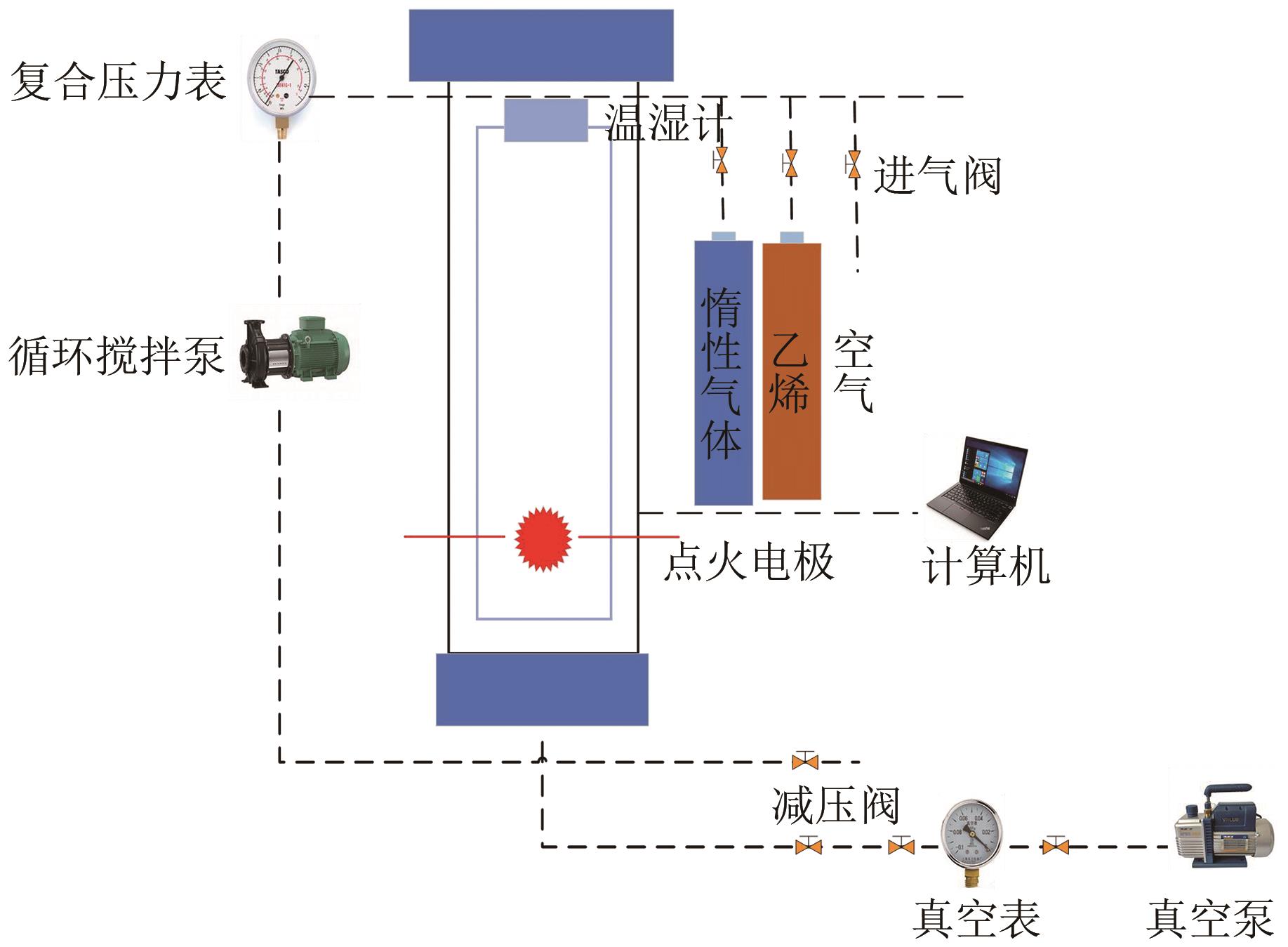
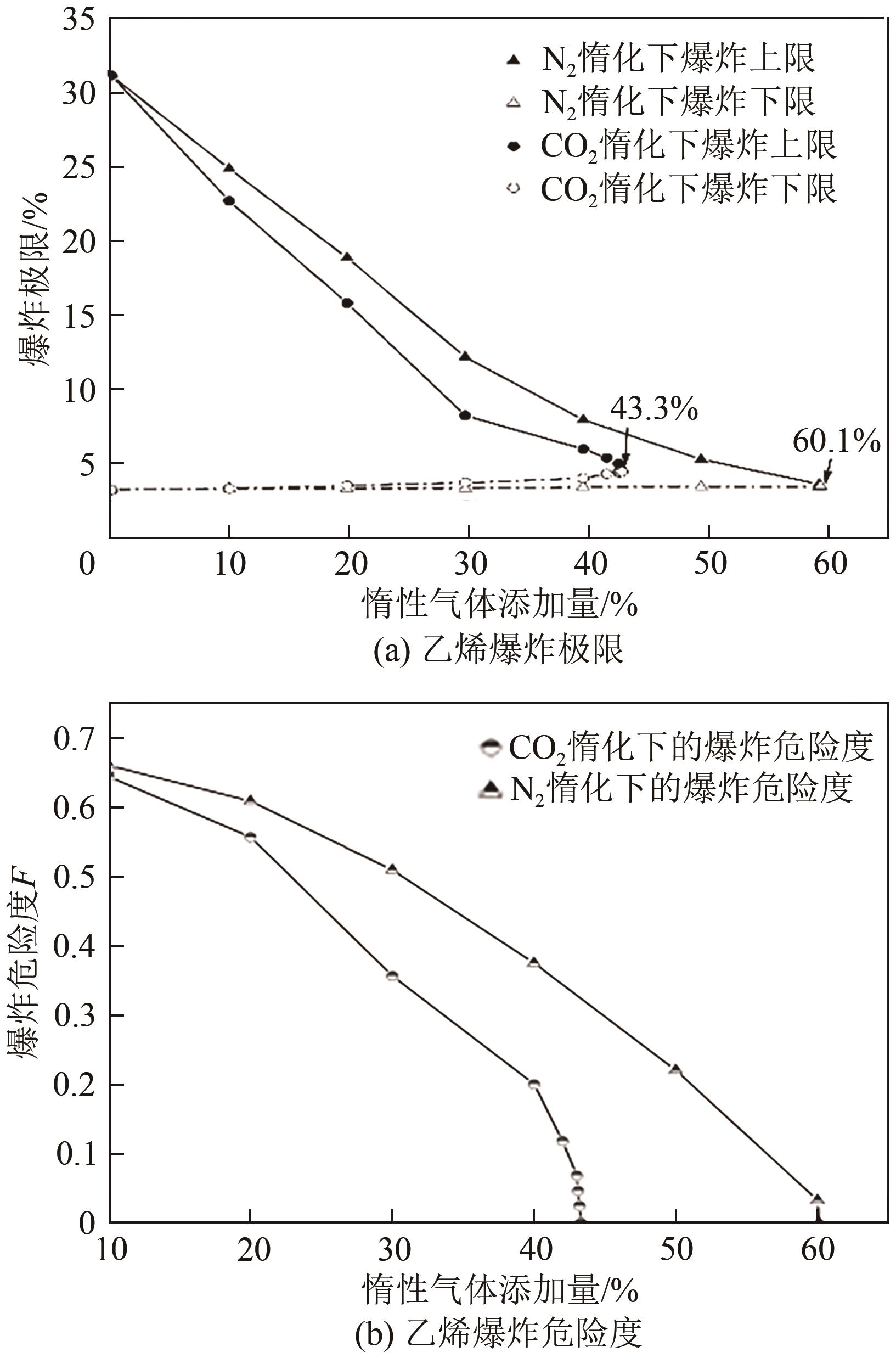
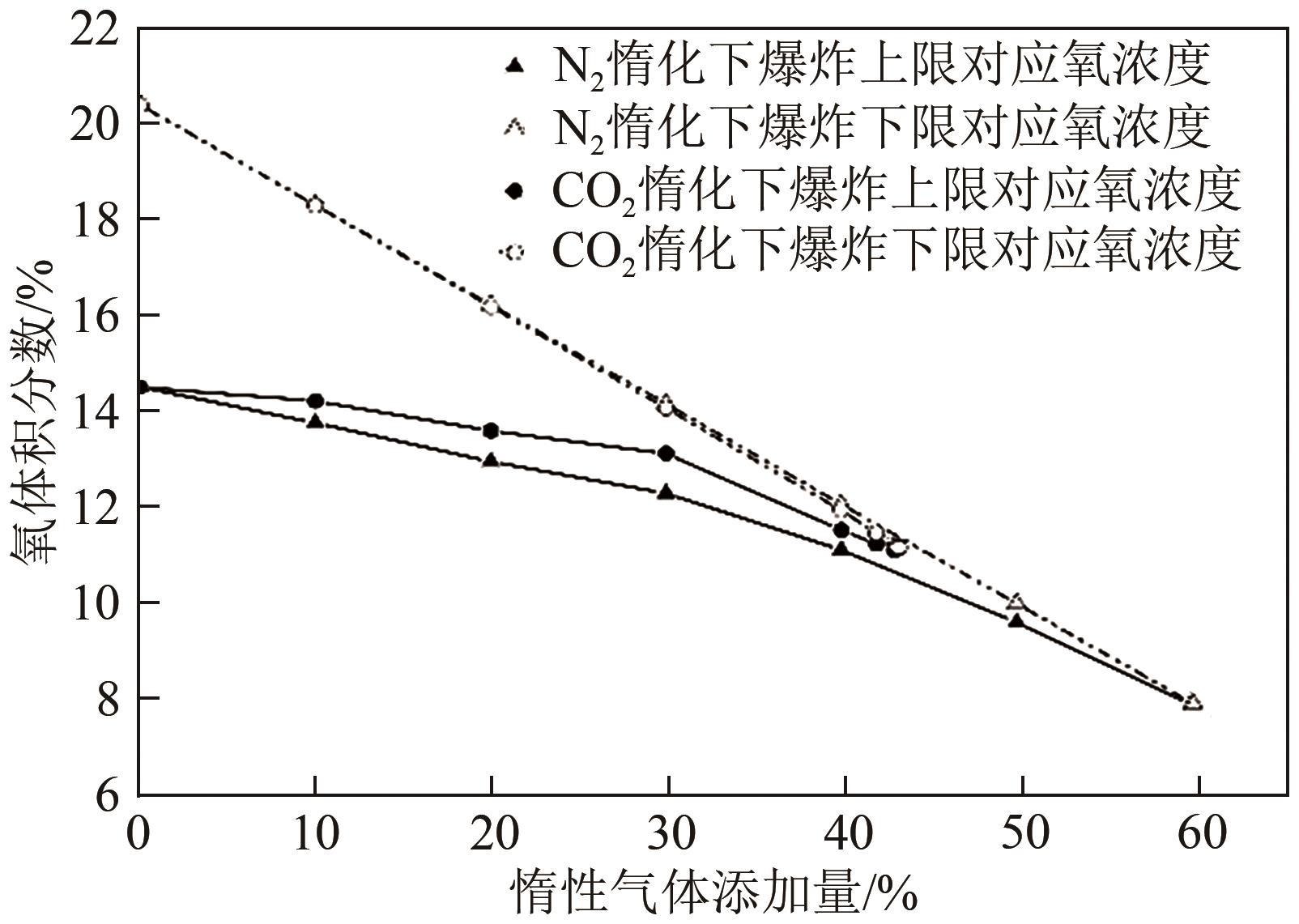
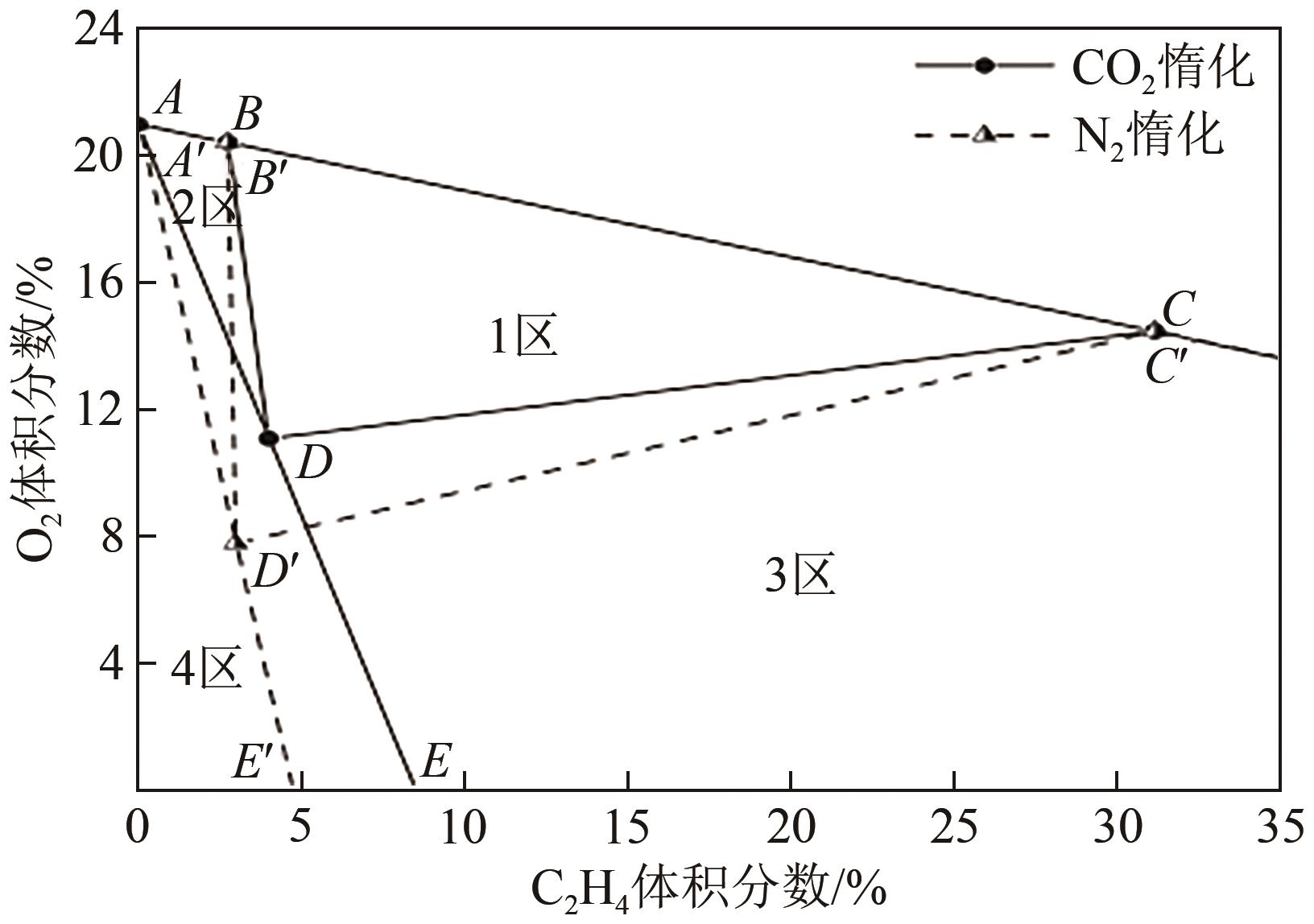
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (9): 4653-4661.DOI: 10.16085/j.issn.1000-6613.2021-2432
• Chemical processes and equipment • Previous Articles Next Articles
Effect of inert gas on ethylene explosion limit parameters and kinetic characteristics
LUO Zhenmin1,2,3( ), LIU Lu1, SU Bin1, SONG Fangzhi1
), LIU Lu1, SU Bin1, SONG Fangzhi1
- 1.School of Safety Science and Engineering, Xi’an University of Science and Technology, Xi’an 710054, Shaanxi, China
2.Shaanxi Provincial Industrial Process Safety and Emergency Rescue Engineering Technology Research Center, Xi’an 710054, Shaanxi, China
3.Shaanxi Key Laboratory of Prevention and Control of Coal Fire, Xi’an 710054, Shaanxi, China
-
Received:2021-11-26Revised:2021-12-21Online:2022-09-27Published:2022-09-25 -
Contact:LUO Zhenmin
惰性气体影响乙烯爆炸极限参数及动力学特性
- 1.西安科技大学安全科学与工程学院,陕西 西安 710054
2.陕西省工业过程安全与应急救援工程技术研究中心,陕西 西安 710054
3.陕西省煤火灾害防治重点实验室,陕西 西安 710054
-
通讯作者:罗振敏 -
作者简介:罗振敏(1976—),女,教授,博士生导师,研究方向为气体与粉尘爆炸防控。E-mail: zmluo@xust.edu.cn。 -
基金资助:陕西省创新能力支撑计划(2020TD-021)
CLC Number:
Cite this article
LUO Zhenmin, LIU Lu, SU Bin, SONG Fangzhi. Effect of inert gas on ethylene explosion limit parameters and kinetic characteristics[J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4653-4661.
罗振敏, 刘璐, 苏彬, 宋方智. 惰性气体影响乙烯爆炸极限参数及动力学特性[J]. 化工进展, 2022, 41(9): 4653-4661.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-2432
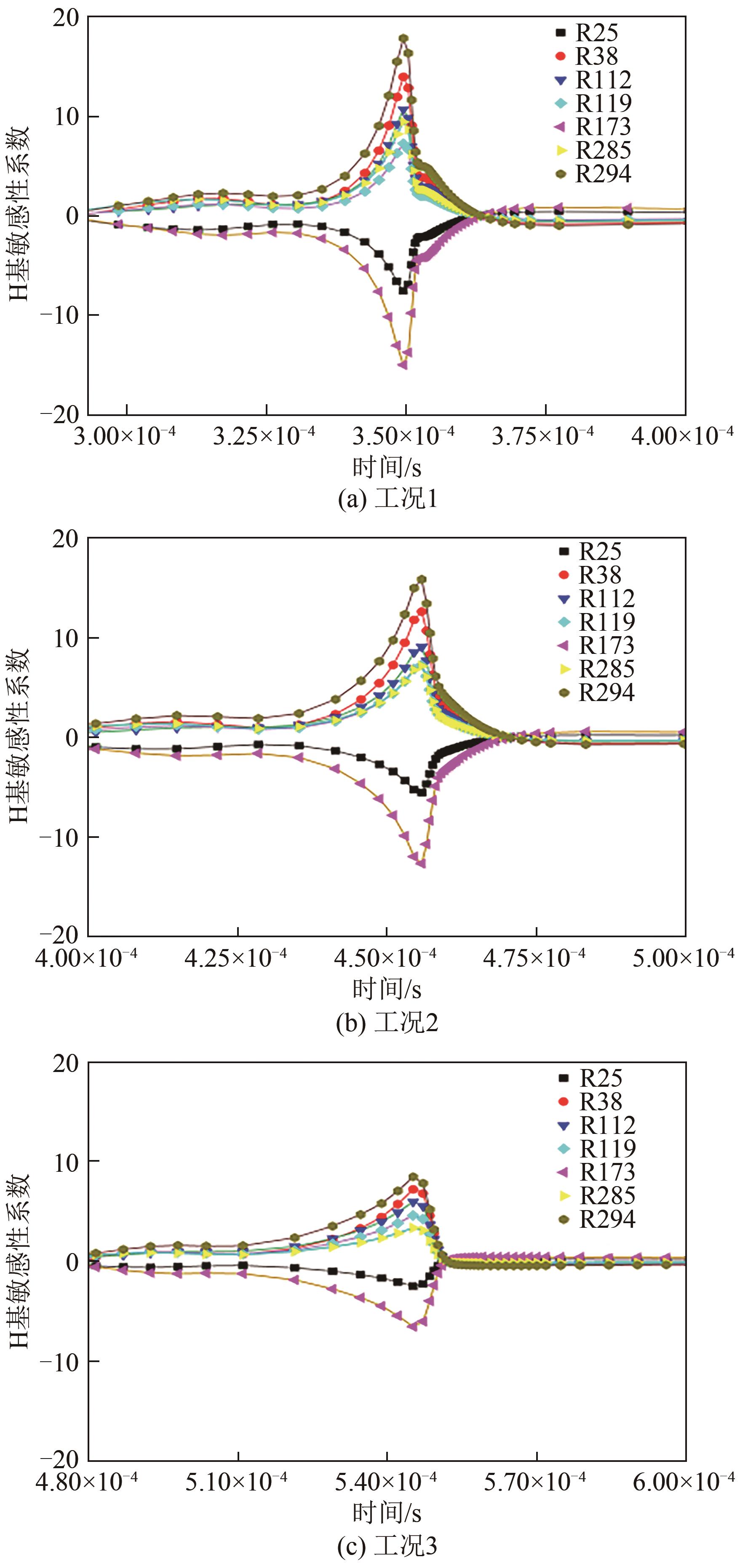
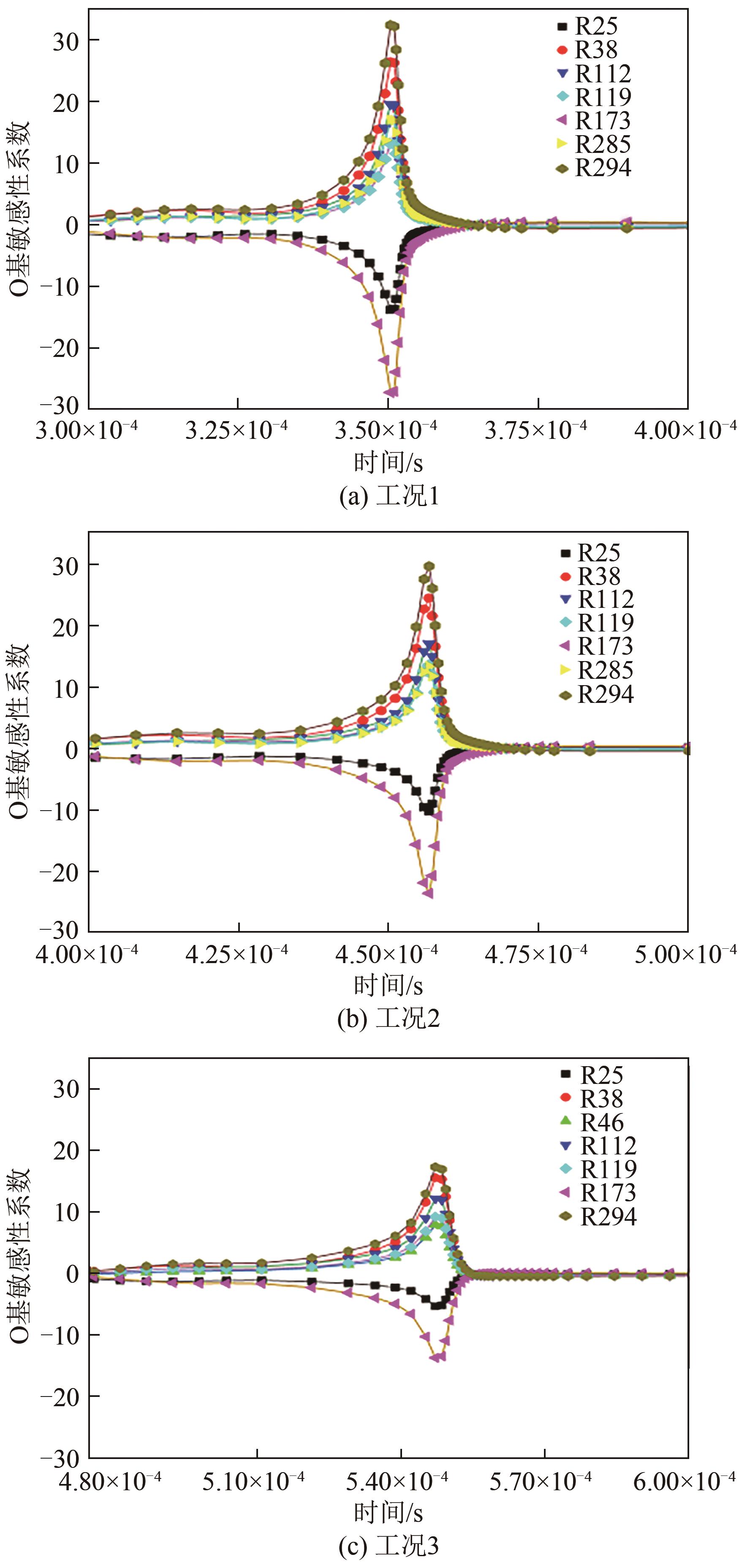
| 反应步 | 方程式 | 指前因子 |
|---|---|---|
| R25 | 1.25×107 | |
| R38 | 2.65×1016 | |
