Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (3): 1252-1261.DOI: 10.16085/j.issn.1000-6613.2020-2137
• Special column:Green biomanufacturing • Previous Articles Next Articles
Advances in the suitability of artificial metabolic pathways
GUO Liang1,2( ), GAO Cong1,2, ZHANG Li3, CHEN Xiulai1,2, LIU Liming1,2(
), GAO Cong1,2, ZHANG Li3, CHEN Xiulai1,2, LIU Liming1,2( )
)
- 1.State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi 214122, Jiangsu, China
2.Key Laboratory of Industrial Biotechnology, Ministry of Education, Jiangnan University, Wuxi 214122, Jiangsu, China
3.School of Marine and Bioengineering, Yancheng Institute of Technology, Yancheng 224051, Jiangsu, China
-
Received:2020-10-26Online:2021-03-17Published:2021-03-05 -
Contact:LIU Liming
人工代谢路径适配性的研究进展
郭亮1,2( ), 高聪1,2, 张丽3, 陈修来1,2, 刘立明1,2(
), 高聪1,2, 张丽3, 陈修来1,2, 刘立明1,2( )
)
- 1.江南大学食品科学与技术国家重点实验室,江苏 无锡 214122
2.江南大学工业生物技术教育部重点实验室,江苏 无锡 214122
3.盐城工学院海洋与生物工程学院,江苏 盐城 224051
-
通讯作者:刘立明 -
作者简介:郭亮(1989—),男,助理研究员,研究方向为合成生物学。E-mail:liangguo@jiangnan.edu.cn 。 -
基金资助:广东省重点领域研发项目(2019B020218001);国家自然科学基金创新研究群体科学基金(32021005);中国博士后科学基金(2019M661727)
CLC Number:
Cite this article
GUO Liang, GAO Cong, ZHANG Li, CHEN Xiulai, LIU Liming. Advances in the suitability of artificial metabolic pathways[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1252-1261.
郭亮, 高聪, 张丽, 陈修来, 刘立明. 人工代谢路径适配性的研究进展[J]. 化工进展, 2021, 40(3): 1252-1261.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-2137
| 1 | LEE S Y, KIM H U. Systems strategies for developing industrial microbial strains[J]. Nature Biotechnology, 2015, 33: 1061-1072. |
| 2 | CHOI K R, SHIN J H, CHO J S, et al. Systems metabolic engineering of Escherichia coli[J]. EcoSal Plus, 2016, 7(1): 1-56. |
| 3 | NIELSEN J, KEASLING J D. Engineering cellular metabolism[J]. Cell, 2016, 164(6): 1185-1197. |
| 4 | GUO L, DIAO W, GAO C, et al. Engineering Escherichia coli lifespan for enhancing chemical production[J]. Nature Catalysis, 2020, 3(3): 307-318. |
| 5 | LEE S Y, KIM H U, CHAE T U, et al. A comprehensive metabolic map for production of bio-based chemicals[J]. Nature Catalysis, 2019, 2(1): 18-33. |
| 6 | GUO L, PANG Z, GAO C, et al. Engineering microbial cell morphology and membrane homeostasis toward industrial applications[J]. Current Opinion in Biotechnology, 2020, 66: 18-26. |
| 7 | CHEN X, GAO C, GUO L, et al. DCEO biotechnology: tools to design, construct, evaluate, and optimize the metabolic pathway for biosynthesis of chemicals[J]. Chemical Reviews, 2017, 118(1): 4-72. |
| 8 | KO Y S, KIM J W, LEE J A, et al. Tools and strategies of systems metabolic engineering for the development of microbial cell factories for chemical production[J]. Chemical Society Reviews, 2020, 48(14): 4615-4636. |
| 9 | HU G, LI Y, YE C, et al. Engineering microorganisms for enhanced CO2 sequestration[J]. Trends in Biotechnology, 2018(5): 532-547. |
