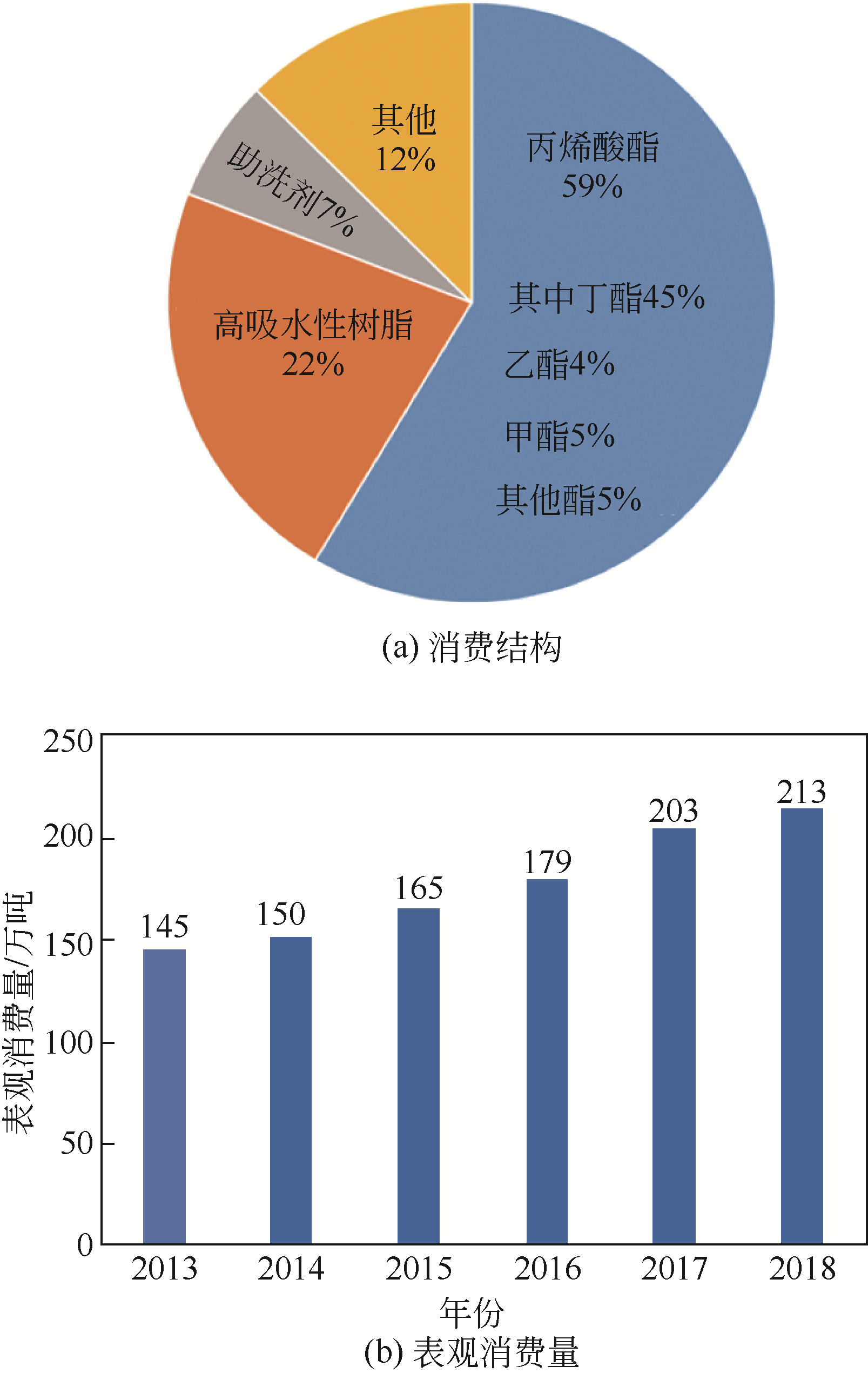
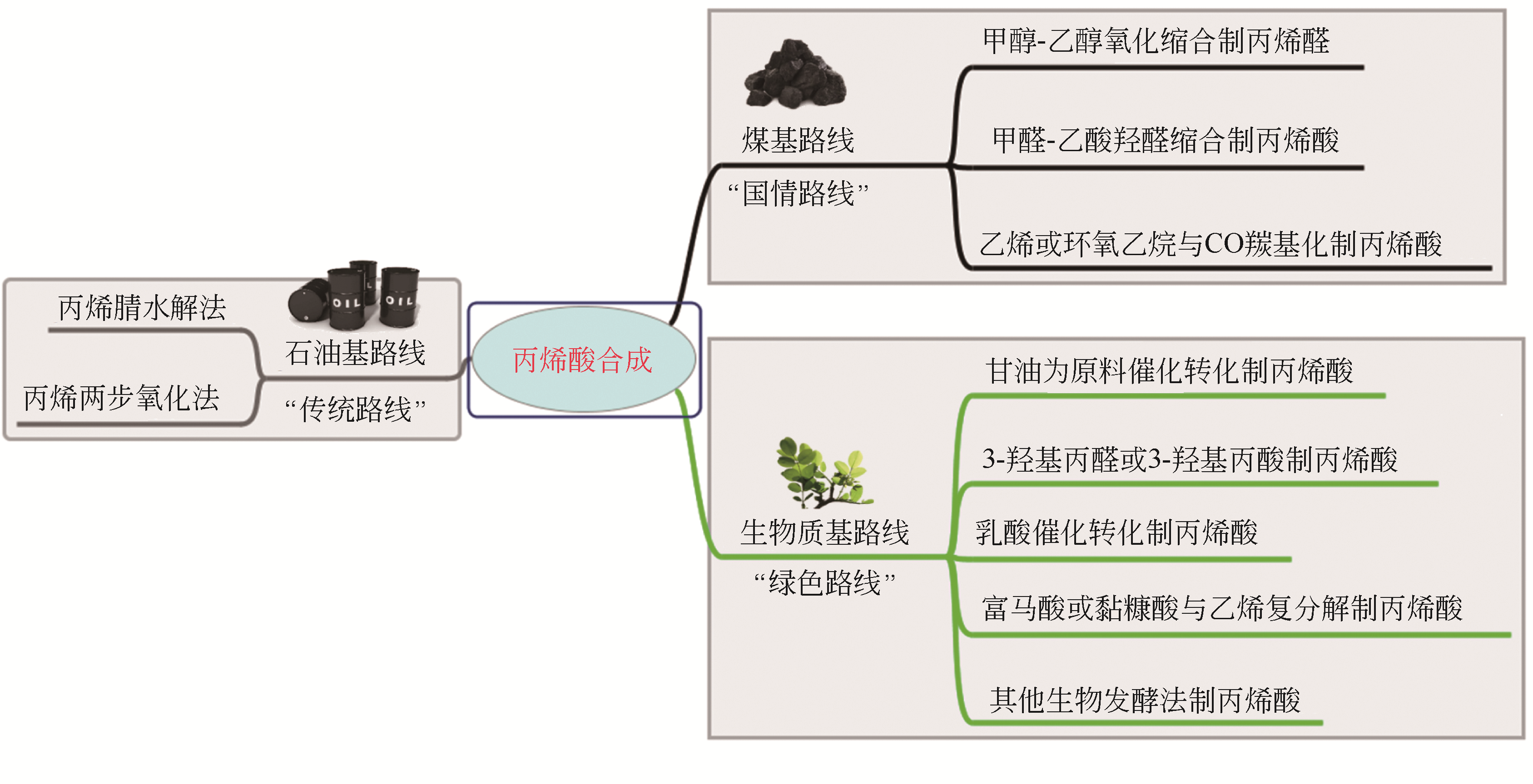
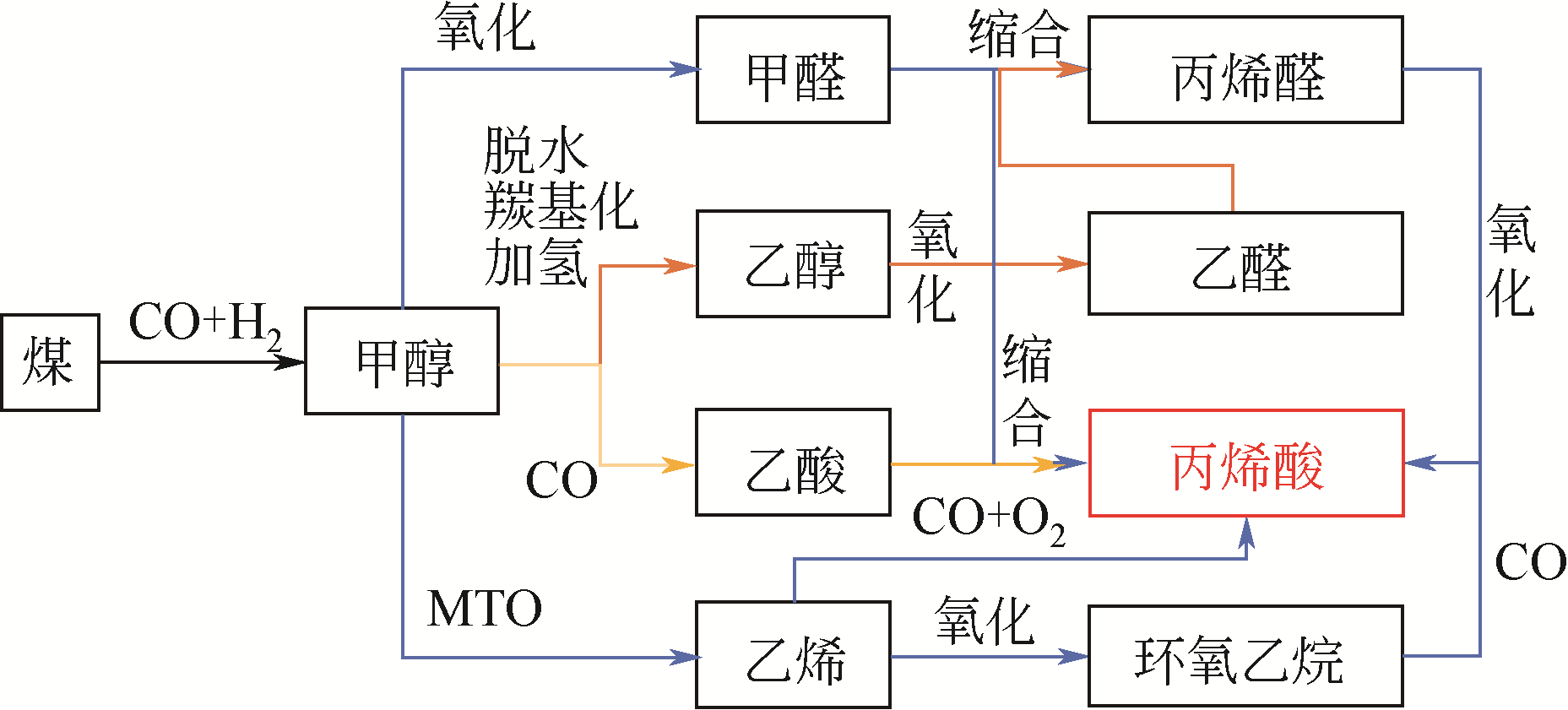
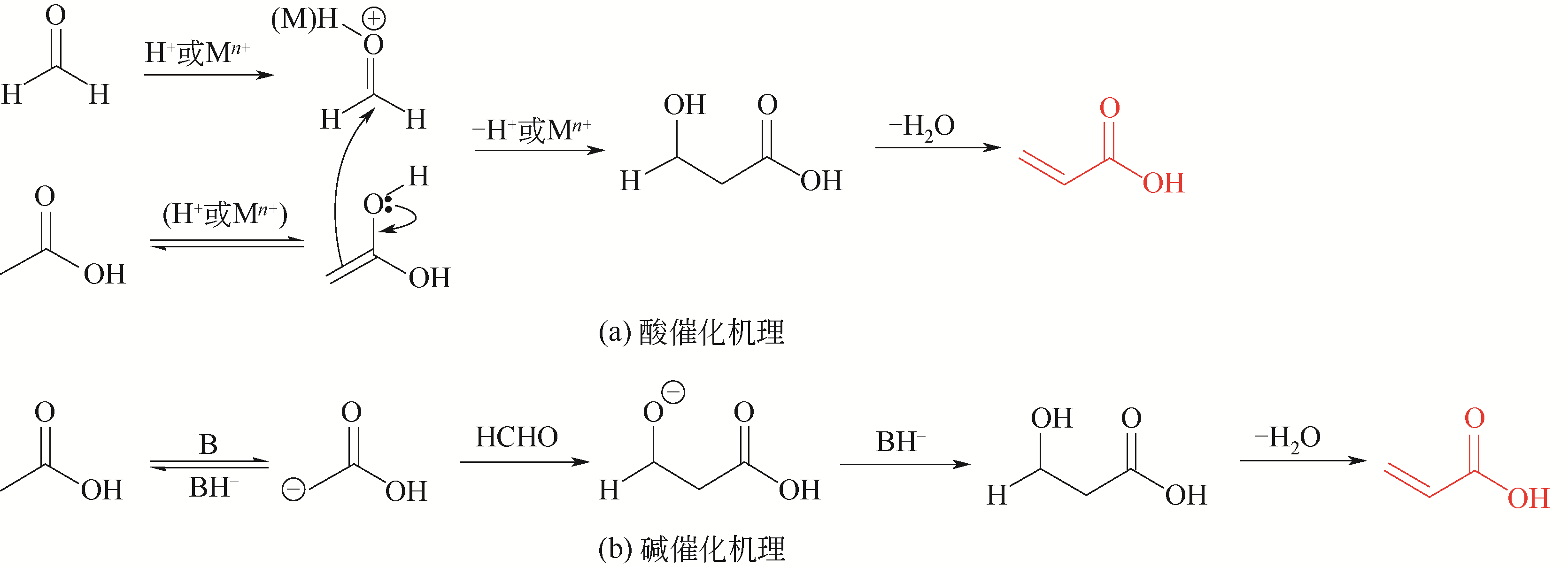
Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (4): 2016-2033.DOI: 10.16085/j.issn.1000-6613.2020-2036
• Special column:Industrial catalysis • Previous Articles Next Articles
New advances in catalytic synthesis of acrylic acid