| R46 | 8.40×1013 | |
| R112 | 3.60×106 | |
| R119 | 3.78×1013 | |
| R173 | 4.58×1016 | |
| R285 | 6.70×106 | |
| R294 | 3.03×1011 |
| 反应步 | 方程式 | 指前因子 |
|---|---|---|
| R25 | 1.25×107 | |
| R38 | 2.65×1016 | |
| R46 | 8.40×1013 | |
| R112 | 3.60×106 | |
| R119 | 3.78×1013 | |
| R173 | 4.58×1016 | |
| R285 | 6.70×106 | |
| R294 | 3.03×1011 |
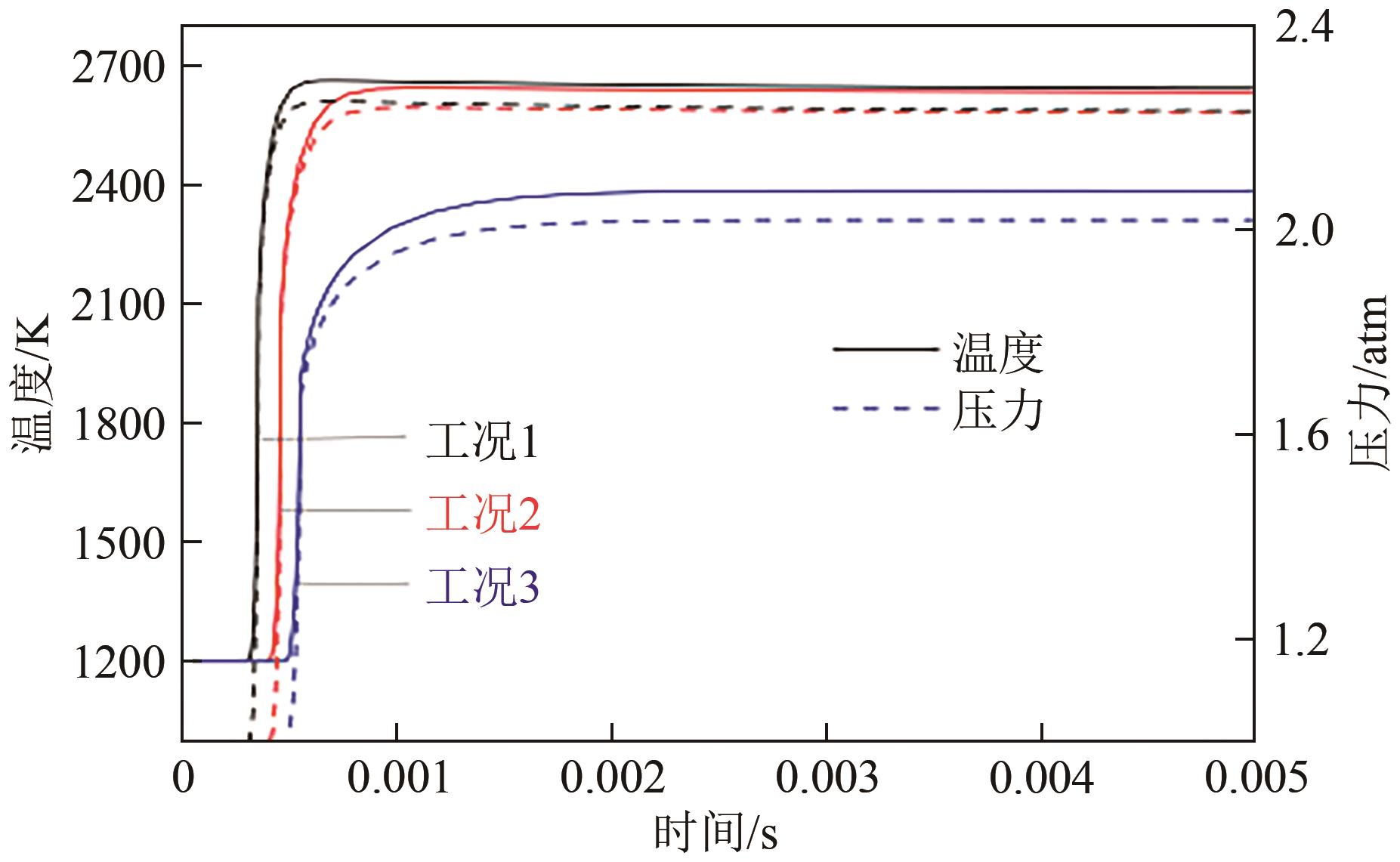
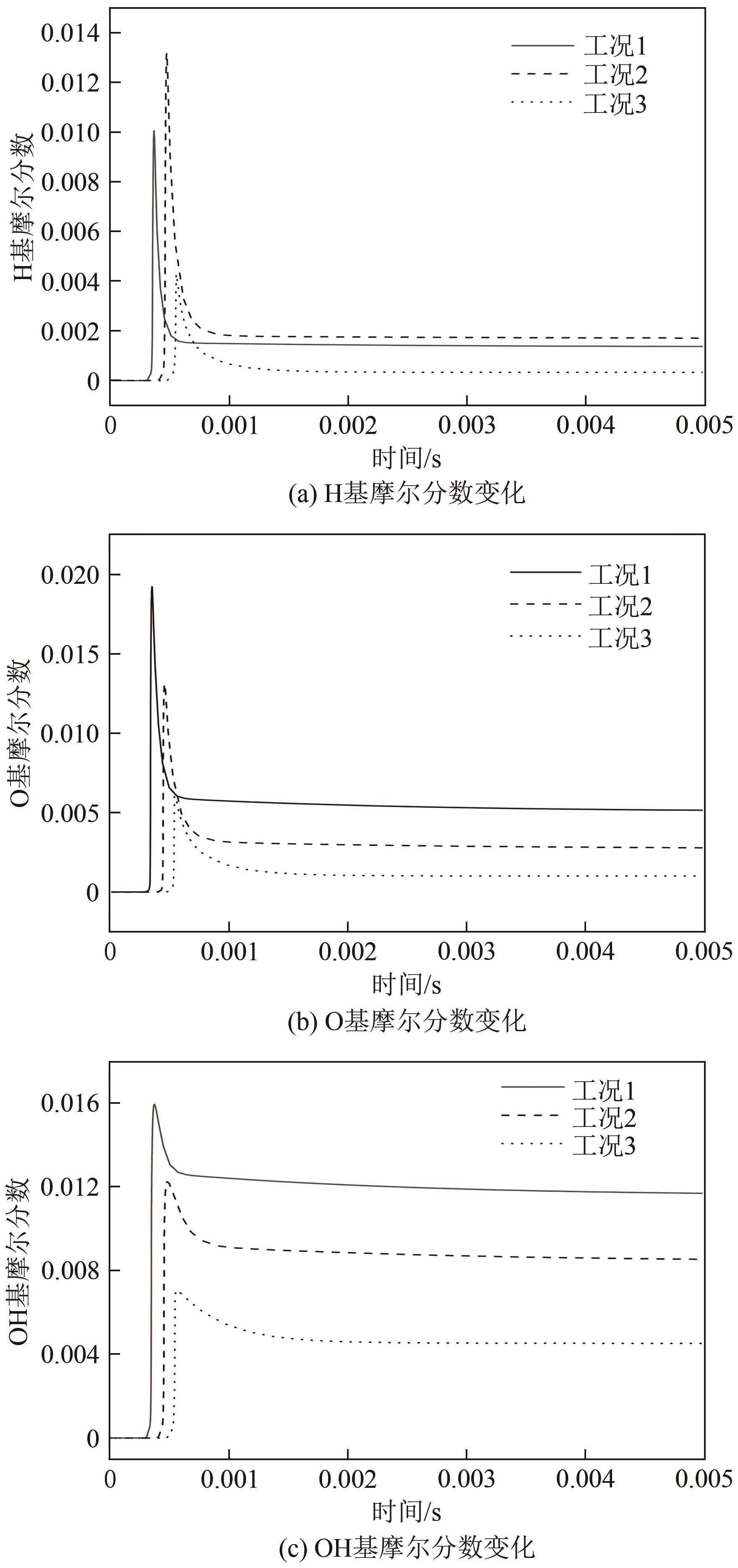
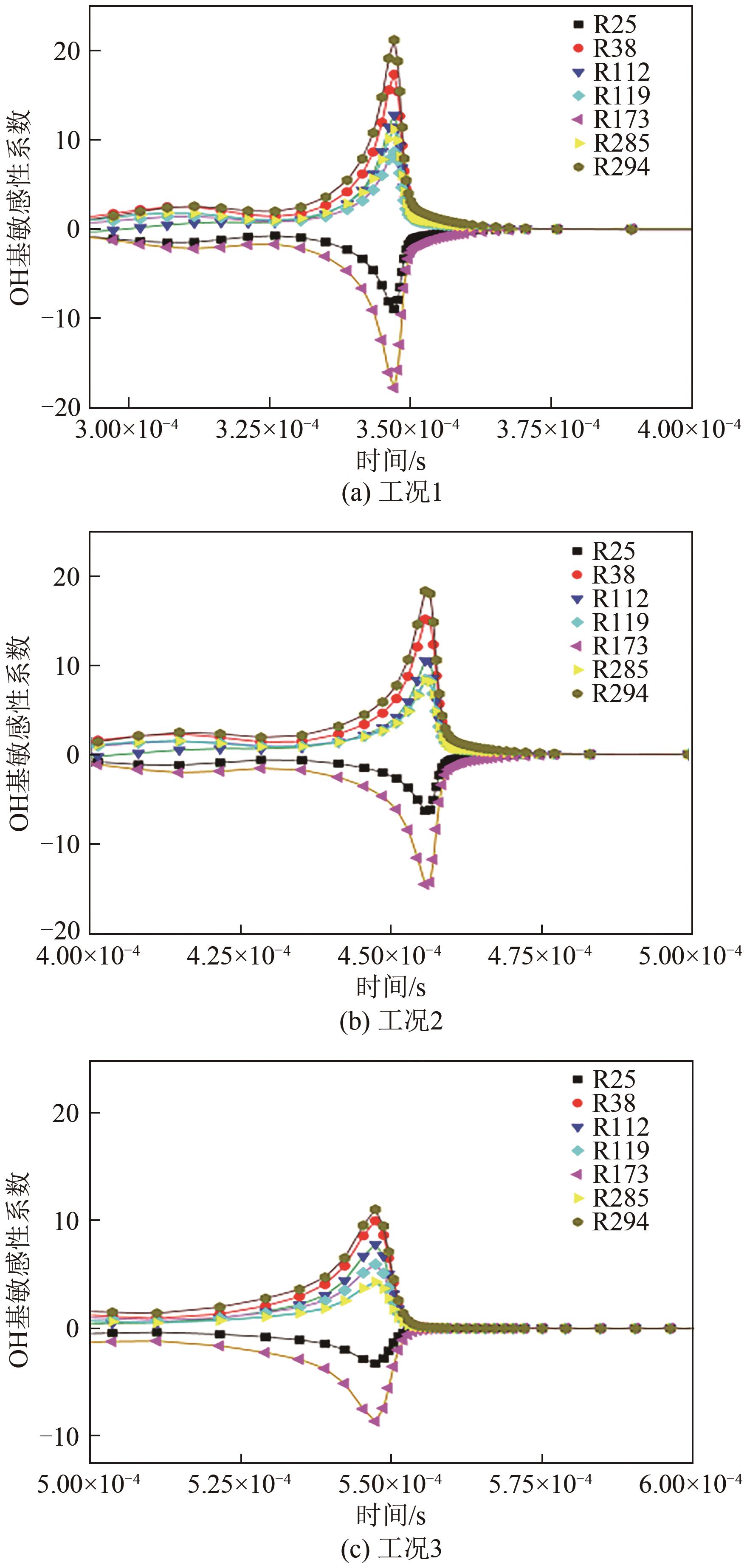
| 序号 | C2H4 | N2 | O2 | N2 | CO2 |
|---|---|---|---|---|---|
| 工况1 | 4 | 76 | 20 | ||
| 工况2 | 4 | 52 | 14 | 30 | |
| 工况3 | 4 | 52 | 14 | 30 |
| 序号 | C2H4 | N2 | O2 | N2 | CO2 |
|---|---|---|---|---|---|
| 工况1 | 4 | 76 | 20 | ||
| 工况2 | 4 | 52 | 14 | 30 | |
| 工况3 | 4 | 52 | 14 | 30 |
| 1 | 罗振敏, 解超, 王九柱, 等. N2和CO2对液化石油气(LPG)惰化抑爆效能对比分析[J]. 化工进展, 2019, 38(6): 2574-2580. |
| LUO Zhenmin, XIE Chao, WANG Jiuzhu, et al. Comparative analysis of the inert effects of N2 and CO2 on LPG explosion[J]. Chemical Industry and Engineering Progress, 2019, 38(6): 2574-2580. | |
| 2 | 罗振敏, 杨勇, 程方明, 等. N2和CO2惰化丙烯爆炸极限参数实验研究[J]. 化工学报, 2020, 71(4): 1922-1928. |
| LUO Zhenmin, YANG Yong, CHENG Fangming, et al. Experimental study on explosion limits parameters of propylene with dilution of nitrogen and carbon dioxide[J]. CIESC Journal, 2020, 71(4): 1922-1928. | |
| 3 | 周宁, 李海涛, 任常兴, 等. 氮气、二氧化碳对液化石油气的惰化抑爆研究[J]. 消防科学与技术, 2016, 35(6): 733-737. |
| ZHOU Ning, LI Haitao, REN Changxing, et al. The liquefied petroleum gas inert gas explosion suppression about of nitrogen and carbon dioxide[J]. Fire Science and Technology, 2016, 35(6): 733-737. | |
| 4 | 周宁, 李海涛, 任常兴, 等. 多元混合气体爆炸特性及惰化防爆研究[J]. 安全与环境学报, 2018, 18(1): 165-171. |
