Chemical Industry and Engineering Progress ›› 2020, Vol. 39 ›› Issue (S2): 319-328.DOI: 10.16085/j.issn.1000-6613.2020-1084
• Resources and environmental engineering • Previous Articles Next Articles
Research and application progress in the technology of water softening by ion exchange
Yong XU( ), Qingbai CHEN, Jianyou WANG(
), Qingbai CHEN, Jianyou WANG( )
)
- TianjinKey Laboratory of Environmental Technology for Complex Trans-Media Pollution, College of Environmental Science and Engineering, NanKai University, Tianjin 300350, China
-
Received:2020-06-15Online:2020-11-17Published:2020-11-20 -
Contact:Jianyou WANG
离子交换水软化技术研究与应用进展
- 南开大学环境科学与工程学院,天津市跨介质复合污染环境治理技术重点实验室,天津 300350
-
通讯作者:王建友 -
作者简介:徐勇(1995—),男,硕士研究生,研究方向为水污染控制与资源化。E-mail:emailxuyong@163.com 。 -
基金资助:国家重点研发计划(2017YFC0404003);中央高校科研业务费专项资金(20180017);天津市生态环境治理科技重大专项(18ZXSZSF00050);天津市科技支撑计划重点项目(19YFZCSF00760)
CLC Number:
Cite this article
Yong XU, Qingbai CHEN, Jianyou WANG. Research and application progress in the technology of water softening by ion exchange[J]. Chemical Industry and Engineering Progress, 2020, 39(S2): 319-328.
徐勇, 陈青柏, 王建友. 离子交换水软化技术研究与应用进展[J]. 化工进展, 2020, 39(S2): 319-328.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-1084
| 树脂名称 | 树脂类型 | 功能基团 | 出厂 型态 | 含水率 /% | 湿视密度 /g·mL-1 | 湿真密度 /g·mL-1 | 粒径范围 /mm | 质量交换容量 /mmol·g-1 | 体积交换 容量 /mmol·mL-1 | 制造商 |
|---|---|---|---|---|---|---|---|---|---|---|
| D001 | 大孔强酸 | 磺酸基 | 钠型 | 45~50 | 0.77~0.85 | 1.25~1.28 | 0.31~1.25(≥95%) | ≥2.17 | ≥0.90 | 苏青(SUQING) |
| D001H | 大孔强酸 | 磺酸基 | 氢型 | 48~58 | 0.74~0.80 | 1.16~1.24 | 0.31~1.25(≥95%) | ≥2.40 | ≥0.85 | |
| 112 | 凝胶弱酸 | 羧酸基 | 氢型 | 40~50 | 0.72~0.82 | 1.15~1.25 | 0.31~1.25(≥95%) | ≥5.00 | ≥2.15 | |
| D113 | 大孔弱酸 | 羧酸基 | 氢型 | 45~52 | 0.72~0.80 | 1.14~1.20 | 0.31~1.25(≥95%) | ≥5.40 | ≥2.15 | |
| D402 | 大孔螯合 | 亚氨二乙酸基 | 钠型 | 52~58 | 0.72~0.78 | 1.15~1.25 | 0.31~1.25(≥95%) | ≥1.95 | ≥0.60 | |
| D402-Ⅱ | 大孔螯合 | 氨基磷酸 | 钠型 | 52~58 | 0.72~0.78 | 1.15~1.25 | 0.31~1.25(≥95%) | ≥1.45 | ≥0.50 | |
| 001×7 | 凝胶强酸 | 磺酸基 | 钠型 | 45~50 | 0.77~0.87 | 1.25~1.29 | 0.31~1.25(≥95%) | ≥2.25 | ≥0.85 | 争光(Hydrolite) |
| D001 | 大孔强酸 | 磺酸基 | 钠型 | 45~55 | 0.77~0.85 | 1.25~1.28 | 0.31~1.25(≥95%) | ≥2.17 | ≥0.90 | |
| D113 | 大孔弱酸 | 羧酸基 | 氢型 | 45~52 | 0.72~0.80 | 1.14~1.20 | 0.31~1.25(≥95%) | ≥5.40 | ≥2.20 | |
| D851 | 大孔螯合 | 亚氨二乙酸基 | 钠型 | 55~65 | 0.70~0.80 | 1.15~1.20 | 0.45~1.25(≥95%) | — | ≥1.00 | |
| D852 | 大孔螯合 | 亚氨二乙酸基 | 钠型 | 45~58 | 0.73~0.83 | 1.15~1.23 | 0.31~1.25(≥95%) | — | ≥1.15 | |
| D860 | 大孔螯合 | 氨基磷酸基 | 钠型 | 46~56 | 0.70~0.80 | 1.08~1.16 | 0.45~1.00 (≥95%) | — | ≥0.60 | |
| 001×7 | 凝胶强酸 | 磺酸基 | 钠型 | 45~50 | 0.77~0.87 | 1.25~1.29 | 0.31~1.25(≥95%) | ≥2.25 | ≥0.85 | 淄博东大(DONGDA) |
| D001 | 大孔强酸 | 磺酸基 | 钠型 | 45~55 | 0.75~0.85 | 1.25~1.28 | 0.31~1.25(≥95%) | ≥2.17 | ≥0.90 | |
| D113 | 大孔弱酸 | 羧酸基 | 氢型 | 45~52 | 0.74~0.82 | 1.14~1.20 | 0.31~1.25(≥95%) | ≥5.40 | ≥2.20 | |
| HPR 1100Na | 凝胶强酸 | 磺酸基 | 钠型 | 42~48 | 0.85 | 1.29 | 0.58±0.05 | — | ≥1.00 | 杜邦(AmberLite) |
