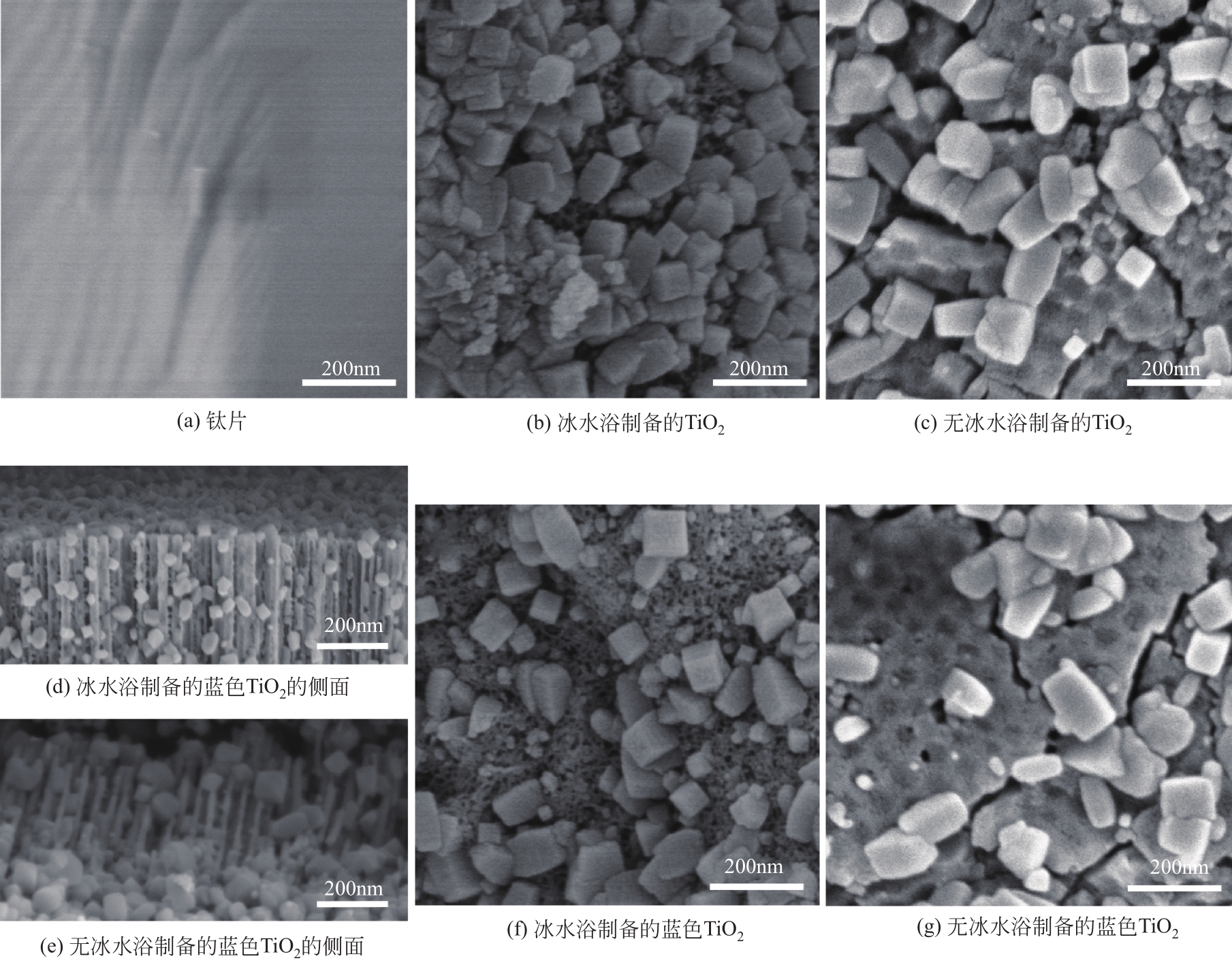
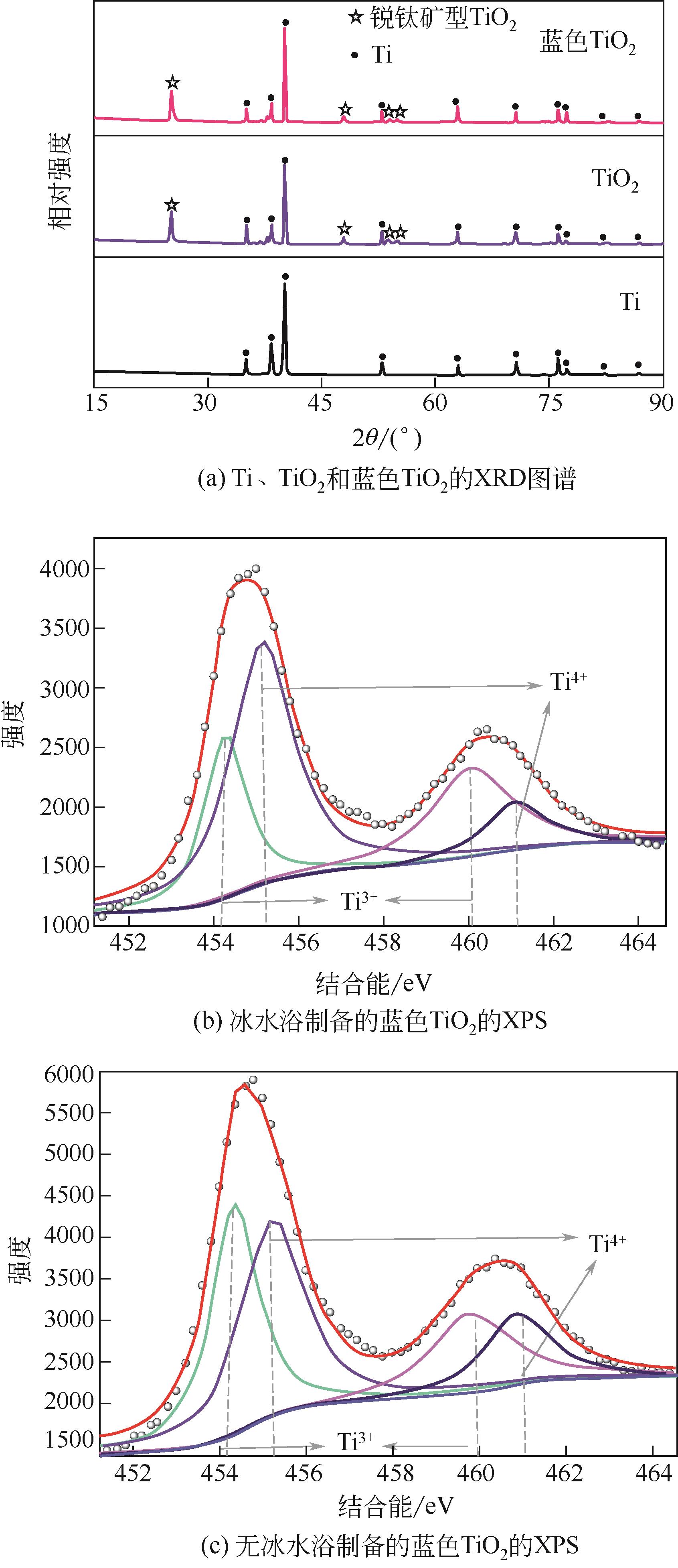
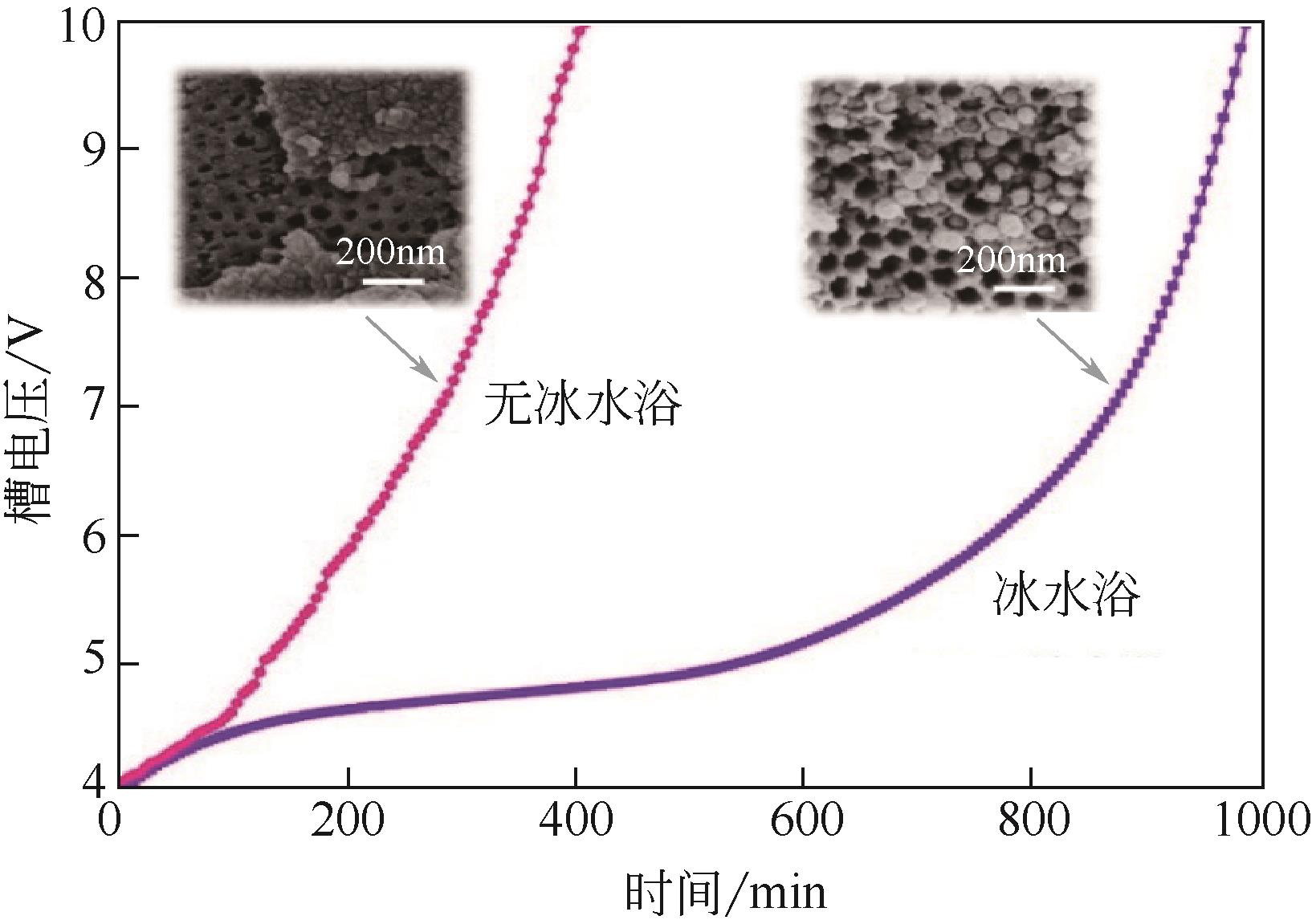
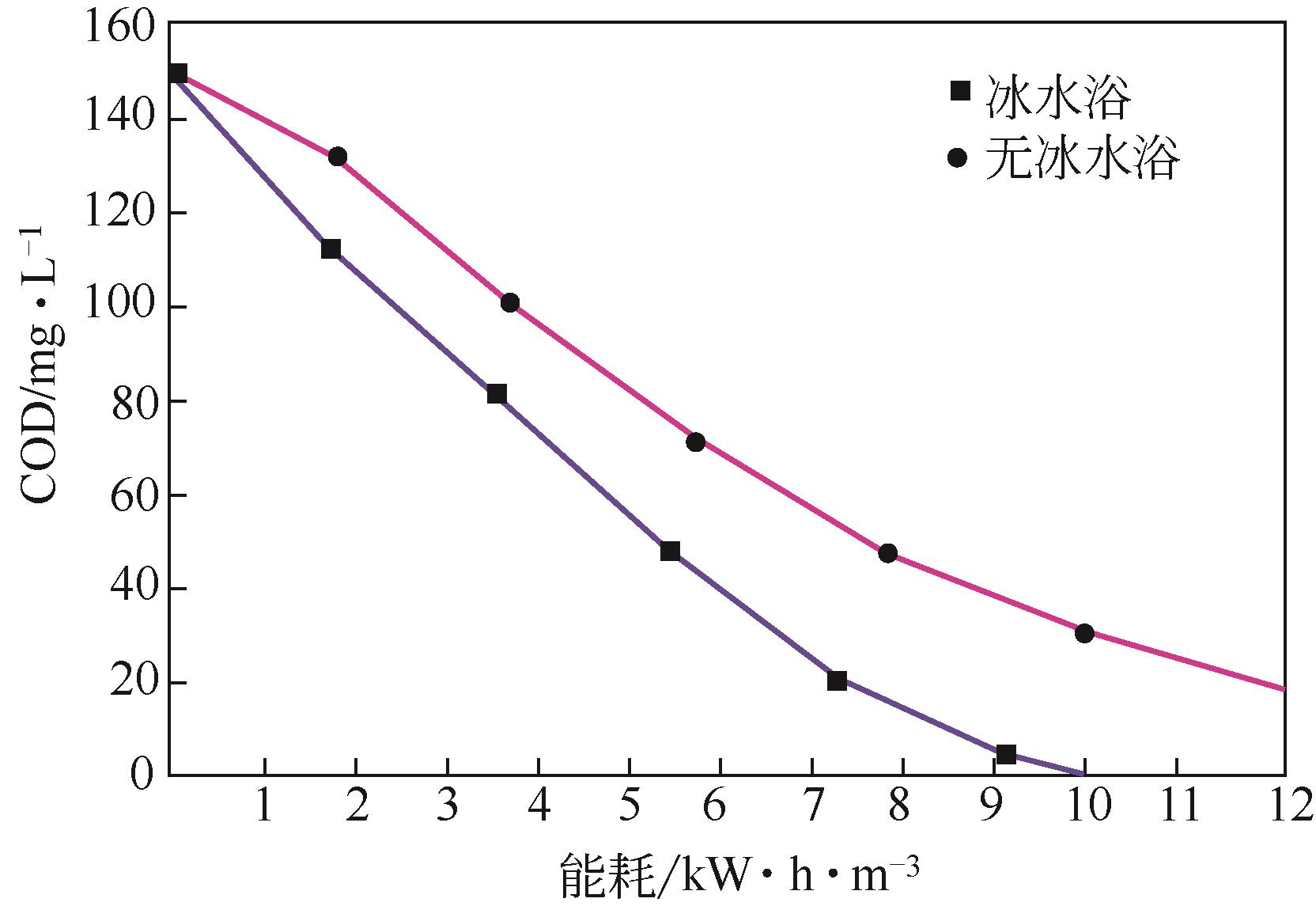
Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (2): 862-873.DOI: 10.16085/j.issn.1000-6613.2021-0463
• Materials science and technology • Previous Articles Next Articles
Hierarchically nanostructured blue TiO2 with enhanced electrochemical oxidation performance and stability
DAI Shaoling( ), YU Zhen, LI Yihang, CHENG Shao’an(
), YU Zhen, LI Yihang, CHENG Shao’an( )
)
- State Key Laboratory of Clean Energy, Department of Energy Engineering, Zhejiang University, Hangzhou 310027, Zhejiang, China
-
Received:2021-03-08Revised:2021-03-25Online:2022-02-23Published:2022-02-05 -
Contact:CHENG Shao’an
多层纳米结构蓝色TiO2的电化学氧化性能和稳定性
- 浙江大学能源工程学院,能源清洁利用国家重点实验室,浙江 杭州 310027
-
通讯作者:成少安 -
作者简介:戴绍铃(1995—),男,硕士研究生,研究方向为电化学高级氧化。E-mail:21827038@zju.edu.cn 。 -
基金资助:国家自然科学基金(51778562);国家重点研发计划(2018YFA0901300)
CLC Number:
Cite this article
DAI Shaoling, YU Zhen, LI Yihang, CHENG Shao’an. Hierarchically nanostructured blue TiO2 with enhanced electrochemical oxidation performance and stability[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 862-873.
