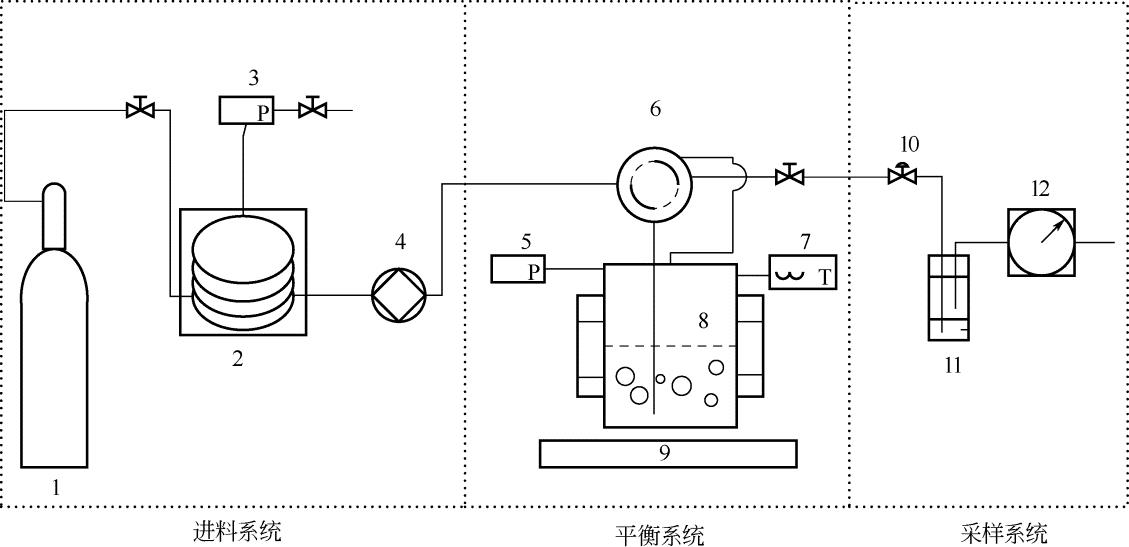
Chemical Industry and Engineering Progress ›› 2019, Vol. 38 ›› Issue (04): 1662-1670.DOI: 10.16085/j.issn.1000-6613.2018-0468
• Chemical processes and equipment • Previous Articles Next Articles
Phase equilibrium for binary system of diphenyl ether-supercritical carbon dioxide
Bowen DU( ),Kang CHEN,Xin DING,Zhao JIANG,Tao FANG(
),Kang CHEN,Xin DING,Zhao JIANG,Tao FANG( )
)
- School of Chemical Engineering and Technology, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
-
Received:2018-03-07Revised:2018-04-23Online:2019-04-05Published:2019-04-05 -
Contact:Tao FANG
超临界二氧化碳与二苯醚相平衡研究
- 西安交通大学化学工程与技术学院,陕西 西安 710049
-
通讯作者:方涛 -
作者简介:杜博文(1993—),男,硕士研究生,研究方向为超临界流体。E-mail:<email>18182428965@163.com</email>。|方涛,教授,博士生导师,研究方向为生物质能、超临界流体、化学储氢、微纳米材料。E-mail:<email>taofang@mail.xjtu.edu.cn</email>。 -
基金资助:陕能源化工过程强化重点实验室项目(20160109-4)
CLC Number:
Cite this article
Bowen DU, Kang CHEN, Xin DING, Zhao JIANG, Tao FANG. Phase equilibrium for binary system of diphenyl ether-supercritical carbon dioxide[J]. Chemical Industry and Engineering Progress, 2019, 38(04): 1662-1670.
