Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (10): 5302-5312.DOI: 10.16085/j.issn.1000-6613.2021-0654
• Special column:Resource recycling and value-added utilization • Previous Articles Next Articles
Status and research progress on recovery of spent hydrogenation catalysts
SHI Zhisheng1( ), DING Yunji1,2(
), DING Yunji1,2( ), ZHANG Shengen1(
), ZHANG Shengen1( )
)
- 1.Institute for Advanced Materials and Technology, University of Science and Technology Beijing, Beijing 100083, China
2.Shunde Graduate School, University of Science and Technology Beijing, Foshan 528399, Guangdong, China
-
Received:2021-03-30Revised:2021-07-22Online:2021-10-25Published:2021-10-10 -
Contact:DING Yunji,ZHANG Shengen
废加氢催化剂的回收现状与研究进展
- 1.北京科技大学新材料技术研究院,北京 100083
2.北京科技大学顺德研究生院,广东 佛山 528399
-
通讯作者:丁云集,张深根 -
作者简介:史志胜(1992—),男,博士研究生,研究方向为废催化剂资源化利用。E-mail:shizhisheng1992@163.com 。 -
基金资助:国家自然科学基金重点项目(U2002212);广东省基础与应用基础研究基金(2020A1515110408);北京科技大学顺德研究生院博士后科研经费项目(2020BH004);佛山市人民政府科技创新专项项目(BK21BE002);中央高校基本科研业务费项目(FRF-TP-20-031A1)
CLC Number:
Cite this article
SHI Zhisheng, DING Yunji, ZHANG Shengen. Status and research progress on recovery of spent hydrogenation catalysts[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5302-5312.
史志胜, 丁云集, 张深根. 废加氢催化剂的回收现状与研究进展[J]. 化工进展, 2021, 40(10): 5302-5312.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-0654
| 加氢过程 | 催化活性对比 |
|---|---|
| 加氢脱硫 | Mo-Co>Mo-Ni>W-Ni |
| 加氢脱氮 | Mo-Ni=W-Ni>Mo-Co |
| 加氢脱氧 | Mo-Ni>Mo-Co>W-Ni |
| 加氢饱和 | W-Ni>Mo-Ni>Mo-Co |
| 加氢过程 | 催化活性对比 |
|---|---|
| 加氢脱硫 | Mo-Co>Mo-Ni>W-Ni |
| 加氢脱氮 | Mo-Ni=W-Ni>Mo-Co |
| 加氢脱氧 | Mo-Ni>Mo-Co>W-Ni |
| 加氢饱和 | W-Ni>Mo-Ni>Mo-Co |
| 废催化剂 组成 | 浸出方法 | 主要工艺参数 | 浸出率 | 参考文献 |
|---|---|---|---|---|
| Mo,Co | HCl | 3mol/L,固体添加量5%(质量分数),90℃,60min | Mo 97%;Co 94% | [ |
| Mo,Ni | H2SO4 | 9mol/L,固液比1/2.54(g/mL),90℃,90min | Mo 99%;Ni 99% | [ |
| Mo,Co | H2SO4 | 20%(体积分数),固体添加量5%(质量分数),90℃,2h | Mo 91%;Co 78% | [ |
| Ni | HNO3 | 5mol/L,固液比1/10(g/mL),90℃,120min | Ni 99% | [ |
| Mo,Ni,V | HNO3∶H2SO4∶HCl=2∶1∶1(体积比) | 固液比为70g/L,70℃,60min | Mo 90%;Ni 99%;V 99% | [ |
| Mo,Ni,Co | HCOOH | 0.6mol/L,固液比1/10(g/mL),80℃,90min | Mo 76%、Ni 93%、Co 97% | [ |
| Mo,Ni | H2C2O4 | 1mol/L,固液比1/10(g/mL),40℃,3h | Mo 92%;Ni 19% | [ |
| Mo,Ni,Co | EDTA | 0.2mol/L,固液比1/15(g/mL),60℃,60min | Mo 90%;Ni 95%;Co 97% | [ |
| Mo,Co | H2SO4+H2O2(pH=1.3) | H2O2浓度3.75mol/L,固液比1/7.5(g/mL),60℃,1h | Mo 90%;Co 83% | [ |
| Mo,Ni,V | H2C2O4+H2O2 | H2C2O4浓度0.5mol/L,H2O2浓度3mol/L,60℃,1h | Mo 90%;Ni 65%;V 94% | [ |
| Mo,V | NaOH加压 | NaOH 30%(g/mL),250℃ | Mo 98%;V 95% | [ |
