Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (4): 2161-2170.DOI: 10.16085/j.issn.1000-6613.2021-0823
• Resources and environmental engineering • Previous Articles Next Articles
Physical and chemical properties analysis of electrolytic manganese residue in different storage times
DENG Yaling1( ), SHU Jiancheng1(
), SHU Jiancheng1( ), CHEN Mengjun1, LEI Tianya1, ZENG Xiangfei1, YANG Yong2, LIU Zuohua2
), CHEN Mengjun1, LEI Tianya1, ZENG Xiangfei1, YANG Yong2, LIU Zuohua2
- 1.School of Environment and Resource, Southwest University of Science and Technology, Mianyang 621000, Sichuan, China
2.School of Chemistry and Chemical Engineering, Chongqing University, Chongqing 401311, China
-
Received:2021-04-19Revised:2021-06-21Online:2022-04-25Published:2022-04-23 -
Contact:SHU Jiancheng
不同堆存时间电解锰渣的理化特性分析
邓亚玲1( ), 舒建成1(
), 舒建成1( ), 陈梦君1, 雷天涯1, 曾祥菲1, 杨勇2, 刘作华2
), 陈梦君1, 雷天涯1, 曾祥菲1, 杨勇2, 刘作华2
- 1.西南科技大学环境与资源学院,四川 绵阳 621000
2.重庆大学化学化工学院,重庆 401331
-
通讯作者:舒建成 -
作者简介:邓亚玲(1997—),女,硕士研究生,研究方向为电解锰渣污染治理。E-mail:2533202812@qq.com 。 -
基金资助:国家自然科学基金(21806132);国家重点研发计划(2018YFC1903500)
CLC Number:
Cite this article
DENG Yaling, SHU Jiancheng, CHEN Mengjun, LEI Tianya, ZENG Xiangfei, YANG Yong, LIU Zuohua. Physical and chemical properties analysis of electrolytic manganese residue in different storage times[J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2161-2170.
邓亚玲, 舒建成, 陈梦君, 雷天涯, 曾祥菲, 杨勇, 刘作华. 不同堆存时间电解锰渣的理化特性分析[J]. 化工进展, 2022, 41(4): 2161-2170.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-0823
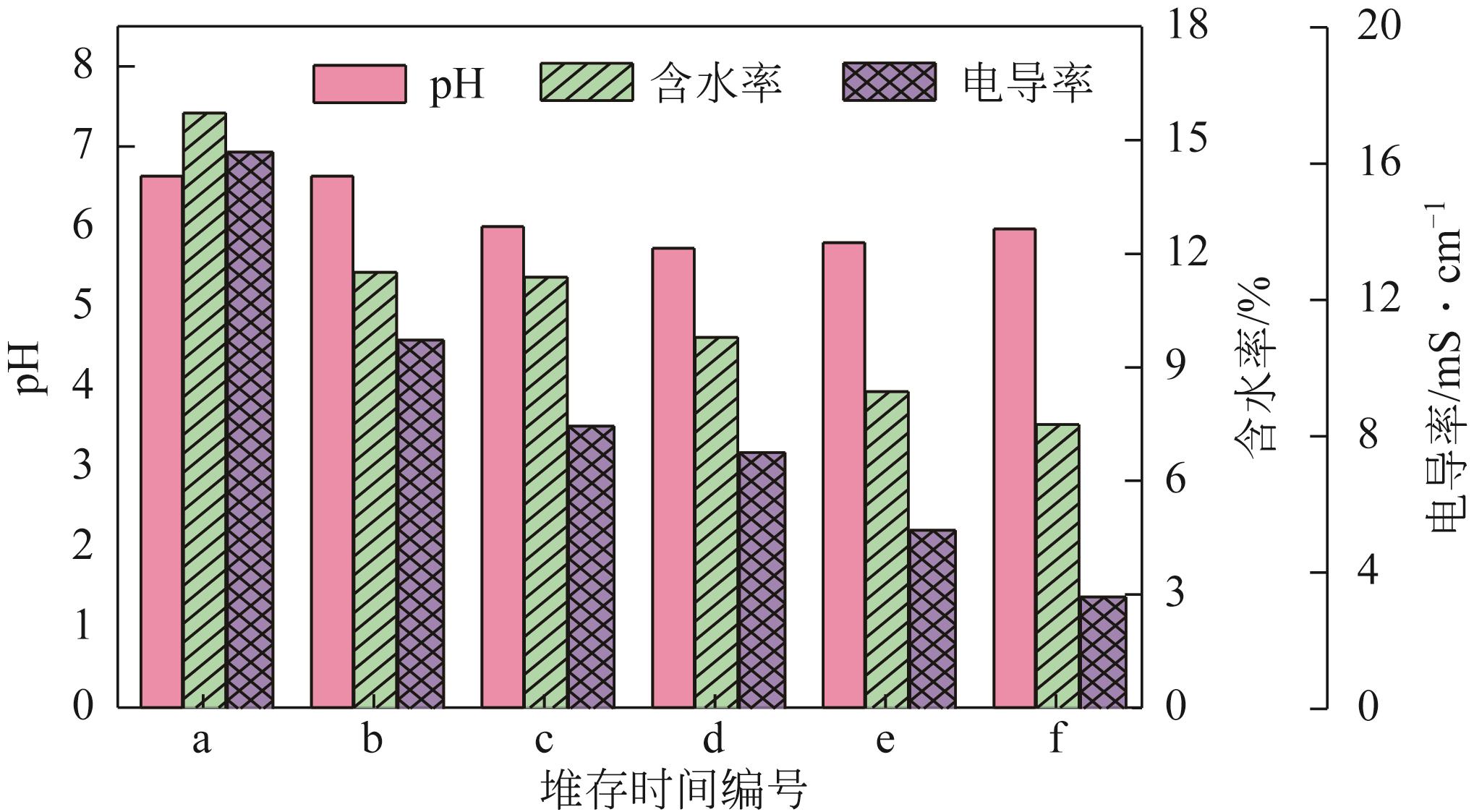
堆存时间 编号 | Mn | Ca | Mg | Se | Cu | Cr | Cd | Pb | Zn | Fe | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | 71700 | 102500 | 18105 | 480 | 550 | 274 | 250 | 350 | 895 | 56400 | 13010 |
| b | 58150 | 90600 | 14955 | 340 | 535 | 295 | 150 | 345 | 1020 | 56600 | 14905 |
| c | 51200 | 74100 | 9960 | 305 | 521 | 240 | 120 | 380 | 695 | 59100 | 19790 |
| d | 37505 | 83250 | 8615 | 290 | 512 | 225 | 135 | 362 | 562 | 56650 | 16275 |
| e | 34960 | 96600 | 8165 | 260 | 523 | 230 | 110 | 350 | 600 | 68550 | 18145 |
| f | 29865 | 81350 | 8205 | 190 | 518 | 200 | 115 | 341 | 553 | 73850 | 22255 |
堆存时间 编号 | Mn | Ca | Mg | Se | Cu | Cr | Cd | Pb | Zn | Fe | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | 71700 | 102500 | 18105 | 480 | 550 | 274 | 250 | 350 | 895 | 56400 | 13010 |
| b | 58150 | 90600 | 14955 | 340 | 535 | 295 | 150 | 345 | 1020 | 56600 | 14905 |
| c | 51200 | 74100 | 9960 | 305 | 521 | 240 | 120 | 380 | 695 | 59100 | 19790 |
| d | 37505 | 83250 | 8615 | 290 | 512 | 225 | 135 | 362 | 562 | 56650 | 16275 |
| e | 34960 | 96600 | 8165 | 260 | 523 | 230 | 110 | 350 | 600 | 68550 | 18145 |
| f | 29865 | 81350 | 8205 | 190 | 518 | 200 | 115 | 341 | 553 | 73850 | 22255 |
| 评价标准 | Cu | Cr | Cd | Pb | Zn | Mn | ||
|---|---|---|---|---|---|---|---|---|
| 土壤质量标准 | Ⅰ | 自然背景 | 35 | 90 | 0.2 | 35 | 100 | — |