| HPR 2900Na | 大孔强酸 | 磺酸基 | 钠型 | 46~52 | 0.785 | 1.28 | 0.55±0.05 | — | ≥0.90 | |
| HPR 8300H | 大孔弱酸 | 羧酸基 | 氢型 | 40~50 | 0.76 | 1.21 | 0.45~0.600 | — | ≥2.35 | |
| HPR 8400H | 大孔弱酸 | 羧酸基 | 氢型 | 40~50 | 0.76 | 1.21 | 0.60~0.800 | — | ≥2.35 | |
| IRC 120Na | 凝胶强酸 | 磺酸基 | 钠型 | 42~49 | 0.82 | 1.27 | 0.30~1.18 | — | ≥1.00 | |
| IRC 200Na | 大孔强酸 | 磺酸基 | 钠型 | 46~52 | 0.80 | 1.24 | 0.60~0.80 | — | ≥0.90 | 杜邦(AmberLite) |
| IRC 83H | 大孔弱酸 | 羧酸基 | 氢型 | 40~50 | 0.76 | 1.21 | 0.50~0.75 | — | ≥2.35 | |
| IRC 747UPS | 大孔螯合 | 氨基磷酸基 | 钠型 | 64~69 | 0.755 | 1.10~1.14 | 0.55±0.05 | — | ≥0.87 | |
| IRC 748UPS | 大孔螯合 | 亚氨二乙酸基 | 钠型 | 60~69 | 0.75 | — | 0.57±0.075 | — | ≥0.67 | |
| C 249 | 凝胶强酸 | 磺酸基 | 钠型 | 45~48 | 0.832 | 1.26 | 0.40~1.25 | — | ≥4.0 | 朗盛(LEWATIT) |
| SP 112H | 大孔强酸 | 磺酸基 | 氢型 | 56~60 | 0.72 | 1.18 | 0.67±0.05 | — | ≥3.2 | |
| S 8227 | 大孔弱酸 | 羧酸基 | 氢型 | 47~53 | 0.77 | 1.20 | 0.40~1.60 | — | ≥2.15 | |
| S 8229 | 大孔弱酸 | 羧酸基 | 氢型 | 48~56 | 0.77 | 1.18 | 0.40~1.60 | — | ≥2.10 | |
| TP 207 | 大孔螯合 | 亚氨二乙酸基 | 钠型 | 48~56 | 0.72 | 1.19 | 0.40~1.25 | — | ≥1.10 | |
| TP 208 | 大孔螯合 | 亚氨二乙酸基 | 钠型 | 59~65 | 0.70 | 1.16 | 0.40~1.25 | — | ≥1.45 | |
| TP 260 | 大孔螯合 | 氨基甲基磷酸 | 钠型 | 63 | 0.74 | 1.21 | 0.40~1.25 | — | ≥1.15 | |
| PK208 | 大孔强酸 | 磺酸基 | 钠型 | 58~68 | 0.765 | — | 0.30~1.18 | — | ≥0.60 | 三菱(DIAION) |
| PK208LH | 大孔强酸 | 磺酸基 | 钠型 | 63~73 | 0.74 | 1.13 | 0.43~1.18 | — | ≥0.55 | |
| SK1B | 凝胶强酸 | 磺酸基 | 钠型 | 43~50 | 0.84 | 1.28 | 0.30~1.18 | — | ≥1.00 | |
| WK40L | 大孔弱酸 | 羧酸基 | 氢型 | 41~48 | 0.77 | 1.19 | 0.425~1.18 | — | ≥2.20 | |
| CR11 | 大孔螯合 | 亚氨二乙酸基 | 钠型 | 55~65 | 0.73 | — | 0.36~1.18 | — | ≥0.50 |
| 树脂名称 | 树脂类型 | 功能基团 | 出厂 型态 | 含水率 /% | 湿视密度 /g·mL-1 | 湿真密度 /g·mL-1 | 粒径范围 /mm | 质量交换容量 /mmol·g-1 | 体积交换 容量 /mmol·mL-1 | 制造商 |
|---|---|---|---|---|---|---|---|---|---|---|
| D001 | 大孔强酸 | 磺酸基 | 钠型 | 45~50 | 0.77~0.85 | 1.25~1.28 | 0.31~1.25(≥95%) | ≥2.17 | ≥0.90 | 苏青(SUQING) |
| D001H | 大孔强酸 | 磺酸基 | 氢型 | 48~58 | 0.74~0.80 | 1.16~1.24 | 0.31~1.25(≥95%) | ≥2.40 | ≥0.85 | |
| 112 | 凝胶弱酸 | 羧酸基 | 氢型 | 40~50 | 0.72~0.82 | 1.15~1.25 | 0.31~1.25(≥95%) | ≥5.00 | ≥2.15 | |
| D113 | 大孔弱酸 | 羧酸基 | 氢型 | 45~52 | 0.72~0.80 | 1.14~1.20 | 0.31~1.25(≥95%) | ≥5.40 | ≥2.15 | |
| D402 | 大孔螯合 | 亚氨二乙酸基 | 钠型 | 52~58 | 0.72~0.78 | 1.15~1.25 | 0.31~1.25(≥95%) | ≥1.95 | ≥0.60 | |
| D402-Ⅱ | 大孔螯合 | 氨基磷酸 | 钠型 | 52~58 | 0.72~0.78 | 1.15~1.25 | 0.31~1.25(≥95%) | ≥1.45 | ≥0.50 | |
| 001×7 | 凝胶强酸 | 磺酸基 | 钠型 | 45~50 | 0.77~0.87 | 1.25~1.29 | 0.31~1.25(≥95%) | ≥2.25 | ≥0.85 | 争光(Hydrolite) |
| D001 | 大孔强酸 | 磺酸基 | 钠型 | 45~55 | 0.77~0.85 | 1.25~1.28 | 0.31~1.25(≥95%) | ≥2.17 | ≥0.90 | |
| D113 | 大孔弱酸 | 羧酸基 | 氢型 | 45~52 | 0.72~0.80 | 1.14~1.20 | 0.31~1.25(≥95%) | ≥5.40 | ≥2.20 | |
| D851 | 大孔螯合 | 亚氨二乙酸基 | 钠型 | 55~65 | 0.70~0.80 | 1.15~1.20 | 0.45~1.25(≥95%) | — | ≥1.00 | |
| D852 | 大孔螯合 | 亚氨二乙酸基 | 钠型 | 45~58 | 0.73~0.83 | 1.15~1.23 | 0.31~1.25(≥95%) | — | ≥1.15 | |
