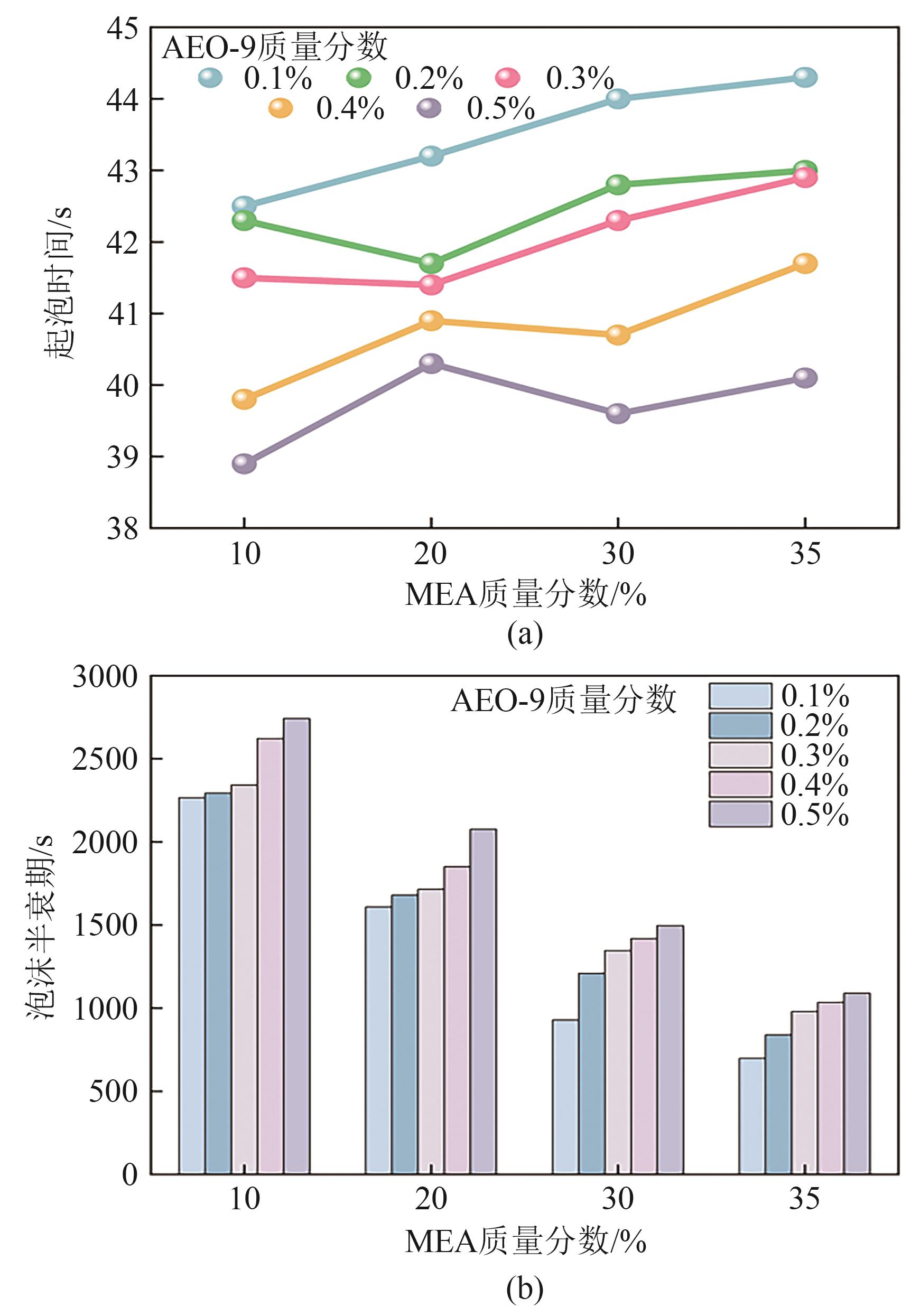
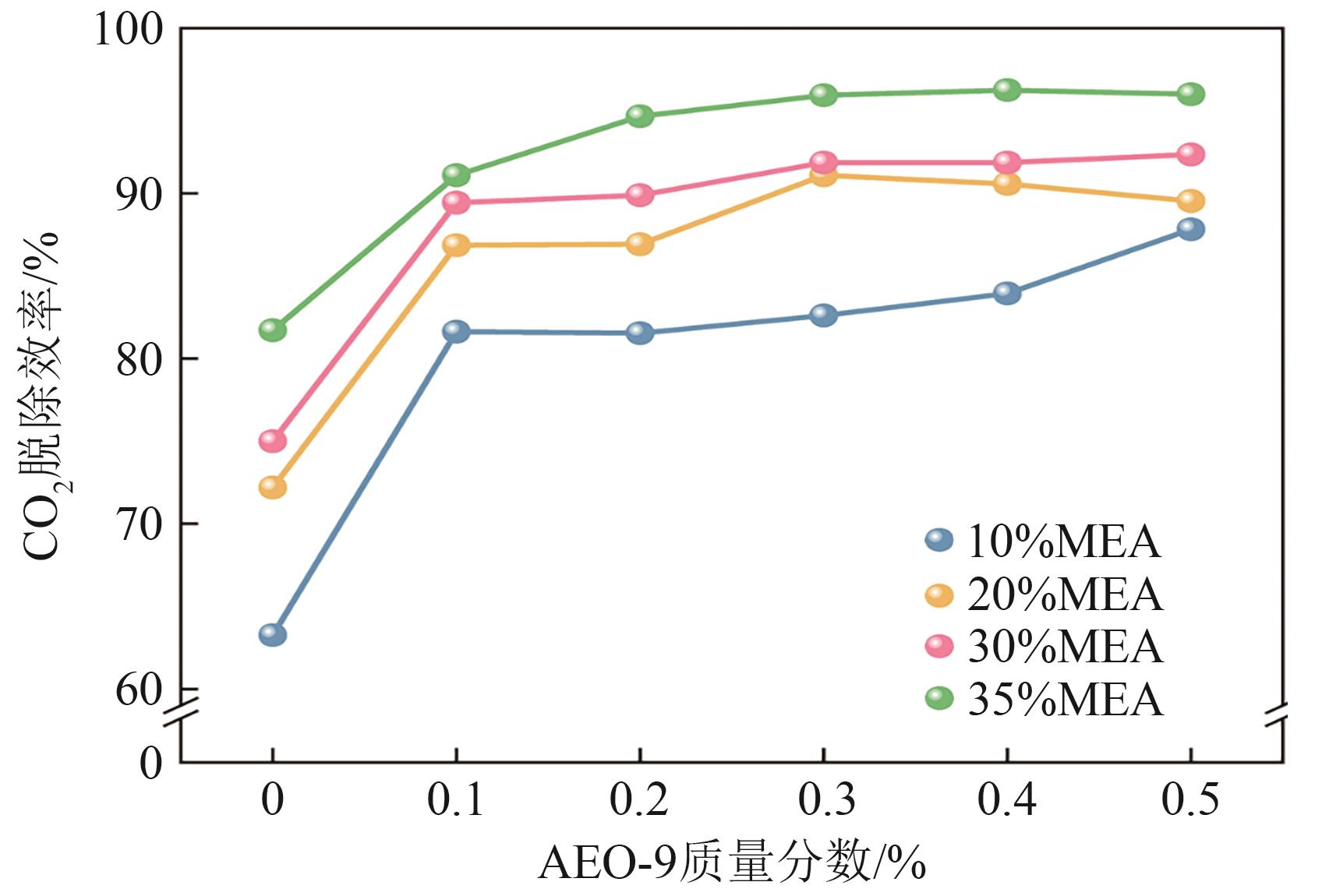
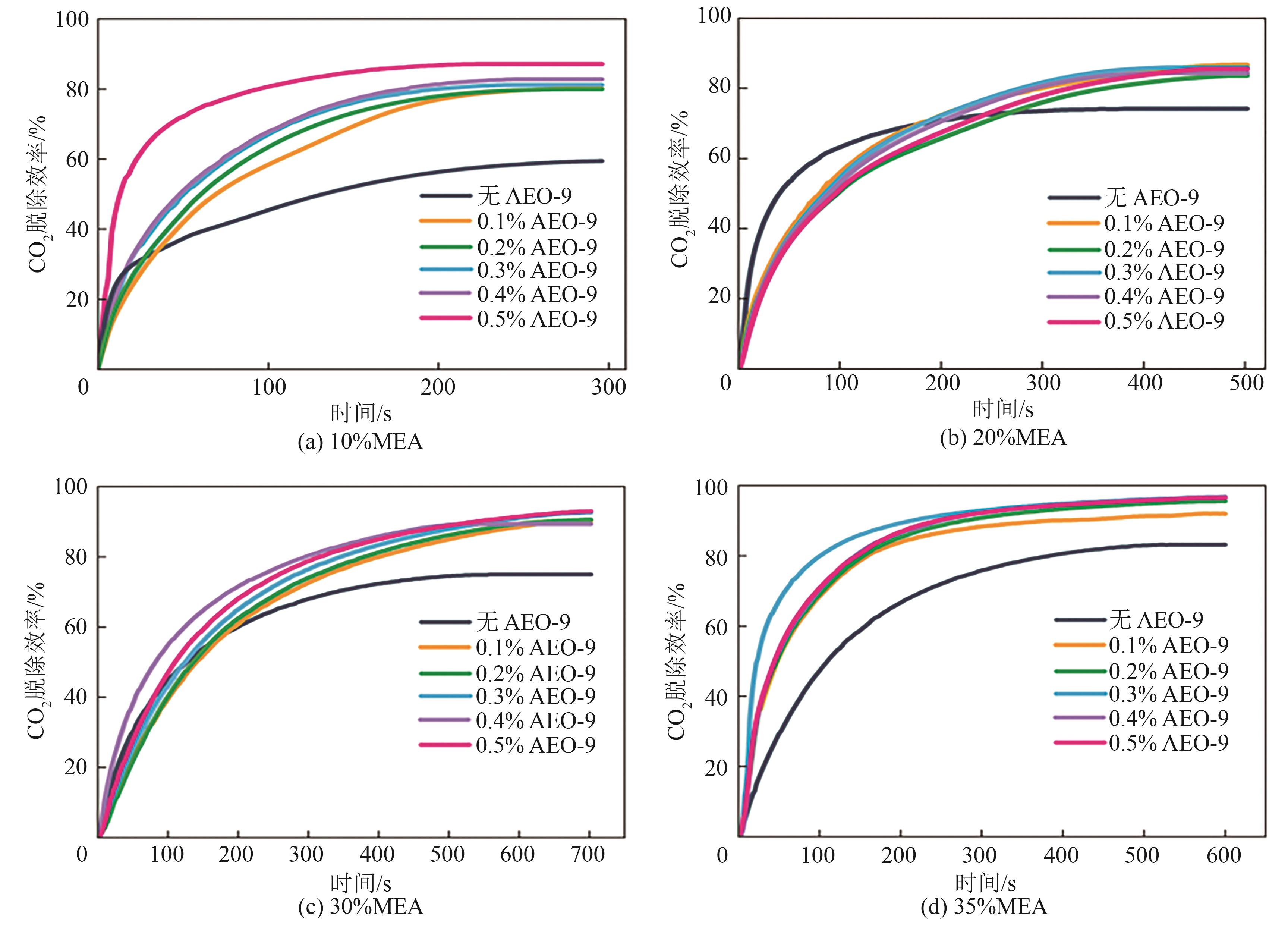
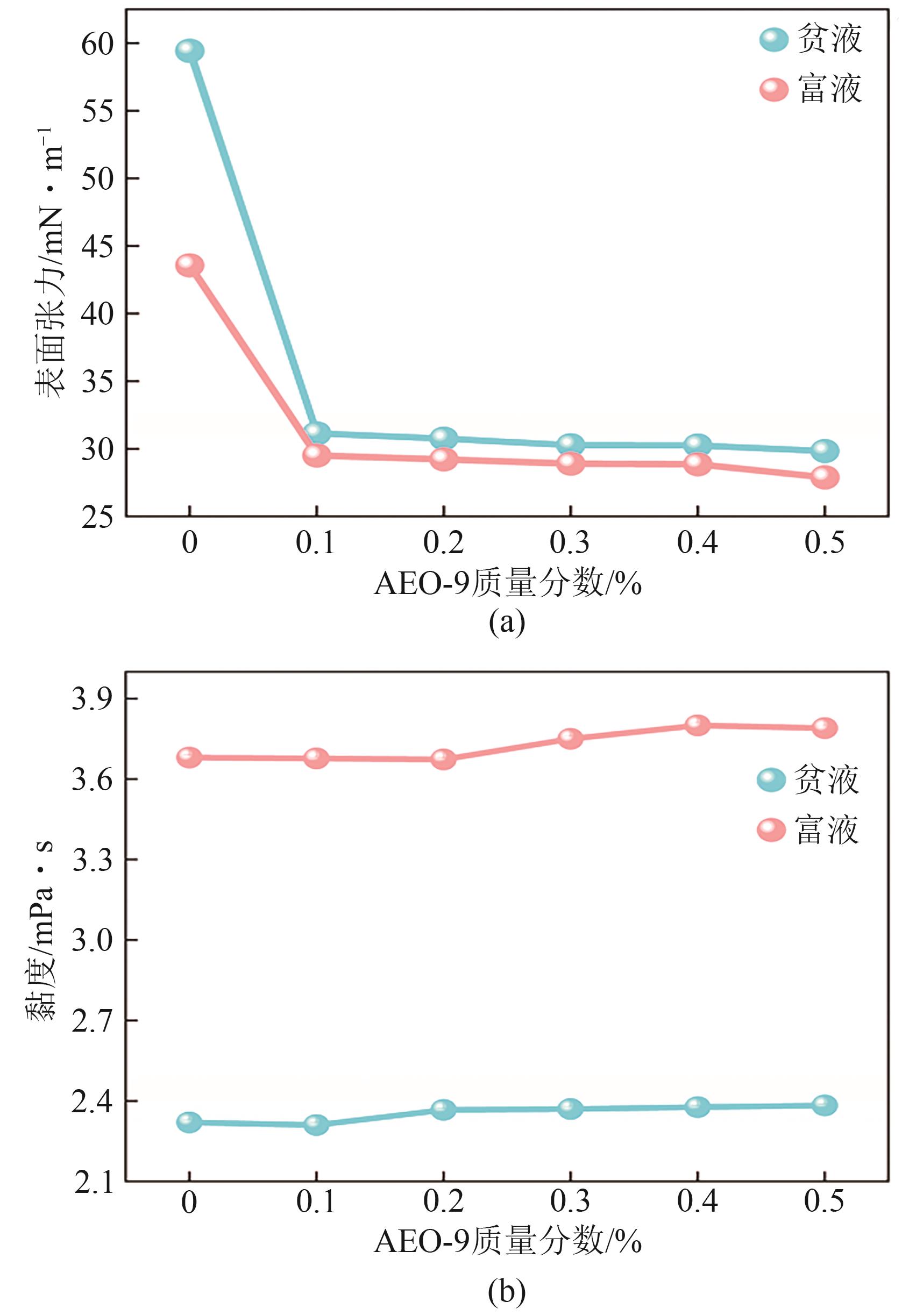
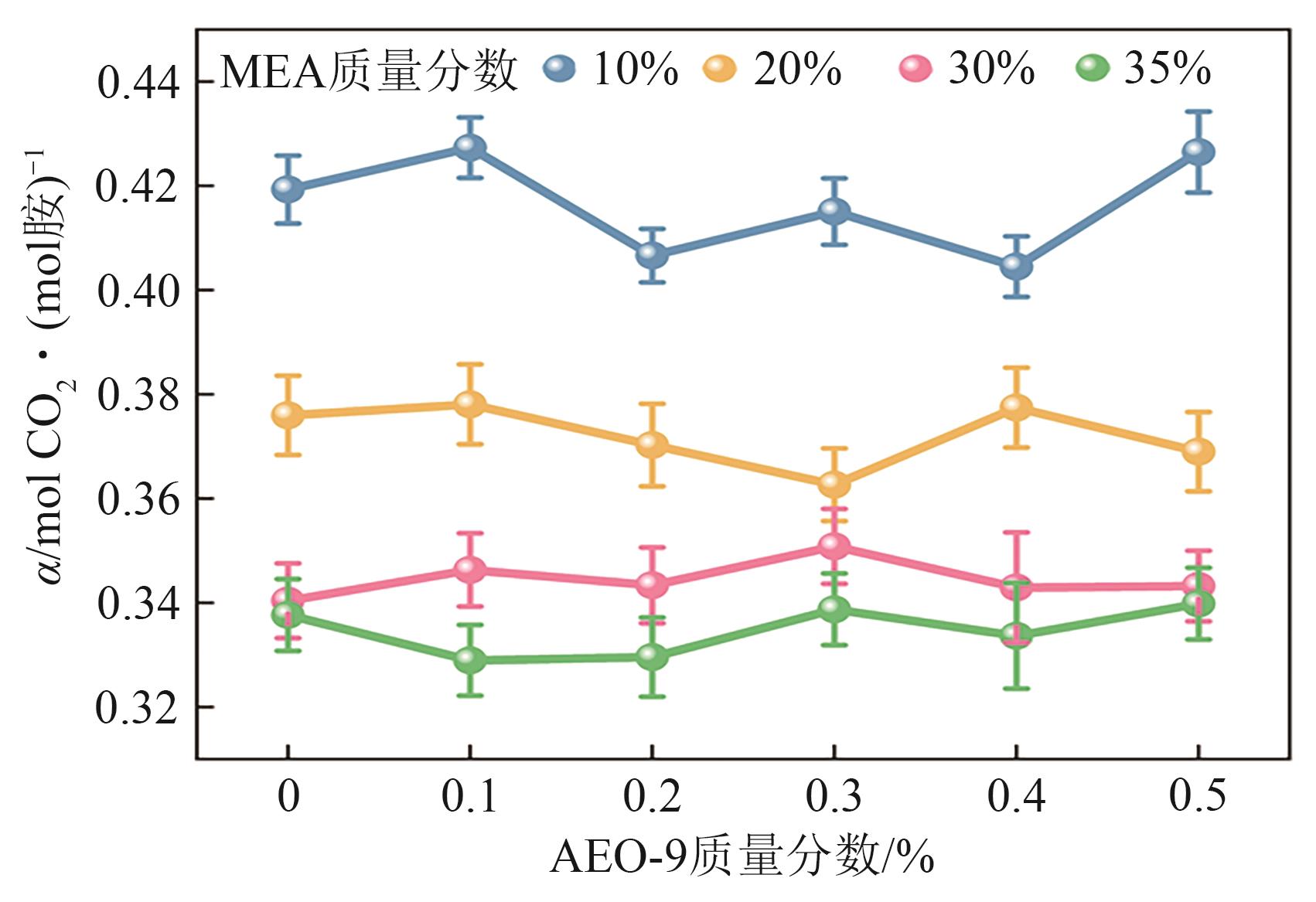
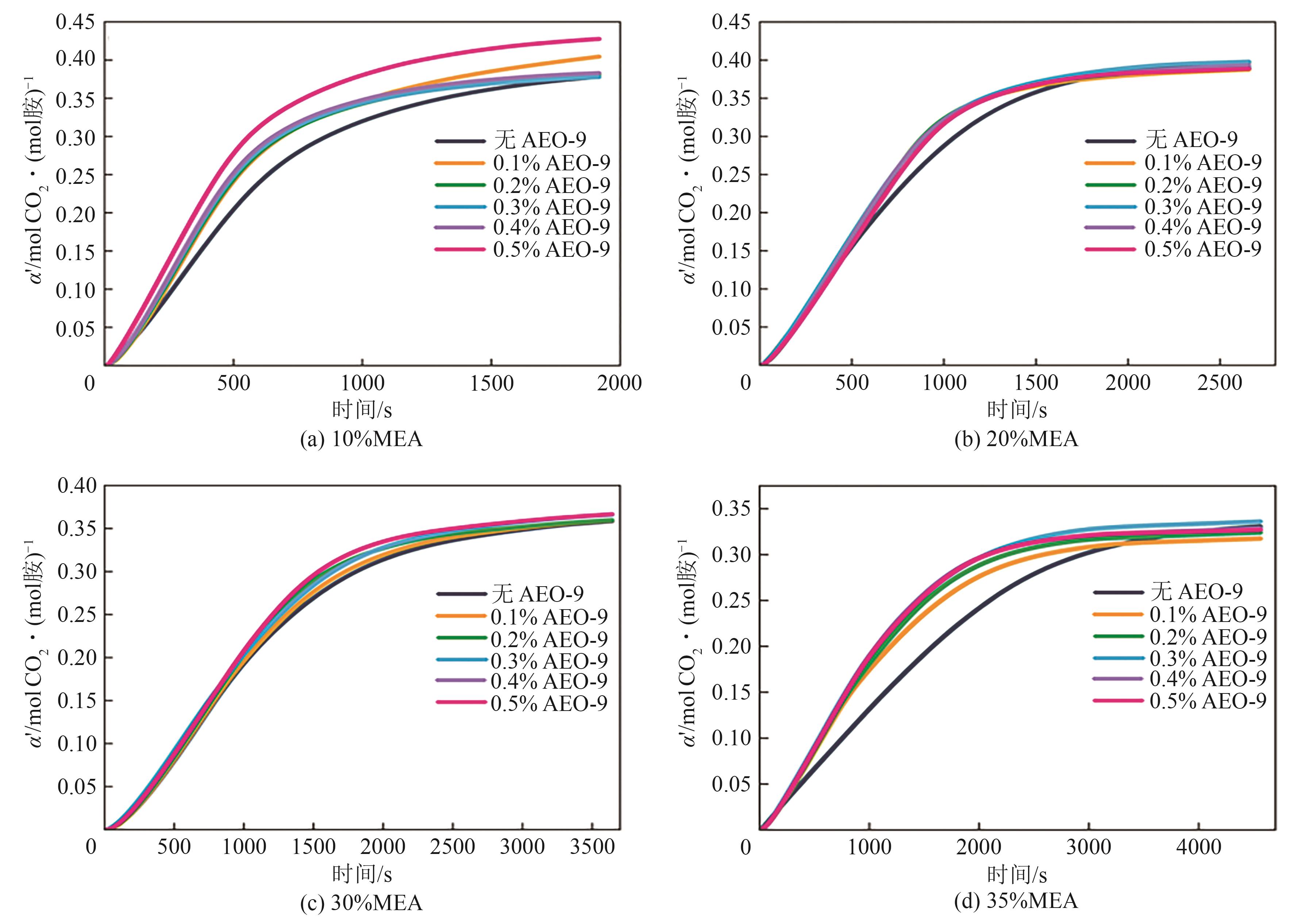
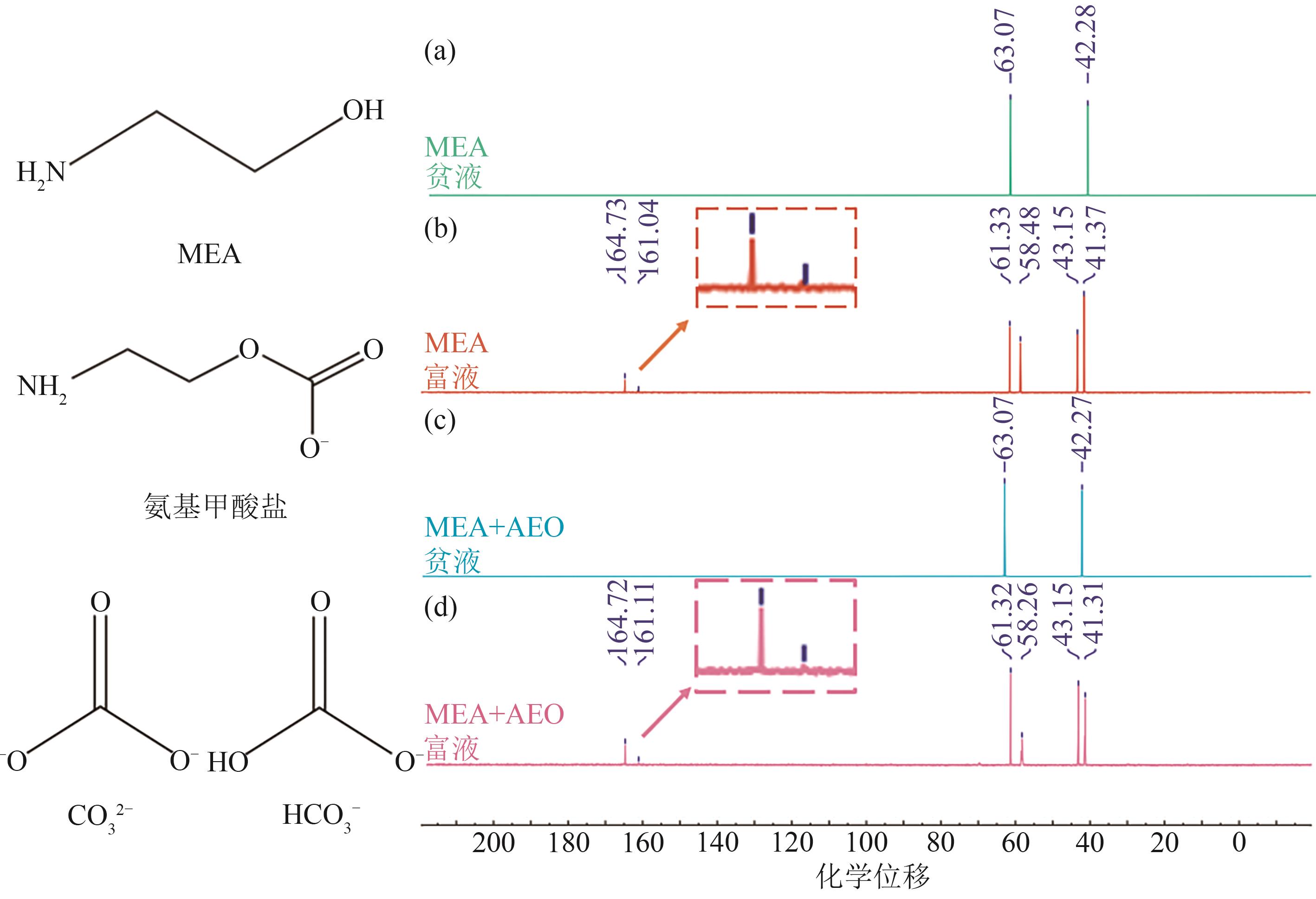
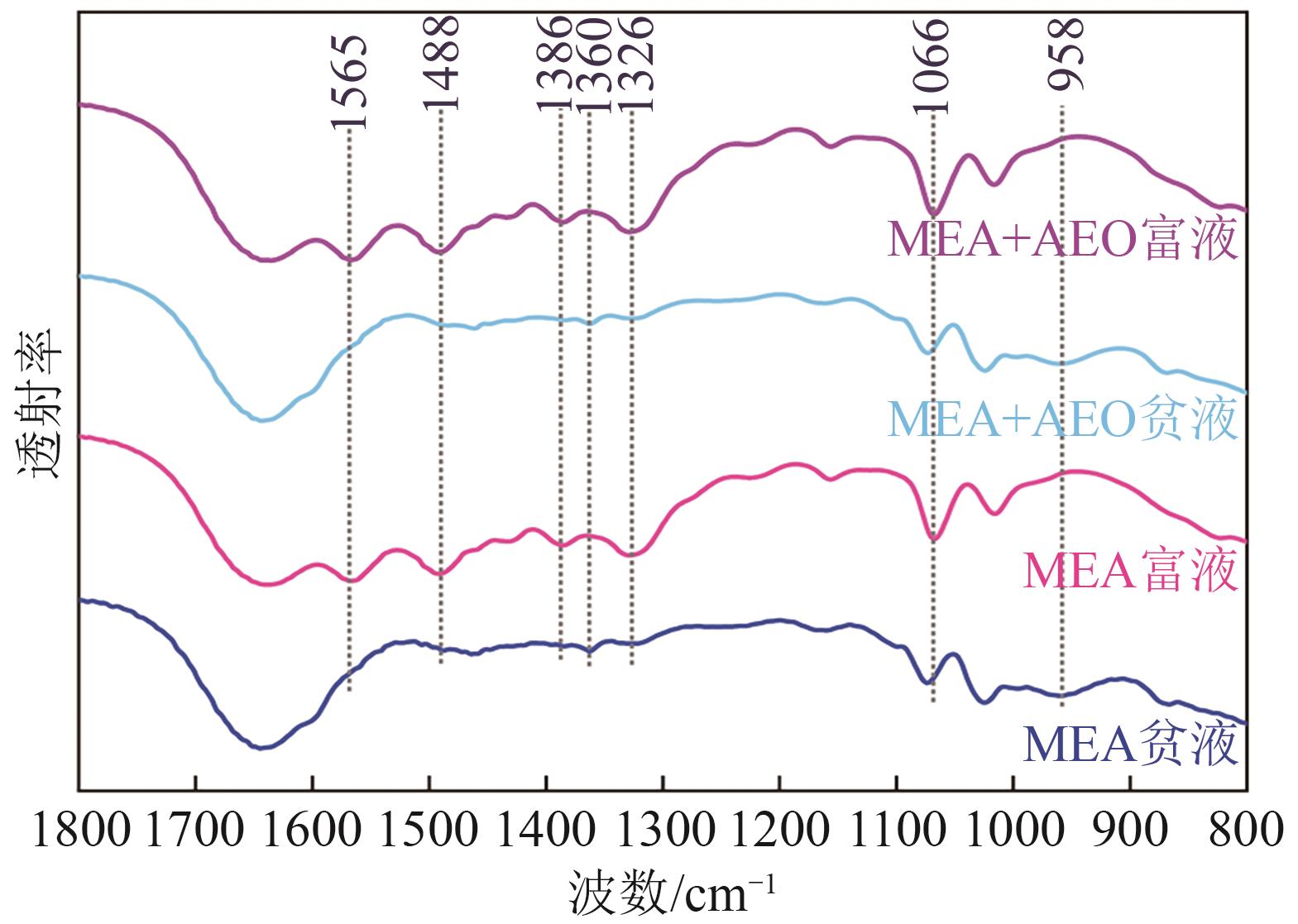