| <6.5 | 50 | 150 | 0.3 | 250 | 200 | — | ||
| Ⅱ | 6.5~7.5 | 100 | 200 | 0.3 | 300 | 250 | — | |
| >7.5 | 100 | 250 | 0.6 | 350 | 300 | — | ||
| Ⅲ | >6.5 | 400 | 300 | 1 | 500 | 500 | — | |
| 广西土壤背景值 | 23.1 | 65.3 | 0.073 | 19.5 | 51.8 | 176 | ||
| 评价标准 | Cu | Cr | Cd | Pb | Zn | Mn | ||
|---|---|---|---|---|---|---|---|---|
| 土壤质量标准 | Ⅰ | 自然背景 | 35 | 90 | 0.2 | 35 | 100 | — |
| <6.5 | 50 | 150 | 0.3 | 250 | 200 | — | ||
| Ⅱ | 6.5~7.5 | 100 | 200 | 0.3 | 300 | 250 | — | |
| >7.5 | 100 | 250 | 0.6 | 350 | 300 | — | ||
| Ⅲ | >6.5 | 400 | 300 | 1 | 500 | 500 | — | |
| 广西土壤背景值 | 23.1 | 65.3 | 0.073 | 19.5 | 51.8 | 176 | ||
| 堆存时间编号 | Mn2+ | NH | Se4+ | Pb2+ | Cd2+ | 总Cr | Cu2+ | Zn2+ | Ni2+ |
|---|---|---|---|---|---|---|---|---|---|
| a | 1533 | 6675 | 8.7 | 0.73 | 0.12 | 0.32 | 45.3 | 38.1 | 3.5 |
| b | 859.3 | 6425 | 6.4 | 0.62 | 0.15 | 0.45 | 36.5 | 30.3 | 3.8 |
| c | 777.4 | 5675 | 15.2 | 0.63 | 0.26 | 0.41 | 37.2 | 27.2 | 4.2 |
| d | 772.5 | 2175 | 11.9 | 0.74 | 0.21 | 0.32 | 38.6 | 26.9 | 4.3 |
| e | 509.6 | 925 | 10.9 | 0.82 | 0.39 | 0.30 | 39.3 | 30.8 | 3.8 |
| f | 204.4 | 675 | 11.4 | 0.76 | 0.35 | 0.25 | 37.5 | 29.8 | 3.1 |
| GB 8978—1996 一级 | 2.0 | 15.0 | — | 1.0 | 0.1 | 1.5 | 0.5 | 2.0 | 1.0 |
| 堆存时间编号 | Mn2+ | NH | Se4+ | Pb2+ | Cd2+ | 总Cr | Cu2+ | Zn2+ | Ni2+ |
|---|---|---|---|---|---|---|---|---|---|
| a | 1533 | 6675 | 8.7 | 0.73 | 0.12 | 0.32 | 45.3 | 38.1 | 3.5 |
| b | 859.3 | 6425 | 6.4 | 0.62 | 0.15 | 0.45 | 36.5 | 30.3 | 3.8 |
| c | 777.4 | 5675 | 15.2 | 0.63 | 0.26 | 0.41 | 37.2 | 27.2 | 4.2 |
| d | 772.5 | 2175 | 11.9 | 0.74 | 0.21 | 0.32 | 38.6 | 26.9 | 4.3 |
| e | 509.6 | 925 | 10.9 | 0.82 | 0.39 | 0.30 | 39.3 | 30.8 | 3.8 |
| f | 204.4 | 675 | 11.4 | 0.76 | 0.35 | 0.25 | 37.5 | 29.8 | 3.1 |
| GB 8978—1996 一级 | 2.0 | 15.0 | — | 1.0 | 0.1 | 1.5 | 0.5 | 2.0 | 1.0 |
| 堆存时间编号 | 总量锰/mg·kg-1 | 可溶性锰/mg·L-1 | 锰溶出率/% |
|---|---|---|---|
| a | 71700 | 1533 | 19.2 |
| b | 58150 | 859.3 | 13.3 |
| c | 51200 | 777.4 | 13.7 |
| d | 37505 | 772.5 | 18.5 |
| e | 34960 | 509.6 | 13.1 |
| f | 29865 | 204.4 | 6.2 |
| 堆存时间编号 | 总量锰/mg·kg-1 | 可溶性锰/mg·L-1 | 锰溶出率/% |
|---|---|---|---|
| a | 71700 | 1533 | 19.2 |
| b | 58150 | 859.3 | 13.3 |
| c | 51200 | 777.4 | 13.7 |
| d | 37505 | 772.5 | 18.5 |
| e | 34960 | 509.6 | 13.1 |
| f | 29865 | 204.4 | 6.2 |
| 项目 | 总量锰 | 可溶性锰 |
|---|---|---|
| 总量锰 | 1 | |
| 可溶性锰 | 0.009** | 1 |
| 项目 | 总量锰 | 可溶性锰 |
|---|---|---|
| 总量锰 | 1 | |
| 可溶性锰 | 0.009** | 1 |
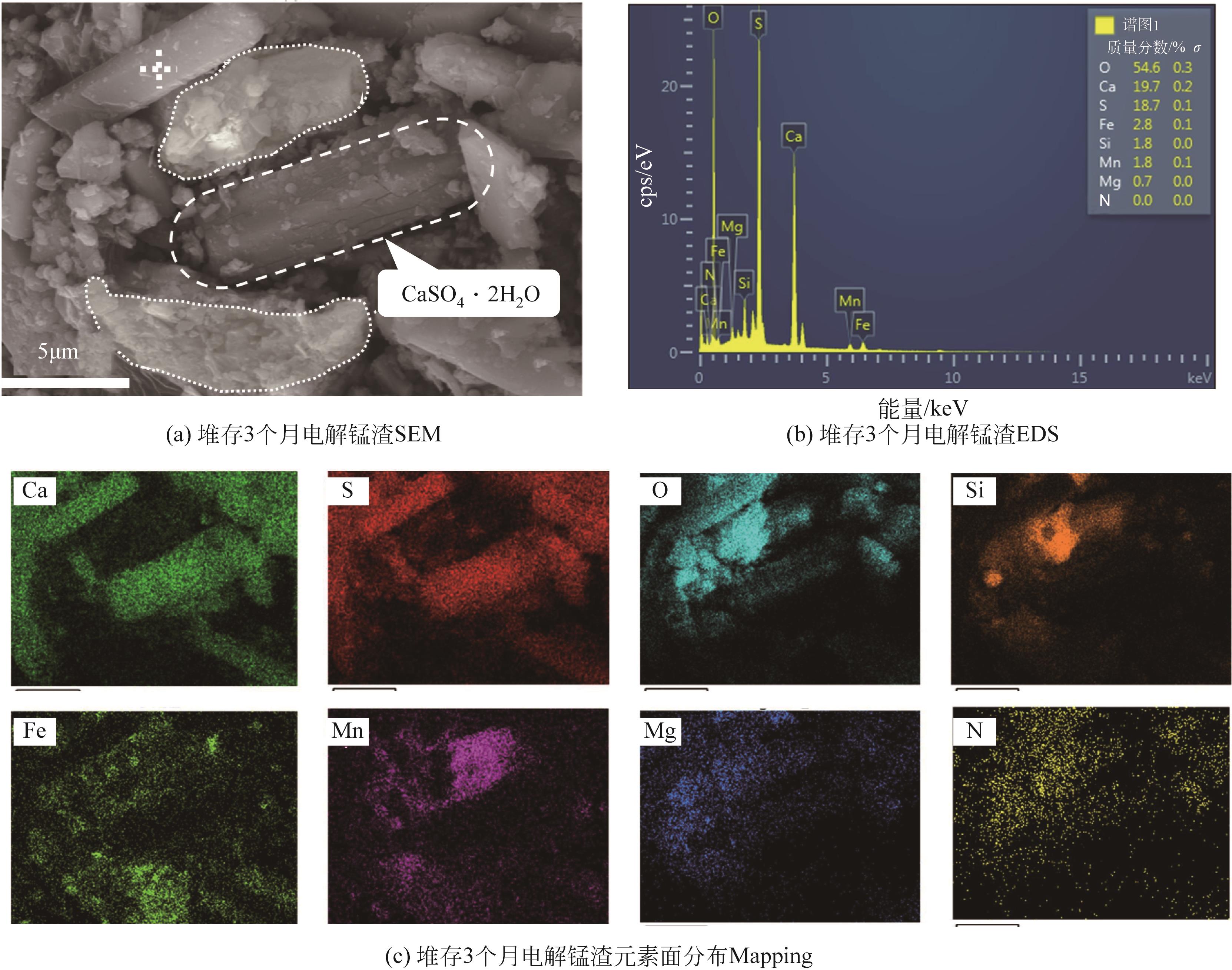
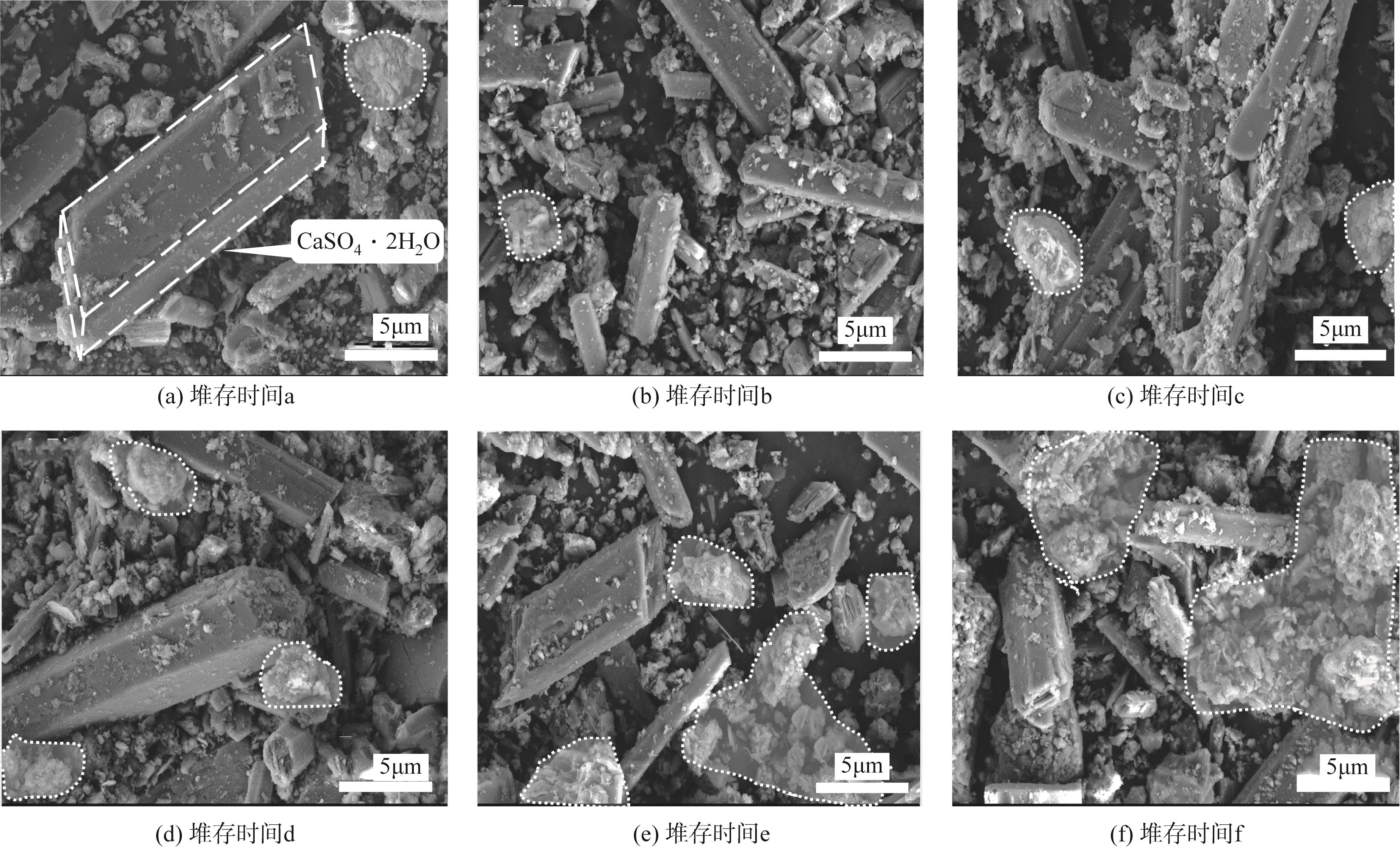
堆存时间 编号 | SO3 | SiO2 | CaO | Fe2O3 | MnO | Al2O3 | MgO | K2O | P2O5 | TiO2 | BaO | 其他 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | 32.43 | 26.6 | 16.23 | 9.78 | 7.97 | 2.82 | 2.48 | 0.74 | 0.33 | 0.17 | 0.18 | 0.27 |
| b | 31.72 | 25.24 | 17.31 | 10.40 | 8.76 | 2.69 | 2.03 | 0.78 | 0.35 | 0.19 | 0.21 | 0.32 |