| ZHOU Ning, LI Haitao, REN Changxing, et al. On the bursting and explosion characteristic features and inert inhibition of the mixed gas[J]. Journal of Safety and Environment, 2018, 18(1): 165-171. | |
| 5 | 周宁, 王宇飞, 赵会军, 等. 惰化系统极限氧浓度变化规律及监测预警研究[J]. 中国安全科学学报, 2018, 28(1): 75-80. |
| ZHOU Ning, WANG Yufei, ZHAO Huijun, et al. Study on change regulation of limit oxygen concentration and monitoring early warning system for inerting system[J]. China Safety Science Journal, 2018, 28(1): 75-80. | |
| 6 | TREVIÑO C, MÉNDEZ F. Reduced kinetic mechanism for methane ignition[J]. Symposium (International) on Combustion, 1992, 24(1): 121-127. |
| 7 | MEDVEDEV V G, TELEGIN V G, TELEGIN G G. Statistical analysis of kinetics of an adiabatic thermal explosion[J]. Combustion, Explosion, and Shock Waves, 2009, 45(3): 274-277. |
| 8 | NIE Baisheng, YANG Longlong, GE Boqing, et al. Chemical kinetic characteristics of methane/air mixture explosion and its affecting factors[J]. Journal of Loss Prevention in the Process Industries, 2017, 49:675-682. |
| 9 | 罗振敏, 张群, 王华, 等. 基于FLACS的受限空间瓦斯爆炸数值模拟[J]. 煤炭学报, 2013, 38(8): 1381-1387. |
| LUO Zhenmin, ZHANG Qun, WANG Hua, et al. Numerical simulation of gas explosion in confined space with FLACS[J]. Journal of China Coal Society, 2013, 38(8): 1381-1387. | |
| 10 | 罗振敏, 邓军, 郭晓波. 基于Gaussian的瓦斯爆炸微观反应机理[J]. 辽宁工程技术大学学报(自然科学版), 2008, 27(3): 325-328. |
| LUO Zhenmin, DENG Jun, GUO Xiaobo. Microcosmic mechanism of gas explosion based on Gaussian[J]. Journal of Liaoning Technical University (Natural Science), 2008, 27(3): 325-328. | |
| 11 | 梁运涛, 曾文. 定容燃烧弹中瓦斯爆炸的反应动力学模拟[J]. 燃烧科学与技术, 2010, 16(4): 375-381. |
| LIANG Yuntao, ZENG Wen. Kinetic simulation of gas explosion in constant volume bomb[J]. Journal of Combustion Science and Technology, 2010, 16(4): 375-381. | |