杜博文, 陈康, 丁鑫, 姜召, 方涛. 超临界二氧化碳与二苯醚相平衡研究[J]. 化工进展, 2019, 38(04): 1662-1670.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2018-0468
| 序号 | 仪器名称和型号 | 生产厂家 |
|---|---|---|
| 1 | 普析G5气相色谱仪 | 北京普析通用仪器有限责任公司 |
| 2 | CP系列先行者TM电子天平 | 奥豪斯仪器(上海)有限公司 |
| 3 | 湿式气体流量计 | 天津市泰斯仪器有限公司 |
| 序号 | 仪器名称和型号 | 生产厂家 |
|---|---|---|
| 1 | 普析G5气相色谱仪 | 北京普析通用仪器有限责任公司 |
| 2 | CP系列先行者TM电子天平 | 奥豪斯仪器(上海)有限公司 |
| 3 | 湿式气体流量计 | 天津市泰斯仪器有限公司 |
| 序号 | 试剂名称 | 规格 | 生产厂家 |
|---|---|---|---|
| 1 | 二氧化碳 | 分析纯 | 西安泰达低温设备有限公司气体厂 |
| 2 | 无水乙醇 | 分析纯 | 国药集团化学试剂有限公司 |
| 3 | 二苯醚 | ≥98% | 阿拉丁科技(中国)有限公司 |
| 序号 | 试剂名称 | 规格 | 生产厂家 |
|---|---|---|---|
| 1 | 二氧化碳 | 分析纯 | 西安泰达低温设备有限公司气体厂 |
| 2 | 无水乙醇 | 分析纯 | 国药集团化学试剂有限公司 |
| 3 | 二苯醚 | ≥98% | 阿拉丁科技(中国)有限公司 |
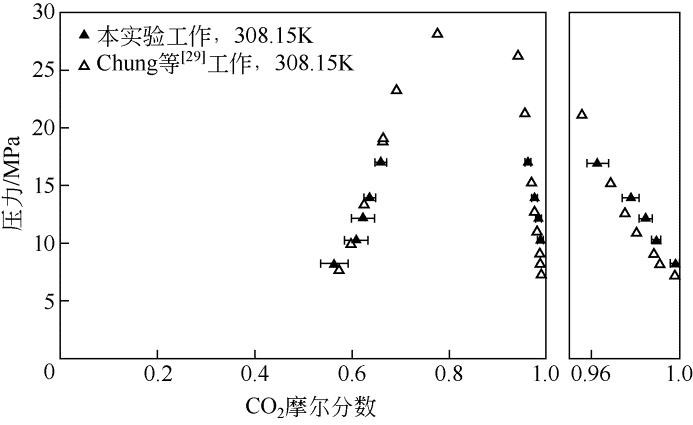
| 数据来源 | 压力 /MPa | x 1 | Sx /% | 压力 /MPa | y 1 | Sy /% |
|---|---|---|---|---|---|---|
| Chung等[ | 7.81 | 0.571 | — | 7.39 | 0.9982 | — |
| 9.89 | 0.599 | — | 8.32 | 0.9911 | — | |
| 13.52 | 0.625 | — | 9.13 | 0.9885 | — | |
| 19.04 | 0.665 | — | 11.09 | 0.9807 | — | |
| 19.26 | 0.665 | — | 12.75 | 0.9751 | — | |
| 23.40 | 0.692 | — | 15.40 | 0.9687 | — | |
| 28.25 | 0.776 | — | 17.69 | 0.9838 | — | |
| 21.33 | 0.9560 | — | ||||
| 26.39 | 0.9419 | — | ||||
| 本文测定 | 8.3 | 0.5646 | 2.82 | 8.3 | 0.9994 | 0.03 |
| 10.3 | 0.60875 | 2.36 | 10.3 | 0.9897 | 0.02 | |
| 12.2 | 0.6228 | 2.49 | 12.2 | 0.9849 | 0.03 | |
| 14.0 | 0.6361 | 1.13 | 14.0 | 0.9779 | 0.04 | |
| 17.0 | 0.6598 | 1.03 | 17.0 | 0.9628 | 0.05 |
| 数据来源 | 压力 /MPa | x 1 | Sx /% | 压力 /MPa | y 1 | Sy /% |
|---|---|---|---|---|---|---|
| Chung等[ | 7.81 | 0.571 | — | 7.39 | 0.9982 | — |
| 9.89 | 0.599 | — | 8.32 | 0.9911 | — | |
| 13.52 | 0.625 | — | 9.13 | 0.9885 | — | |
| 19.04 | 0.665 | — | 11.09 | 0.9807 | — | |
| 19.26 | 0.665 | — | 12.75 | 0.9751 | — | |
| 23.40 | 0.692 | — | 15.40 | 0.9687 | — | |
| 28.25 | 0.776 | — | 17.69 | 0.9838 | — | |
| 21.33 | 0.9560 | — | ||||
| 26.39 | 0.9419 | — | ||||
| 本文测定 | 8.3 | 0.5646 | 2.82 | 8.3 | 0.9994 | 0.03 |
| 10.3 | 0.60875 | 2.36 | 10.3 | 0.9897 | 0.02 | |
| 12.2 | 0.6228 | 2.49 | 12.2 | 0.9849 | 0.03 | |
| 14.0 | 0.6361 | 1.13 | 14.0 | 0.9779 | 0.04 | |
| 17.0 | 0.6598 | 1.03 | 17.0 | 0.9628 | 0.05 |
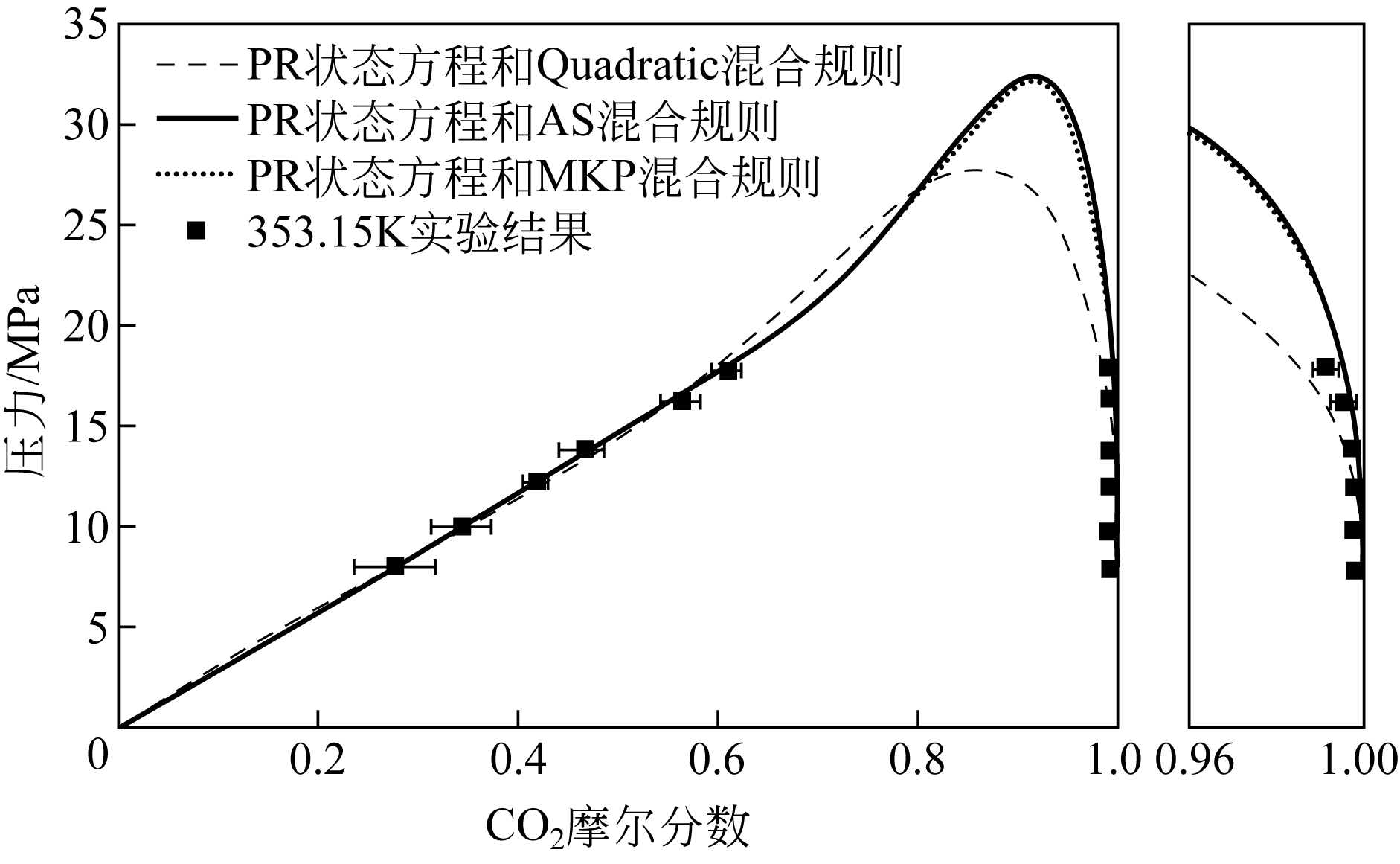
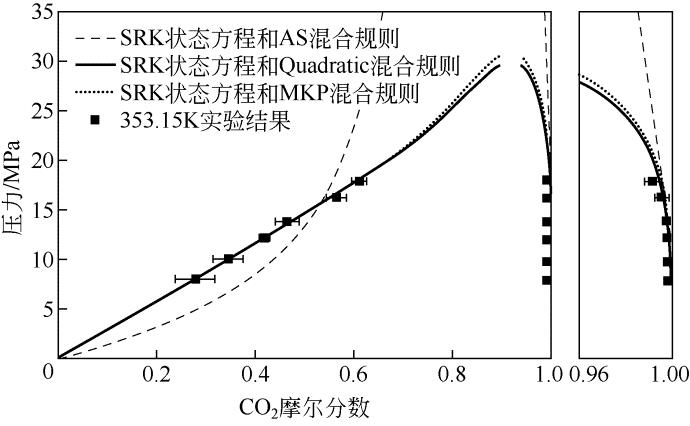
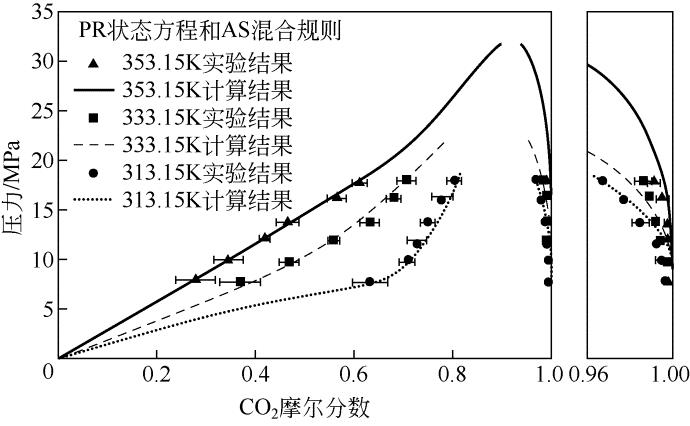
| 温度/K | 压力/MPa | x 1 | Sx /% | y 1/% | Sy /% |
|---|---|---|---|---|---|
| 353.15 | 8.0 | 0.2765 | 8.36 | 0.9994 | 0.04 |
| 10.0 | 0.3433 | 6.67 | 0.9991 | 0.02 | |
| 12.2 | 0.4171 | 1.55 | 0.9983 | 0.01 | |
| 13.8 | 0.4636 | 2.56 | 0.9976 | 0.02 | |
| 16.2 | 0.5628 | 2.42 | 0.9954 | 0.03 | |
| 17.8 | 0.6088 | 2.06 | 0.9913 | 0.03 | |
| 333.15 | 7.8 | 0.3671 | 7.75 | 0.9996 | 0.04 |
| 9.8 | 0.4655 | 4.03 | 0.9985 | 0.02 | |