| c | 30.18 | 25.76 | 16.38 | 13.29 | 7.79 | 3.31 | 1.43 | 0.85 | 0.39 | 0.22 | 0.21 | 0.19 |
| d | 31.29 | 28.07 | 17.52 | 10.37 | 5.95 | 3.32 | 1.65 | 0.89 | 0.35 | 0.17 | 0.22 | 0.20 |
| e | 31.43 | 27.27 | 19.32 | 10.20 | 5.19 | 3.60 | 1.35 | 0.73 | 0.34 | 0.21 | 0.17 | 0.19 |
| f | 28.29 | 33.12 | 16.98 | 9.80 | 4.02 | 4.50 | 1.32 | 1.03 | 0.30 | 0.26 | 0.21 | 0.17 |
堆存时间 编号 | SO3 | SiO2 | CaO | Fe2O3 | MnO | Al2O3 | MgO | K2O | P2O5 | TiO2 | BaO | 其他 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | 32.43 | 26.6 | 16.23 | 9.78 | 7.97 | 2.82 | 2.48 | 0.74 | 0.33 | 0.17 | 0.18 | 0.27 |
| b | 31.72 | 25.24 | 17.31 | 10.40 | 8.76 | 2.69 | 2.03 | 0.78 | 0.35 | 0.19 | 0.21 | 0.32 |
| c | 30.18 | 25.76 | 16.38 | 13.29 | 7.79 | 3.31 | 1.43 | 0.85 | 0.39 | 0.22 | 0.21 | 0.19 |
| d | 31.29 | 28.07 | 17.52 | 10.37 | 5.95 | 3.32 | 1.65 | 0.89 | 0.35 | 0.17 | 0.22 | 0.20 |
| e | 31.43 | 27.27 | 19.32 | 10.20 | 5.19 | 3.60 | 1.35 | 0.73 | 0.34 | 0.21 | 0.17 | 0.19 |
| f | 28.29 | 33.12 | 16.98 | 9.80 | 4.02 | 4.50 | 1.32 | 1.03 | 0.30 | 0.26 | 0.21 | 0.17 |
| XPS扫描区域 | 堆存时间 编号 | 参数 | FeOOH | Fe3O4 | γ-Fe2O3 | 卫星峰 |
|---|---|---|---|---|---|---|
| Fe 2p3/2 | a | 结合能/eV | 711.56 | 714.61 | 717.68 | 720.27 |
| 含量/% | 57.51 | 14.31 | 14.31 | |||
| b | 结合能/eV | 711.47 | 714.35 | 717.21 | 721.14 | |
| 含量/% | 56.68 | 14.54 | 15.82 | |||
| c | 结合能/eV | 711.23 | 713.87 | 716.56 | 720.22 | |
| 含量/% | 56.41 | 15.52 | 16.26 | |||
| d | 结合能/eV | 711.48 | 714.1 | 716.81 | 720.92 | |
| 含量/% | 55.56 | 14.33 | 16.24 | |||
| e | 结合能/eV | 711.39 | 714.21 | 717.21 | 721.96 | |
| 含量/% | 55.04 | 16.23 | 15.72 | |||
| f | 结合能/eV | 711.64 | 714.33 | 718.26 | 721.23 | |
| 含量/% | 49.18 | 24.96 | 14.10 |
| XPS扫描区域 | 堆存时间 编号 | 参数 | FeOOH | Fe3O4 | γ-Fe2O3 | 卫星峰 |
|---|---|---|---|---|---|---|
| Fe 2p3/2 | a | 结合能/eV | 711.56 | 714.61 | 717.68 | 720.27 |
| 含量/% | 57.51 | 14.31 | 14.31 | |||
| b | 结合能/eV | 711.47 | 714.35 | 717.21 | 721.14 | |
| 含量/% | 56.68 | 14.54 | 15.82 | |||
| c | 结合能/eV | 711.23 | 713.87 | 716.56 | 720.22 | |
| 含量/% | 56.41 | 15.52 | 16.26 | |||
| d | 结合能/eV | 711.48 | 714.1 | 716.81 | 720.92 | |
| 含量/% | 55.56 | 14.33 | 16.24 | |||
| e | 结合能/eV | 711.39 | 714.21 | 717.21 | 721.96 | |
| 含量/% | 55.04 | 16.23 | 15.72 | |||
| f | 结合能/eV | 711.64 | 714.33 | 718.26 | 721.23 | |
| 含量/% | 49.18 | 24.96 | 14.10 |
| XPS扫描区域 | 堆存时间编号 | 参数 | Mn2O3 | MnO2 | KMnO4 |
|---|---|---|---|---|---|
| Mn 2p3/2 | a | 结合能/eV | 641.44 | 642.67 | 646.7 |
| 含量/% | 19.53 | 64.16 | 16.31 | ||
| b | 结合能/eV | 641.57 | 643.23 | 646.85 | |
| 含量/% | 42.6 | 42.61 | 14.79 | ||
| c | 结合能/eV | 641.73 | 643.43 | 646.77 | |
| 含量/% | 50.29 | 34.92 | 14.79 | ||
| d | 结合能/eV | 641.5 | 643.28 | 646.82 | |
| 含量/% | 50.42 | 36.39 | 13.19 | ||
| e | 结合能/eV | 641.85 | 644.08 | 647.49 | |
| 含量/% | 54.09 | 29.28 | 16.63 | ||
| f | 结合能/eV | 641.88 | 643.87 | 647.2 | |
| 含量/% | 59.84 | 25.88 | 14.28 |
| XPS扫描区域 | 堆存时间编号 | 参数 | Mn2O3 | MnO2 | KMnO4 |