| W,V | NaOH+Na2CO3加压 | NaOH浓度2mol/L,Na2CO3浓度0.2mol/L,固液比1/20(g/mL),300℃,2h | W>90%;V>90% | [ |
| Mo,V | NH3·H2O+H2O2 | NH3·H2O浓度4.5mol/L,H2O2浓度为1.0mol/L,固液比1/20(g/mL),140℃,2h | Mo 95%;V 46% | [ |
| Mo | Na2CO3+H2O2 | Na2CO3浓度85g/L,H2O2 10%(体积分数),废催化剂添加量20%(质量分数),25℃,1h | Mo 84% | [ |
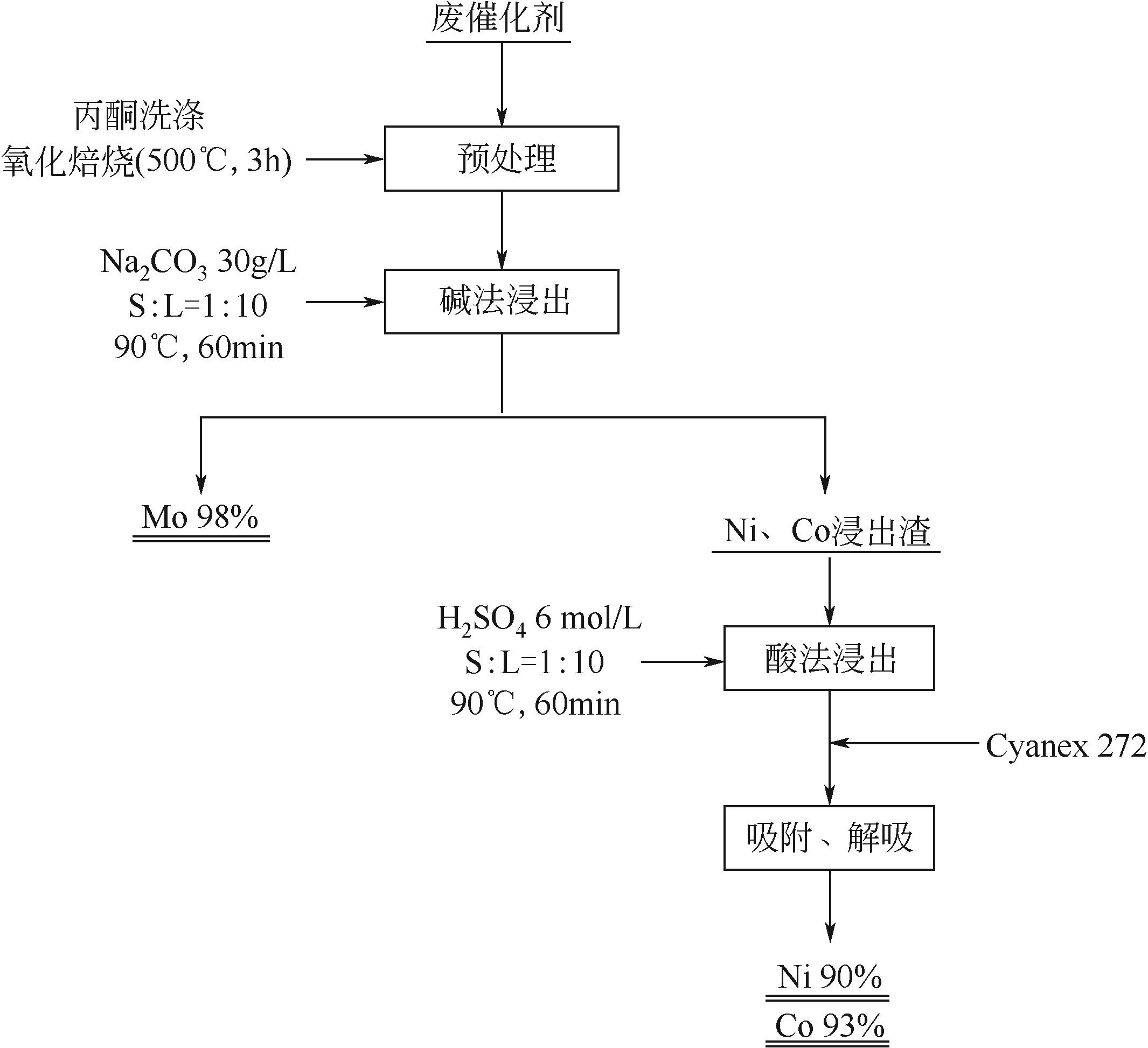
| Mo,Ni,Co | Na2CO3+H2SO4两步浸出 | Na2CO3浓度为30g/L,固液比1/10(g/mL),90℃,60min;H2SO4浓度为6mol/L,固液比1/10(g/mL),90℃,60min | Mo 98%;Ni 90%;Co 93% | [ |
| Mo,V | NaOH微波辅助 | 微波功率600W,NaOH浓度2mol/L,固液比1/5(g/mL),90℃,10min | Mo 96%;V 94% | [ |
| 废催化剂 组成 | 浸出方法 | 主要工艺参数 | 浸出率 | 参考文献 |
|---|---|---|---|---|
| Mo,Co | HCl | 3mol/L,固体添加量5%(质量分数),90℃,60min | Mo 97%;Co 94% | [ |
| Mo,Ni | H2SO4 | 9mol/L,固液比1/2.54(g/mL),90℃,90min | Mo 99%;Ni 99% | [ |
| Mo,Co | H2SO4 | 20%(体积分数),固体添加量5%(质量分数),90℃,2h | Mo 91%;Co 78% | [ |
| Ni | HNO3 | 5mol/L,固液比1/10(g/mL),90℃,120min | Ni 99% | [ |
| Mo,Ni,V | HNO3∶H2SO4∶HCl=2∶1∶1(体积比) | 固液比为70g/L,70℃,60min | Mo 90%;Ni 99%;V 99% | [ |
| Mo,Ni,Co | HCOOH | 0.6mol/L,固液比1/10(g/mL),80℃,90min | Mo 76%、Ni 93%、Co 97% | [ |
| Mo,Ni | H2C2O4 | 1mol/L,固液比1/10(g/mL),40℃,3h | Mo 92%;Ni 19% | [ |
| Mo,Ni,Co | EDTA | 0.2mol/L,固液比1/15(g/mL),60℃,60min | Mo 90%;Ni 95%;Co 97% | [ |
| Mo,Co | H2SO4+H2O2(pH=1.3) | H2O2浓度3.75mol/L,固液比1/7.5(g/mL),60℃,1h | Mo 90%;Co 83% | [ |
| Mo,Ni,V | H2C2O4+H2O2 | H2C2O4浓度0.5mol/L,H2O2浓度3mol/L,60℃,1h | Mo 90%;Ni 65%;V 94% | [ |
| Mo,V | NaOH加压 | NaOH 30%(g/mL),250℃ | Mo 98%;V 95% | [ |
| W,V | NaOH+Na2CO3加压 | NaOH浓度2mol/L,Na2CO3浓度0.2mol/L,固液比1/20(g/mL),300℃,2h | W>90%;V>90% | [ |
| Mo,V | NH3·H2O+H2O2 | NH3·H2O浓度4.5mol/L,H2O2浓度为1.0mol/L,固液比1/20(g/mL),140℃,2h | Mo 95%;V 46% | [ |
| Mo | Na2CO3+H2O2 | Na2CO3浓度85g/L,H2O2 10%(体积分数),废催化剂添加量20%(质量分数),25℃,1h | Mo 84% | [ |
| Mo,Ni,Co | Na2CO3+H2SO4两步浸出 | Na2CO3浓度为30g/L,固液比1/10(g/mL),90℃,60min;H2SO4浓度为6mol/L,固液比1/10(g/mL),90℃,60min | Mo 98%;Ni 90%;Co 93% | [ |
| Mo,V | NaOH微波辅助 | 微波功率600W,NaOH浓度2mol/L,固液比1/5(g/mL),90℃,10min | Mo 96%;V 94% | [ |
| 废催化剂组成 | 回收方法 | 主要工艺参数 | 回收率 | 参考文献 |
|---|---|---|---|---|
| Mo,Co | H2SO4焙烧+H2SO4浸出 | 焙烧条件:300℃,2h; 浸出条件:H2SO4体积分数2%,95℃,60min | Mo>90%;Co>90% | [ |
| Mo,Ni,Co | KHSO4焙烧-水浸 | 焙烧条件:500℃,4h;90℃水浸4h | Mo>90%;Ni>90%;Co>90% | [ |
| Mo,V | Na2CO3焙烧-水浸 | 焙烧条件:750℃,45min;90℃水浸15min | Mo 90%;V 90% | [ |
| Mo | Na2CO3焙烧-水浸 | 焙烧条件:600℃,45min;60℃水浸 | Mo 99% | [ |
| 废催化剂组成 | 回收方法 | 主要工艺参数 | 回收率 | 参考文献 |
|---|---|---|---|---|
| Mo,Co | H2SO4焙烧+H2SO4浸出 | 焙烧条件:300℃,2h; 浸出条件:H2SO4体积分数2%,95℃,60min | Mo>90%;Co>90% | [ |
| Mo,Ni,Co | KHSO4焙烧-水浸 | 焙烧条件:500℃,4h;90℃水浸4h | Mo>90%;Ni>90%;Co>90% | [ |