戴绍铃, 于桢, 李逸航, 成少安. 多层纳米结构蓝色TiO2的电化学氧化性能和稳定性[J]. 化工进展, 2022, 41(2): 862-873.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-0463
| 电极 | 制备方法 | CMB/mg·L-1 | V/L | η/% | i/A·cm-2 | S/cm2 | t/min | Y/×10-3mg·C-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| Ti/SnO2-Sb | 直流电沉积 | 100 | 0.10 | 89.6 | 0.02 | 4 | 240 | 7.78 | [ |
| Ti/SnO2-Sb-Ce | 直流电沉积 | 10 | 0.20 | 89.6 | 0.04 | 6 | 240 | 6.67 | [ |
| Ti/SnO2-Sb | 溶胶-凝胶法 | 50 | 0.05 | 43.8 | 0.02 | 2 | 100 | 4.56 | [ |
| Ti/IrO2-RuO2 | — | 100 | 0.15 | 98.0 | 0.10 | 6 | 30 | 13.61 | [ |
| 石墨阳极 | — | 50 | 0.50 | 72.5 | 0.01 | 22 | 60 | 22.89 | [ |
| 蓝色TiO2 | 电化学还原 | 100 | 0.10 | 96.7 | 0.02 | 4 | 90 | 22.38 | 本研究 |
| 电极 | 制备方法 | CMB/mg·L-1 | V/L | η/% | i/A·cm-2 | S/cm2 | t/min | Y/×10-3mg·C-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| Ti/SnO2-Sb | 直流电沉积 | 100 | 0.10 | 89.6 | 0.02 | 4 | 240 | 7.78 | [ |
| Ti/SnO2-Sb-Ce | 直流电沉积 | 10 | 0.20 | 89.6 | 0.04 | 6 | 240 | 6.67 | [ |
| Ti/SnO2-Sb | 溶胶-凝胶法 | 50 | 0.05 | 43.8 | 0.02 | 2 | 100 | 4.56 | [ |
| Ti/IrO2-RuO2 | — | 100 | 0.15 | 98.0 | 0.10 | 6 | 30 | 13.61 | [ |
| 石墨阳极 | — | 50 | 0.50 | 72.5 | 0.01 | 22 | 60 | 22.89 | [ |
| 蓝色TiO2 | 电化学还原 | 100 | 0.10 | 96.7 | 0.02 | 4 | 90 | 22.38 | 本研究 |
| 44 | DUAN Tigang, WEN Qing, CHEN Ye, et al. Enhancing electrocatalytic performance of Sb-doped SnO2 electrode by compositing nitrogen-doped graphene nanosheets[J]. Journal of Hazardous Materials, 2014, 280: 304-314. |
| 45 | SONG Guanjun, YANG Jian, LI Wenxiang, et al. Electrolytic treatment of methylene blue solution with Ti-based IrO2-RuO2 anode[J]. Environmental Protection of Chemical Industry, 2012, 32(3): 205-208. |
| 46 | JAWAD NOOR H, NAJIM SARMAD T. Removal of methylene blue by direct electrochemical oxidation method using a graphite anode[J]. IOP Conference Series Materials Science and Engineering, 2018, 454(1): 012023. |
| 47 | CAI Jingju, ZHOU Minghua, YANG Weiliu, et al. Degradation and mechanism of 2,4-dichlorophenoxyacetic acid (2,4-D) by thermally activated persulfate oxidation[J]. Chemosphere, 2018, 212: 784-793. |
| 48 | ZHANG Hui, WANG Zhe, LIU Chiachi, et al. Removal of COD from landfill leachate by an electro/Fes/peroxydisulfate process[J]. Chemical Engineering Journal, 2014, 250: 76-82. |
| 49 | LIANG Chengju, WANG Zih Sin, BRUELL Clifford J, et al. Influence of pH on persulfate oxidation of TCE at ambient temperatures[J]. Chemosphere, 2007, 66(1): 106-113. |
| 50 | CHEN Luchuan, LEI Chaojun, LI Zhongjian, et al. Electrochemical activation of sulfate by BDD anode in basic medium for efficient removal of organic pollutants[J]. Chemosphere, 2018, 210: 516-523. |
| 51 | YANG Y, KAO L C, LIU Y, et al. Cobalt-doped black TiO2 nanotube array as a stable anode for oxygen evolution and electrochemical wastewater treatment[J]. ACS Catal., 2018, 8(5): 4278-4287. |
| 1 | 桂新安, 杨海真. 高级氧化技术在垃圾渗滤液处理中的应用[J]. 环境科学与管理, 2007, 32(2): 58-63. |
| GUI Xin’an, YANG Haizhen. Application of advanced oxidation processes in the treatment of landfill leachate[J]. Environmental Science and Management, 2007, 32(2): 58-63. | |
| 2 | GHERNAOUT Djame, ELBOUGHDIRI Noureddine, GHAREBA Saad, et al. Electrochemical advanced oxidation processes (EAOPs) for disinfecting water-fresh perspectives[J]. Open Access Library Journal, 2020, 7(4): 1-12. |
| 3 | Carlos MARTINEZ-HUITLE, RODRIGO Manuel, SIRES Ignasi, et al. Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: a critical review[J]. Chemical Reviews, 2015, 115(24): 13362-13407. |
| 4 | 宋日海, 魏刚, 熊蓉春, 等. 废水处理用催化电极的研究与应用[J]. 水处理技术, 2006, 32(12): 4-9. |
| SONG Rihai, WEI Gang, XIONG Rongchun, et al. Research and application of catalytic electrode for wastewater treatment[J]. Technology of Water Treatment, 2006, 32(12): 4-9. | |