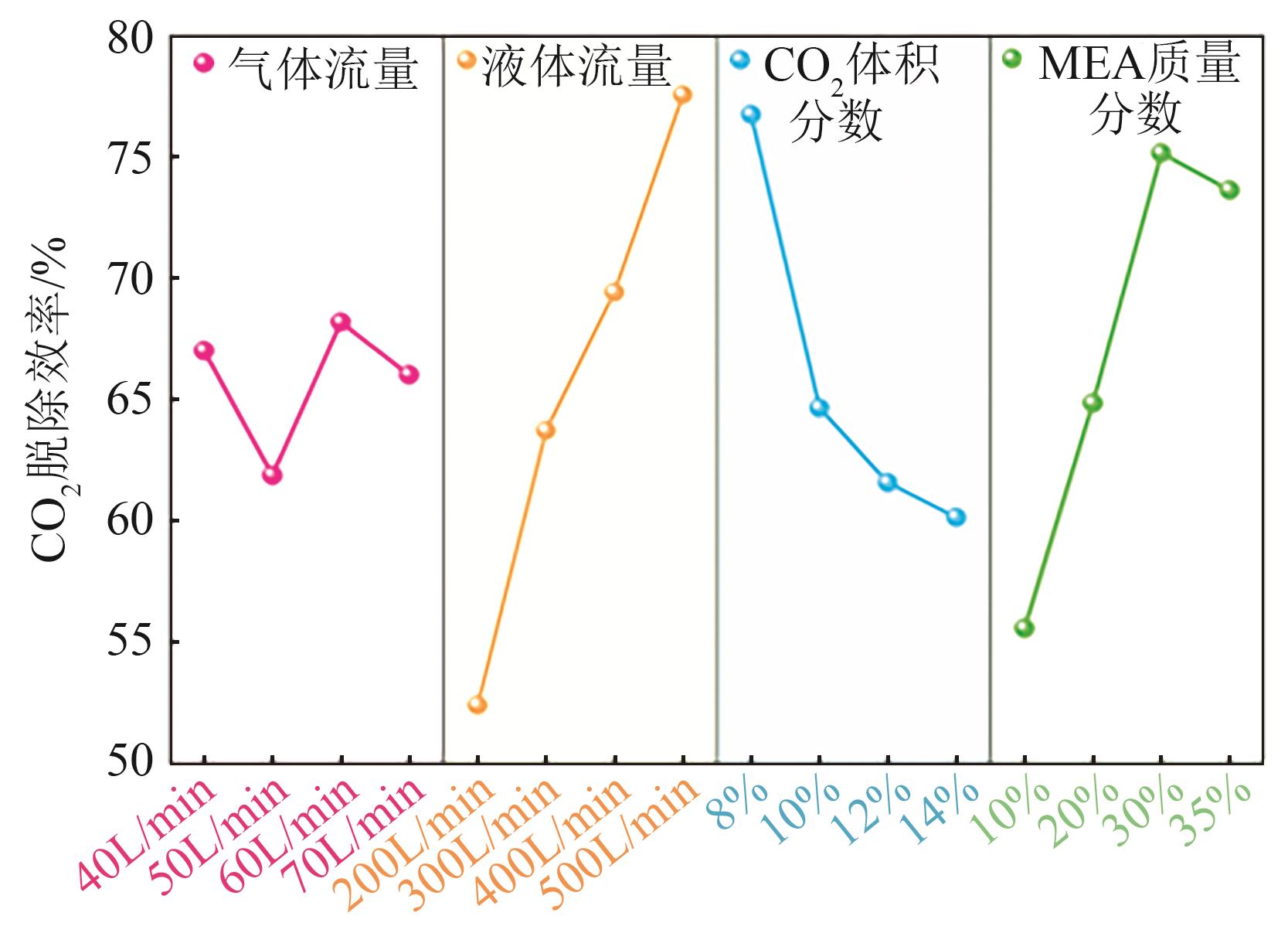
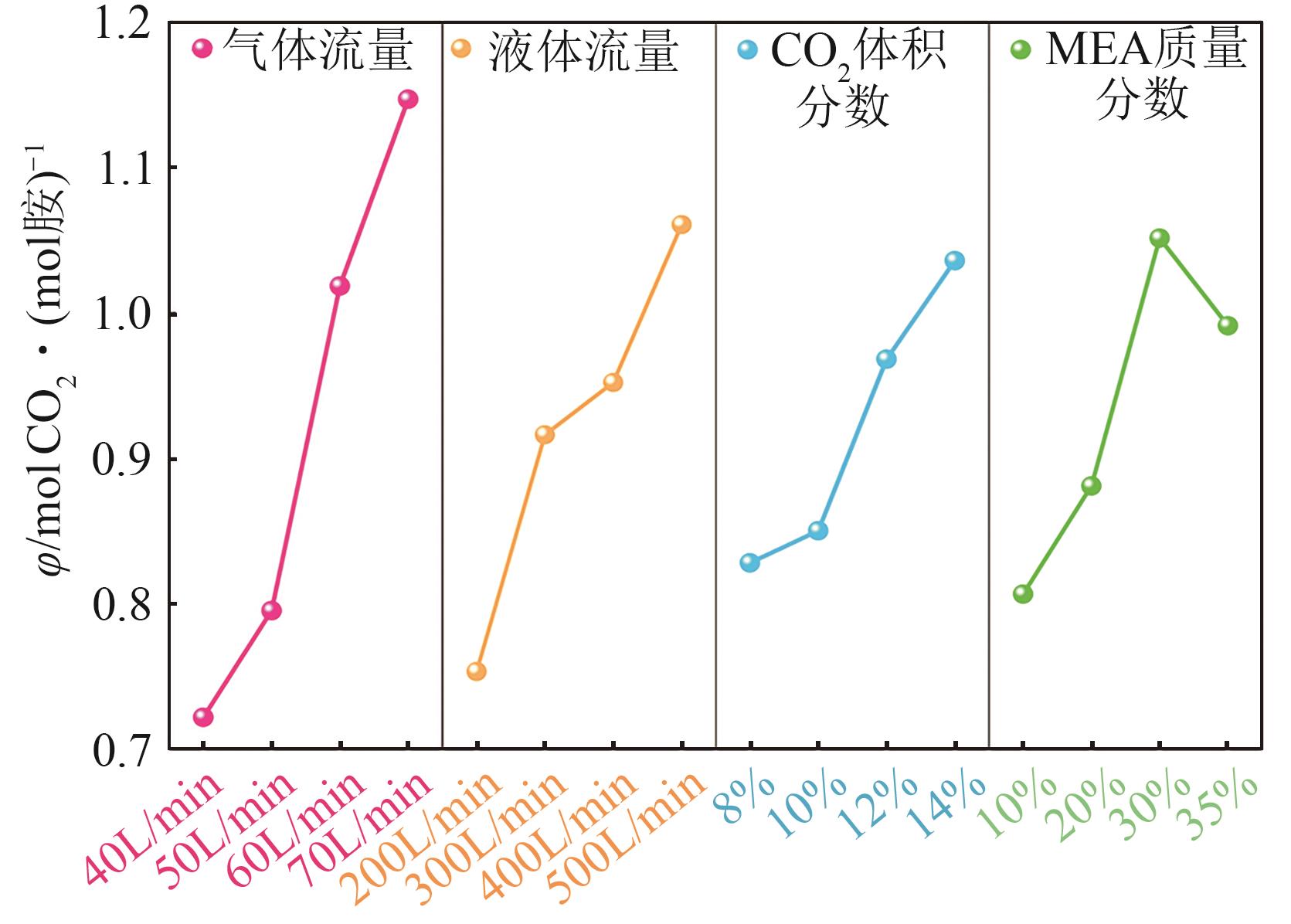
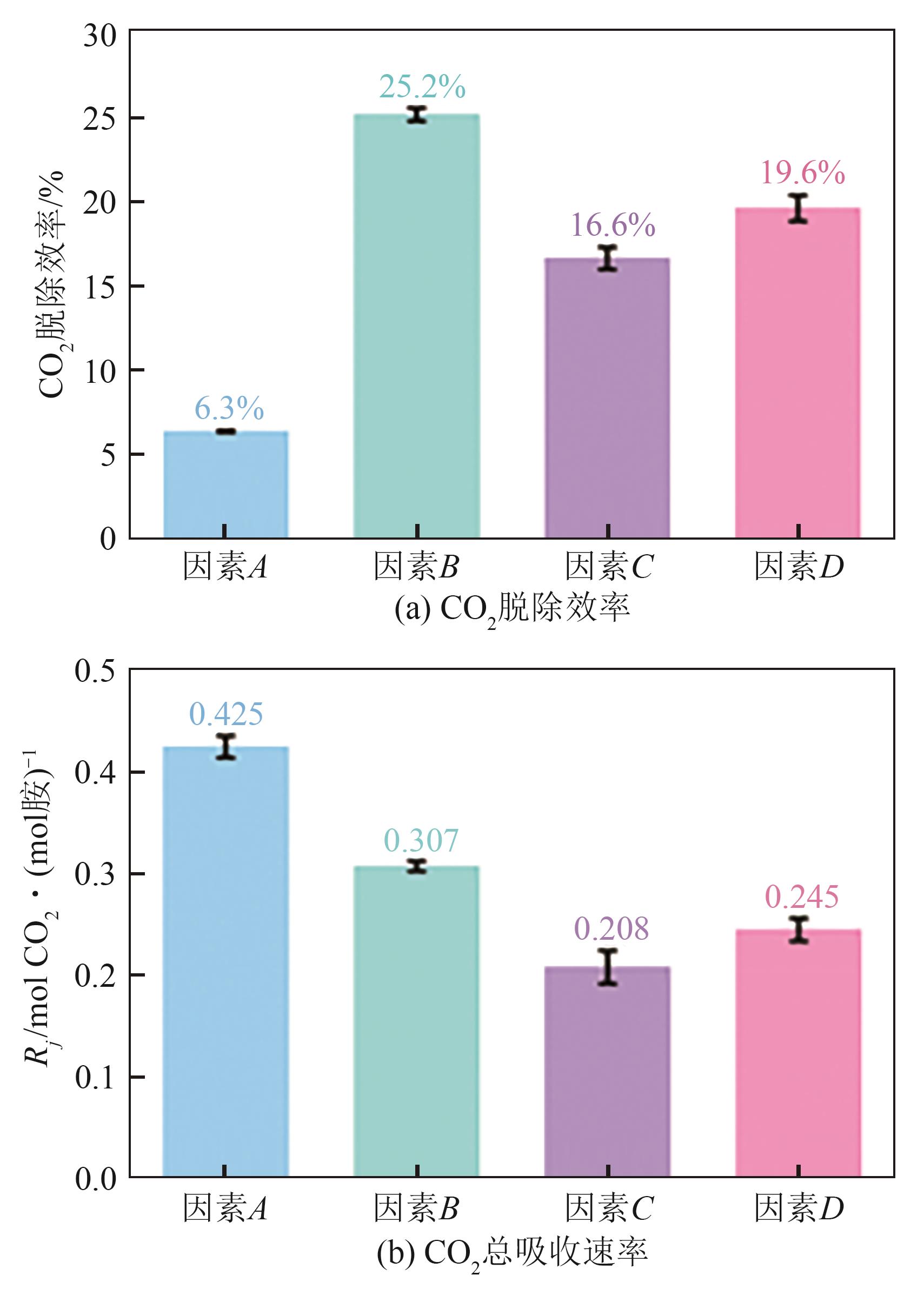
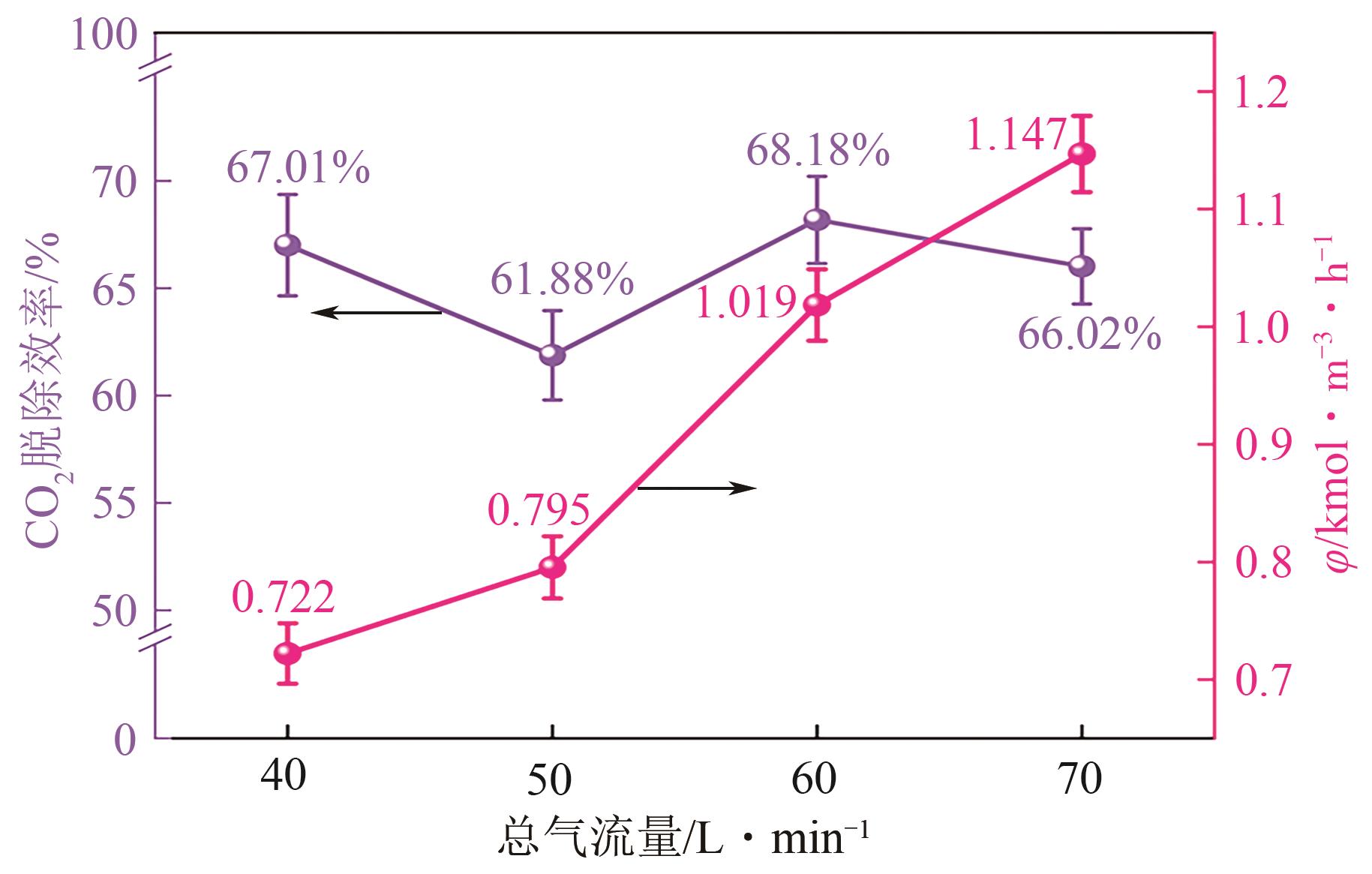
| [1] |
CHU Huaqiang, HUANG Zhen, ZHANG Zekai, et al. Integration of carbon emission reduction policies and technologies: Research progress on carbon capture, utilization and storage technologies[J]. Separation