| Mo,V | Na2CO3焙烧-水浸 | 焙烧条件:750℃,45min;90℃水浸15min | Mo 90%;V 90% | [ |
| Mo | Na2CO3焙烧-水浸 | 焙烧条件:600℃,45min;60℃水浸 | Mo 99% | [ |
| 1 | AKCIL A, VEGLIÒ F, FERELLA F, et al. A review of metal recovery from spent petroleum catalysts and ash[J]. Waste Management, 2015, 45: 420-433. |
| 2 | MARAFI M, RANA M S. Refinery waste: the spent hydroprocessing catalyst and its recycling options[J]. WIT Transactions on Ecology and the Environment, 2016, 202: 219-230. |
| 3 | 任春晓, 吴培, 李振昊, 等. 加氢催化剂预硫化技术现状[J]. 化工进展, 2013, 32(5): 1060-1064. |
| REN Chunxiao, WU Pei, LI Zhenhao, et al. The status of presulfurization technology for hydrogenation catalyst[J]. Chemical Industry and Engineering Progress, 2013, 32(5): 1060-1064. | |
| 4 | 张利波, 王璐, 曲雯雯, 等. Al2O3基石油加氢脱硫催化剂研究现状与进展[J]. 材料导报, 2018, 32(5): 772-779, 795. |
| ZHANG Libo, WANG Lu, QU Wenwen, et al. Research and development of petroleum hydrodesulfurization catalysts with Al2O3-based supports[J]. Materials Review, 2018, 32(5): 772-779, 795. | |
| 5 | GRANGE P, VANHAEREN X. Hydrotreating catalysts, an old story with new challenges[J]. Catalysis Today, 1997, 36(4): 375-391. |
| 6 | DUFRESNE P. Hydroprocessing catalysts regeneration and recycling[J]. Applied Catalysis A: General, 2007, 322: 67-75. |
| 7 | MARAFI M, STANISLAUS A. Spent catalyst waste management: a review: Part Ⅰ—Developments in hydroprocessing catalyst waste reduction and use[J]. Resources, Conservation and Recycling, 2008, 52(6): 859-873. |
| 8 | 刘勇军, 刘晨光. 炼厂加氢废催化剂的综合利用[J]. 化工进展, 2010, 29(6): 1066-1070. |
| LIU Yongjun, LIU Chenguang. Comprehensive utilization of spent hydrotreating catalysts in refinery[J]. Chemical Industry and Engineering Progress, 2010, 29(6): 1066-1070. | |
| 9 | 孙晓雪, 刘仲能, 杨为民. 废弃负载型加氢处理催化剂金属回收技术进展[J]. 化工进展, 2016, 35(6): 1894-1904. |
| SUN Xiaoxue, LIU Zhongneng, YANG Weimin. Research progress of metal recovery in spent supported hydroprocessing catalyst[J]. Chemical Industry and Engineering Progress, 2016, 35(6): 1894-1904. | |
| 10 | PATHAK A, VINOBA M, KOTHARI R. Emerging role of organic acids in leaching of valuable metals from refinery-spent hydroprocessing catalysts, and potential techno-economic challenges: a review[J]. Critical Reviews in Environmental Science and Technology, 2021, 51(1): 1-43. |
| 11 | EIJSBOUTS S, BATTISTON A A, LEERDAM G C VAN. Life cycle of hydroprocessing catalysts and total catalyst management[J]. Catalysis Today, 2008, 130(2/3/4): 361-373. |