| D860 | 大孔螯合 | 氨基磷酸基 | 钠型 | 46~56 | 0.70~0.80 | 1.08~1.16 | 0.45~1.00 (≥95%) | — | ≥0.60 | |
| 001×7 | 凝胶强酸 | 磺酸基 | 钠型 | 45~50 | 0.77~0.87 | 1.25~1.29 | 0.31~1.25(≥95%) | ≥2.25 | ≥0.85 | 淄博东大(DONGDA) |
| D001 | 大孔强酸 | 磺酸基 | 钠型 | 45~55 | 0.75~0.85 | 1.25~1.28 | 0.31~1.25(≥95%) | ≥2.17 | ≥0.90 | |
| D113 | 大孔弱酸 | 羧酸基 | 氢型 | 45~52 | 0.74~0.82 | 1.14~1.20 | 0.31~1.25(≥95%) | ≥5.40 | ≥2.20 | |
| HPR 1100Na | 凝胶强酸 | 磺酸基 | 钠型 | 42~48 | 0.85 | 1.29 | 0.58±0.05 | — | ≥1.00 | 杜邦(AmberLite) |
| HPR 2900Na | 大孔强酸 | 磺酸基 | 钠型 | 46~52 | 0.785 | 1.28 | 0.55±0.05 | — | ≥0.90 | |
| HPR 8300H | 大孔弱酸 | 羧酸基 | 氢型 | 40~50 | 0.76 | 1.21 | 0.45~0.600 | — | ≥2.35 | |
| HPR 8400H | 大孔弱酸 | 羧酸基 | 氢型 | 40~50 | 0.76 | 1.21 | 0.60~0.800 | — | ≥2.35 | |
| IRC 120Na | 凝胶强酸 | 磺酸基 | 钠型 | 42~49 | 0.82 | 1.27 | 0.30~1.18 | — | ≥1.00 | |
| IRC 200Na | 大孔强酸 | 磺酸基 | 钠型 | 46~52 | 0.80 | 1.24 | 0.60~0.80 | — | ≥0.90 | 杜邦(AmberLite) |
| IRC 83H | 大孔弱酸 | 羧酸基 | 氢型 | 40~50 | 0.76 | 1.21 | 0.50~0.75 | — | ≥2.35 | |
| IRC 747UPS | 大孔螯合 | 氨基磷酸基 | 钠型 | 64~69 | 0.755 | 1.10~1.14 | 0.55±0.05 | — | ≥0.87 | |
| IRC 748UPS | 大孔螯合 | 亚氨二乙酸基 | 钠型 | 60~69 | 0.75 | — | 0.57±0.075 | — | ≥0.67 | |
| C 249 | 凝胶强酸 | 磺酸基 | 钠型 | 45~48 | 0.832 | 1.26 | 0.40~1.25 | — | ≥4.0 | 朗盛(LEWATIT) |
| SP 112H | 大孔强酸 | 磺酸基 | 氢型 | 56~60 | 0.72 | 1.18 | 0.67±0.05 | — | ≥3.2 | |
| S 8227 | 大孔弱酸 | 羧酸基 | 氢型 | 47~53 | 0.77 | 1.20 | 0.40~1.60 | — | ≥2.15 | |
| S 8229 | 大孔弱酸 | 羧酸基 | 氢型 | 48~56 | 0.77 | 1.18 | 0.40~1.60 | — | ≥2.10 | |
| TP 207 | 大孔螯合 | 亚氨二乙酸基 | 钠型 | 48~56 | 0.72 | 1.19 | 0.40~1.25 | — | ≥1.10 | |
| TP 208 | 大孔螯合 | 亚氨二乙酸基 | 钠型 | 59~65 | 0.70 | 1.16 | 0.40~1.25 | — | ≥1.45 | |
| TP 260 | 大孔螯合 | 氨基甲基磷酸 | 钠型 | 63 | 0.74 | 1.21 | 0.40~1.25 | — | ≥1.15 | |
| PK208 | 大孔强酸 | 磺酸基 | 钠型 | 58~68 | 0.765 | — | 0.30~1.18 | — | ≥0.60 | 三菱(DIAION) |
| PK208LH | 大孔强酸 | 磺酸基 | 钠型 | 63~73 | 0.74 | 1.13 | 0.43~1.18 | — | ≥0.55 | |
| SK1B | 凝胶强酸 | 磺酸基 | 钠型 | 43~50 | 0.84 | 1.28 | 0.30~1.18 | — | ≥1.00 | |
| WK40L | 大孔弱酸 | 羧酸基 | 氢型 | 41~48 | 0.77 | 1.19 | 0.425~1.18 | — | ≥2.20 | |
| CR11 | 大孔螯合 | 亚氨二乙酸基 | 钠型 | 55~65 | 0.73 | — | 0.36~1.18 | — | ≥0.50 |
| 序号 | TDS/mg·L-1 | 总硬度/mg·L-1 | 软化工艺 |
|---|---|---|---|
| 1 | ≤2000 | ≤100 | 强酸树脂(单床) |
| 2 | 700~5000 | ≤2000 | 强酸树脂(上流式串联) |
| 3 | 5000~10000 | ≤500 | 弱酸树脂(单床) |
| 4 | 5000~10000 | 500~2000 | 强酸树脂+弱酸树脂(串联) |
| 5 | 10000~50000 | ≤2000 | 弱酸树脂(串联) |
| 6 | ≥50000 | ≤500 | 螯合树脂(单床) |
| 序号 | TDS/mg·L-1 | 总硬度/mg·L-1 | 软化工艺 |
|---|---|---|---|
| 1 | ≤2000 | ≤100 | 强酸树脂(单床) |
| 2 | 700~5000 | ≤2000 | 强酸树脂(上流式串联) |
| 3 | 5000~10000 | ≤500 | 弱酸树脂(单床) |
| 4 | 5000~10000 | 500~2000 | 强酸树脂+弱酸树脂(串联) |
| 5 | 10000~50000 | ≤2000 | 弱酸树脂(串联) |
| 6 | ≥50000 | ≤500 | 螯合树脂(单床) |
| 1 | CLAUWAERT P, DE PAEPE J, JIANG F, et al. Electrochemical tap water softening: a zero chemical input approach[J]. Water Research, 2020, 169: 115263. |
| 2 | GABRIELLI C, MAURIN G, FRANCY-CHAUSSON H, et al. Electrochemical water softening: principle and application[J]. Desalination, 2006, 201(1/2/3): 150-163. |