|---|---|---|---|---|---|
| Mn 2p3/2 | a | 结合能/eV | 641.44 | 642.67 | 646.7 |
| 含量/% | 19.53 | 64.16 | 16.31 | ||
| b | 结合能/eV | 641.57 | 643.23 | 646.85 | |
| 含量/% | 42.6 | 42.61 | 14.79 | ||
| c | 结合能/eV | 641.73 | 643.43 | 646.77 | |
| 含量/% | 50.29 | 34.92 | 14.79 | ||
| d | 结合能/eV | 641.5 | 643.28 | 646.82 | |
| 含量/% | 50.42 | 36.39 | 13.19 | ||
| e | 结合能/eV | 641.85 | 644.08 | 647.49 | |
| 含量/% | 54.09 | 29.28 | 16.63 | ||
| f | 结合能/eV | 641.88 | 643.87 | 647.2 | |
| 含量/% | 59.84 | 25.88 | 14.28 |
| 1 | 李涛, 马国华. 电解锰渣中锰组分的粉磨浸取工艺研究[J]. 无机盐工业, 2021, 53(2): 66-70. |
| LI Tao, MA Guohua. Research on milling and leaching process of manganese in electrolytic manganese slag[J]. Inorganic Chemicals Industry, 2021, 53(2): 66-70. | |
| 2 | HE Shichao, WILSON B P, LUNDSTRÖM M, et al. Hazard-free treatment of electrolytic manganese residue and recovery of manganese using low temperature roasting-water washing process[J]. Journal of Hazardous Materials, 2021, 402: 220-227. |
| 3 | SHU Jiancheng, CHEN Mengjun, WU Haiping, et al. An innovative method for synergistic stabilization/solidification of Mn2+, NH4 +-N, PO4 3- and F- in electrolytic manganese residue and phosphogypsum[J]. Journal of Hazardous Materials, 2019, 376: 212-222. |
| 4 | 完么东智, 李玉香, 谭宏斌. 高温还原焙烧法脱除电解锰渣中的硫[J]. 非金属矿, 2017, 40(5): 25-28. |
| WANME Dongzhi, LI Yuxiang, TAN Hongbin. Removal of sulfur from electrolytic manganese residue through high temperature reduction roast[J]. Non-Metallic Mines, 2017, 40(5): 25-28. | |
| 5 | 赵侣璇, 刘凯, 覃楠钧, 等. 电解锰渣锰和硫酸铵资源回收及无害化试验研究[J]. 工业安全与环保, 2019, 45(1): 103-106. |
| ZHAO Lyuxuan, LIU Kai, QIN Nanjun, et al. Research of resource recycling and harmless treatment of electrolytic manganese residue[J]. Industrial Safety and Environmental Protection, 2019, 45(1): 103-106. | |
| 6 | LAN Jirong, DONG Yiqie, XIANG Yuwei, et al. Selective recovery of manganese from electrolytic manganese residue by using water as extractant under mechanochemical ball grinding: mechanism and kinetics[J]. Journal of Hazardous Materials, 2021, 415: 125556. |
| 7 | SHU Jiancheng, WU Haiping, LIU Renlong, et al. Simultaneous stabilization/solidification of Mn2+ and NH4 +-N from electrolytic manganese residue using MgO and different phosphate resource[J]. Ecotoxicology and Environmental Safety, 2018, 148: 220-227. |
| 8 | 程淑君, 陶宗硕, 施学宝. 锰渣作水泥混合材的应用研究[J]. 中国建材科技, 2019, 28(4): 48-49. |
| CHENG Shujun, TAO Zongshuo, SHI Xuebao. Study on application of manganese slag as cement mixture[J]. China Building Materials Science and Technology, 2019, 28(4): 48-49. | |
| 9 | 蒋勇, 文梦媛, 贾陆军. 电解锰渣的预处理及对水泥水化的影响[J]. 非金属矿, 2018, 41(3): 49-52. |
| JIANG Yong, WEN Mengyuan, JIA Lujun. Pretreatment of electrolytic manganese residue and influence on cement hydration[J]. Non-Metallic Mines, 2018, 41(3): 49-52. | |
| 10 | 胡超超, 王里奥, 詹欣源, 等. 城市生活垃圾焚烧飞灰与电解锰渣烧制陶粒[J]. 环境工程学报, 2019, 13(1): 177-185. |