| 12 | BARIK S P, PARK K H, PARHI P K, et al. Direct leaching of molybdenum and cobalt from spent hydrodesulphurization catalyst with sulphuric acid[J]. Hydrometallurgy, 2012, 111/112: 46-51. |
| 13 | 王曰杰, 李玲玲, 何春宏. 炼油废催化剂生物淋滤脱金属研究进展[J]. 化工学报, 2021, 72(2): 901-912. |
| WANG Yuejie, LI Lingling, HE Chunhong. Review on the bioleaching of spent refinery catalysts for metals removal[J]. CIESC Journal, 2021, 72(2): 901-912. | |
| 14 | LE M N, LEE M S. A review on hydrometallurgical processes for the recovery of valuable metals from spent catalysts and life cycle analysis perspective[J]. Mineral Processing and Extractive Metallurgy Review, 2021, 42(5): 335-354. |
| 15 | AL-SALEM S M, CONSTANTINOU A, LEEKE G A, et al. A review of the valorization and management of industrial spent catalyst waste in the context of sustainable practice: the case of the State of Kuwait in parallel to European industry[J]. Waste Management & Research, 2019, 37(11): 1127-1141. |
| 16 | WANG Hongjun, FENG Yali, LI Hailong, et al. Recovery of vanadium from acid leaching solutions of spent oil hydrotreating catalyst using solvent extraction with D2EHPA (P204)[J]. Hydrometallurgy, 2020, 195: 105404. |
| 17 | LE M N, LEE M S. Separation of Al(Ⅲ), Mo(Ⅵ), Ni(Ⅱ), and V(Ⅴ) from model hydrochloric acid leach solutions of spent petroleum catalyst by solvent extraction[J]. Journal of Chemical Technology & Biotechnology, 2020, 95(11): 2886-2897. |
| 18 | WANG Hongjun, FENG Yali, LI Haoran, et al. The kinetics of vanadium extraction from spent hydroprocessing catalyst by leaching with sulfuric acid at atmospheric pressure[J]. Metallurgical Research & Technology, 2019, 116(2): 214. |
| 19 | SHEIK A R, GHOSH M K, SANJAY K, et al. Dissolution kinetics of nickel from spent catalyst in nitric acid medium[J]. Journal of the Taiwan Institute of Chemical Engineers, 2013, 44(1): 34-39. |
| 20 | VUYYURU K R, PANT K K, KRISHNAN V V, et al. Recovery of nickel from spent industrial catalysts using chelating agents[J]. Industrial & Engineering Chemistry Research, 2010, 49(5): 2014-2024. |
| 21 | CHAUHAN G, PANT K K, NIGAM K D P. Metal recovery from hydroprocessing spent catalyst: a green chemical engineering approach[J]. Industrial & Engineering Chemistry Research, 2013, 52(47): 16724-16736. |
| 22 | IMAM D M, EL-NADI Y A. Recovery of molybdenum from alkaline leach solution of spent hydrotreating catalyst by solvent extraction using methyl tricaprylammonium hydroxide[J]. Hydrometallurgy, 2018, 180: 172-179. |