| 3 | LI X, HASSON D, SEMIAT R, et al. Intermediate concentrate demineralization techniques for enhanced brackish water reverse osmosis water recovery—A review[J]. Desalination, 2019, 466: 24-35. |
| 4 | ZHANG X, YE C, PI K, et al. Sustainable treatment of desulfurization wastewater by ion exchange and bipolar membrane electrodialysis hybrid technology[J]. Separation and Purification Technology, 2019, 211: 330-339. |
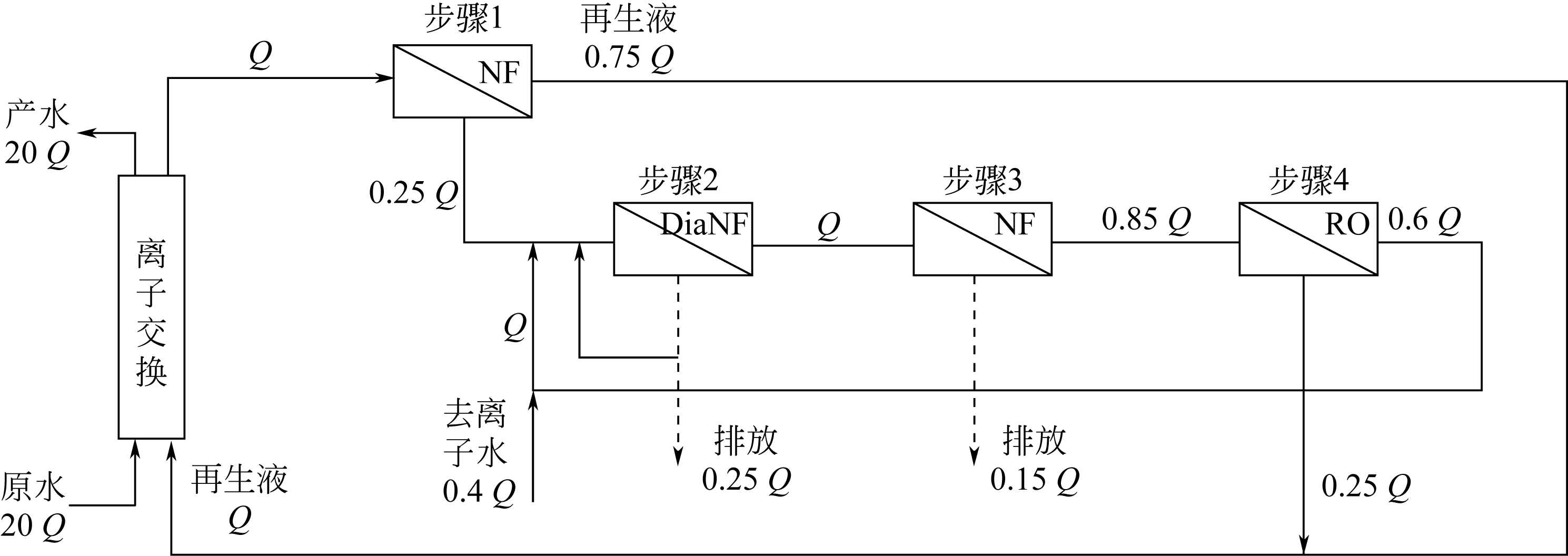
| 5 | 尚言武, 成怀刚, 王铎, 等. 海上油田注水纳滤软化中试研究[J]. 化工进展, 2009, 28(3): 534-538. |
| SHANG Y W, CHENG H G, WANG Y, et al. A pilot study on seawater softening by UF-NF integrated technique[J]. Chemical Industry and Engineering Progress, 2009, 28(3):534-538. | |
| 6 | HARLAND C E. Ion exchange: theory and practice[M]. Royal Society of Chemistry, 1994. |
| 7 | APPLEBAUM S B. Demineralization by ion exchange: in water treatment and chemical processing of other liquids[M]. Elsevier, 2013. |
| 8 | ZAGORODNI A A. Ion exchange materials: properties and applications[M]. Elsevier, 2006. |
| 9 | 钱庭宝,许凤楼. 离子交换树脂的应用技术(Ⅰ)[J]. 化工进展, 1986, 5(4): 48-51. |
| QIAN T B, XU F L. Application technology of ion exchange resin(Ⅰ)[J]. Chemical Industry and Engineering Progress, 1986, 5(4):48-51. | |
| 10 | 钱庭宝,许凤楼. 离子交换树脂的应用技术(Ⅱ)[J]. 化工进展, 1986, 5(5): 44-47. |
| QIAN T B, XU F L. Application technology of ion exchange resin(Ⅱ)[J]. Chemical Industry and Engineering Progress, 1986, 5(5): 44-47. | |
| 11 | 严煦世,范瑾初. 给水工程[M]. 北京: 中国建筑工业出版社, 1999. |
| YAN X S, FAN J C. GEI SHUI GONG CHENG[M]. Beijing: China Construction Industry Press, 1999. | |
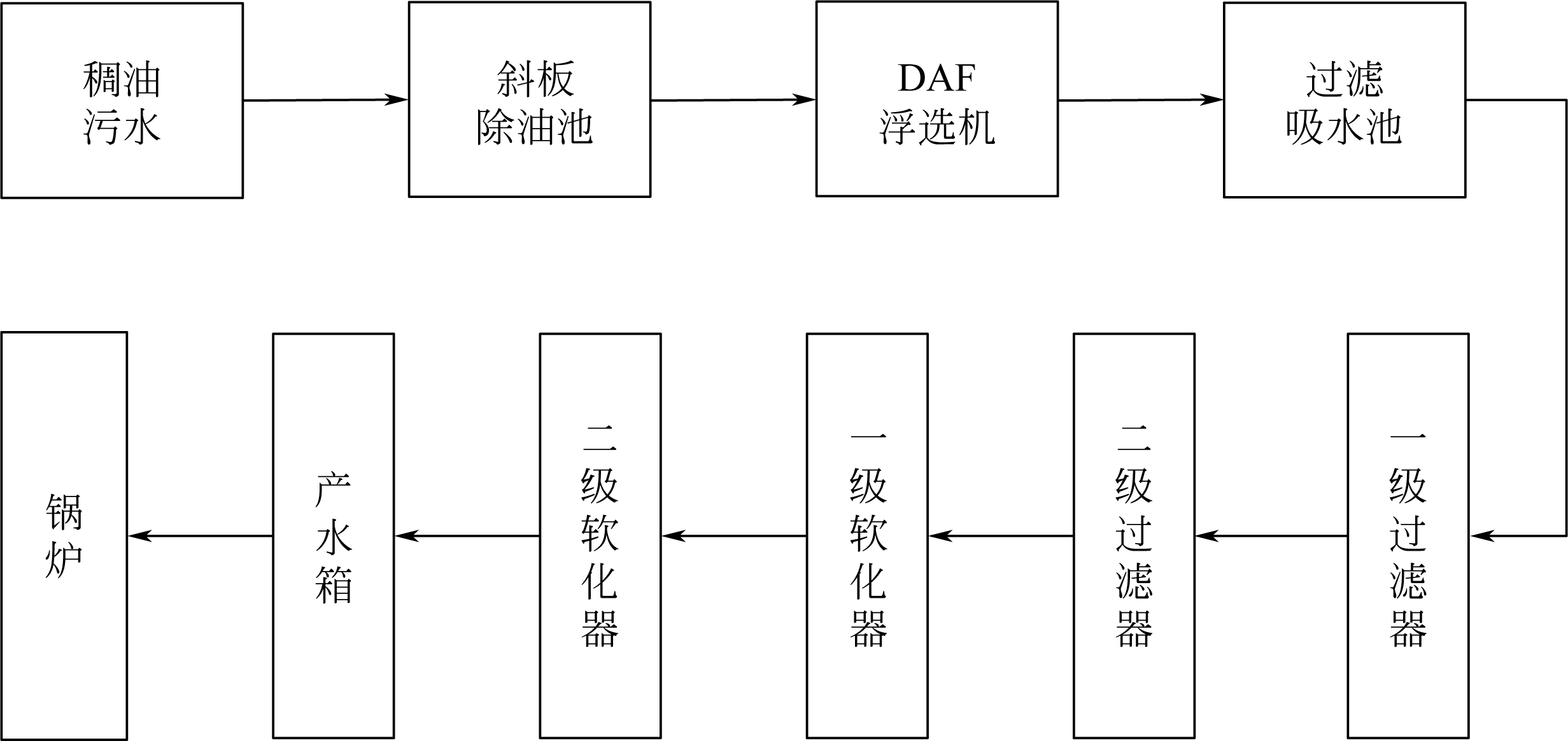
| 12 | 卢建国,王勇. 回用锅炉的热采稠油采出水软化工艺[J]. 油气田环境保护, 1995(3): 53-60. |
| LU J G, WANG Y. Softening process of boiler feed water from produced water of heavy crude thermal recovery[J]. Environmental Protection of Oil & Gas Fields, 1995(3): 53-60. | |
| 13 | YU Z, QI T, QU J, et al. Application of mathematical models for ion-exchange removal of calcium ions from potassium chromate solutions by Amberlite IRC 748 resin in a continuous fixed bed column[J]. Hydrometallurgy, 2015, 158: 165-171. |
| 14 | YU Z, QI T, QU J, et al. Removal of Ca(Ⅱ) and Mg(Ⅱ) from potassium chromate solution on Amberlite IRC 748 synthetic resin by ion exchange[J]. Journal of Hazardous Materials, 2009, 167(1): 406-412. |
| 15 | 黄艳, 章志昕, 韩倩倩, 等. 国内离子交换树脂生产及应用现状与前景[J]. 净水技术, 2010, 29(5): 11-16. |
| HUANG Y, ZHANG Z X, HAN Q Q, et al. Present situation and prospect of production and application of domestic ion exchange resin[J]. Water Purification Technology, 2010, 29(5):11-16. | |
| 16 | DuPont. Data Sheets Download of Ion Exchange Resins[EB/OL]. . |
| 17 | 淄博东大化工股份有限公司. 树脂参数[EB/OL]. . |
| Zibo Dongda Chemical Co., Ltd. Parameters of resin[EB/OL]. . | |
| 18 | 苏青集团. 苏青树脂[EB/OL]. . |
| Group Suqing. Suqing Resins[EB/OL]. . | |
| 19 | 浙江争光实业股份有限公司. 产品中心[EB/OL]. . |