| HU Chaochao, WANG Li’ao, ZHAN Xinyuan, et al. Preparation of ceramsite with MSWI fly ash and electrolytic manganese residues[J]. Chinese Journal of Environmental Engineering, 2019, 13(1): 177-185. | |
| 11 | 王亚光. 粉煤灰/电解锰渣地质聚合物材料的制备及其性能研究[D]. 银川: 北方民族大学, 2018. |
| WANG Yaguang. Study on the preparation and properties of fly ash/electrolytic manganese slag geopolymer materials[D]. Yinchuan: North Minzu University, 2018. | |
| 12 | 李昌新, 喻源, 张庆武, 等. 合成条件对电解锰渣制备水化硅酸钙过程中结构和性能的影响[J]. 中国有色金属学报, 2020, 30(6): 1368-1376. |
| LI Changxin, YU Yuan, ZHANG Qinwu, et al. Effects of synthesis conditions on formation process and property of calcium silicate hydrate prepared from electrolytic manganese residue[J]. The Chinese Journal of Nonferrous Metals, 2020, 30(6): 1368-1376. | |
| 13 | ZHAN Xinyuan, WANG Li’ao, WANG Lei, et al. Co-sintering MSWI fly ash with electrolytic manganese residue and coal fly ash for lightweight ceramisite[J]. Chemosphere, 2021, 263: 127914. |
| 14 | 何德军, 舒建成, 陈梦君, 等. 电解锰渣建材资源化研究现状与展望[J]. 化工进展, 2020, 39(10): 4227-4237. |
| HE Dejun, SHU Jiancheng, CHEN Mengjun, et al. Current status and future prospects of electrolytic manganese residuereused as building materials[J]. Chemical Industry and Engineering Progress, 2020, 39(10): 4227-4237. | |
| 15 | 徐金荣. 电解锰渣无害化处理技术及资源化利用研究进展[J]. 中国锰业, 2020, 38(6): 1-6. |
| XU Jinrong. A research progress on harmless treatment technology and resource utilization of electrolytic manganese residue[J]. China’s Manganese Industry, 2020, 38(6): 1-6. | |
| 16 | 陈红亮, 刘仁龙, 李文生, 等. 电解锰渣的理化特性分析研究[J]. 金属材料与冶金工程, 2014, 42(1): 3-5. |
| CHEN Hongliang, LIU Renlong, LI Wensheng, et al. Physicochemical analysis of electrolytic manganese residue[J]. Metal Materials and Metallurgy Engineering, 2014, 42(1): 3-5. | |
| 17 | 陈红亮. 新鲜电解锰渣和长期堆存渣的矿物成分和毒性特征的差异分析[J]. 贵州师范大学学报(自然科学版), 2016, 34(2): 32-36. |
| CHEN Hongliang. Differences analysis of minerals compositions and toxicity characteristics between the fresh electrolytic manganese residue and the stockpiling residue[J]. Journal of Guizhou Normal University(Natural Sciences), 2016, 34(2): 32-36. | |
| 18 | 周亚武, 陆谢娟, 高明刚, 等. 电解锰渣固结体中重金属浸出毒性及其在模拟酸雨下的淋溶特性分析[J]. 武汉科技大学学报, 2018, 41(2): 127-132. |
| ZHOU Yawu, LU Xiejuan, GAO Minggang, et al. Leaching toxicity and leaching properties in simulated acid rain of heavy metals in solidified electrolytic manganese residue[J]. Journal of Wuhan University of Science and Technology, 2018, 41(2): 127-132. | |
| 19 | 刘荣. 贵州锰矿废渣淋溶条件下重金属迁移特征及淋溶水对植物种苗生长的影响[J]. 中国科技博览, 2015(40): 3. |
| LIU Rong. Migration characteristics of heavy metals under leaching conditions of manganese ore residues in Guizhou and effects of leaching water on plant seedling growth[J]. China Science and Technology Review, 2015(40): 3. | |
| 20 | 杨金秀, 刘方, 杨爱江, 等. 改性生物质炭对电解锰废渣中Mn淋溶迁移的影响[J]. 工业安全与环保, 2016, 42(1): 42-45. |