| 12 | 梁运涛, 王连聪, 罗海珠, 等. 定容燃烧反应器中瓦斯爆炸反应动力学计算模型[J]. 煤炭学报, 2015, 40(8): 1853-1858. |
| LIANG Yuntao, Wang Liancong, Luo Haizhu, et al. Computational model of reaction kinetic for gas explosion in constant volume combustion reactor[J]. Journal of China Coal Society, 2015, 40(8): 1853-1858. | |
| 13 | 贾宝山, 李艳红, 曾文, 等. 定容体系中氮气影响瓦斯爆炸反应的动力学模拟[J]. 过程工程学报, 2011, 11(5): 812-817. |
| JIA Baoshan, LI Yanhong, ZENG Wen, et al. Kinetic simulation for the effect of N2 content on gas explosion in an constant volume system[J]. The Chinese Journal of Process Engineering, 2011, 11(5): 812-817. | |
| 14 | 贾宝山, 温海燕, 梁运涛, 等. 煤矿巷道内N2及CO2抑制瓦斯爆炸的机理特性[J]. 煤炭学报, 2013, 38(3): 361-366. |
| JIA Baoshan, WEN Haiyan, Liang Yuntao, et al. Mechanism characteristics of CO2 and N2 inhibiting methane explosions in coal mine roadways[J]. Journal of China Coal Society, 2013, 38(3): 361-366. | |
| 15 | 贾宝山, 胡如霞, 李春苗, 等. 受限空间内C2H6对瓦斯爆炸的促进机理研究[J]. 中国安全生产科学技术, 2015, 11(10): 17-22. |
| JIA Baoshan, HU Ruxia, LI Chunmiao, et al. Study on promoting mechanism of C2H6 on gas explosion in confined space[J]. Journal of Safety Science and Technology, 2015, 11(10): 17-22. | |
| 16 | 贾宝山, 李艳红, 曾文, 等. 受限空间瓦斯爆炸链式反应机理的敏感性分析[J]. 环境工程, 2011, 29(S1): 318-323. |
| JIA Baoshan, LI Yanhong, ZENG Wen, et al. Sensitive analysis of chain reaction mechanism of gas explosion[J]. Environmental Engineering, 2011, 29(S1): 318-323. | |
| 17 | 贾宝山, 温海燕, 梁运涛, 等. 受限空间瓦斯爆炸与氢气促进机理研究[J]. 中国安全科学学报, 2012, 22(2): 81-87. |
| JIA Baoshan, WEN Haiyan, LIANG Yuntao, et al. Study on the methane explosion in an enclosed space and hydrogen promoting mechanism[J]. China Safety Science Journal, 2012, 22(2): 81-87. | |