ZHANG Zhixin( ), WANG Yehong, ZHANG Chaofeng, WANG Feng(
), WANG Yehong, ZHANG Chaofeng, WANG Feng( )
)
- Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, Liaoning, China
-
Received:2020-10-10Online:2021-04-14Published:2021-04-05 -
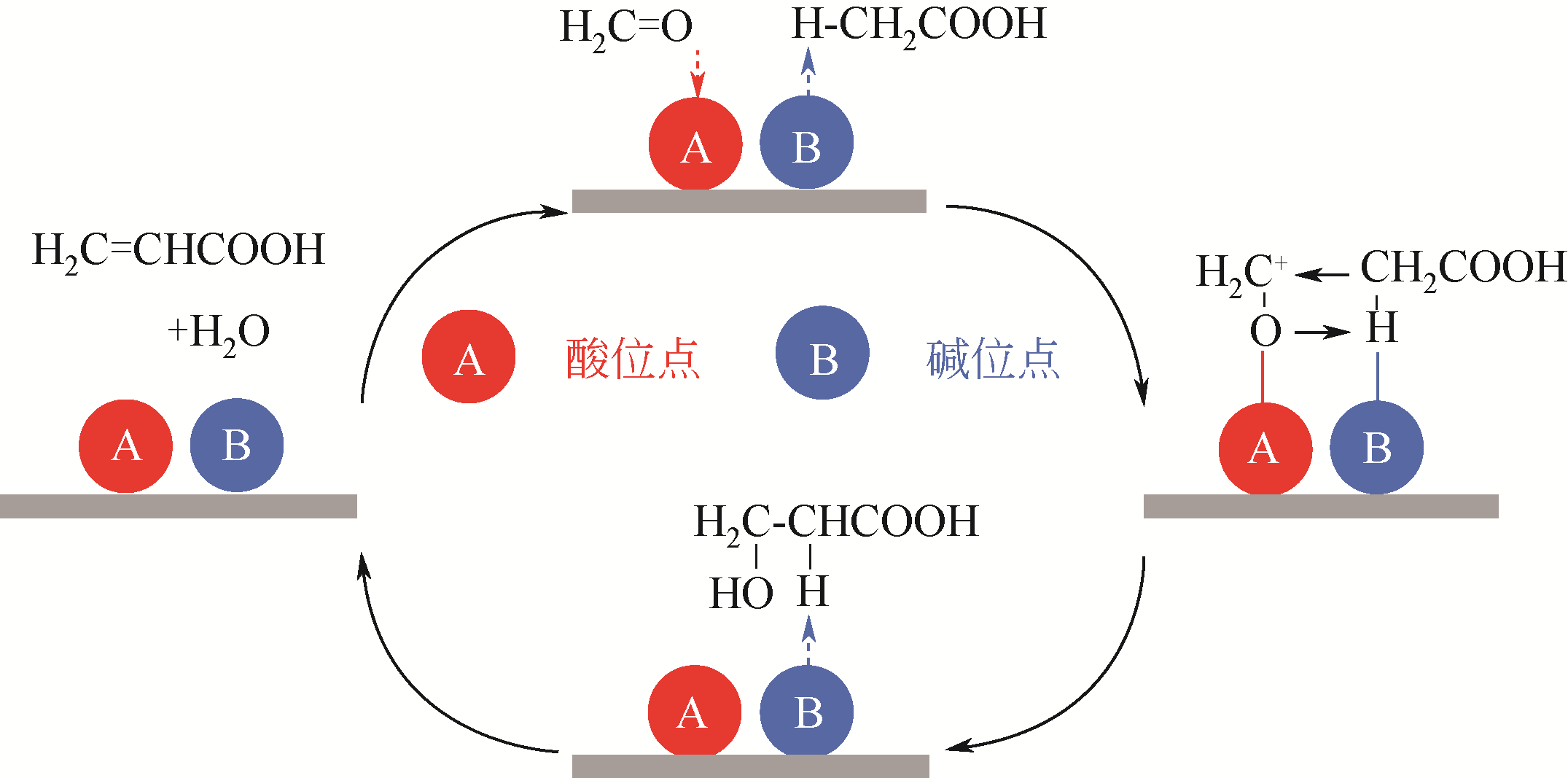
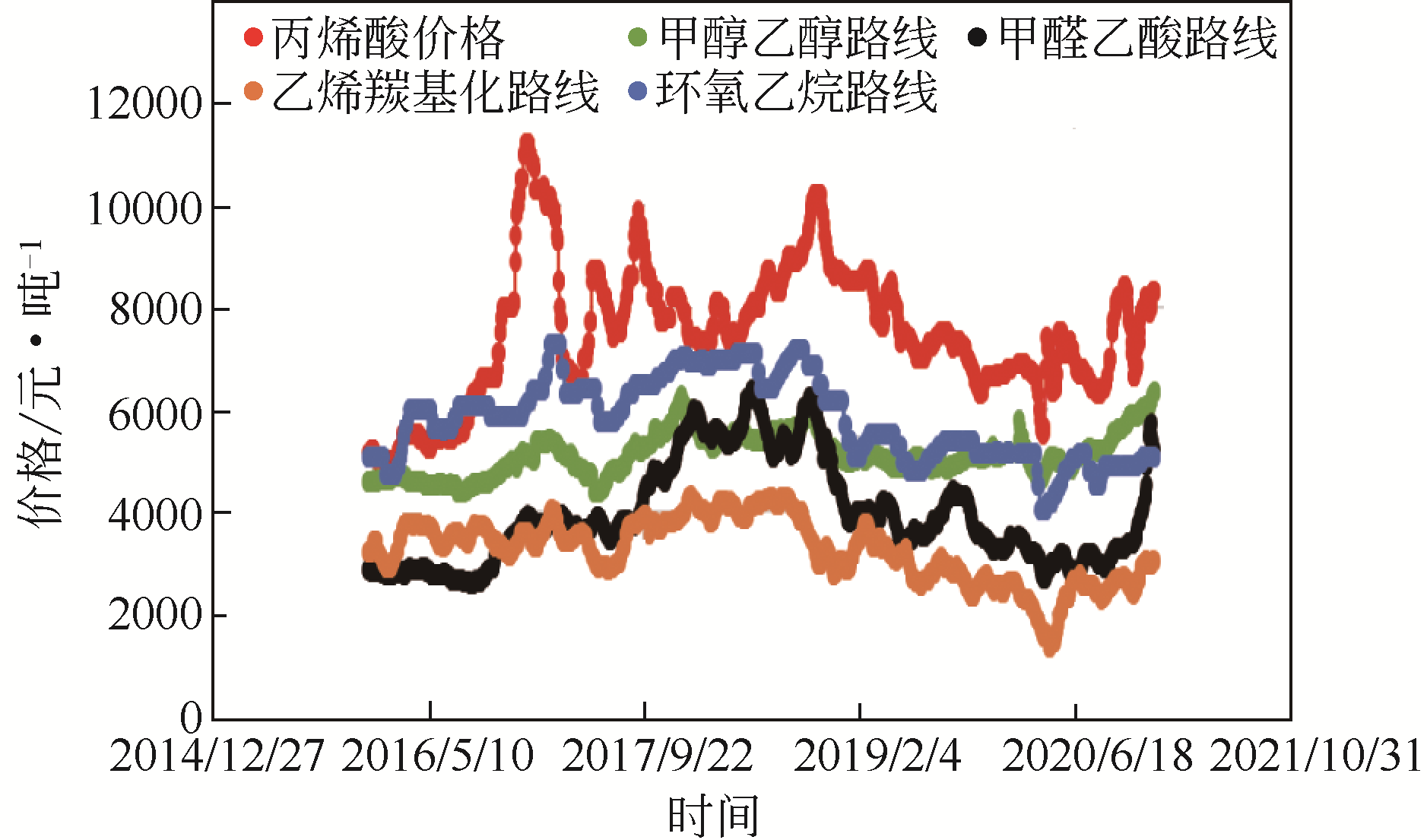
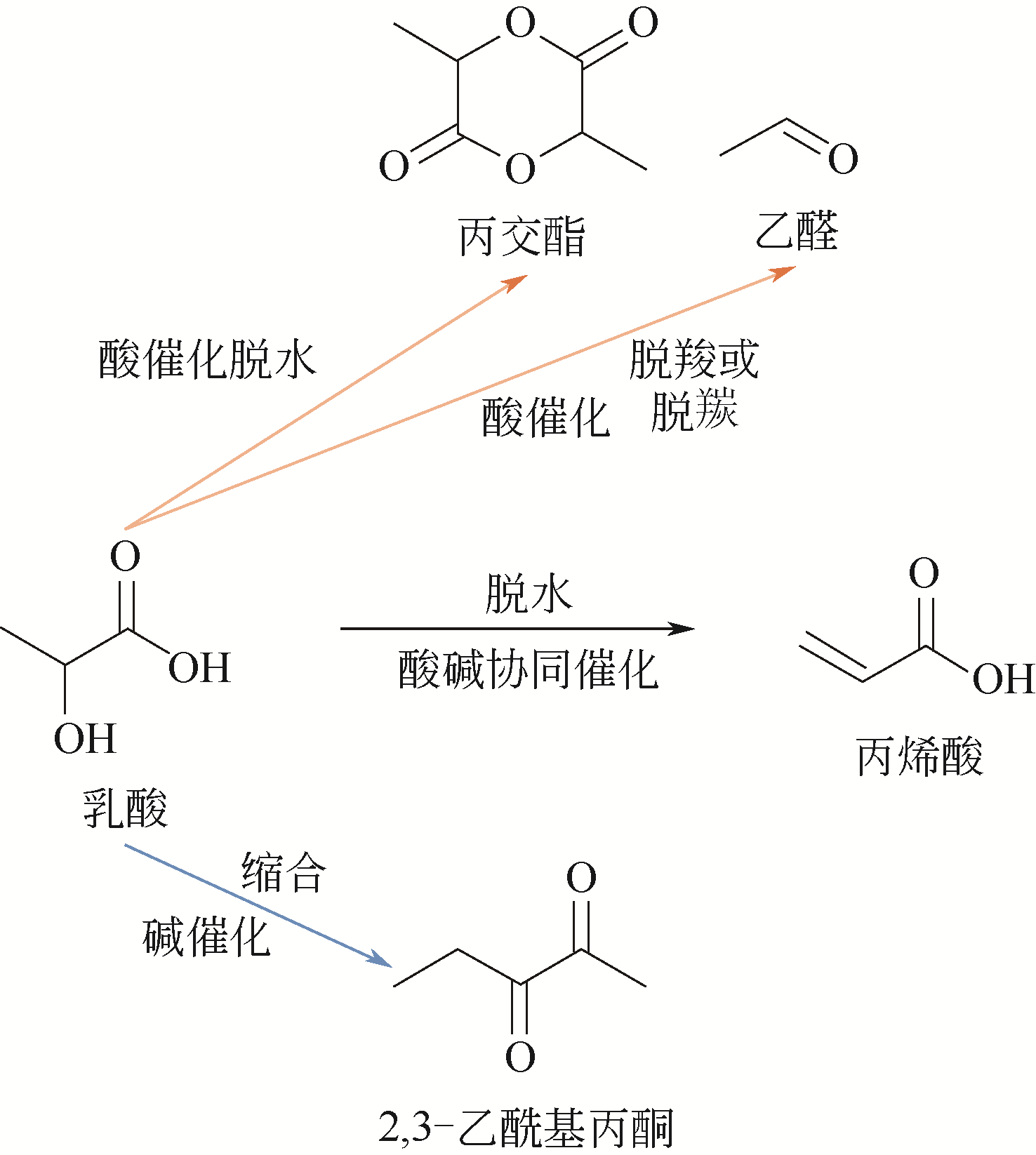
Contact:WANG Feng
丙烯酸催化合成新进展
- 中国科学院大连化学物理研究所,辽宁 大连 116023
-
通讯作者:王峰 -
作者简介:张志鑫(1988—),男,博士,工程师,研究方向为多相催化。E-mail:zhangzhixin@dicp.ac.cn 。 -
基金资助:国家自然科学基金(21706250);辽宁省博士启动基金(2019-BS-244);大连市科技创新基金(2019J11CY009);中国科学院大连化学物理研究所创新基金(DICP I201945)
CLC Number:
Cite this article
ZHANG Zhixin, WANG Yehong, ZHANG Chaofeng, WANG Feng. New advances in catalytic synthesis of acrylic acid[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2016-2033.