| YANG Jingxiu, LIU Fang, YANG Aijiang, et al. The effects of modified biomass charcoal on leaching migration of Mn in manganese slag[J]. Industrial Safety and Environmental Protection, 2016, 42(1): 42-45. | |
| 21 | 罗乐, 王金霞, 周皓. 锰渣中重金属在模拟酸雨环境下的浸出规律[J]. 湿法冶金, 2019, 38(5): 352-357. |
| LUO Le, WANG Jinxia, ZHOU Hao. Leaching regularities of heavy metals in electrolytic manganese residue using simulation acid rain[J]. Hydrometallurgy of China, 2019, 38(5): 352-357. | |
| 22 | 刀谞, 霍晓芹, 张霖琳, 等. 我国土壤中主要元素监测技术及难点[J]. 中国环境监测, 2018, 34(5): 12-21. |
| Xu DAO, HUO Xiaoqin, ZHANG Linlin, et al. Over view of main soil element heavy metal monitoring technology and difficulties in China[J]. Environmental Monitoring in China, 2018, 34(5): 12-21. | |
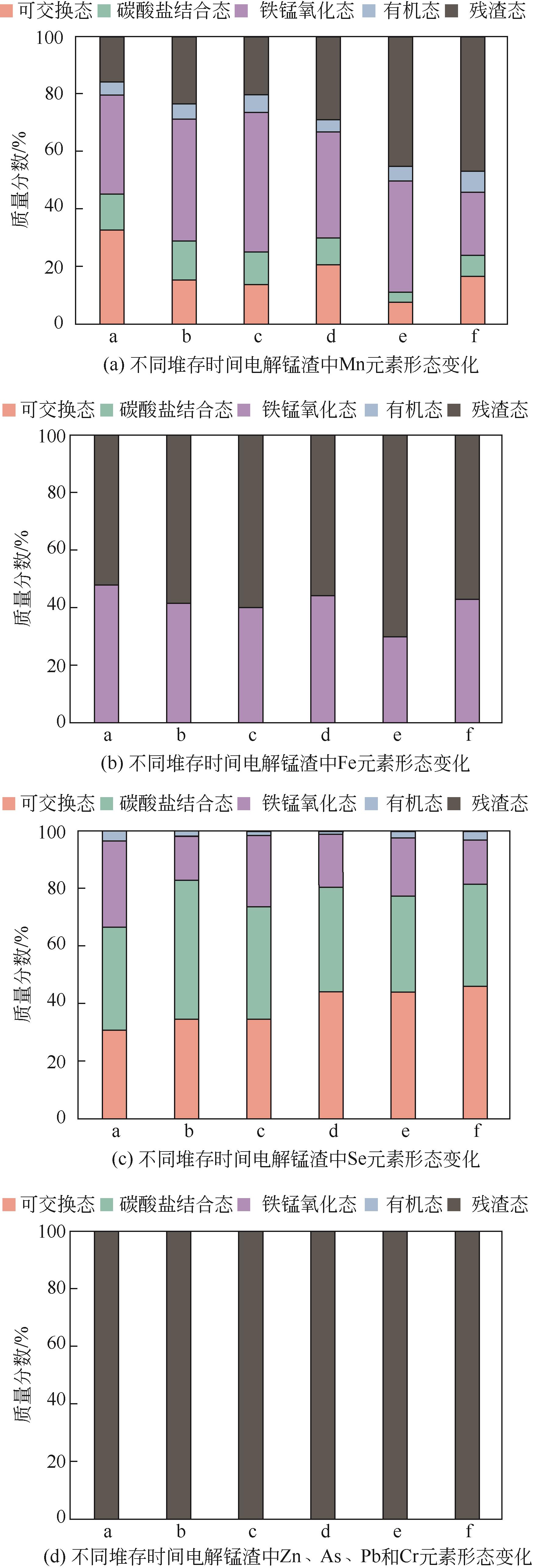
| 23 | 陈莉薇, 陈海英, 武君, 等. 利用Tessier五步法和改进BCR法分析铜尾矿中Cu、Pb、Zn赋存形态的对比研究[J]. 安全与环境学报, 2020, 20(2): 735-740. |
| CHEN Liwei, CHEN Haiying, WU Jun, et al. Comparative study on speciation of Cu, Pb and Zn from mining tailings via Tessier 5-step sequential extraction and improved BCR method[J]. Journal of Safety and Environment, 2020, 20(2): 735-740. | |
| 24 | 黄红铭, 黄增, 韦江慧, 等. 2011—2018年广西酸雨污染变化特征及影响因素分析[J]. 化学工程师, 2019, 33(10): 41-44. |
| HUANG Hongming, HUANG Zeng, WEI Jianghui, et al. Characteristics of acid rain and its influencing factors from 2011 to 2018 in Guangxi[J]. Chemical Engineer, 2019, 33(10): 41-44. | |
| 25 | 杨凡, 蒋良兴, 于枭影, 等. 采用铝阴极从硫酸锰溶液中电沉积金属锰[J]. 中国有色金属学报, 2018, 28(12): 2568-2579. |
| YANG Fan, JIANG Liangxing, YU Xiaoying, et al. Electrodeposition of Mn from manganese sulfate solution by aluminum cathode[J]. The Chinese Journal of Nonferrous Metals, 2018, 28(12): 2568-2579. | |
| 26 | 唐文杰, 黄江波, 余谦, 等. 锰矿区农作物重金属含量及健康风险评价[J]. 环境科学与技术, 2015, 38(S1): 464-468. |
| TANG Wenjie, HUANG Jiangbo, YU Qian, et al. Analysis on the content of heavy metal in the food crops and assessment on human health risk of manganese mine[J]. Environmental Science and Technology, 2015, 38(S1): 464-468. | |
| 27 | 韩春梅, 王林山, 巩宗强, 等. 土壤中重金属形态分析及其环境学意义[J]. 生态学杂志, 2005(12): 1499-1502. |
| HAN Chunmei, WANG Linshan, GONG Zongqiang, et al. Chemical forms of soil heavy metals and their environmental significance[J]. Chinese Journal of Ecology, 2005(12): 1499-1502. | |
| 28 | 鲍丽丽. 典型工矿绿洲As污染农田土壤生物锰氧化物修复技术的适用性[D]. 兰州: 兰州大学, 2020. |
| BAO Lili. Applicability of biogenic Mn oxides remediation for As contaminated agriculturalsoil in a typical mine-based oasis[D]. Lanzhou: Lanzhou University, 2020. | |
| 29 | CHEN Lu, WEN Fang, CHENG Yan, et al. Characteristics of speciation distribution and toxicity leaching of heavy metals in Pb-Zn tailings[J]. Journal of Arid Land Resources and Environment, 2017, 31(3): 89-94. |
| 30 | 胡文. 土壤-植物系统中重金属的生物有效性及其影响因素的研究[D]. 北京: 北京林业大学, 2008. |
| HU Wen. Heavy metal bio-availability and its affecting factors in soil-plant system[D]. Beijing: Beijing Forestry University, 2008. | |