| 10 | LO T M, TEO W S, LING H, et al. Microbial engineering strategies to improve cell viability for biochemical production[J]. Biotechnology Advances, 2013, 31(6): 903-914. |
| 11 | XU P, GU Q, WANG W, et al. Modular optimization of multi-gene pathways for fatty acids production in E. coli[J]. Nature Communications, 2013, 4: 1-7. |
| 12 | WU G, YAN Q, JONES J A, et al. Metabolic burden: cornerstones in synthetic biology and metabolic engineering applications[J]. Trends in Biotechnology, 2016, 34(8): 652-664. |
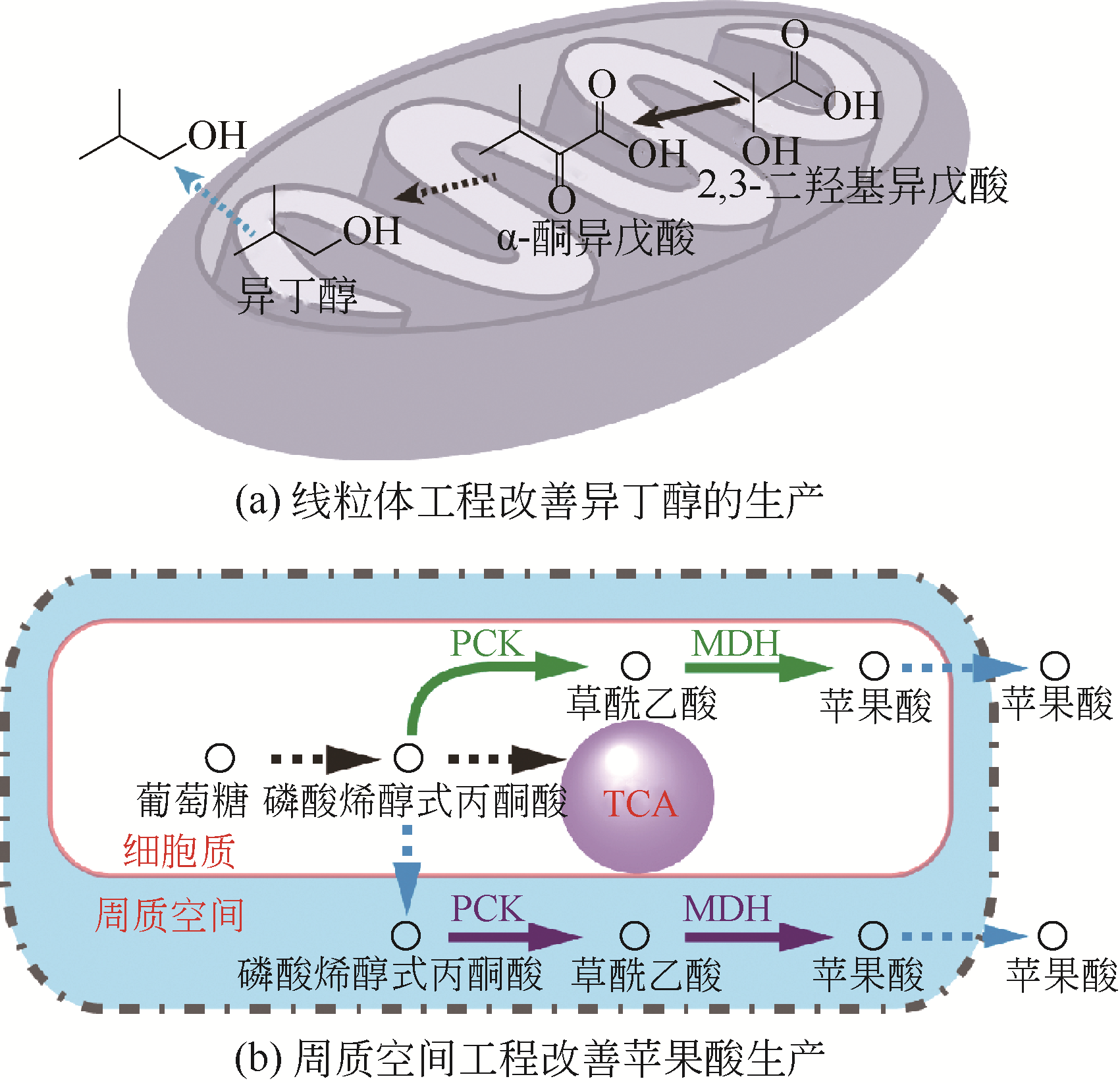
| 13 | AVALOS J L, FINK G R, STEPHANOPOULOS G. Compartmentalization of metabolic pathways in yeast mitochondria improves the production of branched-chain alcohols[J]. Nature Biotechnology, 2013, 31(4): 335-341. |
| 14 | CHEN X, ZHU P, LIU L. Modular optimization of multi-gene pathways for fumarate production[J]. Metabolic Engineering, 2016, 33: 76-85. |
| 15 | AHN J H, SEO H, PARK W, et al. Enhanced succinic acid production by Mannheimia employing optimal malate dehydrogenase[J]. Nature Communications, 2020, 11(1): 1-12. |
| 16 | BASSALO M C, LIU R, GILL R T. Directed evolution and synthetic biology applications to microbial systems[J]. Current Opinion in Biotechnology, 2016, 39: 126-133. |
| 17 | DELEBECQUE C J, LINDNER A B, SILVER P A, et al. Organization of intracellular reactions with rationally designed RNA assemblies[J]. Science, 2011, 333(6041): 470-474. |
| 18 | DUEBER J E, WU G C, MALMIRCHEGINI G R, et al. Synthetic protein scaffolds provide modular control over metabolic flux[J]. Nature Biotechnology, 2009, 27(8): 753-759. |
| 19 | WANG J, JAIN R, SHEN X L, et al. Rational engineering of diol dehydratase enables 1,4-butanediol biosynthesis from xylose[J]. Metabolic Engineering, 2017, 40: 148-156. |
| 20 | WANG F, LV X M, XEI W P, et al. Combining Gal4p-mediated expression enhancement and directed evolution of isoprene synthase to improve isoprene production in Saccharomyces cerevisiae[J]. Metabolic Engineering, 2017, 39: 257-266. |
| 21 | MUKAI C, GAO L, NELSON J L, et al. Biomimicry promotes the efficiency of a 10-step sequential enzymatic reaction on nanoparticles, converting glucose to lactate[J]. Angewandte Chemie: International Edition, 2017, 56(1): 235-238. |
| 22 | LIAN J, HAMEDIRAD M, HU S, et al. Combinatorial metabolic engineering using an orthogonal tri-functional CRISPR system[J]. Nature Communications, 2017, 8: 1-9. |
| 23 | WU S, ZHOU Y, WANG T, et al. Highly regio- and enantioselective multiple oxy- and amino-functionalizations of alkenes by modular cascade biocatalysis[J]. Nature Communications, 2016, 7: 1-13. |
| 24 | AJIKUMAR P K, XIAO W H, TYO K E J, et al. Isoprenoid pathway optimization for taxol precursor overproduction in Escherichia coli[J]. Science, 2010, 330(6000): 70-74. |
| 25 | WU J, ZHOU T, DU G, et al. Modular optimization of heterologous pathways for de novo synthesis of (2S)-naringenin in Escherichia coli[J]. PLoS One, 2014, 9(7): e101492. |