| 18 | 贾宝山, 王小云, 张师一, 等. 受限空间中CO与水蒸汽阻尼瓦斯爆炸的反应动力学模拟研究[J]. 火灾科学, 2013, 22(3): 131-139. |
| JIA Baoshan, WANG Xiaoyun, ZHANG Shiyi, et al. Kinetic simulation of CO and water vapor damping the gas explosion in an enclosed space[J]. Fire Safety Science, 2013, 22(3): 131-139. | |
| 19 | 李孝斌, 李会荣, 何昆, 等. 甲烷爆炸感应期内CN/CH/CHO/CH2O/NCN特征光谱分析[J]. 煤炭学报, 2014, 39(10): 2042-2046. |
| LI Xiaobin, LI Huirong, HE Kun, et al. Analysis of characteristic spectrum of CN/CH/CHO/CH2O/NCN in induction period of methane explosion[J]. Journal of China Coal Society, 2014, 39(10): 2042-2046. | |
| 20 | 杨春丽, 刘艳, 胡玢, 等. 氮气和水蒸气对瓦斯爆炸基元反应的影响及抑爆机理分析[J]. 高压物理学报, 2017, 31(3): 301-308. |
| YANG Chunli, LIU Yan, HU Bin, et al. Effect of nitrogen and water vapor on methane-air mixture explosion elementary reaction and suppression mechanism[J]. Chinese Journal of High Pressure Physics, 2017, 31(3): 301-308. | |
| 21 | YU Xiaozhe, YAN Xingqing, JI Wentao, et al. Effect of super-ambient conditions on the upper explosion limit of ethane/oxygen and ethylene/oxygen mixtures[J]. Journal of Loss Prevention in the Process Industries, 2019, 59: 100-105. |
| 22 | 罗灿. 高温高压下乙烷、乙烯在纯氧中爆炸极限研究[D]. 大连: 大连理工大学, 2018. |
| LUO Can. Research on the explosion limit of ethane-oxygen and ethylene-oxygen mixtures at elevated temperatures and pressures[D]. Dalian: Dalian University of Technology, 2018. | |
| 23 | 喻健良, 纪文涛, 孙会利, 等. 乙烯/聚乙烯两相体系爆炸特性[J]. 化工学报, 2017, 68(12): 4841-4847. |
| YU Jianliang, JI Wentao, SUN Huili, et al. Explosibility of hybrid mixtures of ethylene and polyethylene dust[J]. CIESC Journal, 2017, 68(12): 4841-4847. | |
| 24 | 杨理, 饶国宁, 解立峰, 等. 扩散时间对乙烯-空气燃爆特性的影响[J]. 高压物理学报, 2015, 29(5): 369-376. |