| Ningbo Zhengguang Resin Co., Ltd. Main products[EB/OL]. . | |
| 20 | 三菱化学控股集团. DIAION Product Line Brochure [EB/OL]. . |
| Mitsubishi Chemical Holdings. DIAION Product Line Brochure [EB/OL]. | |
| 21 | LENNTECH. Lanxess Lewatit ion exchange resins[EB/OL]. . |
| 22 | Van der BRUGGEN B, GOOSSENS H, EVERARD P A, et al. Cost-benefit analysis of central softening for production of drinking water[J]. Journal of Environmental Management, 2009, 91(2): 541-549. |
| 23 | GODSKESEN B, HAUSCHILD M, RYGAARD M, et al. Life cycle assessment of central softening of very hard drinking water[J]. Journal of Environmental Management, 2012, 105: 83-89. |
| 24 | COTRUVO J A, BARTRAM J. Calcium and magnesium in drinking-water: public health significance[M]. World Health Organization, 2009. |
| 25 | MENTE A, O'DONNELL M, RANGARAJAN S, et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies[J]. The Lancet, 2016, 388(10043): 465-475. |
| 26 | POMERANZ A, DOLFIN T, KORZETS Z, et al. Increased sodium concentrations in drinking water increase blood pressure in neonates[J]. Journal of Hypertension, 2002, 20(2): 203-207. |
| 27 | BIRNHACK L, KELLER O, TANG S C, et al. A membrane-based recycling process for minimizing environmental effects inflicted by ion-exchange softening applications[J]. Separation and Purification Technology, 2019, 223: 24-30. |
| 28 | LI J, KONER S, GERMAN M, et al. Aluminum-cycle ion exchange process for hardness removal: a new approach for sustainable softening[J]. Environmental Science & Technology, 2016, 50(21): 11943-11950. |
| 29 | BARKER R E, NUTTALL K, MILLAR G J. Softening of coal seam gas associated water with aluminium exchanged resins[J]. Journal of Water Process Engineering, 2018, 21: 27-43. |
| 30 | COMSTOCK S E H, BOYER T H. Combined magnetic ion exchange and cation exchange for removal of DOC and hardness[J]. Chemical Engineering Journal, 2014, 241: 366-375. |
| 31 | APELL J N, BOYER T H. Combined ion exchange treatment for removal of dissolved organic matter and hardness[J]. Water Research, 2010, 44(8): 2419-2430. |
| 32 | SAJJAD A, YUNUS M Y B M, AZODDEIN A A M, et al. Electrodialysis desalination for water and wastewater: a review[J]. Chemical Engineering Journal, 2020, 380: 122231. |
| 33 | HOEK C VAN, KAAKINEN J W, HAUGSETH L A. Ion exchange pretreatment using desalting plant concentrate for regeneration[J]. Desalination, 1976, 19(1): 471-479. |
| 34 | WILF M, KONSTANTIN M, CHENCINSKY A. Evaluation of an ion exchange system regenerated with seawater for the increase of product recovery of reverse osmosis brackish water plant[J]. Desalination, 1980, 34(3): 189-197. |
| 35 | DOBREVSKY I, ZVEZDOV A, DASARE B D, et al. Possibility for selective removal of calcium ions from black sea water using the continuous counter-current ion-exchange softening process with Wofatit KS-10[J]. Journal of Chromatography A, 1980, 201: 377-381. |