张志鑫, 王业红, 张超锋, 王峰. 丙烯酸催化合成新进展[J]. 化工进展, 2021, 40(4): 2016-2033.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-2036
| 合成路线 | 反应步数 (反应类型) | 原子 经济性 | 研究阶段 |
|---|---|---|---|
| 甲醇-乙醇氧化缩合制丙烯醛,丙烯醛氧化制丙烯酸 | 3(氧化-缩合-氧化) | 57% | 基础研究初期、文献较少 |
| 甲醛-乙酸羟醛缩合制丙烯酸 | 1(缩合) | 80% | 基础研究较为充分、文献较多 |
| 乙烯或环氧乙烷与CO羰基化制丙烯酸 | 1(氧化羰基化/羰基化) | 100% | 仅有少量专利报道、文献较少 |
| 合成路线 | 反应步数 (反应类型) | 原子 经济性 | 研究阶段 |
|---|---|---|---|
| 甲醇-乙醇氧化缩合制丙烯醛,丙烯醛氧化制丙烯酸 | 3(氧化-缩合-氧化) | 57% | 基础研究初期、文献较少 |
| 甲醛-乙酸羟醛缩合制丙烯酸 | 1(缩合) | 80% | 基础研究较为充分、文献较多 |
| 乙烯或环氧乙烷与CO羰基化制丙烯酸 | 1(氧化羰基化/羰基化) | 100% | 仅有少量专利报道、文献较少 |
| 催化剂 | 反应条件 | 催化性能 | 参考文献 |
|---|---|---|---|
| FeVO(FeOx/FeVO4) | O2,300℃,10h | C=100%,S=14% | [ |
| WNbVO | O2,285℃ | C=100%,S=46% | [ |
| H3PO4/WNbVO | O2,285℃,1~2h | C=100%,S=59% | [ |
| WVO | O2,318℃,2h | C=100%,S=26% | [ |
| WNbVO | O2,265℃,37h | C=100%,S=51% | [ |
| WMoVO | O2,290℃,69h | C=100%,S=42% | [ |
| H0.1Cs(VO)0.2(PMo12O40)0.5(PW12O40)0.5 | O2,340℃,1h | C=100%,S=60% | [ |
| VSiO | O2,320℃,1h | C=94%,S=85% | [ |
| H-Fe-MCM-22 | O2,320℃,10h | Y=53% | [ |
| Cs2.5H0.5PW12O40/Nb2O5+MoVO-SiC | O2,300℃,70h | C=100%,S=75% | [ |
| V-H3SiW12O40/HZSM-5 | H2O2,90℃,6h | Y=36% | [ |
| Cu/SiO2-MnO2 | H2O2,70℃,30h | C=77%,S=75% | [ |
| 催化剂 | 反应条件 | 催化性能 | 参考文献 |
|---|---|---|---|
| FeVO(FeOx/FeVO4) | O2,300℃,10h | C=100%,S=14% | [ |
| WNbVO | O2,285℃ | C=100%,S=46% | [ |
| H3PO4/WNbVO | O2,285℃,1~2h | C=100%,S=59% | [ |
| WVO | O2,318℃,2h | C=100%,S=26% | [ |
| WNbVO | O2,265℃,37h | C=100%,S=51% | [ |
| WMoVO | O2,290℃,69h | C=100%,S=42% | [ |
| H0.1Cs(VO)0.2(PMo12O40)0.5(PW12O40)0.5 | O2,340℃,1h | C=100%,S=60% | [ |
| VSiO | O2,320℃,1h | C=94%,S=85% | [ |
| H-Fe-MCM-22 | O2,320℃,10h | Y=53% | [ |
| Cs2.5H0.5PW12O40/Nb2O5+MoVO-SiC | O2,300℃,70h | C=100%,S=75% | [ |
| V-H3SiW12O40/HZSM-5 | H2O2,90℃,6h | Y=36% | [ |
| Cu/SiO2-MnO2 | H2O2,70℃,30h | C=77%,S=75% | [ |
| 催化剂 | 反应条件 | 催化性能 | 参考文献 |
|---|---|---|---|
| WO3/ZrO2 | 280℃,10h | C=100%,S=65% | [ |
| WO3/TiO2 | 280℃,14h | C=100%,S=73% | [ |
| Nb2O5 | 315℃,10h | C=88%,S=51% | [ |
| Nb2O5/SiO2-ZrO2 | 325℃,8h | C=77%,S=45% | [ |
| HY | 250℃,10h | C=89%,S=100% | [ |
| 丝光沸石 | 250℃,10h | C=92%,S=100% | [ |
| FePO4 | 280℃,5h | C=100%,S=92% | [ |
| VPO | 320℃,2h | C=100%,S=70% | [ |
| Nd4(P2O7)3 | 320℃,7~8h | C=96%,S=83% | [ |
| H3PW12O40/ZrO2 | 315℃,10h | C=76%,S=71% | [ |
| H4SiW12O40/SiO2 | 275℃,5h | C=98%,S=86% | [ |
| Cs2.5H0.5PW12O40 | 275℃,1h | C=100%,S=98% | [ |
| CsSiW12O40/Al2O3 | 250℃,3h | Y=96%,S=96% | [ |
| 催化剂 | 反应条件 | 催化性能 | 参考文献 |
|---|---|---|---|
| WO3/ZrO2 | 280℃,10h | C=100%,S=65% | [ |
| WO3/TiO2 | 280℃,14h | C=100%,S=73% | [ |
| Nb2O5 | 315℃,10h | C=88%,S=51% | [ |
| Nb2O5/SiO2-ZrO2 | 325℃,8h | C=77%,S=45% | [ |
| HY | 250℃,10h | C=89%,S=100% | [ |
| 丝光沸石 | 250℃,10h | C=92%,S=100% | [ |
| FePO4 | 280℃,5h | C=100%,S=92% | [ |
| VPO | 320℃,2h | C=100%,S=70% | [ |
| Nd4(P2O7)3 | 320℃,7~8h | C=96%,S=83% | [ |
| H3PW12O40/ZrO2 | 315℃,10h | C=76%,S=71% | [ |
| H4SiW12O40/SiO2 | 275℃,5h | C=98%,S=86% | [ |
| Cs2.5H0.5PW12O40 | 275℃,1h | C=100%,S=98% | [ |
| CsSiW12O40/Al2O3 | 250℃,3h | Y=96%,S=96% | [ |
| 催化剂 | 反应条件 | 催化性能 | 参考文献 |
|---|---|---|---|
| 硅胶 | 15% 3-HPA水溶液,300℃,WHSV=1h-1 | C=100%,Y>99% | [ |
| TiO2 | — | Y=95% | [ |
| SiO2 | 20% 3-HPA水溶液,250℃ | C=100%,Y=97% | [ |
| Al2O3 | 60%~80% 3-HPA水溶液,250℃ | C=100%,Y=97% | [ |
| 催化剂 | 反应条件 | 催化性能 | 参考文献 |
|---|---|---|---|
| 硅胶 | 15% 3-HPA水溶液,300℃,WHSV=1h-1 | C=100%,Y>99% | [ |
| TiO2 | — | Y=95% | [ |
| SiO2 | 20% 3-HPA水溶液,250℃ | C=100%,Y=97% | [ |
| Al2O3 | 60%~80% 3-HPA水溶液,250℃ | C=100%,Y=97% | [ |
| 催化剂 | 反应条件 | 催化性能或效果 | 参考文献 |
|---|---|---|---|
| HAP | 360℃,乳酸WHSV=1.4~2.1h-1, 8h | S=71%~74%,Y=50%~62% | [ |
| Rb0.95Na0.05β | 360℃,乳酸WHSV=2.1h-1, 10h | S=70%,Y=60%~65% | [ |
| K0.97Na0.03ZSM-5 | 360℃,乳酸WHSV=2.1h-1, 10h | S=80%,Y=74%~78% | [ |
| Na2HPO4/NaY | 340℃ | Y=58%~74% | [ |
| Ca2P2O7 | 25%(质量分数),乳酸,375℃,WHSV=3 | C=100%,Y=78% | [ |
| BaSO4 | 400℃ | Y=74% | [ |
| 催化剂 | 反应条件 | 催化性能或效果 | 参考文献 |
|---|---|---|---|
| HAP | 360℃,乳酸WHSV=1.4~2.1h-1, 8h | S=71%~74%,Y=50%~62% | [ |
| Rb0.95Na0.05β | 360℃,乳酸WHSV=2.1h-1, 10h | S=70%,Y=60%~65% | [ |
| K0.97Na0.03ZSM-5 | 360℃,乳酸WHSV=2.1h-1, 10h | S=80%,Y=74%~78% | [ |
| Na2HPO4/NaY | 340℃ | Y=58%~74% | [ |
| Ca2P2O7 | 25%(质量分数),乳酸,375℃,WHSV=3 | C=100%,Y=78% | [ |
| BaSO4 | 400℃ | Y=74% | [ |
| 合成路线 | 反应步数(反应类型) | 原子经济性,原料成本(以丙烯酸为基准) | 研究阶段 |
|---|---|---|---|
| 甘油为原料催化转化制丙烯酸 | 1~2(脱水、氧化/氨氧化等) | <67%,6327CNY·t-1 | 基础研究充分,大量文献报道 |
| 3-羟基丙醛或3-羟基丙酸催化脱水制丙烯酸 | 1(脱水) | 80%,— | 基础研究较为充分,大量文献报道 |
| 乳酸催化脱水制丙烯酸 | 1(脱水) | 80%,10750CNY·t-1 | 基础研究较为充分,大量文献报道 |
| 富马酸与乙烯复分解制丙烯酸 | 1(烯烃复分解) | 100%,8396CNY·t-1 | 仅有少量专利报道、文献较少 |
| 黏糠酸与乙烯复分解制丙烯酸 | 1(烯烃复分解) | 100%,23620CNY·t-1 | 仅有少量专利报道、文献较少 |
| 合成路线 | 反应步数(反应类型) | 原子经济性,原料成本(以丙烯酸为基准) | 研究阶段 |
|---|---|---|---|
| 甘油为原料催化转化制丙烯酸 | 1~2(脱水、氧化/氨氧化等) | <67%,6327CNY·t-1 | 基础研究充分,大量文献报道 |
| 3-羟基丙醛或3-羟基丙酸催化脱水制丙烯酸 | 1(脱水) | 80%,— | 基础研究较为充分,大量文献报道 |
| 乳酸催化脱水制丙烯酸 | 1(脱水) | 80%,10750CNY·t-1 | 基础研究较为充分,大量文献报道 |
| 富马酸与乙烯复分解制丙烯酸 | 1(烯烃复分解) | 100%,8396CNY·t-1 | 仅有少量专利报道、文献较少 |
| 黏糠酸与乙烯复分解制丙烯酸 | 1(烯烃复分解) | 100%,23620CNY·t-1 | 仅有少量专利报道、文献较少 |
| 1 | BEERTHUIS R, ROTHENBERG G, SHIJU N R. Catalytic routes towards acrylic acid, adipic acid and ε-caprolactam starting from biorenewables[J]. Green Chemistry, 2015, 17(3): 1341-1361. |
| 2 | GAUTAM P, NEHA, UPADHYAY S N, et al. Bio-methanol as a renewable fuel from waste biomass: current trends and future perspective[J]. Fuel, 2020, 273: 117783. |
| 3 | HAHN-HAGERDAL B, GALBE M, GORWA-GRAUSLUND M F, et al. Bio-ethanol - the fuel of tomorrow from the residues of today[J]. Trends in Biotechnology, 2006, 24(12): 549-556. |
| 4 | BOROWIEC A, DEVAUX J F, DUBOIS J L, et al. An acrolein production route from ethanol and methanol mixtures over FeMo-based catalysts[J]. Green Chemistry, 2017, 19(11): 2666-2674. |
| 5 | BOROWIEC A, LILIĆ A, MORIN J C, et al. Acrolein production from methanol and ethanol mixtures over La- and Ce-doped FeMo catalysts[J]. Applied Catalysis B: Environmental, 2018, 237: 149-157. |