| 5 | BRILLAS Enric, MARTINEZ-HUITLE Carlos A. Synthetic diamond films: preparation, electrochemistry, characterization, and applications[M]. Germany: John Wiley & Sons, Inc., 2011. |
| 6 | TRASATTI Sergio. Electrocatalysis: understanding the success of DSA[J]. Electrochimica Acta, 2000, 45(15): 2377-85. |
| 7 | 孔德生, 吕文华, 冯媛媛, 等. DSA电极电催化性能研究及尚待深入探究的几个问题[J]. 化学进展, 2009, 21(6): 1107-1117. |
| KONG Desheng, Wenhua LYU, FENG Yuanyuan, et al. Advances and some problems in electrocatalysis of DSA electrodes[J]. Progress in Chemistry, 2009, 21(6): 1107-1117. | |
| 8 | 刘峻峰, 冯玉杰, 吕江维, 等. 含Mn中间层提高钛基SnO2电催化电极的稳定性[J]. 材料研究学报, 2008, 22(6): 593-598. |
| LIU Junfeng, FENG Yujie, Jiangwei LYU, et al. Enhancing service life of SnO2 electrode by introducing an interlayer containing Mn element[J]. Chinese Journal of Materials Resarch, 2008, 22(6): 593-598. | |
| 9 | KIM Choonsoo, KIM Seonghwan, CHOI Jusol, et al. Blue TiO2 nanotube array as an oxidant generating novel anode material fabricated by simple cathodic polarization[J]. Electrochimica Acta, 2014, 141: 113-119. |
| 10 | GAN Ling, WU Yifan, SONG Haiou, et al. Self-doped TiO2 nanotube arrays for electrochemical mineralization of phenols[J]. Chemosphere, 2019, 226: 329-339. |
| 11 | FANG Wenzhang, XING Mingyang, ZHANG Jinlong, et al. A new approach to prepare Ti3+ self-doped TiO2via NaBH4 reduction and hydrochloric acid treatment[J]. Applied Catalysis B: Environmental, 2014, 160/161(1): 240-246. |
| 12 | WANG Zhou, YANG Chongyin, LIN Tianquan, et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium-reduced black titania[J]. Energy & Environmental Science, 2013, 6(10): 3007-3014. |
| 13 | MAO Chengyu, ZUO Fan, HOU Yang, et al. In situ preparation of a Ti3+ self-doped TiO2 film with enhanced activity as photoanode by N2H4 reduction[J]. Angewandte Chemie International Edition, 2014, 53(39): 10485-10489. |
| 14 | CHEN Xiaobo, LIU Lei, YU Peter Y, et al. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals[J]. Science, 2011, 331(6018): 746-750. |
| 15 | LI Zhen, DING Youting, KANG Weijun, et al. Reduction mechanism and capacitive properties of highly electrochemically reduced TiO2 nanotube arrays[J]. Electrochimica Acta, 2015, 161: 40-47. |
| 16 | ZHANG Aiqin, GONG Feilong, XIAO Yuanhua, et al. Electrochemical reductive doping and interfacial impedance of TiO2 nanotube arrays in aqueous and aprotic solvents[J]. Journal of the Electrochemical Society, 2017, 164(2): 91-96. |
| 17 | GENG Ping, CHEN Guohua. Antifouling ceramic membrane electrode modified by Magnéli Ti4O7 for electro-microfiltration of humic acid[J]. Separation and Purification Technology, 2017, 185: 61-71. |
| 18 | WANG Fang, DING Xian, SHI Ruyue, et al. Facile synthesis of Ti4O7 on hollow carbon spheres with enhanced polysulfide binding for high-performance lithium–sulfur batteries[J]. Journal of Materials Chemistry A, 2019, 7(17): 10494-10504. |
| 19 | YOU Shijie, LIU Bo, GAO Yifan, et al. Monolithic porous Magnéli-phase Ti4O7 for electro-oxidation treatment of industrial wastewater[J]. Electrochimica Acta, 2016, 214: 326-335. |
| 20 | MOHAJERNIA S, HEJAZI S, MAZARE A, et al. Photoelectrochemical H2 generation from suboxide TiO2 nanotubes: visible-light absorption versus conductivity[J]. Chemistry, 2017, 23: 12406-12411. |
| 21 | LIU Gang, YANG Huagui, PAN Jian, et al. Titanium dioxide crystals with tailored facets[J]. Chemical Reviews, 2014, 114(19): 9559-9612. |
| 22 | CHANG Xin, THIND Sapanbir S, CHEN Aicheng, et al. Electrocatalytic enhancement of salicylic acid oxidation at electrochemically reduced TiO2 nanotubes[J]. ACS Catalysis, 2014, 4(8): 2616-2622. |