| 36 | VERMEULEN T, TLEIMAT B W, KLEIN G. Ion-exchange pretreatment for scale prevention in desalting systems[J]. Desalination, 1983, 47(1/2/3): 149-159. |
| 37 | SHAIN P, KLEIN G, VERMEULEN T. A mathematical model of the cyclic operation of desalination-feedwater softening by ion-exchange with Fluidized-bed regeneration[J]. Desalination, 1988, 69(2): 135-146. |
| 38 | 葛宵. 大孔弱酸氢型树脂Ca2+吸附特性与深度软化处理稠油污水研究[D]. 赣州: 江西理工大学, 2015. |
| GE X. Study on Adsorption Characteristics and Depth Softening Treatment to Viscous Oil Sewage of Macroporous Weak Acid Hydrogen Type Resin[D]. Ganzhou: Jiangxi University of Science and Technology, 2015. | |
| 39 | 王璟, 毛进, 赵剑强, 等. 稠油热采废水回用电站锅炉补给水工艺[J]. 化工进展, 2015, 34(12): 4407-4414. |
| WANG J, MAO J, ZHAO J Q, et al. Process of heavy oil thermal recovery wastewater reused as power plant boiler make-up water[J]. Chemical Industry and Engineering Progress, 2015, 34(12): 4407-4414. | |
| 40 | 许根福, 冯勇, 陆诗洁, 等. 离子交换技术软化处理稠油污水的研究:Ⅰ.离子交换技术方案探讨[J]. 湿法冶金, 2005(3): 159-167. |
| XU G F, FEN Y, LU S J, et al. Softening of thick oil wastewater by ion exchange technique Ⅰ. Discussion on process technology of ion exchange[J]. Hydrometallurgy of China, 2005(3):159-167. | |
| 41 | 许根福, 冯勇, 张国甫, 等. 离子交换技术软化处理稠油污水的研究:Ⅱ. 大孔弱酸性树脂-密实移动床工业装置的试运行[J]. 铀矿冶, 2006(2): 97-102. |
| XU G F, FEN Y, ZHANG G F, et al. Softening of thick oil wastewater by ion exchange technique Ⅱ. The test run of macroporous weak acid resin packed moving bed industrial installation[J]. Uranium Mining and Metallurgy, 2006(2): 97-102. | |
| 42 | 许根福, 汤为龙, 张国甫, 等. 密实移动床离子交换法软化处理稠油污水[J]. 湿法冶金, 2000(2): 57-64. |
| XU G F, TANG W L, ZHANG G F, et al. Softening of oil drilling waste in non-porous moving bed adsorber by Ion exchange[J]. Hydrometallurgy of China, 2000(2): 57-64. | |
| 43 | 詹咏, 亓燕, 董滨, 等. 高含硅稠油废水深度软化树脂中试实验研究[J]. 水资源与水工程学报, 2013, 24(1): 81-83. |
| ZHAN Y, YUAN Y, DONG B, et al. Pilot experimental research on heavy oil waste water contained high silicon depth softening resin[J]. Journal of Water Resources & Water Engineering, 2013, 24(1): 81-83. | |
| 44 | 詹咏, 许颖, 董滨, 等. 树脂对稠油污水中 Ca2+, Mg2+ 的吸附动力学及机理研究[J]. 水资源与水工程学报, 2012(4): 102-106. |
| ZHAN Y, XU Y, DONG B, et al. Study on kinetics and mechanism of adsorption for Ca2+ and Mg2+ in the wastewater by resins[J]. Journal of Water Resources & Water Engineering, 2012(4):102-106. | |
| 45 | 王丽丽. 油田污水回用采暖锅炉软化工艺技术研究[D]. 北京: 中国石油大学, 2008. |
| WANG L L. Research on softening technology in process of reusing oily sewage for heating boiler[D]. Beijing: China University of Petroleum, 2008. | |
| 46 | 费红丽. 国内氯碱行业盐水精制工艺状况调查报告(2016—2017年)[J]. 氯碱工业, 2017, 53(11): 8-13. |
| FEI H L. Survey report of status of brine refining process in China between 2016 and 2017[J]. Chlor-Alkali Industry, 2017, 53(11):8-13. | |
| 47 | 孙俊艳, 张军, 周龙东, 等. 二次盐水精制树脂塔运行周期延长技术的应用[J]. 中国氯碱, 2016(3): 16-19. |
| SUN J Y, ZHANG J, ZHOU L D, et al. Application of the secondary brine refined resin tower running cycle extended technology[J]. China Chlor-Alkali, 2016(3): 16-19. | |