| 31 | DENG Yaling, SHU Jiancheng, LEI Tianya, et al. A green method for Mn2+ and NH4 +-N removal in electrolytic manganese residue leachate by electric field and phosphorus ore flotation tailings[J]. Separation and Purification Technology, 2021, 270: 118820. |
| 32 | 和森. 铜渣基磷酸盐胶凝材料的制备及固化电解锰渣的基础研究[D]. 昆明: 昆明理工大学, 2019. |
| HE Seng. Basic research on preparation copper slag-based phosphate cementitious material and the solidification of electrolytic manganese residue[D]. Kunming: Kunming University of Science and Technology, 2019. | |
| 33 | 杜兵. 电解锰废渣化学稳定化处理及固锰机理研究[D]. 北京: 中国科学院大学, 2015. |
| DU Bing. Immobilization mechanism on soluble Mn from electrolytic manganese solid waste with chemical stabilization treatment[D]. Beijing: University of Chinese Academy of Sciences, 2015. | |
| 34 | 李志清, 沈鑫, 戚志宇, 等. 基于压汞法与气体吸附法的页岩孔隙结构特征对比研究[J]. 工程地质学报, 2017, 25(6): 1405-1413. |
| LI Zhiqing, SHEN Xin, QI Zhiyu, et al. Comparations between mercury intrusion and gas adsorption for pore structure characteristics of shale[J]. Journal of Engineering Geology, 2017, 25(6): 1405-1413. | |
| 35 | 曹昕. 铁锰复合氧化物催化氧化去除地下水中氨氮研究[D]. 西安: 西安建筑科技大学, 2015. |
| CAO Xin. The study on catalytic oxidation of ammonia in groundwater by co-oxides of iron and manganese[D]. Xi’an: Xi’an University of Architecture and Technology, 2015. | |
| 36 | DONG Lu, WANG Hai, HUANG Yaji, et al. Elemental mercury removal from coal-fired flue gas using recyclable magnetic Mn-Fe based attapulgite sorbent[J]. Chemical Engineering Journal, 2020, 407: 127182. |
| 37 | 黄廷林, 郑娜, 曹昕. 滤料表面活性滤膜对水中锰的吸附特性与机理研究[J]. 水处理技术, 2013, 39(3): 39-43. |
| HUANG Tinglin, ZHEN Na, CAO Xin. Study on adsorption characteristics and mechanism of manganese by active filter membranes in solution[J]. Technology of Water Treatment, 2013, 39(3): 39-43. | |
| 38 | 孙燕, 蓝际荣, 郭莉, 等. 利用电解锰渣制备As(Ⅲ)吸附材料及其性能研究[J]. 化工学报, 2019, 70(6): 2377-2385. |
| SUN Yan, LAN Jirong, GUO Li, et al. Preparation of As(Ⅲ) adsorbent material by electrolytic manganese slag and its properties[J]. CIESC Journal, 2019, 70(6): 2377-2385. |
| [1] | JIANG Jing, CHEN Xiaoyu, ZHANG Ruiyan, SHENG Guangyao. Research progress of manganese-loaded biochar preparation and its application in environmental remediation [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4385-4397. |
| [2] | ZHAN Yong, WANG Hui, WEI Tingting, ZHU Xingyu, WANG Xiankai, CHEN Sisi, DONG Bin. In situ reduction effect of Mn2+ enhanced ozone conditioning on sludge in biological treatment process [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3253-3260. |
| [3] | GUO Xiaoyu, LI Dongchen, ZHAO Wei, DU Zhenyi, LI Xiaoliang. Preparation of Au-Pd/MnO2 catalyst and its catalytic performance for benzyl alcohol oxidation [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5223-5231. |