| YANG Li, RAO Guoning, XIE Lifeng, et al. Effect of diffusion time on deflagration and detonation parameters of ethene-air[J]. Chinese Journal of High Pressure Physics, 2015, 29(5): 369-376. | |
| 25 | 乐慧慧, 张浩, 朱中南, 等. 乙烯-氧-醋酸-氮气-二氧化碳系统爆炸极限的测定研究[J]. 化学反应工程与工艺, 1995, 11(1): 80-85. |
| LE Huihui, ZHANG Hao, ZHU Zhongnan, et al. A study on the explosion limits for the system of ethylene-oxygen-acetic acid nitrogen-carbon dioxide[J].Chemical Reaction Engineering and Technology, 1995, 11(1): 80-85. | |
| 26 | HOLTAPPELS K, BRINKMANN C, DIETLEN S, et al. Measurement and prediction of the inert gas influence on explosion limits for ethylene/nitrogen/air and ethylene/carbon-dioxide/air mixtures at elevated pressures[J]. Chemical Engineering & Technology, 2001, 24(12): 1263-1267. |
| 27 | 张欣, 任常兴, 张琰, 等. 三种测试装置与判定标准对比研究可燃气体爆炸[J]. 消防科学与技术, 2018, 37(7): 863-866. |
| ZHANG Xin, REN Changxing, ZHANG Yan, et al. Research on flammable gas by three test devices and three criterions[J]. Fire Science and Technology, 2018, 37(7): 863-866. | |
| 28 | LUTZ A E, KEE R J, MILLER J A. SENKIN: A Fortran program for predicting homogeneous gas phase chemical kinetics with sensitivity analysis[R]. Sandia National Laboratories Report, 1987. |
| 29 | RODAT S, ABANADES S, COULIÉ J, et al. Kinetic modelling of methane decomposition in a tubular solar reactor[J]. Chemical Engineering Journal, 2009, 146(1): 120-127. |
| 30 | CARRASCO N, ALCARAZ C, DUTUIT O, et al. Sensitivity of a Titan ionospheric model to the ion-molecule reaction parameters[J]. Planetary and Space Science, 2008, 56(12): 1644-1657. |
| 31 | RASMUSSEN C L, RASMUSSEN A E, GLARBORG P. Sensitizing effects of NO x on CH4 oxidation at high pressure[J]. Combustion and Flame, 2008, 154(3): 529-545. |
