chemical structures and performance of perovskite oxides[J]. Chemical Reviews, 2001, 101(7): 1981-2018. |
| 62 | SIMBÖCK J, GHIASI M, SCHÖNEBAUM S, et al. Electronic parameters in cobalt-based perovskite-type oxides as descriptors for chemocatalytic reactions[J]. Nature Communications, 2020, 11(1): 652. |
| 63 | KAMMER K. Studies of Fe-Co based perovskite cathodes with different A-site cations[J]. Solid State Ionics, 2006, 177(11/12): 1047-1051. |
| 64 | BANSAL N P, WISE B. Sol-gel synthesis of La0.6Sr0.4CoO3-x and Sm0.5Sr0.5CoO3-x cathode nanopowders for solid oxide fuel cells[J]. Ceramics International, 2012, 38(7): 5535-5541. |
| 65 | FUKUNAGA H, KOYAMA M, TAKAHASHI N, et al. Reaction model of dense Sm0.5Sr0.5CoO3 as SOFC cathode[J]. Solid State Ionics, 2000, 132(3/4): 279-285. |
| 66 | XIA C R, RAUCH W, CHEN F L, et al. Sm0.5Sr0.5CoO3 cathodes for low-temperature SOFCs[J]. Solid State Ionics, 2002, 149(1/2): 11-19. |
| 67 | JIANG W, WEI B, LV Z, et al. Performance and stability of co-synthesized Sm0.5Sr0.5CoO3-Sm0.2Ce0.8O1.9 oxygen electrode for reversible solid oxide cells[J]. Electrochimica Acta, 2015, 180: 1085-1093. |
| 68 | KHAN M S, XU X Y, LI M R, et al. Evaluation of SrCo0.8Nb0.2O3-δ, SrCo0.8Ta0.2O3-δ and SrCo0.8Nb0.1Ta0.1O3-δ as air electrode materials for solid oxide electrolysis and reversible solid oxide cells[J]. Electrochimica Acta, 2019, 321: 134654. |
| 69 | ZHOU X W, YAN N, CHUANG K T, et al. Progress in La-doped SrTiO3 (LST)-based anode materials for solid oxide fuel cells[J]. RSC Advances, 2014, 4(1): 118. |
| 70 | ATTA N F, GALAL A, EL-ADS E H. Effect of B-site doping on Sr2PdO3 perovskite catalyst activity for non-enzymatic determination of glucose in biological fluids[J]. Journal of Electroanalytical Chemistry, 2019, 852: 113523. |
| 71 | YE X-F, WEN Y B, YANG S J, et al. Study of CaZr0.9In0.1O3-δ based reversible solid oxide cells with tubular electrode supported structure[J]. International Journal of Hydrogen Energy, 2017, 42(36): 23189–23197. |
| 72 | FAN H, KEANE M, SINGH P, et al. Electrochemical performance and stability of lanthanum strontium cobalt ferrite oxygen electrode with gadolinia doped ceria barrier layer for reversible solid oxide fuel cell[J]. Journal of Power Sources, 2014, 268: 634-639. |
| 73 | SUMI H, SUDA E, MORI M. Blocking layer for prevention of current leakage for reversible solid oxide fuel cells and electrolysis cells with ceria-based electrolyte[J]. International Journal of Hydrogen Energy, 2017, 42(7): 4449-4455. |
| 74 | SHIMURA K, NISHINO H, KAKINUMA K, et al. Effect of Samaria-doped ceria (SDC) interlayer on the performance of La0.6Sr0.4Co0.2Fe0.8O3-δ/SDC composite oxygen electrode for reversible solid oxide fuel cells[J]. Electrochimica Acta, 2017, 225: 114-120. |
| 75 | FAN H, KEANE M, LI N, et al. Electrochemical stability of La0.6Sr0.4Co0.2Fe0.8O3-δ-infiltrated YSZ oxygen electrode for reversible solid oxide fuel cells[J]. International Journal of Hydrogen Energy, 2014, 39(26): 14071-14078. |
| 76 | BRITO M E, MORISHITA H, YAMADA J, et al. Further improvement in performances of La0.6Sr0.4Co0.2Fe0.8O3-δ-doped ceria composite oxygen electrodes with infiltrated doped ceria nanoparticles for reversible solid oxide cells[J]. Journal of Power Sources, 2019, 427: 293-298. |
| 77 | HE Z L, ZHANG L, HE S, et al. Cyclic polarization enhances the operating stability of La0.57Sr0.38Co0.18Fe0.72Nb0.1O3-δ oxygen electrode of reversible solid oxide cells[J]. Journal of Power Sources, 2018, 404: 73-80. |
| 78 | MOLERO-SÁNCHEZ B, PRADO-GONJAL J, ÁVILA-BRANDE D, et al. High performance La0.3Ca0.7Cr0.3Fe0.7O3-δ air electrode for reversible solid oxide fuel cell applications[J]. International Journal of Hydrogen Energy, 2015, 40(4): 1902-1910. |