| 6 | LILIĆ A, BENNICI S, DEVAUX J F, et al. Influence of catalyst acid/base properties in acrolein production by oxidative coupling of ethanol and methanol[J]. ChemSusChem, 2017, 10(9): 1916-1930. |
| 7 | LILIĆ A, WEI T T, BENNICI S, et al. A comparative study of basic, amphoteric, and acidic catalysts in the oxidative coupling of methanol and ethanol for acrolein production[J]. ChemSusChem, 2017, 10(17): 3459-3472. |
| 8 | STOŠIĆ D, HOSOGLU F, BENNICI S, et al. Methanol and ethanol reactivity in the presence of hydrotalcites with Mg/Al ratios varying from 2 to 7[J]. Catalysis Communications, 2017, 89: 14-18. |
| 9 | VITCHA J F, SIMS V A. Vapor phase aldol reaction. Acrylic acid by reaction of acetic acid and formaldehyde[J]. I&EC Product Research and Development, 1966, 5(1): 50-53. |
| 10 | GUO Xinpeng, YANG Dan, ZUO Cuncun, et al. Catalysts, process optimization, and kinetics for the production of methyl acrylate over vanadium phosphorus oxide catalysts[J]. Industrial & Engineering Chemistry Research, 2017, 56(20): 5860-5871. |
| 63 | LI Xiukai, ZHANG Yugen. Highly efficient process for the conversion of glycerol to acrylic acid via gas phase catalytic oxidation of an allyl alcohol intermediate[J]. ACS Catalysis, 2016, 6(1): 143-150. |
| 64 | SHIRAMIZU M, TOSTE F D. Deoxygenation of biomass-derived feedstocks: oxorhenium-catalyzed deoxydehydration of sugars and sugar alcohols[J]. Angewandte Chemie International Edition, 2012, 51(32): 8082-8086. |
| 11 | ZUO Cuncun, LI Chunshan, GE Tingting, et al. Spherical P-modified catalysts for heterogeneous cross-aldol condensation of formaldehyde with methyl acetate for methyl acrylate production[J]. Canadian Journal of Chemical Engineering, 2017, 95(11): 2104-2111. |
| 12 | MA Zhanling, MA Xiangang, LIU Hongchao, et al. A green route to methyl acrylate and acrylic acid by an aldol condensation reaction over H-ZSM-35 zeolite catalysts[J]. Chemical Communications, 2017, 53(65): 9071-9074. |
| 13 | YAN Jianbiao, ZHANG Chunlei, NING Chunli, et al. Vapor phase condensation of methyl acetate with formaldehyde to preparing methyl acrylate over cesium supported SBA-15 catalyst[J]. Journal of Industrial and Engineering Chemistry, 2015, 25: 344-351. |
| 14 | AI M. Vapor-phase aldol condensation of formaldehyde with acetic acid on V2O5-P2O5 catalysts[J]. Journal of Catalysis, 1987, 107(1): 201-208. |
| 15 | AI M. Effects of organic-compounds used in preparing V/Ti binary phosphate catalysts[J]. Journal of Catalysis, 1988, 113(2): 562-566. |
| 16 | FENG Xinzhen, SUN Bo, YAO Yao, et al. Renewable production of acrylic acid and its derivative: new insights into the aldol condensation route over the vanadium phosphorus oxides[J]. Journal of Catalysis, 2014, 314: 132-141. |
| 17 | LIU Jun, WANG Pengcheng, FENG Yina, et al. Precisely phase-modulated VPO catalysts with enhanced inter-phase conjunction for acrylic acid production through the condensation of acetic acid and formaldehyde[J]. Journal of Catalysis, 2019, 374: 171–182. |
| 18 | YANG Dan, LI Dan, YAO Haoyu, et al. Reaction of formalin with acetic acid over vanadium-phosphorus oxide bifunctional catalyst[J]. Industrial & Engineering Chemistry Research, 2015, 54(27): 6865-6873. |
| 19 | HU Jing, LU Zhipeng, YIN Hengbo, et al. Aldol condensation of acetic acid with formaldehyde to acrylic acid over SiO2-, SBA-15-, and HZSM-5-supported V-P-O catalysts[J]. Journal of Industrial and Engineering Chemistry, 2016, 40: 145-151. |
| 20 | ZHAO Hui, ZUO Cuncun, YANG Dan, et al. Effects of support for vanadium phosphorus oxide catalysts on vapor-phase aldol condensation of methyl acetate with formaldehyde[J]. Industrial & Engineering Chemistry Research, 2016, 55(50): 12693-12702. |
| 21 | WANG Yumeng, WANG Zhenlu, HAO Xue, et al. Nb-doped vanadium phosphorus oxide catalyst for the aldol condensation of acetic acid with formaldehyde to acrylic acid[J]. Industrial & Engineering Chemistry Research, 2018, 57(36): 12055-12060. |
| 22 | WANG Gang, SARARUK C, LI Zengxi, et al. Studies on mild catalytic synthesis of methyl acrylate via one-step aldol reaction[J]. AIChE Journal, 2018, 64(4): 1359-1372. |
| 23 | WANG Gang, LI Zengxi, LI Chunshan, et al. Preparation of methyl acrylate from methyl acetate and methanol with mild catalysis of cobalt complex[J]. Chemical Engineering Journal, 2019, 359: 863-873. |
| 24 | WANG Xiao, WANG Hui, SUN Yuhan. Synthesis of acrylic acid derivatives from CO2 and ethylene[J]. Chem, 2017, 3(2): 211-228. |
| 25 | SUN Daolai, YAMADA Y, SATO S, et al. Glycerol as a potential renewable raw material for acrylic acid production[J]. Green Chemistry, 2017, 19(14): 3186-3213. |
| 26 | GRASSELLI R K, TRIFIRO F. Acrolein and acrylic acid from biomass[J]. Rendiconti Lincei-Scienze Fisiche E: Naturali, 2017, 28: 59-67. |
| 27 | 花东龙, 庄晓煜, 童东绅, 等.催化甘油胶水氧化连串反应制丙烯酸[J]. 化学进展, 2016, 28(S2): 375-390. |
| HUA Donglong, ZHUANG Xiaoyu, TONG Dongshen, et al. Catalytic oxidehydration of glycerol to acrylic acid[J]. Progress in Chemistry, 2016, 28(S2): 375-390. | |
| 28 | WANG Feng, XU Jie, DUBOIS J L, et al. Catalytic oxidative dehydration of glycerol over a catalyst with iron oxide domains embedded in an iron orthovanadate phase[J]. ChemSusChem, 2010, 3(12): 1383-1389. |
| 29 | OMATA K, MATSUMOTO K, MURAYAMA T, et al. Direct oxidative transformation of glycerol into acrylic acid over phosphoric acid-added W-V-Nb complex metal oxide catalysts[J]. Chemistry Letters, 2014, 43(4): 435-437. |
| 30 | OMATA K, MATSUMOTO K, MURAYAMA T, et al. Direct oxidative transformation of glycerol to acrylic acid over Nb-based complex metal oxide catalysts[J]. Catalysis Today, 2016, 259: 205-212. |
| 31 | CHIEREGATO A, SORIANO M D, BASILE F, et al. One-pot glycerol oxidehydration to acrylic acid on multifunctional catalysts: Focus on the influence of the reaction parameters in respect to the catalytic performance[J]. Applied Catalysis B: Environmental, 2014, 150: 37-46. |