| 23 | AL-SHEEHA H, MARAFI M, RAGHAVAN V, et al. Recycling and recovery routes for spent hydroprocessing catalyst waste[J]. Industrial & Engineering Chemistry Research, 2013, 52(36): 12794-12801. |
| 24 | SZYMCZYCHA-MADEJA A. Kinetics of Mo, Ni, V and Al leaching from a spent hydrodesulphurization catalyst in a solution containing oxalic acid and hydrogen peroxide[J]. Journal of Hazardous Materials, 2011, 186(2/3): 2157-2161. |
| 25 | MA Zhiyuan, LIU Yong, ZHOU Jikui, et al. Recovery of vanadium and molybdenum from spent petrochemical catalyst by microwave-assisted leaching[J]. International Journal of Minerals, Metallurgy, and Materials, 2019, 26(1): 33-40. |
| 26 | BANDA R, NGUYEN T H, SOHN S H, et al. Recovery of valuable metals and regeneration of acid from the leaching solution of spent HDS catalysts by solvent extraction[J]. Hydrometallurgy, 2013, 133: 161-167. |
| 27 | VALVERDE I M, PAULINO J F, AFONSO J C. Hydrometallurgical route to recover molybdenum, nickel, cobalt and aluminum from spent hydrotreating catalysts in sulphuric acid medium[J]. Journal of Hazardous Materials, 2008, 160(2/3): 310-317. |
| 28 | KIM H I, PARK K H, MISHRA D. Sulfuric acid baking and leaching of spent Co-Mo/Al2O3 catalyst[J]. Journal of Hazardous Materials, 2009, 166(2/3): 1540-1544. |
| 29 | LAI Y C, LEE W J, HUANG Kuolin, et al. Metal recovery from spent hydrodesulfurization catalysts using a combined acid-leaching and electrolysis process[J]. Journal of Hazardous Materials, 2008, 154(1/2/3): 588-594. |
| 30 | ARSLANOĞLU H, YARAŞ A. Recovery of precious metals from spent Mo-Co-Ni/Al2O3 catalyst in organic acid medium: process optimization and kinetic studies[J]. Petroleum Science and Technology, 2019, 37(19): 2081-2093. |
| 31 | ILHAN S. Extraction of molybdenum, nickel and aluminium from spent Ni-Mo hydrodesulphurization (HDS) catalyst in oxalic acid solutions[J]. Canadian Metallurgical Quarterly, 2020, 59(1): 26-35. |
| 32 | ALPASLAN O, YARAS A, ARSLANOĞLU H. A kinetic model for chelating extraction of metals from spent hydrodesulphurization catalyst by complexing agent[J]. Transactions of the Indian Institute of Metals, 2020, 73(7): 1925-1937. |
| 33 | RUIZ V, MEUX E, SCHNEIDER M, et al. Hydrometallurgical treatment for valuable metals recovery from spent CoMo/Al2O3Catalyst. 2. Oxidative leaching of an unroasted catalyst using H2O2[J]. Industrial & Engineering Chemistry Research, 2011, 50(9): 5307-5315. |
| 34 | MULAK W, SZYMCZYCHA A, LESNIEWICZ A, et al. Preliminary results of metals leaching from a spent hydrodesulphurization (HDS) catalyst[J]. Physicochemical Problems of Mineral Processing, 2006, 40: 69-76. |