| 48 | 高旭东. 2012年国内氯碱形势综述[J]. 氯碱工业, 2013, 49(6): 1-11. |
| GAO X D. Review on China's chlor-alkali situation in 2012[J]. Chlor-Alkali Industry, 2013, 49(6): 1-11. | |
| 49 | 王强,王靖宇. 用离子交换树脂软化含盐废水试验研究[J]. 湿法冶金, 2020(1): 69-73. |
| WANG Q, WANG J Y. Softening of saline wastewater by ion exchange resin[J]. Hydrometallurgy of China, 2020(1):69-73. | |
| 50 | 王丽, 袁建军. 改性凹凸棒土对钾, 钙, 镁离子交换作用的研究[J]. 中国矿业, 2008, 17(1): 84-88. |
| WANG L, YUAN J J. Study on the Ion exchange of potassium, calcium and magnesium by alter-attapulgite[J]. China Mining Magazine, 2008, 17(1): 84-88. | |
| 51 | 耿卫东, 杨雪静, 杨亚玲. 改性层状结晶硅酸钠对钙镁离子交换的动力学研究[J]. 无机盐工业, 2011, 43(2): 14-16. |
| GENG W D, YANG X J, YANG Y L. Study on exchange kinetics of layered modified sodium silicate crystal to Ca2+/Mg2+[J]. Inorgnic Chemicals Industry, 2011, 43(2): 14-16. | |
| 52 | BEKRI-ABBES I, BAYOUDH S, BAKLOUTI M. The removal of hardness of water using sulfonated waste plastic[J]. Desalination, 2008, 222(1/2/3): 81-86. |
| 53 | 苗威. 羧酸及酰胺类共聚物交联树脂和改性活性炭材料的制备及在硬水软化中的应用[D]. 上海: 上海师范大学, 2018. |
| MIAO W. Preparation of carboxylic acid and amide copolymer cross-linked resin and modified activated carbon material and its application in hard water softening[D]. Shanghai: Shanghai Normal University, 2018. | |
| 54 | 张亚峰, 安路阳, 王宇楠, 等. 水中硬度去除方法研究进展[J]. 煤炭加工与综合利用, 2017(12): 16. |
| ZHANG Y F, AN L Y, WANG Y N, et al. Research progress of water hardness removal methods[J]. Coal Processing & Comprehensive Utilization, 2017(12):16. | |
| 55 | FLODMAN H R, DVORAK B I. Brine reuse in ion-exchange softening: salt discharge, hardness leakage, and capacity tradeoffs[J]. Water Environment Research, 2012, 84(6): 535-543. |
| 56 | MICARI M, CIPOLLINA A, TAMBURINI A, et al. Combined membrane and thermal desalination processes for the treatment of ion exchange resins spent brine[J]. Applied Energy, 2019, 254: 113699. |
| 57 | CHANDRASEKARA N P G N, PASHLEY R M. Study of a new process for the efficient regeneration of ion exchange resins[J]. Desalination, 2015, 357: 131-139. |
| 58 | 金欣荻, 李天均, 颜亦磊, 等. 离子交换树脂的电再生机理研究[J]. 工业水处理, 2019, 39(2): 81-85. |
| JIN X D, LI T J, YAN Y L, et al. Research on the electrical regeneration mechanisms of ion exchange resins[J]. Industrial Water Treatment, 2019, 39(2): 81-85. | |
| 59 | 王方, 王明亚, 王明太. 离子交换树脂电再生技术的研究新进展[J]. 离子交换与吸附, 2015, 31(2): 187-192. |
| WANG F, WANG M Y, WANG M T. New research progress on electric regeneration of ion exchange resins.[J] Ion Exchange and Adsorption, 2015, 31(2):187-192. | |
| 60 | HU J, CHEN Y, GUO L, et al. Chemical-free ion exchange and its application for desalination[J]. Desalination, 2015, 365: 144-150. |
| [1] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [2] | SONG Weitao, SONG Huiping, FAN Zhenlian, FAN Biao, XUE Fangbin. Research progress of fly ash in anti-corrosion coatings [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4894-4904. |