| 26 | LIU Z, ZHANG Y, JIA X, et al. In vitro reconstitution and optimization of the entire pathway to convert glucose into fatty acid[J]. ACS Synthetic Biology, 2017, 6(4): 701-709. |
| 27 | OPGENORTH P H, KORMAN T P, BOWIE J U. A synthetic biochemistry module for production of bio-based chemicals from glucose[J]. Nature Chemical Biology, 2016, 12(6): 393-395. |
| 28 | GAO C, WANG S, HU G, et al. Engineering Escherichia coli for malate production by integrating modular pathway characterization with CRISPRi-guided multiplexed metabolic tuning[J]. Biotechnology and Bioengineering, 2018, 115(3): 661-672. |
| 29 | AGAPAKIS C M, BOYLE P M, SILVER P A. Natural strategies for the spatial optimization of metabolism in synthetic biology[J]. Nature Chemical Biology, 2012, 8(6): 527-535. |
| 30 | GAO C, XU P, YE C, et al. Genetic circuit-assisted smart microbial engineering[J]. Trends in Microbiology, 2019, 27(12): 1011-1124. |
| 31 | CHEN X, LIU L. Gene circuits for dynamically regulating metabolism[J]. Trends in Biotechnology, 2018, 36(8): 751-754. |
| 32 | WANG M, CHEN B, FANG Y, et al. Cofactor engineering for more efficient production of chemicals and biofuels[J]. Biotechnology Advances, 2017, 35(8): 1032-1039. |
| 33 | MCCARTY N S, LEDESMA-AMARO R. Synthetic biology tools to engineer microbial communities for biotechnology[J]. Trends in Biotechnology, 2019, 37(2):181-197. |
| 34 | BAILEY J E, SBURLATI A, HATZIMANIKATIS V, et al. Inverse metabolic engineering: a strategy for directed genetic engineering of useful phenotypes[J]. Biotechnology and Bioengineering, 2002, 79(5): 568-579. |
| 35 | YE C, XU N, GAO C, et al. Comprehensive understanding of Saccharomyces cerevisiae phenotypes with whole-cell model WM_S288C[J]. Biotechnology and Bioengineering, 2020, 117(5): 1562-1574. |
| 36 | WANG S, HOU Y, CHEN X, et al. Kick-starting evolution efficiency with an autonomous evolution mutation system[J]. Metabolic Engineering, 2019, 54: 127-136. |
| 37 | LEE H, DELOACHE W C, DUEBER J E. Spatial organization of enzymes for metabolic engineering[J]. Metabolic Engineering, 2012, 14(3): 242-251. |
| 38 | DELOACHE W C, RUSS Z N, DUEBER J E. Towards repurposing the yeast peroxisome for compartmentalizing heterologous metabolic pathways[J]. Nature Communications, 2016, 7: 1-11. |
| 39 | ZHOU Y J, BUIJS N A, ZHU Z, et al. Harnessing yeast peroxisomes for biosynthesis of fatty-acid-derived biofuels and chemicals with relieved side-pathway competition[J]. Journal of America Chemistry Society, 2016, 138(47): 15368-15377. |
| 40 | KUMAR S, HAHN F M, BAIDOO E, et al. Remodeling the isoprenoid pathway in tobacco by expressing the cytoplasmic mevalonate pathway in chloroplasts[J]. Metabolic Engineering, 2012, 14(1): 19-28. |