| 35 | KIM J W, LEE W G, HWANG I S, et al. Recovery of tungsten from spent selective catalytic reduction catalysts by pressure leaching[J]. Journal of Industrial and Engineering Chemistry, 2015, 28: 73-77. |
| 36 | ZHAO Zhipeng, GUO Min, ZHANG Mei. Extraction of molybdenum and vanadium from the spent diesel exhaust catalyst by ammonia leaching method[J]. Journal of Hazardous Materials, 2015, 286: 402-409. |
| 37 | PARK K H, MOHAPATRA D, REDDY B R. Selective recovery of molybdenum from spent HDS catalyst using oxidative soda ash leach/carbon adsorption method[J]. Journal of Hazardous Materials, 2006, 138(2): 311-316. |
| 38 | PARK K H, MOHAPATRA D, NAM C W. Two stage leaching of activated spent HDS catalyst and solvent extraction of aluminium using organo-phosphinic extractant, Cyanex 272[J]. Journal of Hazardous Materials, 2007, 148(1/2): 287-295. |
| 39 | MARAFI M, STANISLAUS A. Spent hydroprocessing catalyst management: a review: Part Ⅱ. Advances in metal recovery and safe disposal methods[J]. Resources, Conservation and Recycling, 2008, 53(1/2): 1-26. |
| 40 | SÁMANO V, RANA M S, ANCHEYTA J. An easy approach based on textural properties to evaluate catalyst deactivation during heavy oil hydrotreating[J]. Catalysis Communications, 2020, 133: 105823. |
| 41 | LE M N, LEE M S. Selective dissolution of vanadium(Ⅴ) from spent petroleum catalysts by oxalic acid solution[J]. Journal of Mining and Metallurgy, Section B: Metallurgy, 2020, 56(1): 127-133. |
| 42 | YANG Yue, XU Shengming, LI Zhen, et al. Oil removal of spent hydrotreating catalyst CoMo/Al2O3via a facile method with enhanced metal recovery[J]. Journal of Hazardous Materials, 2016, 318: 723-731. |
| 43 | FU Pengbo, WANG Hualin, LI Jianping, et al. Cyclonic gas stripping deoiling and gas flow acceleration classification for the resource utilization of spent catalysts in residue hydrotreating process[J]. Journal of Cleaner Production, 2018, 190: 689-702. |
| 44 | DE SOUZA PEREIRA A L, SILVA C N D, AFONSO J C, et al. The importance of pre-treatment of spent hydrotreating catalysts on metals recovery[J]. Química Nova, 2011, 34(1): 145-150. |
| 45 | MENOUFY M F, AHMED H S. Treatment and reuse of spent hydrotreating catalyst[J]. Energy Sources A: Recovery, Utilization, and Environmental Effects, 2008, 30(13): 1213-1222. |
| 46 | ZHANG Di, LIU Yunqing, HU Qizhao, et al. Sustainable recovery of nickel, molybdenum, and vanadium from spent hydroprocessing catalysts by an integrated selective route[J]. Journal of Cleaner Production, 2020, 252: 119763. |