| 79 | XU J, ZHOU X L, CHENG J H, et al. Electrochemical performance of highly active ceramic symmetrical electrode La0.3Sr0.7Ti0.3Fe0.7O3-δ-CeO2 for reversible solid oxide cells[J]. Electrochimica Acta, 2017, 257: 64-72. |
| 80 | ZHOU N, YIN Y M, LI J C, et al. A robust high performance cobalt-free oxygen electrode La0.5Sr0.5Fe0.8Cu0.15Nb0.05O3-δ for reversible solid oxide electrochemical cell[J]. Journal of Power Sources, 2017, 340: 373-379. |
| 81 | GUAN C Z, WANG Y D, CHEN K F, et al. Molten salt synthesis of Nb-doped (La, Sr)FeO3 as the oxygen electrode for reversible solid oxide cells[J]. Materials Letters, 2019, 245: 114-117. |
| 82 | ZAPATA-RAMÍREZ V, MATHER G C, AZCONDO M T, et al. Electrical and electrochemical properties of the Sr(Fe, Co, Mo)O3-δsystem as air electrode for reversible solid oxide cells[J]. Journal of Power Sources, 2019, 437: 226895. |
| 83 | LÓPEZ-ROBLEDO M J, LAGUNA-BERCERO M A, LARREA A, et al. Reversible operation of microtubular solid oxide cells using La0.6Sr0.4Co0.2Fe0.8O3-δ-Ce0.9Gd0.1O2-δ oxygen electrodes[J]. Journal of Power Sources, 2018, 378: 184-189. |
| 84 | GLISENTI A, BEDON A, CAROLLO G, et al. Reversible, all-perovskite SOFCs based on La, Sr gallates[J]. International Journal of Hydrogen Energy, 2020, 45(53): 29155-29165. |
| 85 | BIAN L Z, LIU C Y, LI S T, et al. Highly stable La0.5Sr0.5Fe0.9Mo0.1O3-δ electrode for reversible symmetric solid oxide cells[J]. International Journal of Hydrogen Energy, 2020, 45(38): 19813-19822. |
| 86 | TIAN Y F, WANG W J, LIU Y, et al. Cobalt-free perovskite oxide La0.6Sr0.4Fe0.8Ni0.2O3–δ as active and robust oxygen electrode for reversible solid oxide cells[J]. ACS Applied Energy Materials, 2019, 2(5): 3297-3305. |
| 87 | ZUO X D, CHEN Z Y, GUAN C Z, et al. Molten salt synthesis of high-performance, nanostructured La0.6Sr0.4FeO3–δ oxygen electrode of a reversible solid oxide cell[J]. Materials, 2020, 13(10): 2267. |
| 88 | YANG Y, WU Y J, BAO H, et al. An efficient and prospective self-assembled hybrid electrocatalyst for symmetrical and reversible solid oxide cells[J]. Electrochimica Acta, 2020, 362: 137171. |
| 89 | GUO Y Y, GUO T M, ZHOU S J, et al. Characterization of Sr2Fe1.5Mo0.5O6-δ -Gd0.1Ce0.9O1.95 symmetrical electrode for reversible solid oxide cells[J]. Ceramics International, 2019, 45(8): 10969-10975. |
| 90 | LI Y H, ZOU S X, JU J W, et al. Characteristics of nano-structured SFM infiltrated onto YSZ backbone for symmetrical and reversible solid oxide cells[J]. Solid State Ionics, 2018, 319: 98-104. |
| 91 | TIAN Y F, LI J, LIU Y Y, et al. Preparation and properties of PrBa0.5Sr0.5Co1.5Fe0.5O5+δ as novel oxygen electrode for reversible solid oxide electrochemical cell[J]. International Journal of Hydrogen Energy, 2018, 43(28): 12603-12609. |
| 92 | TIAN Y F, LIU Y, WANG W J, et al. High performance and stability of double perovskite-type oxide NdBa0.5Ca0.5Co1.5Fe0.5O5+δ as an oxygen electrode for reversible solid oxide electrochemical cell[J]. Journal of Energy Chemistry, 2020, 43: 108-115. |
| 93 | WANG S R, HAO X, ZHAN W T. Research on a low temperature reversible solid oxide cell[J]. International Journal of Hydrogen Energy, 2017, 42(50): 29881-29887. |
| 94 | YANG S J, LU Y, WANG Q, et al. Effects of porous support microstructure enabled by the carbon microsphere pore former on the performance of proton-conducting reversible solid oxide cells[J]. International Journal of Hydrogen Energy, 2018, 43(43): 20050-20058. |