| 12.0 | 0.5556 | 2.49 | 0.9935 | 0.02 | |
| 13.8 | 0.6296 | 3.53 | 0.9873 | 0.02 | |
| 16.4 | 0.6785 | 2.78 | 0.9861 | 0.01 | |
| 18.0 | 0.7039 | 3.37 | 0.9818 | 0.04 | |
| 313.15 | 7.8 | 0.6298 | 6.63 | 0.9982 | 0.02 |
| 10.0 | 0.7064 | 3.14 | 0.9949 | 0.03 | |
| 11.6 | 0.7239 | 3.55 | 0.9928 | 0.02 | |
| 13.8 | 0.7463 | 2.96 | 0.9820 | 0.04 | |
| 16.0 | 0.7757 | 4.63 | 0.9769 | 0.01 | |
| 18.0 | 0.8002 | 3.03 | 0.9669 | 0.02 |
| 温度/K | 压力/MPa | x 1 | Sx /% | y 1/% | Sy /% |
|---|---|---|---|---|---|
| 353.15 | 8.0 | 0.2765 | 8.36 | 0.9994 | 0.04 |
| 10.0 | 0.3433 | 6.67 | 0.9991 | 0.02 | |
| 12.2 | 0.4171 | 1.55 | 0.9983 | 0.01 | |
| 13.8 | 0.4636 | 2.56 | 0.9976 | 0.02 | |
| 16.2 | 0.5628 | 2.42 | 0.9954 | 0.03 | |
| 17.8 | 0.6088 | 2.06 | 0.9913 | 0.03 | |
| 333.15 | 7.8 | 0.3671 | 7.75 | 0.9996 | 0.04 |
| 9.8 | 0.4655 | 4.03 | 0.9985 | 0.02 | |
| 12.0 | 0.5556 | 2.49 | 0.9935 | 0.02 | |
| 13.8 | 0.6296 | 3.53 | 0.9873 | 0.02 | |
| 16.4 | 0.6785 | 2.78 | 0.9861 | 0.01 | |
| 18.0 | 0.7039 | 3.37 | 0.9818 | 0.04 | |
| 313.15 | 7.8 | 0.6298 | 6.63 | 0.9982 | 0.02 |
| 10.0 | 0.7064 | 3.14 | 0.9949 | 0.03 | |
| 11.6 | 0.7239 | 3.55 | 0.9928 | 0.02 | |
| 13.8 | 0.7463 | 2.96 | 0.9820 | 0.04 | |
| 16.0 | 0.7757 | 4.63 | 0.9769 | 0.01 | |
| 18.0 | 0.8002 | 3.03 | 0.9669 | 0.02 |
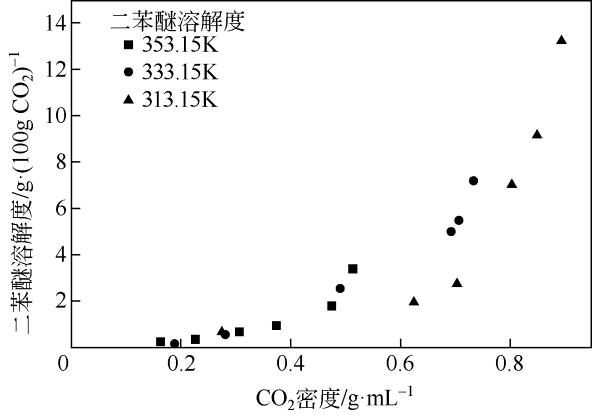
| 温度/K | 压力 /MPa | CO2密度 /g·mL-1 | 二苯醚溶解度 /g·(100g CO2)-1 |
|---|---|---|---|
| 353.15 | 8.0 | 0.1640 | 0.2322 |
| 10.0 | 0.2266 | 0.3484 | |
| 12.2 | 0.3078 | 0.6586 | |
| 13.8 | 0.3731 | 0.9304 | |
| 16.2 | 0.4764 | 1.7873 | |
| 17.8 | 0.5138 | 3.3940 | |
| 333.15 | 7.8 | 0.1884 | 0.1547 |
| 9.8 | 0.2820 | 0.5810 | |
| 12.0 | 0.4918 | 2.5304 | |
| 13.8 | 0.6926 | 4.9752 | |
| 16.4 | 0.7080 | 5.4519 | |
| 18.0 | 0.7350 | 7.1698 | |
| 313.15 | 7.8 | 0.2753 | 0.6974 |
| 10.0 | 0.6259 | 1.9826 | |
| 11.6 | 0.7051 | 2.8049 | |
| 13.8 | 0.8052 | 7.0895 | |
| 16.0 | 0.8512 | 9.1457 | |
| 18.0 | 0.8949 | 13.2405 |
| 温度/K | 压力 /MPa | CO2密度 /g·mL-1 | 二苯醚溶解度 /g·(100g CO2)-1 |
|---|---|---|---|
| 353.15 | 8.0 | 0.1640 | 0.2322 |
| 10.0 | 0.2266 | 0.3484 | |
| 12.2 | 0.3078 | 0.6586 | |
| 13.8 | 0.3731 | 0.9304 | |
| 16.2 | 0.4764 | 1.7873 | |
| 17.8 | 0.5138 | 3.3940 | |
| 333.15 | 7.8 | 0.1884 | 0.1547 |
| 9.8 | 0.2820 | 0.5810 | |
| 12.0 | 0.4918 | 2.5304 | |
| 13.8 | 0.6926 | 4.9752 | |
| 16.4 | 0.7080 | 5.4519 | |
| 18.0 | 0.7350 | 7.1698 | |
| 313.15 | 7.8 | 0.2753 | 0.6974 |
| 10.0 | 0.6259 | 1.9826 | |
| 11.6 | 0.7051 | 2.8049 | |
| 13.8 | 0.8052 | 7.0895 | |
| 16.0 | 0.8512 | 9.1457 | |
| 18.0 | 0.8949 | 13.2405 |
| 状态方程 | 混合规则 | 交互参数个数 | 优化后的参数 | Δx 1 /% | Δy 1 /% | ||
|---|---|---|---|---|---|---|---|
| k 1,2 | l 1,2 | λ 1,2 | |||||
| PR | Quadratic | 2 | 0.0670 | ?0.056 | — | 6.05 | 1.28 |