| 47 | FERELLA F, OGNYANOVA A, DE MICHELIS I, et al. Extraction of metals from spent hydrotreating catalysts: physico-mechanical pre-treatments and leaching stage[J]. Journal of Hazardous Materials, 2011, 192(1): 176-185. |
| 48 | HUANG Shaobo, ZHAO Zhongwei, CHEN Xingyu, et al. Alkali extraction of valuable metals from spent Mo-Ni/Al2O3 catalyst[J]. International Journal of Refractory Metals and Hard Materials, 2014, 46: 109-116. |
| 49 | DELIA ROJAS-RODRÍGUEZ A, FLORES-FAJARDO O, SELENE ALCÁNTAR GONZÁLEZ F, et al. Chemical treatment to recover molybdenum and vanadium from spent heavy gasoil hydrodesulfurization catalyst[J]. Advances in Chemical Engineering and Science, 2012, 2(3): 408-412. |
| 50 | PARK K H, REDDY B R, MOHAPATRA D, et al. Hydrometallurgical processing and recovery of molybdenum trioxide from spent catalyst[J]. International Journal of Mineral Processing, 2006, 80(2/3/4): 261-265. |
| 51 | PINTO I S S, SOARES H M V M. Selective leaching of molybdenum from spent hydrodesulphurisation catalysts using ultrasound and microwave methods[J]. Hydrometallurgy, 2012, 129/130: 19-25. |
| 52 | WANG Wenqiang, ZHANG Lei, HAN Yu, et al. Cleaner recycling of spent Ni-Mo/γ-Al2O3 catalyst based on mineral phase reconstruction[J]. Journal of Cleaner Production, 2019, 232: 266-273. |
| 53 | ZHANG Jialiang, YANG Cheng, CHEN Yongqiang, et al. Efficient phase transformation of γ-Al2O3 to α-Al2O3 in spent hydrodesulphurization catalyst by microwave roasting method[J]. Industrial & Engineering Chemistry Research, 2019, 58(4): 1495-1501. |
| 54 | LIU Qi, WANG Wenqiang, YANG Yue, et al. Recovery and regeneration of Al2O3 with a high specific surface area from spent hydrodesulfurization catalyst CoMo/Al2O3[J]. Rare Metals, 2019, 38(1): 1-13. |
| 55 | BUSNARDO R G, BUSNARDO N G, SALVATO G N, et al. Processing of spent NiMo and CoMo/Al2O3 catalysts via fusion with KHSO4[J]. Journal of Hazardous Materials, 2007, 139(2): 391-398. |
| 56 | CHEN Yun, FENG Qiming, SHAO Yanhai, et al. Investigations on the extraction of molybdenum and vanadium from ammonia leaching residue of spent catalyst[J]. International Journal of Mineral Processing, 2006, 79(1): 42-48. |
| 57 | YE Xiaolei, GUO Shenghui, QU Wenwen, et al. Microwave sodium roasting (MWSR) spent HDS catalysts for recovery Mo and in situ sulfur fixation[J]. Journal of the Taiwan Institute of Chemical Engineers, 2019, 97: 146-157. |