| 95 | YANG S J, WEN Y B, ZHANG J C, et al. Electrochemical performance and stability of cobalt-free Ln1.2Sr0.8NiO4 (Ln=La and Pr) air electrodes for proton-conducting reversible solid oxide cells[J]. Electrochimica Acta, 2018, 267: 269-277. |
| 96 | PIKALOVA E, KOLCHUGIN A, KOROLEVA M, et al. Functionality of an oxygen Ca3Co4O9+δ electrode for reversible solid oxide electrochemical cells based on proton-conducting electrolytes[J]. Journal of Power Sources, 2019, 438: 226996. |
| 97 | CAROLLO G, GARBUJO A, BEDON A, et al. Cu/CGO cermet based electrodes for symmetric and reversible solid oxide fuel cells[J]. International Journal of Hydrogen Energy, 2020, 45(25): 13652-13658. |
| 98 | MORALES-ZAPATA M A, LARREA A, LAGUNA-BERCERO M A. Reversible operation performance of microtubular solid oxide cells with a nickelate-based oxygen electrode[J]. International Journal of Hydrogen Energy, 2020, 45(8): 5535-5542. |
| 99 | YOO Y-S, CHOI M, J-H HWANG. La2NiO4+δ as oxygen electrode in reversible solid oxide cells[J]. Ceramics International, 2015, 41(5): 6448-6454. |
| [1] | CHEN Kuangyin, LI Ruilan, TONG Yang, SHEN Jianhua. Structure design of gas diffusion layer in proton exchange membrane fuel cell [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 246-259. |
| [2] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | HU Xi, WANG Mingshan, LI Enzhi, HUANG Siming, CHEN Junchen, GUO Bingshu, YU Bo, MA Zhiyuan, LI Xing. Research progress on preparation and sodium storage properties of tungsten disulfide composites [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 344-355. |
| [5] | ZHANG Jie, BAI Zhongbo, FENG Baoxin, PENG Xiaolin, REN Weiwei, ZHANG Jingli, LIU Eryong. Effect of PEG and its compound additives on post-treatment of electrolytic copper foils [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 374-381. |
| [6] | XU Jiaheng, LI Yongsheng, LUO Chunhuan, SU Qingquan. Optimization of methanol steam reforming process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 41-46. |
| [7] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [8] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [9] | WANG Yaogang, HAN Zishan, GAO Jiachen, WANG Xinyu, LI Siqi, YANG Quanhong, WENG Zhe. Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4043-4057. |
| [10] | LIU Yi, FANG Qiang, ZHONG Dazhong, ZHAO Qiang, LI Jinping. Cu facets regulation of Ag/Cu coupled catalysts for electrocatalytic reduction of carbon dioxide [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4136-4142. |
| [11] | ZHANG Yajuan, XU Hui, HU Bei, SHI Xingwei. Preparation of NiCoP/rGO/NF electrocatalyst by eletroless plating for efficient hydrogen evolution reaction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4275-4282. |
| [12] | WANG Shuaiqing, YANG Siwen, LI Na, SUN Zhanying, AN Haoran. Research progress on element doped biomass carbon materials for electrochemical energy storage [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4296-4306. |
| [13] | LI Haidong, YANG Yuankun, GUO Shushu, WANG Benjin, YUE Tingting, FU Kaibin, WANG Zhe, HE Shouqin, YAO Jun, CHEN Shu. Effect of carbonization and calcination temperature on As(Ⅲ) removal performance of plant-based Fe-C microelectrolytic materials [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3652-3663. |
| [14] | XU Wei, LI Kaijun, SONG Linye, ZHANG Xinghui, YAO Shunhua. Research progress of photocatalysis and co-electrochemical degradation of VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3520-3531. |
| [15] | WANG Yunqing, YANG Guorui, YAN Wei. Transition metal phosphide modification and its applications in electrochemical hydrogen evolution reaction [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3532-3549. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||