and Purification Technology, 2024, 343: 127153.
|
| [2] |
王振, 李玥, 李胜. 化学吸收法捕集CO2的研究进展、性能对比与展望[J]. 洁净煤技术, 2025, 31(4): 120-139.
|
|
WANG Zhen, LI Yue, LI Sheng. Chemical absorption method for CO2 capture: Research progress, performance comparison and prospects[J]. Clean Coal Technology, 2025, 31(4): 120-139.
|
| [3] |
YANG Li, LIU Fang, LIU Yang, et al. Deep regeneration of activated carbon catalyst and autothermal analysis for chemical looping methane thermo-catalytic decomposition process[J]. International Journal of Hydrogen Energy, 2018, 43(37): 17633-17642.
|
| [4] |
陆诗建, 张娟娟, 刘玲, 等. 工业源二氧化碳捕集技术进展与发展趋势[J]. 现代化工, 2022, 42(11): 59-64.
|
|
LU Shijian, ZHANG Juanjuan, LIU Ling, et al. Progress and development trend of industry-sourced carbon dioxide capture technology[J]. Modern Chemical Industry, 2022, 42(11): 59-64.
|
| [5] |
DOU Binlin, WU Kai, ZHANG Hua, et al. Sorption-enhanced chemical looping steam reforming of glycerol with CO2 in situ capture and utilization[J]. Chemical Engineering Journal, 2023, 452: 139703.
|
| [6] |
LIU Fang, CHEN Liangyong, YANG Li, et al. Application of chemical looping process for continuous high purity hydrogen production by methane thermocatalytic decomposition[J]. International Journal of Hydrogen Energy, 2016, 41(8): 4592-4602.
|
| [7] |
向国育, 申长俊, 陆诗建, 等. 二氧化碳捕集、利用与封存示范工程进展[J]. 低碳化学与化工, 2025, 50(3): 113-122.