| 32 | CHIERGATO A, SORIANO M D, GARÍA-GONZÁLEZ E, et al. Multielement crystalline and pseudocrystalline oxides as efficient catalysts for the direct transformation of glycerol into acrylic acid[J]. ChemSusChem, 2015, 8(2): 398-406. |
| 33 | YUN Yang Sik, Kyung Rok LEE, PARK Hongseok, et al. Rational design of a bifunctional catalyst for the oxydehydration of glycerol: a combined theoretical and experimental study[J]. ACS Catalysis, 2015, 5(1): 82-94. |
| 34 | LI Xiukai, ZHANG Yugen. Oxidative dehydration of glycerol to acrylic acid over vanadium-substituted cesium salts of Keggin-type heteropolyacids[J]. ACS Catalysis, 2016, 6(5): 2785-2791. |
| 35 | PAULA A S, POSSATO L G, RATERO D R, et al. One-step oxidehydration of glycerol to acrylic acid using ETS-10-like vanadosilicates[J]. Microporous and Mesoporous Materials, 2016, 232: 151-160. |
| 36 | SANTOS M B DOS, ANDRADE H M C, MASCARENHAS A J S. Oxidative dehydration of glycerol over alternative H,Fe-MCM-22 catalysts: sustainable production of acrylic acid[J]. Microporous and Mesoporous Materials, 2019, 278: 366-377. |
| 37 | LIU Rong, WANG Tiefeng, LIU Chang, et al. Highly selective and stable CsPW/Nb2O5 catalysts for dehydration of glycerol to acrolein[J]. Chinese Journal of Catalysis, 2013, 34(12): 2174–2182. |
| 38 | THANASILP S, SCHWANK J W, MEEYOO V, et al. One-pot oxydehydration of glycerol to value-added compounds over metal-doped SiW/HZSM-5 catalysts: effect of metal type and loading[J]. Chemical Engineering Journal, 2015, 275: 113-124. |
| 39 | SARKAR B, PENDEM C, SIVAKUMAR KONATHALA L N, et al. Cu nanoclusters supported on nanocrystalline SiO2-MnO2: a bifunctional catalyst for the one-step conversion of glycerol to acrylic acid[J]. Chemical Communications, 2014, 50(68): 9707-9710. |
| 40 | SORIANO M D, CONCEPCIÓN P, NIETO J M L, et al. Tungsten-vanadium mixed oxides for the oxidehydration of glycerol into acrylic acid[J]. Green Chemistry, 2011, 13(10): 2954-2962. |
| 41 | KATRYNIOK B, PAUL S, CAPRON M, et al. Towards the sustainable production of acrolein by glycerol dehydration[J]. ChemSusChem, 2009, 2(8): 719-730. |
| 42 | KATRYNIOK B, PAUL S, DUMEIGNIL F. Recent developments in the field of catalytic dehydration of glycerol to acrolein[J]. ACS Catalysis, 2013, 3(8): 1819-1834. |
| 43 | WANG Zichun, WANG Leizhi, JIANG Yijiao, et al. Cooperativity of Brønsted and Lewis acid sites on zeolite for glycerol dehydration[J]. ACS Catalysis, 2014, 4(4): 1144-1147. |
| 44 | YUN Danim, YUN Yang Sik, KIM Tae Yong, et al. Mechanistic study of glycerol dehydration on Brønsted acidic amorphous aluminosilicate[J]. Journal of Catalysis, 2016, 341: 33-43. |
| 45 | CHAI Songhai, WANG Haopeng, LIANG Yu, et al. Sustainable production of acrolein: investigation of solid acid-base catalysts for gas-phase dehydration of glycerol[J]. Green Chemistry, 2007, 9(10): 1130-1136. |
| 46 | DALIL M, DE CARNEVALI D, EDAKE M, et al. Gas phase dehydration of glycerol to acrolein: coke on WO3/TiO2 reduces by-products[J]. Journal of Molecular Catalysis A: Chemical, 2016, 421: 146-155. |
| 47 | CHAI Songhai, WANG Haopeng, LIANG Yu, et al. Sustainable production of acrolein: gas-phase dehydration of glycerol over Nb2O5 catalyst[J]. Journal of Catalysis, 2007, 250(2): 342-349. |
| 48 | GARCÍA-SANCHO C, CECILIA J A, MORENO-RUIZ A, et al. Influence of the niobium supported species on the catalytic dehydration of glycerol to acrolein[J]. Applied Catalysis B: Environmental, 2015, 179: 139-149. |
| 49 | DE OLIVEIRA A S, VASCONCELOS S J S, DE SOUSA J R, et al. Catalytic conversion of glycerol to acrolein over modified molecular sieves: activity and deactivation studies[J]. Chemical Engineering Journal, 2011, 168(2): 765-774. |
| 50 | DECOLATTI H P, COSTA B O D, QUERINI C A. Dehydration of glycerol to acrolein using H-ZSM5 zeolite modified by alkali treatment with NaOH[J]. Microporous and Mesoporous Materials, 2015, 204: 180-189. |
| 51 | DELEPLANQUE J, DUBOIS J L, DEVAUX J F, et al. Production of acrolein and acrylic acid through dehydration and oxydehydration of glycerol with mixed oxide catalysts[J]. Catalysis Today, 2010, 157(1/2/3/4): 351-358. |
| 52 | FENG Xinzhen, YAO Yao, SU Qin, et al. Vanadium pyrophosphate oxides: the role of preparation chemistry in determining renewable acrolein production from glycerol dehydration[J]. Applied Catalysis B: Environmental, 2015, 164: 31-39. |
| 53 | LIU Qingbo, ZHANG Zhen, DU Ying, et al. Rare earth pyrophosphates: effective catalysts for the production of acrolein from vapor-phase dehydration of glycerol[J]. Catalysis Letters, 2009, 127(3/4): 419-428. |
| 54 | CHAI Songhai, WANG Haopeng, LIANG Yu, et al. Sustainable production of acrolein: preparation and characterization of zirconia-supported 12-tungstophosphoric acid catalyst for gas-phase dehydration of glycerol[J]. Applied Catalysis A: General, 2009, 353(2): 213-222. |
| 55 | TSUKUDA E, SATO S, TAKAHASHI R, et al. Production of acrolein from glycerol over silica-supported heteropoly acids[J]. Catalysis Communications, 2007, 8(9): 1349-1353. |
| 56 | ALHANASH A, KOZHEVNIKOVA E F, KOZHEVNIKOV I V. Gas-phase dehydration of glycerol to acrolein catalysed by caesium heteropoly salt[J]. Applied Catalysis A: General, 2010, 378(1): 11-18. |
| 57 | HAIDER M H, DUMMER N F, ZHANG Dazhi, et al. Rubidium- and caesium-doped silicotungstic acid catalysts supported on alumina for the catalytic dehydration of glycerol to acrolein[J]. Journal of Catalysis, 2012, 286: 206-213. |
| 58 | CHAI Songhai, TAO Lizhi, YAN Bo, et al. Sustainable production of acrolein: effects of reaction variables, modifiers doping and ZrO2 origin on the performance of WO3/ZrO2 catalyst for the gas-phase dehydration of glycerol[J]. RSC Advances, 2014, 4(9): 4619-4630. |
| 59 | SUN Daolai, YAMADA Y, SATO S, et al. Glycerol hydrogenolysis into useful C3 chemicals[J]. Applied Catalysis B: Environmental, 2016, 193: 75-92. |