| Adachi-Sugie | 3 | 1.4667 | 0.7892 | ?1.0467 | 4.86 | 0.89 | |
| Mathias-Klotz-Prausnitz | 3 | 1.4709 | 0.7922 | 2.0988 | 4.89 | 0.97 | |
| SRK | Quadratic | 2 | 0.1502 | 0.0471 | — | 51.61 | 1.03 |
| Adachi-Sugie | 3 | 1.4449 | 0.7498 | ?1.0597 | 4.90 | 0.87 | |
| Mathias-Klotz-Prausnitz | 3 | 1.5036 | 0.7832 | 2.2060 | 4.96 | 0.96 | |
| 状态方程 | 混合规则 | 交互参数个数 | 优化后的参数 | Δx 1 /% | Δy 1 /% | ||
|---|---|---|---|---|---|---|---|
| k 1,2 | l 1,2 | λ 1,2 | |||||
| PR | Quadratic | 2 | 0.0670 | ?0.056 | — | 6.05 | 1.28 |
| Adachi-Sugie | 3 | 1.4667 | 0.7892 | ?1.0467 | 4.86 | 0.89 | |
| Mathias-Klotz-Prausnitz | 3 | 1.4709 | 0.7922 | 2.0988 | 4.89 | 0.97 | |
| SRK | Quadratic | 2 | 0.1502 | 0.0471 | — | 51.61 | 1.03 |
| Adachi-Sugie | 3 | 1.4449 | 0.7498 | ?1.0597 | 4.90 | 0.87 | |
| Mathias-Klotz-Prausnitz | 3 | 1.5036 | 0.7832 | 2.2060 | 4.96 | 0.96 | |
| 温度/K | k | l | λ | Δy 1/% | Δx 1/% |
|---|---|---|---|---|---|
| 313.15 | 1.6097 | 0.8897 | ?1.127817 | 4.76 | 1.02 |
| 333.15 | ?0.0410 | ?0.1187 | 0.0815 | 4.77 | 1.09 |
| 353.15 | 1.4667 | 0.7892 | ?1.0467 | 4.86 | 0.95 |
| 温度/K | k | l | λ | Δy 1/% | Δx 1/% |
|---|---|---|---|---|---|
| 313.15 | 1.6097 | 0.8897 | ?1.127817 | 4.76 | 1.02 |
| 333.15 | ?0.0410 | ?0.1187 | 0.0815 | 4.77 | 1.09 |
| 353.15 | 1.4667 | 0.7892 | ?1.0467 | 4.86 | 0.95 |
| a | —— | 状态方程的能量参数 |
| a 0 | —— | 能量项的参数 |
| B | —— | 状态方程的体积参数 |
| c 1 | —— | 能量项的参数 |
| kij | —— | 二元交互因子 |
| lij | —— | 二元交互因子 |
| p | —— | 压力,MPa |
| p c | —— | 临界压力,MPa |
| R | —— | 气体常数,J/(mol·K) |
| T | —— | 温度,K |
| T c | —— | 临界温度,K |
| T r | —— | 对比温度,K |
| v | —— | 摩尔体积,m3/mol |
| x | —— | 液相摩尔分数 |
| Δx | —— | 液相标准误差 |
| y | —— | 气相摩尔分数 |
| Δy | —— | 气相标准误差 |
| γ | —— | 逸度系数 |
| λij | —— | 二元交互因子 |
| ρ | —— | 密度 |
| ω | —— | 偏心因子 |
| 下角标 | ||
| c | —— | 临界值 |
| i | —— | 组分指数 |
| j | —— | 组分指数 |
| r | —— | 对比 |
| 缩写 | ||
| AS | —— | Adachi-Sugie |
| EOS | —— | Equation of State状态方程 |
| MKP | —— | Mathias-Klotz-Prausnitz |
| PR | —— | Peng-Robinson |
| Q | —— | Quadratic 二次型 |
| SRK | —— | Soave-Redlich-Kwong |
| a | —— | 状态方程的能量参数 |
| a 0 | —— | 能量项的参数 |
| B | —— | 状态方程的体积参数 |
| c 1 | —— | 能量项的参数 |
| kij | —— | 二元交互因子 |
| lij | —— | 二元交互因子 |
| p | —— | 压力,MPa |
| p c | —— | 临界压力,MPa |
| R | —— | 气体常数,J/(mol·K) |
| T | —— | 温度,K |
| T c | —— | 临界温度,K |
| T r | —— | 对比温度,K |
| v | —— | 摩尔体积,m3/mol |
| x | —— | 液相摩尔分数 |
| Δx | —— | 液相标准误差 |
| y | —— | 气相摩尔分数 |
| Δy | —— | 气相标准误差 |
| γ | —— | 逸度系数 |
| λij | —— | 二元交互因子 |
| ρ | —— | 密度 |
| ω | —— | 偏心因子 |
| 下角标 | ||
| c | —— | 临界值 |
| i | —— | 组分指数 |
| j | —— | 组分指数 |
| r | —— | 对比 |
| 缩写 | ||
| AS | —— | Adachi-Sugie |
| EOS | —— | Equation of State状态方程 |
| MKP | —— | Mathias-Klotz-Prausnitz |
| PR | —— | Peng-Robinson |
| Q | —— | Quadratic 二次型 |