| 32 | GREGORY P, SMITH D G. Gri-Mech at 3.0[EB/OL]. , 2007. |
| 33 | 韩美. 新的爆炸危险度F值[J]. 低温与特气, 1995, 13(2): 57-60. |
| HAN Mei. A new explosive hazard degree F [J]. Low Temperature and Specialty Gases, 1995, 13(2): 57-60. |
| [1] | ZHAO Chen, MIAO Tianze, ZHANG Chaoyang, HONG Fangjun, WANG Dahai. Heat transfer characteristics of ethylene glycol aqueous solution in slit channel under negative pressure [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 148-157. |
| [2] | YANG Hanyue, KONG Lingzhen, CHEN Jiaqing, SUN Huan, SONG Jiakai, WANG Sicheng, KONG Biao. Decarbonization performance of downflow tubular gas-liquid contactor of microbubble-type [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 197-204. |
| [3] | ZHENG Qian, GUAN Xiushuai, JIN Shanbiao, ZHANG Changming, ZHANG Xiaochao. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. |
| [4] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [5] | WANG Peng, ZHANG Yang, FAN Bingqiang, HE Dengbo, SHEN Changshuai, ZHANG Hedong, ZHENG Shili, ZOU Xing. Process and kinetics of hydrochloric acid leaching of high-carbon ferrochromium [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 510-517. |
| [6] | SUN Yuyu, CAI Xinlei, TANG Jihai, HUANG Jingjing, HUANG Yiping, LIU Jie. Optimization and energy-saving of a reactive distillation process for the synthesis of methyl methacrylate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 56-63. |
| [7] | BAI Zhihua, ZHANG Jun. Oxidative removal of NO in DTPMPA/Fenton system [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4967-4973. |