| 58 | KIM H I, PARK K H, MISHRA D. Influence of sulfuric acid baking on leaching of spent Ni-Mo/Al2O3 hydro-processing catalyst[J]. Hydrometallurgy, 2009, 98(1/2): 192-195. |
| 59 | YE Xiaolei, GUO Shenghui, QU Wenwen, et al. Microwave field: high temperature dielectric properties and heating characteristics of waste hydrodesulfurization catalysts[J]. Journal of Hazardous Materials, 2019, 366: 432-438. |
| 60 | 张邦胜, 刘贵清, 王芳, 等. 一种从废镍钼催化剂中回收镍、钼的方法: CN108467939A[P]. 2018-08-31. |
| ZHANG Bangsheng, LIU Guiqing, WANG Fang, et al. Method for recovering nickel and molybdenum from waste nickel-molybdenum catalyst: CN108467939A[P]. 2018-08-31. | |
| 61 | 朱兆鹏, 杨夫清, 梁宗跃, 等. 用等离子炉处理含钼废催化剂回收有价金属的研究[J]. 中国钼业, 2003, 27(3): 14-16. |
| ZHU Zhaopeng, YANG Fuqing, LIANG Zongyue, et al. Study on recovering valuable metals by treating molybdenum-containing waste catalysts using plasma furnace[J]. China Molybdenum Industry, 2003, 27(3): 14-16. | |
| 62 | 王成彦, 杨成, 张家靓, 等. 废加氢催化剂还原熔炼回收有价金属试验[J]. 有色金属(冶炼部分), 2019(9): 12-17. |
| WANG Chengyan, YANG Cheng, ZHANG Jialiang, et al. Study on recovery of valuable metals from spent hydrodesulphurization catalysts by reduction smelting[J]. Nonferrous Metals (Extractive Metallurgy), 2019(9): 12-17. | |
| 63 | YAO Zan, MA Xiaodong, Sha LYU. Phase equilibria of the Al2O3-CaO-SiO2-(0%, 5%, 10%)MgO slag system for non-metallic inclusions control[J]. Calphad, 2021, 72: 102227. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [6] | WANG Peng, ZHANG Yang, FAN Bingqiang, HE Dengbo, SHEN Changshuai, ZHANG Hedong, ZHENG Shili, ZOU Xing. Process and kinetics of hydrochloric acid leaching of high-carbon ferrochromium [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 510-517. |
| [7] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [8] | SHAO Boshi, TAN Hongbo. Simulation on the enhancement of cryogenic removal of volatile organic compounds by sawtooth plate [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 84-93. |
| [9] | MA Yi, CAO Shiwei, WANG Jiajun, LIN Liqun, XING Yan, CAO Tengliang, LU Feng, ZHAO Zhenlun, ZHANG Zhijun. Research progress in recovery of spent cathode materials for lithium-ion batteries using deep eutectic solvents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 219-232. |
| [10] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [11] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [12] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [13] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [14] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [15] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||