|
|
XIANG Guoyu, SHEN Changjun, LU Shijian, et al. Progress of carbon dioxide capture, utilization and storage demonstration engineerings[J]. Low-Carbon Chemistry and Chemical Engineering, 2025, 50(3): 113-122.
|
| [8] |
陆诗建, 刘苗苗, 刘玲, 等. 烟气胺法CO2捕集技术进展与未来发展趋势[J]. 化工进展, 2023, 42(1): 435-444.
|
|
LU Shijian, LIU Miaomiao, LIU Ling, et al. Progress and future development trend of amine method of CO2 capture technology from flue gas[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 435-444.
|
| [9] |
HE Xinwei, HE Hang, BARZAGLI Francesco, et al. Analysis of the energy consumption in solvent regeneration processes using binary amine blends for CO2 capture[J]. Energy, 2023, 270: 126903.
|
| [10] |
杜延年, 王乐, 马骏, 等. CO2捕集过程醇胺吸收剂降解路径及腐蚀特性分析[J]. 炼油技术与工程, 2025, 55(5): 34-37.
|
|
DU Yannian, WANG Le, MA Jun, et al. Analysis of degradation pathways and corrosion characteristics of alcoholamine absorbents in CO2 capture processes[J]. Petroleum Refinery Engineering, 2025, 55(5): 34-37.
|
| [11] |
MENG Fanzhi, MENG Yuan, JU Tongyao, et al. Research progress of aqueous amine solution for CO2 capture: A review[J]. Renewable and Sustainable Energy Reviews, 2022, 168: 112902.
|
| [12] |
刘飞, 祁志福, 方梦祥, 等. 少水胺吸收剂CO2捕集工艺的中试试验与技术经济性评价[J]. 中国电机工程学报, 2024, 44(10): 3897-3905.
|
|
LIU Fei, QI Zhifu, FANG Mengxiang, et al. Pilot test and techno-economic assessment on water-lean amine-based CO2 capture process[J]. Proceedings of the CSEE, 2024, 44(10): 3897-3905.
|
| [13] |
孟钦辉, 陈斌, 季腾辉, 等.烟气CO2吸收捕集中试实验及工艺优化[J]. 化工进展.DOI:10.16085/j.issn.1000-6613. 2025-0384 .
|
|
MENG Qinhui, CHEN Bin, JI Tenghui, et al. Pilot-scale experiment and process optimization of CO₂ absorption capture from flue gas [J]. Chemical Industry and Engineering Progress.DOI:10.16085/j.issn.1000-6613. 2025-0384 .
|
| [14] |
ZHANG Zhenzhen, GE Yucong, YANG Li, et al. Enhancing CO2 absorption with compact multiflow absorber: Evaluation of operational factors[J]. Industrial & Engineering Chemistry Research, 2024, 63(13): 5618-5628.
|
| [15] |
YIN Yihan, QIU Aoqian, GAO Hongxia, et al. Experimental study of the mass transfer behavior of carbon dioxide absorption into ternary phase change solution in a packed tower[J]. Chinese Journal of Chemical Engineering, 2022, 43: 135-142.
|
| [16] |
夏宁, 何堤, 陈水宣, 等. 醇胺法CO2捕集双塔耦合工艺及参数优化[J]. 动力工程学报, 2025, 45(9): 1527-1535.
|
|
XIA Ning, HE Di, CHEN Shuixuan, et al. Dual-tower coupling process and parameter optimization for CO2 capture using the alkanol amine method[J]. Journal of Chinese Society of Power Engineering, 2025, 45(9): 1527-1535.
|
| [17] |
XU Xiongwen, LIU Yuehui, ZHU Yeming, et al. Liquid-film formation in a hole under the effect of gravity[J]. Industrial & Engineering Chemistry Research, 2022, 61(6): 2600-2614.
|
| [18] |
LIU Shengyu, LIU Zongyang, YANG Jie, et al. Parametric study and mechanism analysis of NO removal by cobalt ethylenediamine[J]. Fuel, 2021, 289: 119936.
|
| [19] |
CEJPEK Ondrej, MALY Milan, KUMAR DHINASEKARAN Vignesh, et al. Novel atomizer concept for CCS applications: Impinging effervescent atomizer[J]. Separation and Purification Technology, 2023, 311: 123259.
|
| [20] |
KUNTZ Jeffery, AROONWILAS Adisorn. Performance of spray column for CO2 capture application[J]. Industrial & Engineering Chemistry Research, 2008, 47(1): 145-153.
|
| [21] |
JAVED K H, MAHMUD T, PURBA E. The CO2 capture performance of a high-intensity vortex spray scrubber[J]. Chemical Engineering Journal, 2010, 162(2): 448-456.
|
| [22] |
WU Xiaomei, YU Yunsong, QIN Zhen, et al. Performance of CO2 absorption in a diameter-varying spray tower[J]. Chinese Journal of Chemical Engineering, 2017, 25(8): 1109-1114.
|
| [23] |
MEI Zhongkai, CHENG Xu. Modeling of interfacial area for single deformed bubble based on VOF method[J]. Nuclear Engineering and Design, 2022, 395: 111864.
|
| [24] |
PANJIPOUR Rasoul, KARAMOOZIAN Mohammad, ALBIJANIC Boris. Investigations of gas holdup, interfacial area of bubbles and bubble size distributions in a pilot plant flotation column[J]. Minerals Engineering, 2021, 164: 106819.
|
| [25] |
PRAKASH Ritesh, KUMAR MAJUMDER Subrata, SINGH Anugrah. Bubble size distribution and specific bubble interfacial area in two-phase microstructured dense bubbling bed[J]. Chemical Engineering Research and Design, 2020, 156: 108-130.
|
| [26] |
PICHETWANIT Panatda, KUNGSANANT Suratsawadee, SUPAP Teeradet. Effects of surfactant type and structure on properties of amines for carbon dioxide capture[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 622: 126602.
|
| [27] |
GE Yucong, ZHANG Zhenzhen, YANG Li, et al. Surfactant-modified monoethanolamine for better foaming to enhance CO2 removal efficiency[J]. Chemical Engineering Journal, 2024, 498: 155440.
|
| [28] |
LIAO Huiying, GAO Hongxia, XU Bin, et al. Mass transfer performance studies of aqueous blended DEEA-MEA solution using orthogonal array design in a packed column[J]. Separation and Purification Technology, 2017, 183: 117-126.
|
| [29] |
BORKOWSKI M, KOSIOR D, ZAWALA J. Effect of initial adsorption coverage and dynamic adsorption layer formation at bubble surface in stability of single foam films[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 589: 124446.
|
| [30] |
BRYANT Jonathan J, LIPPERT Cameron, QI Guojie, et al. Enhanced carbon capture through incorporation of surfactant additives[J]. Industrial & Engineering Chemistry Research, 2016, 55(27): 7456-7461.
|
| [31] |
TIWARI Surya Prakash, STECKEL Janice A, SARMA Moushumi, et al. Foaming dependence on the interface affinities of surfactant-like molecules[J]. Industrial & Engineering Chemistry Research, 2019, 58(43): 19877-19889.
|
| [32] |
盛荟霖, 胡大鹏, 魏炜. 混合醇胺法吸收电厂烟气中CO2的性能研究[J]. 现代化工, 2024, 44(S2): 177-183, 189.
|
|
SHENG Huilin, HU Dapeng, WEI Wei. Study on performance of mixed alcohol amine method to absorb CO2 in flue gas of power plant[J]. Modern Chemical Industry, 2024, 44(S2): 177-183, 189.
|
| [33] |
CHEN Zhilv, WANG Tao, LI Chao, et al. Research on CO2 capture performance of DMEDA water-lean absorbents based on molecular dynamics[J]. Separation and Purification Technology, 2025, 354: 128924.
|
| [34] |
ZHANG Rui, HE Xinwei, LIU Tianzi, et al. Thermodynamic studies for improving the prediction of CO2 equilibrium solubility in aqueous 2-dimethylamino-2-methyl-1-propanol[J]. Separation and Purification Technology, 2022, 295: 121292.