| SRK | —— | Soave-Redlich-Kwong |
| 1 | BRUNNER G . Supercritical process technology related to energy and future directions — An introduction[J]. Journal of Supercritical Fluids, 2015, 96: 11-20. |
| 2 | SAVAGE P E . Organic chemical reactions in supercritical water[J]. Chemical Reviews, 1999, 99(2): 603-621. |
| 3 | GOTO M , NADA T , KAWAJIRI S , et al . Decomposition of municipal sludge by supercritical water oxidation[J]. Journal of Chemical Engineering of Japan, 1997, 30(5): 813-818. |
| 4 | GOTO M , NADA T , OGATA A , et al . Supercritical water oxidation for the destruction of municipal excess sludge and alcohol distillery wastewater of molasses[J]. Journal of Supercritical Fluids, 1998, 13(1/2/3): 277-282. |
| 5 | WAHYUDIONO, SASAKI M , GOTO M . Kinetic study for liquefaction of tar in sub- and supercritical water[J]. Polymer Degradation and Stability, 2008, 93(6): 1194-1204. |
| 6 | WAHYUDIONO, FUJINAGA S , SASAKI M , et al . Recovery of phenol through the decomposition of tar under hydrothermal alkaline conditions[J]. Chemical Engineering & Technology, 2006, 29(7): 882-889. |
| 7 | NICOLAOU K C , BODDY C N C , BRASE S , et al . Chemistry, biology, and medicine of the glycopeptide antibiotics[J]. Angewandte Chemie: International Edition, 1999, 38(15): 2096-2152. |
| 8 | AZPIROZ M D G , BLANCO C G , BANCIELLA C . The use of solvents for purifying industrial naphthalene from coal tar distilled oils[J]. Fuel Processing Technology, 2008, 89(2): 111-117. |
| 9 | YAMAMOTO Y , SATO Y , EBINA T , et al . Separation of high-purity indole from coal-tar by high-pressure crystallization[J]. Fuel, 1991, 70(4): 565-566. |
| 10 | 王汝成, 王宁波, 王明峰, 等 . 中低温煤焦油中酚类化合物的柱层析分离[J]. 煤化工, 2013, 41(6): 53-56. |
| WANG R C , WANG N B , WANG M F , et al . Column chromatography isolation of phenolic compounds in the low temperature coal tar[J]. Coal Chemical Industry, 2013, 41(6): 53-56. | |
| 11 | 姜广策, 张生娟, 王永刚, 等 . 低温煤焦油中特定芳烃组分的选择性分离[J]. 化工学报, 2015, 66(6): 2131-2138. |
| JIANG G C , ZHANG S J , WANG Y G , et al . Selective separation of aromatic hydrocarbons from low temperature coal tar[J]. CIESC Journal, 2015, 66(6): 2131-2138. | |
| 12 | 张生娟, 高亚男, 李晓宏, 等 . 煤焦油组分分离与分析技术研究进展[J]. 煤化工, 2017, 45(1): 45-49. |
| ZHANG S J , GAO Y N , LI X H , et al . Research progress of the separation and analysis technology of coal tar composition[J]. Coal Chemical Industry, 2017, 45(1): 45-49. | |
| 13 | ASIABI H , YAMINI Y , LATIFEH F , et al . Solubilities of four macrolide antibiotics in supercritical carbon dioxide and their correlations using semi-empirical models[J]. Journal of Supercritical Fluids, 2015, 104: 62-69. |
| 14 | CELIK H T , GURU M . Extraction of oil and silybin compounds from milk thistle seeds using supercritical carbon dioxide[J]. Journal of Supercritical Fluids, 2015, 100: 105-109. |