| 60 | ARCEO E, MARSDEN P, BERGMAN R G, et al. An efficient didehydroxylation method for the biomass-derived polyols glycerol and erythritol. Mechanistic studies of a formic acid-mediated deoxygenation[J]. Chemical Communications, 2009(23): 3357-3359. |
| 61 | ARCEO E, ELLMAN J A, BERGMAN R G. A direct, biomass-based synthesis of benzoic acid: formic acid-mediated deoxygenation of the glucose-derived materials quinic acid and shikimic acid[J]. ChemSusChem, 2010, 3(7): 811-813. |
| 62 | KAMM O, MARVEL C S. Allyl alcohol[J]. Organic Syntheses, 1921, 1: 15-19. |
| 65 | YI Jing, LIU Shuo, ABU-OMAR M M. Rhenium-catalyzed transfer hydrogenation and deoxygenation of biomass-derived polyols to small and useful organics[J]. ChemSusChem, 2012, 5(8): 1401-1404. |
| 66 | CANALE V, TONUCCI L, BRESSAN M, et al. Deoxydehydration of glycerol to allyl alcohol catalyzed by rhenium derivatives[J]. Catalysis Science & Technology, 2014, 4(10): 3697-3704. |
| 67 | LIU Yong, TÜYSÜZ H, JIA Chunjiang, et al. From glycerol to allyl alcohol: iron oxide catalyzed dehydration and consecutive hydrogen transfer[J]. Chemical Communications, 2010, 46(8): 1238-1240. |
| 68 | KONAKA A, TAGO T, YOSHIKAWA T, et al. Conversion of biodiesel-derived crude glycerol into useful chemicals over a zirconia-iron oxide catalyst[J]. Industrial & Engineering Chemistry Research, 2013, 52(44): 15509-15515. |
| 69 | KONAKA A, TAGO T, YOSHIKAWA T, et al. Conversion of glycerol into allyl alcohol over potassium-supported zirconia-iron oxide catalyst[J]. Applied Catalysis B: Environmental, 2014, 146: 267-273. |
| 70 | SANTOS R C R, BRAGA D M V, PINHEIRO A N, et al. Role of Cu, Ni and Co metals in the acidic and redox properties of Mo catalysts supported on Al2O3 spheres for glycerol conversion[J]. Catalysis Science & Technology, 2016, 6(13): 4986-5002. |
| 71 | TAZAWA S, OTA N, TAMURA M, et al. Deoxydehydration with molecular hydrogen over ceria-supported rhenium catalyst with gold promoter[J]. ACS Catalysis, 2016, 6(10): 6393-6397. |
| 72 | YANG Sungpil, KIM Minsu, YANG Sungeun, et al. Production of acrylic acid from biomass-derived allyl alcohol by selective oxidation using Au/ceria catalysts[J]. Catalysis Science & Technology, 2016, 6(10): 3616-3622. |
| 73 | KIM Minsu, Hyunjoo LEE. Highly selective production of acrylic acid from glycerol via two steps using Au/CeO2 catalysts[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(12): 11371-11376. |
| 74 | SERESHKI B R, BALAN S J, PATIENCE G S, et al. Reactive vaporization of crude glycerol in a fluidized bed reactor[J]. Industrial & Engineering Chemistry Research, 2010, 49(3): 1050-1056. |
| 75 | LIU Rong, Shuting LYU, WANG Tiefeng. Sustainable production of acrolein from biodiesel-derived crude glycerol over H3PW12O40 supported on Cs-modified SBA-15[J]. Journal of Industrial and Engineering Chemistry, 2016, 37: 354-360. |
| 76 | VALDEHUESA K N G, LIU Huaiwei, NISOLA G M, et al. Recent advances in the metabolic engineering of microorganisms for the production of 3-hydroxypropionic acid as C3 platform chemical[J]. Applied Microbiology and Biotechnology, 2013, 97(8): 3309-3321. |
| 77 | CHEN Yun, NIELSEN J. Advances in metabolic pathway and strain engineering paving the way for sustainable production of chemical building blocks[J]. Current Opinion in Biotechnology, 2013, 24(6): 965-972. |
| 78 | CHEN Zhen, LIU Dehua. Toward glycerol biorefinery: metabolic engineering for the production of biofuels and chemicals from glycerol[J]. Biotechnology for Biofuels, 2016, 9: 205. |
| 79 | LI Chao, ZHU Qiangqiang, CUI Ziheng, et al. Highly efficient and selective production of acrylic acid from 3-hydroxypropionic acid over acidic heterogeneous catalysts[J]. Chemical Engineering Science, 2018, 183: 288-294. |
| 80 | TAM M S, CRACIUN R, MILLER D J, et al. Reaction and kinetic studies of lactic acid conversion over alkali-metal salts[J]. Industrial & Engineering Chemistry Research, 1998, 37(6): 2360-2366. |
| 81 | DUSSELIER M, WOUWE P VAN, DEWAELE A, et al. Shape-selective zeolite catalysis for bioplastics production[J]. Science, 2015, 349(6243): 78-80. |
| 82 | KATRYNIOK B, PAUL S, DUMEIGNIL F. Highly efficient catalyst for the decarbonylation of lactic acid to acetaldehyde[J]. Green Chemistry, 2010, 12(11): 1910-1913. |
| 83 | MÄKI-ARVELA P, SIMAKOVA I L, SALMI T, et al. Production of lactic acid/lactates from biomass and their catalytic transformations to commodities[J]. Chemical Reviews, 2014, 114(3): 1909-1971. |
| 84 | MATSUURA Y, ONDA A, OGO S, et al. Acrylic acid synthesis from lactic acid over hydroxyapatite catalysts with various cations and anions[J]. Catalysis Today, 2014, 226: 192-197. |
| 85 | MATSUURA Y, ONDA A, YANAGISAWA K. Selective conversion of lactic acid into acrylic acid over hydroxyapatite catalysts[J]. Catalysis Communications, 2014, 48: 5-10. |
| 86 | BLANCO E, DELICHERE P, MILLET J M M, et al. Gas phase dehydration of lactic acid to acrylic acid over alkaline-earth phosphates catalysts[J]. Catalysis Today, 2014, 226: 185-191. |
| 87 | YAN Bo, TAO Lizhi, LIANG Yu, et al. Sustainable production of acrylic acid: catalytic performance of hydroxyapatites for gas-phase dehydration of lactic acid[J]. ACS Catalysis, 2014, 4(6): 1931-1943. |
| 88 | YAN Bo, TAO Lizhi, LIANG Yu, et al. Sustainable production of acrylic acid: alkali-ion exchanged beta zeolite for gas-phase dehydration of lactic acid[J]. Chemsuschem, 2014, 7(6): 1568-1578. |
| 89 | YAN Bo, MAHMOOD A, LIANG Yu, et al. Sustainable production of acrylic acid: Rb+- and Cs+-exchanged beta zeolite catalysts for catalytic gas-phase dehydration of lactic acid[J]. Catalysis Today, 2016, 269: 65-73. |
| 90 | NÄFE G, L􀆕PEZ-MARTÍNEZ M A, DYBALLA M,et al. Deactivation behavior of alkali-metal zeolites in the dehydration of lactic acid to acrylic acid[J]. Journal of Catalysis, 2015, 329: 413-424. |
