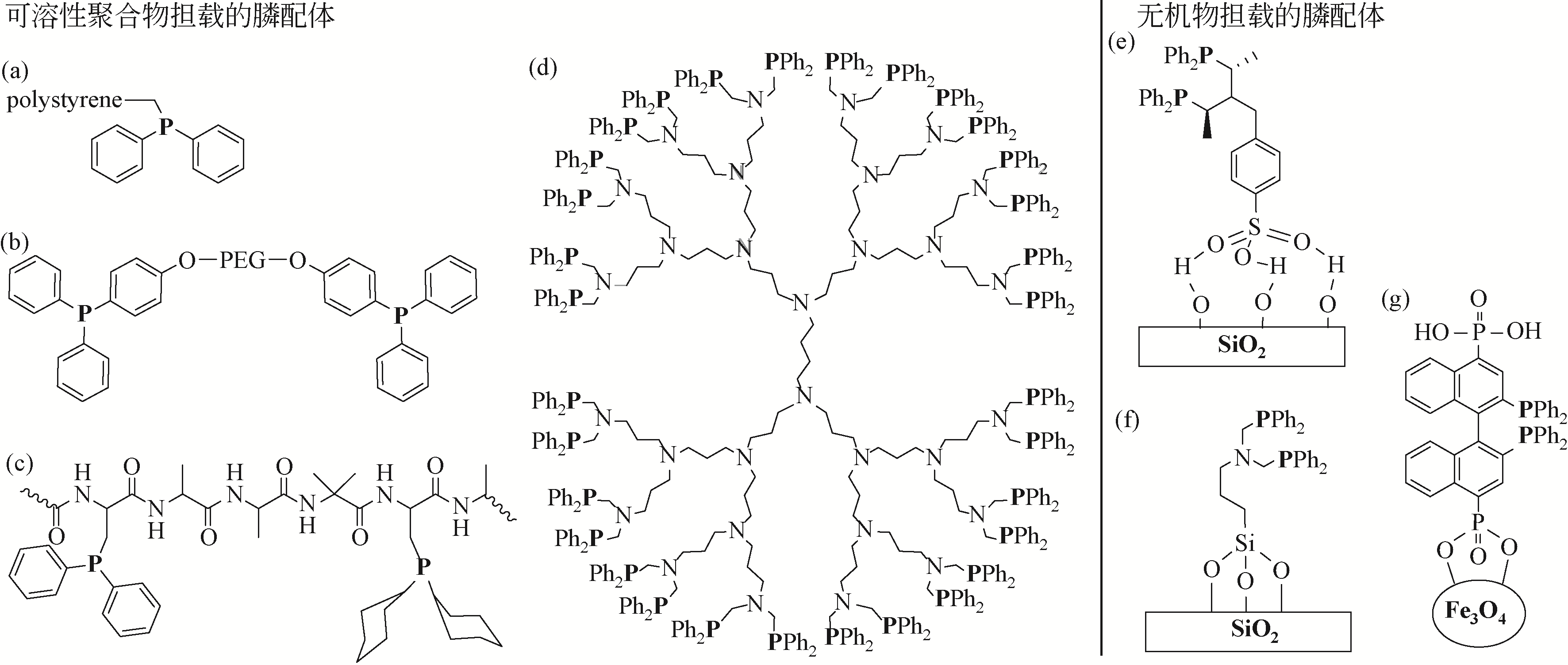
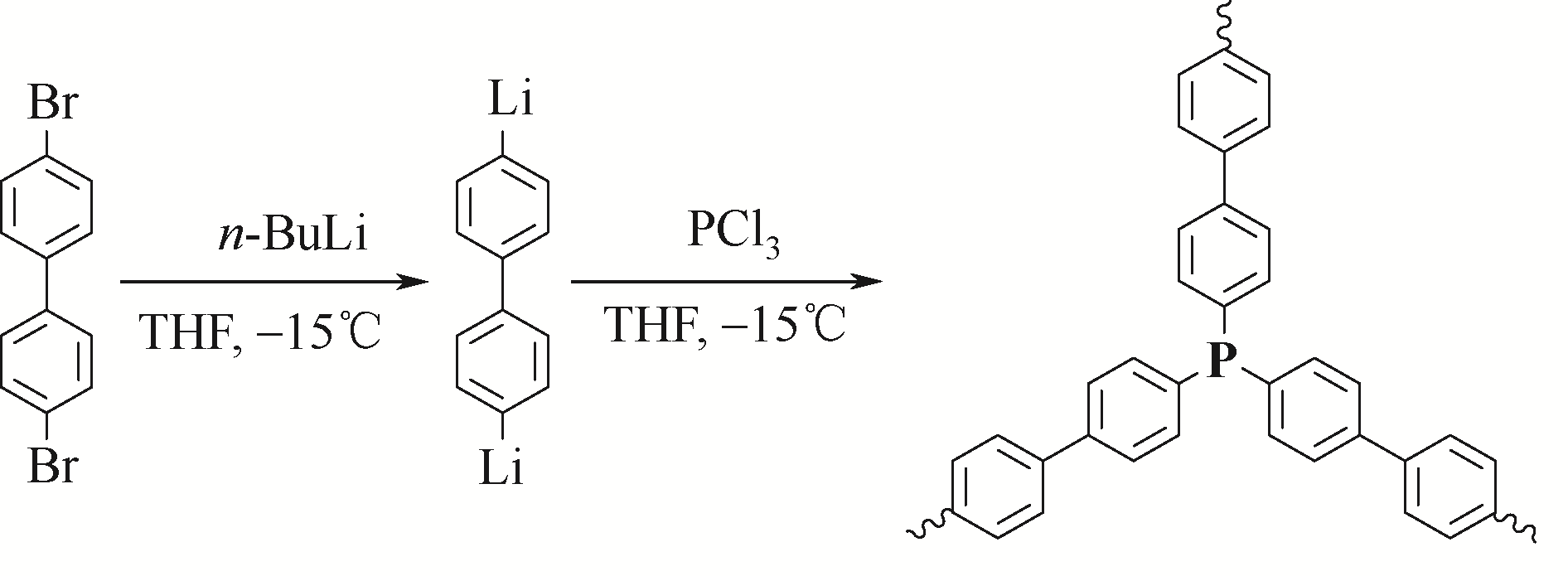