| 41 | BAYER T S, WIDMAIER D M, TEMME K, et al. Synthesis of methyl halides from biomass using engineered microbes[J]. Journal of the American Chemical Society, 2009, 131(18): 6508-6515. |
| 42 | MURAKAMI S, SHIMAMOTO T, NAGANO H, et al. Producing human ceramide-NS by metabolic engineering using yeast Saccharomyces cerevisiae[J]. Scientific Reports, 2015, 5: 1-11. |
| 43 | LV X, WANG F, ZHOU P, et al. Dual regulation of cytoplasmic and mitochondrial acetyl-CoA utilization for improved isoprene production in Saccharomyces cerevisiae[J]. Nature Communications2016, 7: 1-12. |
| 44 | MADIGAN M. Brock biology of microorganisms[M]. 13th ed. Upper Saddle River: Prientice Hall, Inc., 2012. |
| 45 | CHOI S Y, SI J P, KIM W J, et al. One-step fermentative production of poly(lactate-co-glycolate) from carbohydrates in Escherichia coli[J]. Nature Biotechnology, 2016, 34(4): 435-440. |
| 46 | SHIN H D, YOON S H, WU J, et al. High-yield production of meso-2,3-butanediol from cellodextrin by engineered E. coli biocatalysts[J]. Bioresource Technology, 2012, 118: 367-373. |
| 47 | JESCHEK M, REUTER R, HEINISCH T, et al. Directed evolution of artificial metalloenzymes for in vivo metathesis[J]. Nature, 2016, 537(7622): 661-665. |
| 48 | LEE K B, NAM D H, NUHN J A M, et al. Direct expression of active human tissue inhibitors of metalloproteinases by periplasmic secretion in Escherichia coli[J]. Microbial Cell Factories, 2017, 16(1): 1-12. |
| 49 | YANG Y, WU Y, HU Y, et al. Harnessing the periplasm of bacteria to develop biocatalyst for biosynthesis of highly pure chemicals[J]. Applied and Environmental Microbiology. 2017, 84(1): e01693-e01617. |
| 50 | GUO L, ZHANG F, ZHANG C, et al. Enhancement of malate production through engineering of the periplasmic rTCA pathway in Escherichia coli[J]. Biotechnology and Bioengineering, 2018, 115(6): 1571-1580. |
| 51 | TAN Z, YOON J M, NIELSEN D R, et al. Membrane engineering via trans unsaturated fatty acids production improves Escherichia coli robustness and production of biorenewables[J]. Metabolic Engineering, 2016, 35: 105-113. |
| 52 | XU P. Production of chemicals using dynamic control of metabolic fluxes[J]. Current Opinion in Biotechnology, 2018, 53: 12-19. |
| 53 | SHEN X, WANG J, LI C, et al. Dynamic gene expression engineering as a tool in pathway engineering[J]. Current Opinion in Biotechnology, 2019, 59: 122-129. |
| 54 | ZHAO E M, ZHANG Y, MEHL J, et al. Optogenetic regulation of engineered cellular metabolism for microbial chemical production[J]. Nature, 2018, 555: 682-687. |
| 55 | GUPTA A, REIZMAN I M, REISCH C R, et al. Dynamic regulation of metabolic flux in engineered bacteria using a pathway-independent quorum-sensing circuit[J]. Nature Biotechnology, 2017, 35(3): 273-279. |
| 56 | DOONG S J, GUPTA A, PRATHER K L J. Layered dynamic regulation for improving metabolic pathway productivity in Escherichia coli[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(12): 2964-2969. |