| [4] | ZENG Yifan, SHU Jiancheng, YANG Huimin, ZHAO Zhisheng, CHEN Mengjun, YANG Yong, LIU Renlong. Transformation law of gypsum from electrolytic manganese residue in ammonium salt system [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 5115-5121. |
| [5] | WANG Zhen, YAN Ting, HUO Yingjie. Performance of thermochemical sorption heat storage using manganese chloride/ammonia [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4425-4431. |
| [6] | LI Juanjuan, ZHANG Tianyong, LI Xianggao. Construction of high-quality iron-manganese black nano-dispersion system for electrophoretic display [J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3178-3185. |
| [7] | LI Ao, WANG Hongyang, SUN Yuwei, WANG Xu, WANG Xia, ZHU Guangcan. Model and optimization of flow electrode capacitive deionization for ammonium ion removal [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2123-2131. |
| [8] | PENG Chao, YU Di, WANG Lanyi, ZHANG Chunlei, YU Xuehua, ZHAO Zhen. Preparation of manganese oxide catalysts by self-propagating combustion method and their catalytic performance for soot combustion [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 770-780. |
| [9] | NIE Zimeng, YANG Dian, XIONG Yulu, LI Yingjie, TIAN Senlin, NING Ping. Performance and mechanism of electrolytic manganese slag slurry for flue gas desulfurization [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 1063-1072. |
| [10] | GUO Yingming, ZHANG Yuhong, MA Ben, YUAN Shengchen, QIU Wenxuan, YANG Jing. Removal of CODMn in water by potassium ferrate enhanced iron-manganese oxide film filtration and its influencing factors [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 6130-6138. |
| [11] | XIONG Yulu, XU Zihao, LI Yingjie, TIAN Senlin, NING Ping. Removal of sulfur from electrolytic manganese slag by high-temperature reduction roasting in an inert atmosphere [J]. Chemical Industry and Engineering Progress, 2021, 40(S1): 319-325. |
| [12] | YU Minghao, GU Mengxuan, WU Zhengying, SUN Linbing. Advances in the synthesis and application of manganese oxides as anode materials for lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2021, 40(9): 5012-5028. |
| [13] | WANG Jing, NI Jinying, WANG Liqun, QING Qing, YAN Shenghu, ZHANG Yue. Screening of a lignin degrading bacterium and its degradation pathway [J]. Chemical Industry and Engineering Progress, 2021, 40(7): 4021-4026. |
| [14] | DING Xin, GAO Kechang, HAO Erguo, HAN Yanhui, WU Yazhao, JIAO Weizhou, LIU Youzhi. Treatment of low concentration ammonia nitrogen wastewater by high gravity enhanced breakpoint chlorination [J]. Chemical Industry and Engineering Progress, 2021, 40(7): 4083-4090. |
| [15] | CHEN Qizhi, ZHOU Yilin, LIU Zuohua, CHEN Nanxiong, YANG Yong, WEI Hongjun, TONG Zhangfa. Manganese ore leaching and iron removal intensified by variable frequency rigid-flexible stirred reactor [J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3083-3090. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||