| 23 | CHANG Xin, ZALM Joshua Van Der, THIND Sapanbir S, et al. Electrochemical oxidation of lignin at electrochemically reduced TiO2 nanotubes[J]. Journal of Electroanalytical Chemistry, 2020, 863: 114049. |
| 24 | WU Hui, LI Dongdong, ZHU Xufei, et al. High-performance and renewable supercapacitors based on TiO2 nanotube array electrodes treated by an electrochemical doping approach[J]. Electrochimica Acta, 2014, 116: 129-136. |
| 25 | YANG Yang, HOFFMANN Michael R. Synthesis and stabilization of blue-black TiO2 nanotube arrays for electrochemical oxidant generation and wastewater treatment[J]. Environ. Sci. Technol., 2016, 50(21): 11888-11894. |
| 26 | CAI Jingju, ZHOU Minghua, PAN Yuwei, et al. Extremely efficient electrochemical degradation of organic pollutants with co-generation of hydroxyl and sulfate radicals on blue-TiO2 nanotubes anode[J]. Applied Catalysis B: Environmental, 2019, 257(15): 117902. |
| 27 | 宁成云, 王玉强, 郑华德, 等. 阳极氧化法制备二氧化钛纳米管阵列的研究[J]. 化学研究与应用, 2010, 22(1): 14-17. |
| NING Chengyun, WANG Yuqiang, ZHENG Huade, et al. Study on preparation of TiO2 nanotube arrays by anodizing process[J]. Chemical Research and Application, 2010, 22(1): 14-17. | |
| 28 | WANG Jun, LIN Zhiqun. Anodic formation of ordered TiO2 nanotube arrays: effects of electrolyte temperature and anodization potential[J]. Journal of Physical Chemistry C, 2009, 113(10): 4026-4030. |
| 29 | TRASATTI Sergio, PETRII Oleg. Real surface area measurements in electrochemistry[J]. Journal of Electroanalytical Chemistry, 1992, 327(1): 353-376. |
| 30 | VOIRY Damien, CHHOWALLA Manish, GOGOTSI Yury, et al. Best practices for reporting electrocatalytic performance of nanomaterials[J]. ACS Nano, 2018, 12(10): 9635-9638. |
| 31 | SANTOS D, PACHECO M J, GOMES A, et al. Preparation of Ti/Pt/SnO2–Sb2O4 electrodes for anodic oxidation of pharmaceutical drugs[J]. Journal of Applied Electrochemistry, 2013, 43: 407-416 |
| 32 | HYAM Rajeshkumar S, CHOI Dukhyun. Effects of titanium foil thickness on TiO2 nanostructures synthesized by anodization[J]. RSC Advances, 2013, 3(19): 7057-7063. |
| 33 | ZHANG S Y, YU D L, LI D D, et al. Forming process of anodic TiO2 nanotubes under a preformed compact surface layer[J]. Journal of the Electrochemical Society, 2014, 161(10): 135-141. |
| 34 | SKELDON P, THOMPSON G E, GARCIA-VERGARA S J, et al. A tracer study of porous anodic alumina[J]. Electrochemical & Solid State Letters, 2006, 9(11): B47. |
| 35 | GARCIA-VERGARA S J, SKELDON P, THOMPSON G E, et al. A flow model of porous anodic film growth on aluminium[J]. Electrochimica Acta, 2007, 52(2): 681-687. |
| 36 | ALBELLA J M, MONTERO I, MARTINEZ-DUART J M, et al. A theory of avalanche breakdown during anodic oxidation[J]. Electrochimica Acta, 1987, 32(2): 255-258. |
| 37 | MAZZAROLO A, CURIONI M, VICENZO A, et al. Anodic growth of titanium oxide: electrochemical behaviour and morphological evolution[J]. Electrochimica Acta, 2012, 75: 288-295. |
| 38 | XU Xin, CAI Jingju, ZHOU Minghua, et al. Photoelectrochemical degradation of 2,4-dichlorophenoxyacetic acid using electrochemically self-doped blue TiO2 nanotube arrays with formic acid as electrolyte[J]. Journal of Hazardous Materials, 2019, 382: 121096. |
| 39 | MACAK J M, GONG B G, HUEPPE M, et al. Filling of TiO2 nanotubes by self-doping and electrodeposition[J]. Advanced Materials, 2007, 19(19): 3027-3031. |
| 40 | GHICOV Andrei, TSUCHIYA Hiroaki, HAHN Robert, et al. TiO2 nanotubes: H+ insertion and strong electrochromic effects[J]. Electrochemistry Communications, 2006, 8(4): 528-532. |