| [8] | LAI Shini, JIANG Lixia, LI Jun, HUANG Hongyu, KOBAYASHI Noriyuki. Research progress of ammonia blended fossil fuel [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4603-4615. |
| [9] | ZHANG Zhichen, ZHU Yunfeng, CHENG Weishu, MA Shoutao, JIANG Jie, SUN Bing, ZHOU Zichen, XU Wei. Research advances on runaway decomposition of high pressure polyethylene: Reaction mechanism, initiation system and model [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3979-3989. |
| [10] | WANG Yaogang, HAN Zishan, GAO Jiachen, WANG Xinyu, LI Siqi, YANG Quanhong, WENG Zhe. Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4043-4057. |
| [11] | LIU Yi, FANG Qiang, ZHONG Dazhong, ZHAO Qiang, LI Jinping. Cu facets regulation of Ag/Cu coupled catalysts for electrocatalytic reduction of carbon dioxide [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4136-4142. |
| [12] | GUO Jin, ZHANG Geng, CHEN Guohua, ZHU Ming, TAN Yue, LI Wei, XIA Li, HU Kun. Research progress on vehicle liquid hydrogen cylinder design [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4221-4229. |
| [13] | HUANG Yufei, LI Ziyi, HUANG Yangqiang, JIN Bo, LUO Xiao, LIANG Zhiwu. Research progress on catalysts for photocatalytic CO2 and CH4 reforming [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4247-4263. |
| [14] | LI Yanling, ZHUO Zhen, CHI Liang, CHEN Xi, SUN Tanglei, LIU Peng, LEI Tingzhou. Research progress on preparation and application of nitrogen-doped biochar [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3720-3735. |
| [15] | LI Jia, FAN Xing, CHEN Li, LI Jian. Research progress of simultaneous removal of NO x and N2O from the tail gas of nitric acid production [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3770-3779. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||