| 15 | MARKOVI Z , MARKOVI S , ENGELBRECHT J P , et al . Extraction of coal-tar pitch by supercritical carbon dioxide. Dependence of chemical composition of the extracts on temperature, pressure and extraction time[J]. South African Journal of Chemistry-Suid-Afrikaanse Tydskrif Vir Chemie, 2000, 53: 1-12. |
| 16 | KWIATKOWSKI J , LISICKI Z , MAJEWSKI W . An experimental-method for measuring solubilities of solids in supercritical fluids[J]. Berichte Der Bunsen-Gesellschaft-Physical Chemistry Chemical Physics, 1984, 88(9): 865-869. |
| 17 | GOODARZNIA I , ESMAEILZADEH F . Solubility of an anthracene, phenanthrene, and carbazole mixture in supercritical carbon dioxide[J]. Journal of Chemical & Engineering Data, 2002, 47(2): 333-338. |
| 18 | BAGHERI H , ABDUL MANAP M Y B , SOLATI Z . Response surface methodology applied to supercritical carbon dioxide extraction of Piper nigrum L. essential oil[J]. LWT—Food Science and Technology, 2014, 57(1): 149-155. |
| 19 | 陈华, 骆赞椿, 章寿华, 等 . 混合规则对超临界流体相平衡计算的影响——低挥发性液体体系[J]. 华东化工学院学报, 1993(3): 280-284. |
| CHEN H , LUO Z C , ZHANG S H , et al . Effects of mixing rules on phase equilibrium calculations for supercritical fluid—Low volatile liquid systems[J]. Journal of East China Institute of Chemical Technology, 1993(3): 280-284. | |
| 20 | LUCAS M A , BORGES G R , ROCHA I C C DA , et al . Use of real crude oil fractions to describe the high pressure phase behavior of crude oil in carbon dioxide[J]. Journal of Supercritical Fluids, 2016, 118: 140-147. |
| 21 | AUGELLETTI R , FRATTARI S , GIRONI F , et al . Phase equilibria and thermodynamic modeling of systems CO2 - bergamot oil and CO2 - linalyl acetate[J]. Journal of Supercritical Fluids, 2016, 116: 1-9. |
| 22 | PENG D , ROBINSON D B . A new two-constant equation of state[J]. Industrial & Engineering Chemistry Fundamentals, 1976, 15(1): 59-64. |
| 23 | VALDERRAMA J O . The state of the cubic equations of state[J]. Industrial & Engineering Chemistry Research, 2003, 42(8): 1603-1618. |
| 24 | COSTA G M N , CARDOSO S G , SOARES R O , et al . Modeling high pressure vapor-liquid equilibrium of ternary systems containing supercritical CO2 and mixed organic solvents using Peng-Robinson equation of state[J]. Journal of Supercritical Fluids, 2014, 93: 82-90. |
| 25 | HIGASHI H , FURUYA T , ISHIDAO T , et al . An exponent-type mixing rule for energy parameters[J]. Journal of Chemical Engineering of Japan, 1994, 27(5): 677-679. |
| 26 | ADACHI Y , SUGIE H . A new mixing rule modified conventional mixing rule[J]. Fluid Phase Equilibria, 1986, 28(2): 103-118. |