| 57 | GAO C, HOU J, XU P, et al. Programmable biomolecular switches for rewiring flux in Escherichia coli[J]. Nature Communications, 2019, 10(1): 1-12. |
| 58 | CHEN X, LI S, LIU L. Engineering redox balance through cofactor systerms[J]. Trends in Biotechnology, 2014, 32(6): 337-343. |
| 59 | CHEN X, LI Y, TONG T, et al. Spatial modulation and cofactor engineering of key pathway enzymes for fumarate production in Candida glabrata[J]. Biotechnology and Bioengineering, 2019, 116(3): 622-630. |
| 60 | LIM J H, SEO S W, KIM S Y, et al. Refactoring redox cofactor regeneration for high-yield biocatalysis of glucose to butyric acid in Escherichia coli[J]. Bioresource Technology, 2013, 135: 568-573. |
| 61 | QIAO K, WASYLENKO T M, ZHOU K, et al. Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism[J]. Nature Biotechnology, 2017, 35(2): 173-177. |
| 62 | GUO J, SUASTEGUI M, SAKIMOTO K K, et al. Light-driven fine chemical production in yeast biohybrids[J]. Science, 2018, 362(6416): 813-816. |
| 63 | CHEN X, XU G, XU N, et al. Metabolic engineering of Torulopsis glabrata for malate production[J]. Metabolic Engineering, 2013, 19: 10-16. |
| 64 | YE C, LUO Q, GUO L, et al. Improving lysine production through construction of an Escherichia coli enzyme-constrained model[J]. Biotechnology and Bioengineering, 2020, 117(11): 3533-3544. |
| 65 | KIM H M, CHAE T U, CHOI S Y, et al. Engineering of an oleaginous bacterium for the production of fatty acids and fuels[J]. Nature Chemical Biology, 2019, 15(7): 721-729. |
| 66 | ZHOU K, QIAO K J, EDGAR S, et al. Distributing a metabolic pathway among a microbial consortium enhances production of natural products[J]. Nature Biotechnology, 2015, 33(4): 377-783. |
| 67 | KONG W, MELDGIN D R, COLLINS J J, et al. Designing microbial consortia with defined social interactions[J]. Nature Chemical Biology, 2018, 14(8): 821-829. |
| 68 | KIM S, LINDNER S N, ASLAN S, et al. Growth of E. coli on formate and methanol via the reductive glycine pathway[J]. Nature Chemical Biology, 2020, 16: 538-545. |
| 69 | CHOE D, LEE J H, YOO M, et al. Adaptive laboratory evolution of a genome-reduced Escherichia coli[J]. Nature Communications, 2019, 10(1): 1-14. |
| 70 | WANG W, LI S, LI Z, et al. Harnessing the intracellular triacylglycerols for titer improvement of polyketides in Streptomyces[J]. Nature Biotechnology, 2020, 38(1): 76-83. |
| 71 | ZHU Z, HU Y, TEIXEIRA P G, et al. Multidimensional engineering of Saccharomyces cerevisiae for efficient synthesis of medium-chain fatty acids[J]. Nature Catalysis, 2020, 3(1): 64-74. |
| [1] | GAO Cong, CHEN Chenghu, CHEN Xiulai, LIU Liming. Progress and challenges of engineering microorganisms to produce biobased monomers [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4123-4135. |
| [2] | GUO Feng, ZHANG Shangjie, JIANG Yujia, JIANG Wankui, XIN Fengxue, ZHANG Wenming, JIANG Min. Biotransformation of one-carbon resources by yeast [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 30-39. |