| [3] | FENG Jianghan, SONG Fang. Research progress of anion exchange membrane water electrolysis cells [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3501-3509. |
| [4] | YANG Hongmei, GAO Tao, YU Tao, QU Chengtun, GAO Jiapeng. Treatment of refractory organics sulfonated phenolic resin with ferrate [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3302-3308. |
| [5] | HE Yang, LI Siying, LI Chuanqiang, YUAN Xiaoya, ZHENG Xuxu. Anticorrosion performance of thermal reduction graphene oxide /epoxy resin composite coating [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1983-1994. |
| [6] | ZHAO Wangrui, LIU Yan, ZHANG Wei, DENG Huining. Fe3+ ions induced rapid electrodeposition of polydopamine-polyethyleneimine for monovalent selective membrane fabrication [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1508-1514. |
| [7] | ZHANG Yingjie, LU Jiayue, WANG Fanggang. Synthesis of a new MCER and its performance in removing Cu(Ⅱ) from water [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5558-5566. |
| [8] | LIU Yulong, HU Nan, CHEN Xiangbiao, CHEN Sencai, ZENG Bingyong, DING Dexin. Circulating adsorption-desorption properties and kinetic analysis of uranium by strong basic anion resins [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5574-5583. |
| [9] | TIAN Yazhou, HU Yujing, LI Jiyou, REN Jiangyan, WANG Liwei, WANG Xiuli, DING Ying, CHENG Jue, ZHANG Junying. Synthesis, curing kinetics and properties of vanilla alcohol-based epoxy resin [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 477-484. |
| [10] | ZHANG Hongming, LU Jiongyuan, WANG Sanfan. Research progress on molecular structure of anion exchange membrane for fuel cells [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 318-330. |
| [11] | LIU Dachen, DU Minghui, WANG Heng. Bromination modification of phenolic hydroxyl sites of crosslinked teroctyl phenolic resin [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 382-388. |
| [12] | GUO Rui, LI Ping’an, ZHAO Yunfei. Synthesis and performance of silicon modified BPA-PA phenolic epoxy resin conductive adhesive [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4473-4480. |
| [13] | ZU Liwu, BI Ying, ZHAO Binhui, LI Jidong, YANG Qing, CONG Shanshan. Research progress in benzoxazine resin [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4224-4240. |
| [14] | LI Zhibin, TANG Hui, LUO Dawei, YING Qiao. Progress in chemical recycling of waste PET and preparation of unsaturated polyester resins [J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3279-3292. |
| [15] | CAI Sichao, ZHOU Jing, DU Jinze, LI Fangzhou, LI Yuansen, HE Lin, LI Xingang, WANG Chengyang. Process analysis of resource utilization of phenol-based distillation residue from coal chemical industry [J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3360-3371. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||