| 27 | FANG T , GOTO M , SASAKI M , et al . Phase equilibria for the ternary system methyl oleate + tocopherol + supercritical CO2 [J]. Journal of Chemical & Engineering Data, 2005, 50(2): 390-397. |
| 28 | MEIER U , GROSS F , TREPP C . High pressure phase equilibrium studies for the carbon dioxide/α-tocopherol (vitamin E) system[J]. Fluid Phase Equilibria, 1994, 92: 289-302. |
| 29 | CHUNG S T , SHING K S . Multiphase behavior of binary and ternary systems of heavy aromatic hydrocarbons with supercritical carbon dioxide: Part I. Experimental results[J]. Fluid Phase Equilibria, 1992, 81: 321-341. |
| 30 | 陈华, 骆赞椿, 章寿华, 等 . 超临界二氧化碳萃取低挥发性液体混合物的相平衡[J]. 高校化学工程学报, 1994(1): 46-54. |
| CHEN H , LUO Z C , ZHANG S H , et al . Extraction of low volatility liquid mixtures with supercritical carbon dioxide[J]. Journal of Chemical Engineering of Chinese Universities, 1994(1): 46-54. | |
| 31 | LATSKY C , KOUAKOU A C , SCHWARZ C E . Phase equilibria of CO2 with components in the light naphtha cut of tyre derived oil[J]. Journal of Supercritical Fluids, 2018, 131: 58-65. |
| 32 | KORDIKOWSKI A , SCHNEIDER G M . Fluid phase equilibria of binary and ternary mixtures of supercritical carbon dioxide with low-volatility organic substances up to 100 MPa and 393 K[J]. Fluid Phase Equilibria, 1993, 90(1): 149-162. |
| 33 | PENG D Y , ROBINSON D B . A new two-constant equation of state[J]. Industrial and Engineering Chemistry, Fundamentals, 1976, 15(1): 59-64. |
| 34 | ADACHI Y , SUGIE H . A new mixing rule — Modified conventional mixing rule[J]. Fluid Phase Equilibria, 1986, 28(2): 103-118. |
| 35 | MATHIAS P M , KLOTZ H C , PRAUSNITZ J M . Equation-of-State mixing rules for multicomponent mixtures: the problem of invariance[J]. Fluid Phase Equilibria, 1991, 67: 31-44. |
| 36 | WEBER W , PETKOV S , BRUNNER G . Vapour-liquid-equilibria and calculations using the Redlich-Kwong-Aspen-equation of state for tristearin, tripalmitin, and triolein in CO2 and propane[J]. Fluid Phase Equilibria, 1999, 158/159/160: 695-706. |
| [1] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [2] | LUO Cheng, FAN Xiaoyong, ZHU Yonghong, TIAN Feng, CUI Louwei, DU Chongpeng, WANG Feili, LI Dong, ZHENG Hua’an. CFD simulation of liquid distribution in different distributors in medium-low temperature coal tar hydrogenation reactor [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4538-4549. |
| [3] | TAN Lipeng, SHEN Jun, WANG Yugao, LIU Gang, XU Qingbai. Research progress on blending modification of coal tar pitch and petroleum asphalt [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3749-3759. |
| [4] | XU Xian, CUI Louwei, LIU Jie, SHI Junhe, ZHU Yonghong, LIU Jiaojiao, LIU Tao, ZHENG Hua’an, LI Dong. Effect of raw material composition on the development of semicoke mesophase structure [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2343-2352. |