| [3] | TAO Yuxuan, ZHANG Shangjie, JING Yiwen, XIN Fengxue, DONG Weiliang, ZHOU Jie, JIANG Yujia, ZHANG Wenming, JIANG Min. Recent advances in the construction strategy of methylotrophic Escherichia coli [J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3932-3941. |
| [4] | WANG Ying, QU Junze, LIANG Nan, HAO He, YUAN Yingjin. Rapid construction and directed evolution of cell factories for carotenoid biosynthesis [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1187-1201. |
| [5] | LIU Weibing, YE Bangce. Progress of synthetic biology research and biological manufacturing of actinomycetes polyketides [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1226-1237. |
| [6] | MA Yueyuan, CHEN Jinchun, CHEN Guoqiang. Halophilic microorganisms as microbial chassis: applications and prospects [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1178-1186. |
| [7] | SUN Wentao, LI Chun. Design and construction of microbial cell factory for biosynthesis of plant natural products [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1202-1214. |
| [8] | GAO Cong, GUO Liang, HU Guipeng, CHEN Xiulai, LIU Liming. Advances of metabolic flux regulation in microbial cell factories [J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6807-6817. |
| [9] | SHANG Juan, LU Yanghui, ZHENG Jinyang, SUN Chen, HUA Zhengli, YU Wentao, ZHANG Yiwei. Research status-in-situ and key challenges in pipeline transportation of hydrogen-natural gas mixtures [J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5499-5505. |
| [10] | Chen WANG, Meng ZHAO, Mingzhu DING, Ying WANG, Mingdong YAO, Wenhai XIAO. Application of biological scaffold system on synthetic biology [J]. Chemical Industry and Engineering Progress, 2020, 39(11): 4557-4567. |
| [11] | Pengcheng CHANG, Yang YU, Ying WANG, Chun LI. Combinatorial regulation strategies for efficient synthesis of terpenoids in Saccharomyces cerevisiae [J]. Chemical Industry and Engineering Progress, 2019, 38(01): 598-605. |
| [12] | YANG Kun, WANG Ying, LI Chun. Cell transporter protein and engineered applications [J]. Chemical Industry and Engineering Progress, 2017, 36(04): 1410-1417. |
| [13] | LIU Dingyu, MENG Jiao, WANG Zhiwen, CHEN Tao, ZHAO Xueming. Progress and application on multivariate modular metabolic engineering in metabolic engineering [J]. Chemical Industry and Engineering Progress, 2016, 35(11): 3619-3626. |
| [14] | XIAO Wenhai, ZHOU Sijie, WANG Ying, YUAN Yingjin. How to make biology more “engineering” [J]. Chemical Industry and Engineering Progree, 2016, 35(06): 1827-1836. |
| [15] | WAN Tao1,QI Haishan1,CHEN Yunlin2,WEN Jianping1. Research progress of biosynthesis of daptomycin and its derivative [J]. Chemical Industry and Engineering Progree, 2012, 31(07): 1581-1586. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||