| 91 | ZHANG Xianghui, LIN Lu, ZHANG Tong, et al. Catalytic dehydration of lactic acid to acrylic acid over modified ZSM-5 catalysts[J]. Chemical Engineering Journal, 2016, 284: 934-941. |
| 92 | GUO Zhen, THENG De Sheng, TANG K Yuanting, et al. Dehydration of lactic acid to acrylic acid over lanthanum phosphate catalysts: the role of Lewis acid sites[J]. Physical Chemistry Chemical Physics, 2016, 18(34): 23746-23754. |
| 93 | Shuting LÜ, WANG Tiefeng. Efficient production of acrylic acid by dehydration of lactic acid over BaSO4 with crystal defects[J]. RSC Advances, 2017, 7(17): 10278-10286. |
| 94 | YAN Bo, TAO Lizhi, MAHMOOD A, et al. Potassium-ion-exchanged zeolites for sustainable production of acrylic acid by gas-phase dehydration of lactic acid[J]. ACS Catalysis, 2017, 7(1): 538-550. |
| 95 | ZHANG Junfeng, ZHAO Yuling, FENG Xinzhen, et al. Na2HPO4-modified NaY nanocrystallites: efficient catalyst for acrylic acid production through lactic acid dehydration[J]. Catalysis Science & Technology, 2014, 4(5): 1376-1385. |
| 96 | ZHANG Junfeng, ZHAO Yuling, PAN Min, et al. Efficient acrylic acid production through bio lactic acid dehydration over NaY zeolite modified by alkali phosphates[J]. ACS Catalysis, 2011, 1(1): 32-41. |
| 97 | SUN Junming, WANG Yong. Recent advances in catalytic conversion of ethanol to chemicals[J]. ACS Catalysis, 2014, 4(4): 1078-1090. |
| 98 | 孟青青, 杨建国, 王凤寰. 生物法合成丙烯酸的研究进展[J]. 中国生物工程杂志, 2012, 32(10): 119-127. |
| MENG Qingqing, YANG Jianguo, WANG Fenghuan. Advances in the research of biological production of acrylic acid[J]. China Biotechnology, 2012, 32(10): 119-127. | |
| 99 | O’BRIEN D J, PANZER C C, EISELE W P. Biological production of acrylic-acid from cheese whey by resting cells of clostridium-propionicum[J]. Biotechnology Progress, 1990, 6(4): 237-242. |
| 100 | DANNER H, ÜRMÖS M, GARTNER M, et al. Biotechnological production of acrylic acid from biomass[J]. Applied Biochemistry and Biotechnology, 1998, 70/71/72(1): 887-894. |
| 101 | CHU Hun Su, Jin-Ho AHN, YUN Jiae, et al. Direct fermentation route for the production of acrylic acid[J]. Metabolic Engineering, 2015, 32: 23-29. |
| 102 | LIU Zhijie, LIU Tiangang. Production of acrylic acid and propionic acid by constructing a portion of the 3-hydroxypropionate/ 4-hydroxybutyrate cycle from Metallosphaera sedula in Escherichia coli[J]. Journal of Industrial Microbiology & Biotechnology, 2016, 43(12): 1659-1670. |
| 103 | Yoo-Sung KO, KIM Je Woong, CHAE Tong Un, et al. A novel biosynthetic pathway for the production of acrylic acid through beta-alanine route in Escherichia coli[J]. ACS Synthetic Biology, 2020, 9(5): 1150-1159. |
| 104 | KAMAL A, KUMAR M S, KUMAR C G, et al. Bioconversion of acrylonitrile to acrylic acid by rhodococcus ruber strain AKSH-84[J]. Journal of Microbiology and Biotechnology, 2011, 21(1): 37-42. |
| 105 | ZHU Linqi, CHEN Hao, HUANG Lei, et al. Electrochemical analysis of clostridium propionicum and its acrylic acid production in microbial fuel cells[J]. Engineering in Life Sciences, 2011, 11(3): 238-244. |
| 106 | STRAATHOF A J J, SIE S, FRANCO T T, et al. Feasibility of acrylic acid production by fermentation[J]. Applied Microbiology and Biotechnology, 2005, 67(6): 727-734. |
| [1] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [2] | ZHENG Qian, GUAN Xiushuai, JIN Shanbiao, ZHANG Changming, ZHANG Xiaochao. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. |
| [3] | ZHAO Wei, ZHAO Deyin, LI Shihan, LIU Hongda, SUN Jin, GUO Yanqiu. Synthesis and application of triazine drag reducing agent for nature gas pipeline [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 391-399. |
| [4] | WANG Zhengkun, LI Sifang. Green synthesis of gemini surfactant decyne diol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 400-410. |
| [5] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [6] | GENG Yuanze, ZHOU Junhu, ZHANG Tianyou, ZHU Xiaoyu, YANG Weijuan. Homogeneous/heterogeneous coupled combustion of heptane in a partially packed bed burner [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4514-4521. |
| [7] | LUO Cheng, FAN Xiaoyong, ZHU Yonghong, TIAN Feng, CUI Louwei, DU Chongpeng, WANG Feili, LI Dong, ZHENG Hua’an. CFD simulation of liquid distribution in different distributors in medium-low temperature coal tar hydrogenation reactor [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4538-4549. |
| [8] | LAI Shini, JIANG Lixia, LI Jun, HUANG Hongyu, KOBAYASHI Noriyuki. Research progress of ammonia blended fossil fuel [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4603-4615. |
| [9] | ZHU Jie, JIN Jing, DING Zhenghao, YANG Huipan, HOU Fengxiao. Modification of CaSO4 oxygen carrier by Zhundong coal ash in chemical looping gasification and its mechanism [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4628-4635. |
| [10] | GAO Yanjing. Analysis of international research trend of single-atom catalysis technology [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4667-4676. |
| [11] | LI Dongze, ZHANG Xiang, TIAN Jian, HU Pan, YAO Jie, ZHU Lin, BU Changsheng, WANG Xinye. Research progress of NO x reduction by carbonaceous substances for denitration in cement kiln [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4882-4893. |
| [12] | WANG Chen, BAI Haoliang, KANG Xue. Performance study of high power UV-LED heat dissipation and nano-TiO2 photocatalytic acid red 26 coupling system [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4905-4916. |
| [13] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [14] | ZHANG Lihong, JIN Yaoru, CHENG Fangqin. Resource utilization of coal gasification slag [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4447-4457. |
| [15] | WU Haibo, WANG Xilun, FANG Yanxiong, JI Hongbing. Progress of the development and application of 3D printing catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3956-3964. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||