| [5] | ZHANG Lele, QIAN Yundong, ZHU Huatong, FENG Silong, YANG Xiuna, YU Ying, YANG Qiang, LU Hao. Study on optimization limits of dehydration and desalination pretreatment of hydrogenated coal tar [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2298-2305. |
| [6] | YANG Xin, XU Hong, HU Weixun, LIU Hongzuo, LONG Quanzhi, ZHU Liye. Regeneration of waste lubricant oil by supercritical carbon dioxide extraction [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5399-5405. |
| [7] | YUE Zihan, LONG Zhen, ZHOU Xuebing, ZANG Xiaoya, LIANG Deqing. State of the art on hydrogen storage of sⅡ clathrate hydrate [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5121-5134. |
| [8] | JIA Wenlong, SONG Shuoshuo, LI Changjun, WU Xia, YANG Fan, ZHANG Yuanrui. Progress of oily sludge extraction by supercritical CO2 [J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6573-6585. |
| [9] | YANG Yongbin, DONG Yinrui, ZHONG Qiang, LI Qian, WANG Lin, JIANG Tao. Application and research progress of carbonization consolidation of high temperature coal tar pitch binder in formed carbon material [J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6419-6429. |
| [10] | WANG Yiwei, LIU Zhiqi, SUN Qiang, LIU Aixian, YANG Lanying, GONG Jing, GUO Xuqiang. Thermodynamics and kinetics of structure Ⅰ hydrate formation in presence of poly(sodium 4-styrenesulfonate) [J]. Chemical Industry and Engineering Progress, 2021, 40(S1): 168-181. |
| [11] | WANG Junliang, YANG Lili, LIN Chunmian, PAN Zhiyan. Research progress of supercritical carbon dioxide in chemical reactions [J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4127-4134. |
| [12] | Wenliang HAN, Yu LIU. Spatiotemporal differentiation of total organic carbon and black carbon in sediments of urban water source reservoir and its inflowing river: impacts on polybrominated diphenyl ethers [J]. Chemical Industry and Engineering Progress, 2021, 40(2): 1085-1096. |
| [13] | YANG Dong, CHEN Lin. Visualization of transient density field in multiphase jet flow under transcritical/supercritical conditions [J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6432-6440. |
| [14] | Zhenshuai WANG, Baolin XING, Xuefeng HAN, Huihui ZENG, Lei HOU, Hui GUO, Chuanxiang ZHANG, Zhihang YUE. Preparation of coal tar pitch-based microcrystal carbons and their lithium storage properties [J]. Chemical Industry and Engineering Progress, 2021, 40(1): 313-323. |
| [15] | Zhenyu ZHAO, Hong LI, Xingang LI, Xin GAO. Microwave-induced enhancement of distillation separation based on dielectric properties difference [J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2275-2283. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||