| 41 | YANG Yang, Licheng KAO, LIU Yuanyue, et al. Cobalt-doped black TiO2 nanotube array as a stable anode for oxygen evolution and electrochemical wastewater treatment[J]. ACS Catalysis, 2018, 8(5): 4278-4287. |
| 42 | SUN Yi, CHENG Shaoan, MAO Zhengzhong, et al. High electrochemical activity of a Ti/SnO2-Sb electrode electrodeposited using deep eutectic solvent[J]. Chemosphere, 2019, 239: 124715. |
| 43 | YANG Kun, Liu Yuyu, Qiao Jinli, et al. Electrodeposition preparation of Ce-doped Ti/SnO2-Sb electrodes by using selected addition agents for efficient electrocatalytic oxidation of methylene blue in water[J]. Separation & Purification Technology, 2017, 189(22): 459-466. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | HU Xi, WANG Mingshan, LI Enzhi, HUANG Siming, CHEN Junchen, GUO Bingshu, YU Bo, MA Zhiyuan, LI Xing. Research progress on preparation and sodium storage properties of tungsten disulfide composites [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 344-355. |
| [3] | ZHANG Jie, BAI Zhongbo, FENG Baoxin, PENG Xiaolin, REN Weiwei, ZHANG Jingli, LIU Eryong. Effect of PEG and its compound additives on post-treatment of electrolytic copper foils [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 374-381. |
| [4] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [5] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [6] | WANG Yaogang, HAN Zishan, GAO Jiachen, WANG Xinyu, LI Siqi, YANG Quanhong, WENG Zhe. Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4043-4057. |
| [7] | LIU Yi, FANG Qiang, ZHONG Dazhong, ZHAO Qiang, LI Jinping. Cu facets regulation of Ag/Cu coupled catalysts for electrocatalytic reduction of carbon dioxide [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4136-4142. |
| [8] | ZHANG Yajuan, XU Hui, HU Bei, SHI Xingwei. Preparation of NiCoP/rGO/NF electrocatalyst by eletroless plating for efficient hydrogen evolution reaction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4275-4282. |
| [9] | WANG Shuaiqing, YANG Siwen, LI Na, SUN Zhanying, AN Haoran. Research progress on element doped biomass carbon materials for electrochemical energy storage [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4296-4306. |
| [10] | WANG Xin, WANG Bingbing, YANG Wei, XU Zhiming. Anti-scale and anti-corrosion properties of PDA/PTFE superhydrophobic coating on metal surface [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4315-4321. |
| [11] | LI Haidong, YANG Yuankun, GUO Shushu, WANG Benjin, YUE Tingting, FU Kaibin, WANG Zhe, HE Shouqin, YAO Jun, CHEN Shu. Effect of carbonization and calcination temperature on As(Ⅲ) removal performance of plant-based Fe-C microelectrolytic materials [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3652-3663. |
| [12] | CHU Tiantian, LIU Runzhu, DU Gaohua, MA Jiahao, ZHANG Xiao’a, WANG Chengzhong, ZHANG Junying. Preparation and chemical degradability of organoguanidine-catalyzed dehydrogenation type RTV silicone rubbers [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3664-3673. |
| [13] | LU Yang, ZHOU Jinsong, ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao. Leaching mechanism of Hg-absorption products on CeO2/TiO2 sorbentsin syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3875-3883. |
| [14] | WU Zhanhua, SHENG Min. Pitfalls of accelerating rate calorimeter for reactivity hazard evaluation and risk assessment [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3374-3382. |
| [15] | XU Wei, LI Kaijun, SONG Linye, ZHANG Xinghui, YAO Shunhua. Research progress of photocatalysis and co-electrochemical degradation of VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3520-3531. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||