|
| [35] |
WONG M K, BUSTAM M A, SHARIFF A M. In situ measurement of physical solubility of carbon dioxide in loaded aqueous monoethanolamine by Raman spectroscopy[J]. Journal of Natural Gas Science and Engineering, 2016, 36: 305-313.
|
| [36] |
ARSHAD Muhammad Waseem, FOSBOL Philip Loldrup, VON SOLMS Nicolas, et al. Equilibrium solubility of CO2 in alkanolamines[C]// 7th Trondheim Conference on CO2 Capture, Transport and Storage (TCCS), Trondheim, NORWAY, 2013: 217-223.
|
| [37] |
GUO Hui, SHI Xiaoqin, SHEN Shufeng. Solubility of N2O and CO2 in non-aqueous systems of monoethanolamine and glycol ethers: Measurements and model representation[J]. The Journal of Chemical Thermodynamics, 2019, 137: 76-85.
|
| [38] |
CHEN Guangying, CHEN Guangjie, PERUZZINI Maurizio, et al. Understanding the potential benefits of blended ternary amine systems for CO2 capture processes through 13C NMR speciation study and energy cost analysis[J]. Separation and Purification Technology, 2022, 291: 120939.
|
| [39] |
CHEN Guangjie, CHEN Guangying, PERUZZINI Maurizio, et al. Investigating the performance of ethanolamine and benzylamine blends as promising sorbents for postcombustion CO2 capture through 13C NMR speciation and heat of CO2 absorption analysis[J]. Energy & Fuels, 2022, 36(16): 9203-9212.
|
| [40] |
LIU Helei, LI Moxia, LUO Xiao, et al. Investigation mechanism of DEA as an activator on aqueous MEA solution for postcombustion CO2 capture[J]. AIChE Journal, 2018, 64(7): 2515-2525.
|
| [41] |
CHOI Jeong Ho, Seong Geun OH, KIM Young Eun, et al. Carbon dioxide absorption characteristics of aqueous alkanolamine using nuclear magnetic resonance spectroscopy[J]. Environmental Engineering Science, 2012, 29(5): 328-334.
|
| [42] |
CIFTJA Arlinda F, HARTONO Ardi, SVENDSEN Hallvard F. Amine neutralized amino acid as CO2 absorbents: A quantitative 13C-NMR study[J]. International Journal of Greenhouse Gas Control, 2014, 27: 169-177.
|
| [43] |
BARZAGLI Francesco, GIORGI Claudia, MANI Fabrizio, et al. Comparative study of CO2 capture by aqueous and nonaqueous 2-amino-2-methyl-1-propanol based absorbents carried out by 13C NMR and enthalpy analysis[J]. Industrial & Engineering Chemistry Research, 2019, 58(11): 4364-4373.
|
| [44] |
WADA Sakurako, KUSHIDA Takayuki, ITAGAKI Haruna, et al. 13C NMR study on carbamate hydrolysis reactions in aqueous amine/CO2 solutions[J]. International Journal of Greenhouse Gas Control, 2021, 104: 103175.
|
| [45] |
ZHANG Rui, ZHANG Xiaowen, YANG Qi, et al. Analysis of the reduction of energy cost by using MEA-MDEA-PZ solvent for post-combustion carbon dioxide capture (PCC)[J]. Applied Energy, 2017, 205: 1002-1011.
|
| [46] |
BARZAGLI Francesco, MANI Fabrizio, PERUZZINI Maurizio. A 13C NMR study of the carbon dioxide absorption and desorption equilibria by aqueous 2-aminoethanol and N-methyl-substituted 2-aminoethanol[J]. Energy & Environmental Science, 2009, 2(3): 322-330.
|
| [47] |
LV Tong, FANG Mengxiang, ZENG Xi, et al. Carbon structure of coal from the CP/MAS 13C NMR spectra: Effect of contact time and potential quantitative modification[J]. Energy & Fuels, 2024, 38(5): 3740-3754.
|
| [48] |
HOSSEINZADEH Mohammad Taghi, HOSSEINIAN Ayoob. Novel thin film composite nanofiltration membrane using monoethanolamine (MEA) and diethanolamine (DEA) with m-phenylenediamine (MPD)[J]. Journal of Polymers and the Environment, 2018, 26(4): 1745-1753.
|
| [49] |
BOSSA Jean-Baptiste, BORGET Fabien, DUVERNAY Fabrice, et al. Formation of neutral methylcarbamic acid (CH3NHCOOH) and methylammonium methylcarbamate [CH3NH3 +] [CH3NHCO2 -] at low temperature[J]. The Journal of Physical Chemistry A, 2008, 112(23): 5113-5120.
|
| [50] |
QIAO Zhihua, WANG Zhi, YUAN Shuangjie, et al. Preparation and characterization of small molecular amine modified PVAm membranes for CO2/H2 separation[J]. Journal of Membrane Science, 2015, 475: 290-302.
|
| [51] |
VAIDYA Prakash D, KENIG Eugeny Y. Kinetics of carbonyl sulfide reaction with alkanolamines: A review[J]. Chemical Engineering Journal, 2009, 148(2/3): 207-211.
|
| [52] |
SHARIF Maimoona, GE Chunliang, WANG Tao, et al. Computational investigation of co-solvent influence on CO2 absorption and diffusion in water lean solvents[J]. Processes, 2024, 12(8): 1588.
|
| [53] |
MA Daofan, ZHU Chunying, FU Taotao, et al. An effective hybrid solvent of MEA/DEEA for CO2 absorption and its mass transfer performance in microreactor[J]. Separation and Purification Technology, 2020, 242: 116795.
|
| [54] |
SHARIF Maimoona, HAN Tao, WANG Tao, et al. Investigation of rational design of amine solvents for CO2 capture: A computational approach[J]. Chemical Engineering Research and Design, 2024, 204: 524-535.
|
| [55] |
MA Yiyi, GUO Linjiang, XIAO Yuanhao, et al. Bubble mass transfer in fluids under gravity: A review of theoretical models and intensification technologies in industry[J]. Frontiers in Physics, 2024, 12: 1383537.
|
| [56] |
YAN Shenglin, WANG Xuqing, ZHU Litao, et al. Mechanisms and modeling of bubble dynamic behaviors and mass transfer under gravity: A review[J]. Chemical Engineering Science, 2023, 277: 118854.
|
| [57] |
SAITO Takayuki, TORIU Masahiko. Effects of a bubble and the surrounding liquid motions on the instantaneous mass transfer across the gas-liquid interface[J]. Chemical Engineering Journal, 2015, 265: 164-175.
|
| [58] |
ZHANG Kai, WANG Shuai, WU Qiang, et al. Investigation of bubble-to-emulsion phase mass transfer at non-isothermal conditions via a coupled CFD-DEM approach[J]. Chemical Engineering Science, 2021, 231: 116284.
|
| [59] |
SEMA Teerawat, NAAMI Abdulaziz, FU Kaiyun, et al. Comprehensive mass transfer and reaction kinetics studies of CO2 absorption into aqueous solutions of blended MDEA-MEA[J]. Chemical Engineering Journal, 2012, 209: 501-512.
|
| [60] |
HOFFMAN John R, BAUMANN Avery E, STAFFORD Christopher M. Thickness dependent CO2 adsorption of poly(ethyleneimine) thin films for direct air capture[J]. Chemical Engineering Journal, 2024, 481: 148381.
|
| [61] |
HAARHOFF J, EDZWALD J K. Modelling of floc-bubble aggregate rise rates in dissolved air flotation[J]. Water Science and Technology, 2001, 43(8): 175-184.
|
 ), GE Yucong, YANG Li(
), GE Yucong, YANG Li( ), ZHANG Zhenzhen, HENG Xunxuan, LIU Fang, YANG Xiao
), ZHANG Zhenzhen, HENG Xunxuan, LIU Fang, YANG Xiao
 ), 葛煜聪, 杨丽(
), 葛煜聪, 杨丽( ), 张真真, 衡迅瑄, 刘方, 杨霄
), 张真真, 衡迅瑄, 刘方, 杨霄