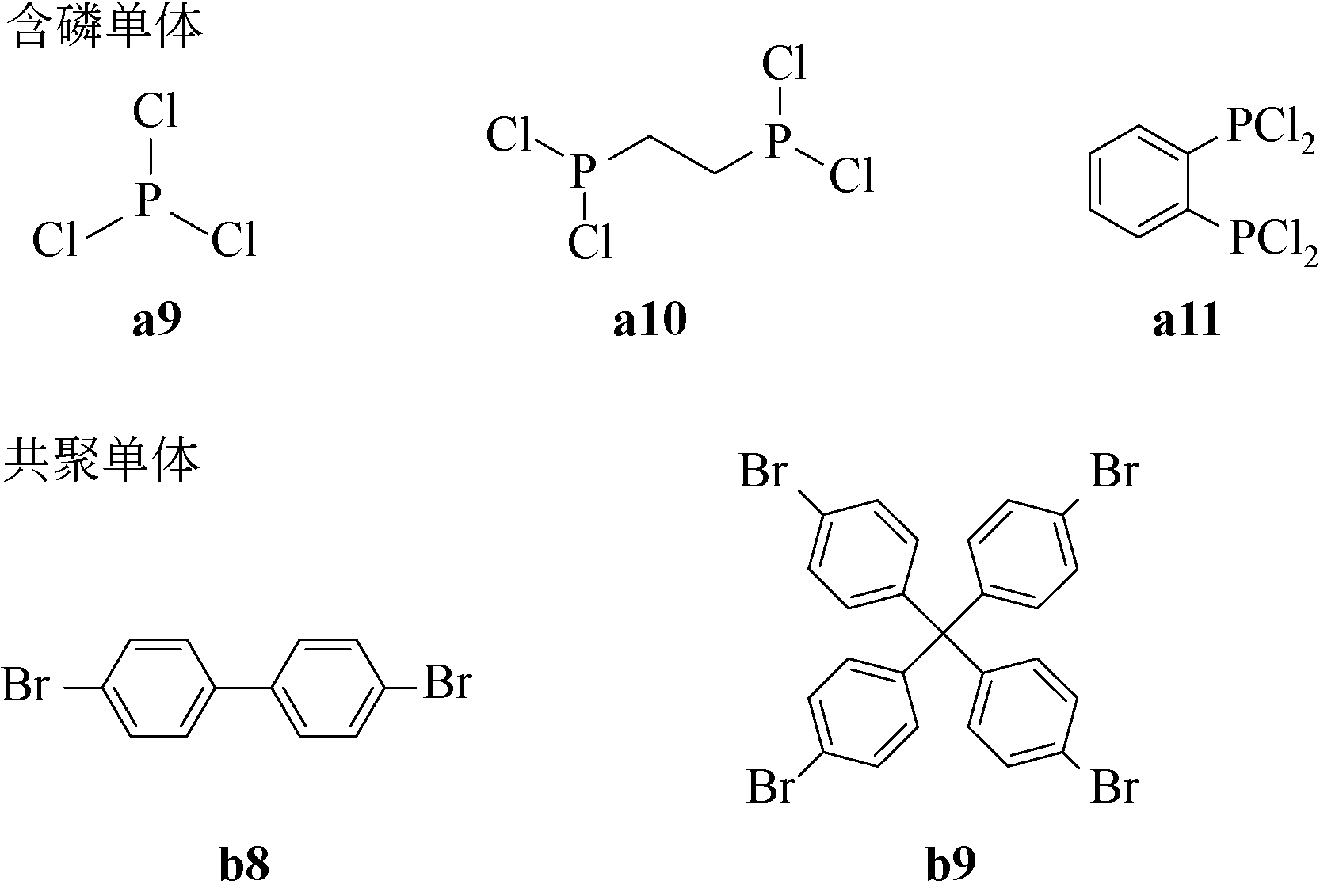
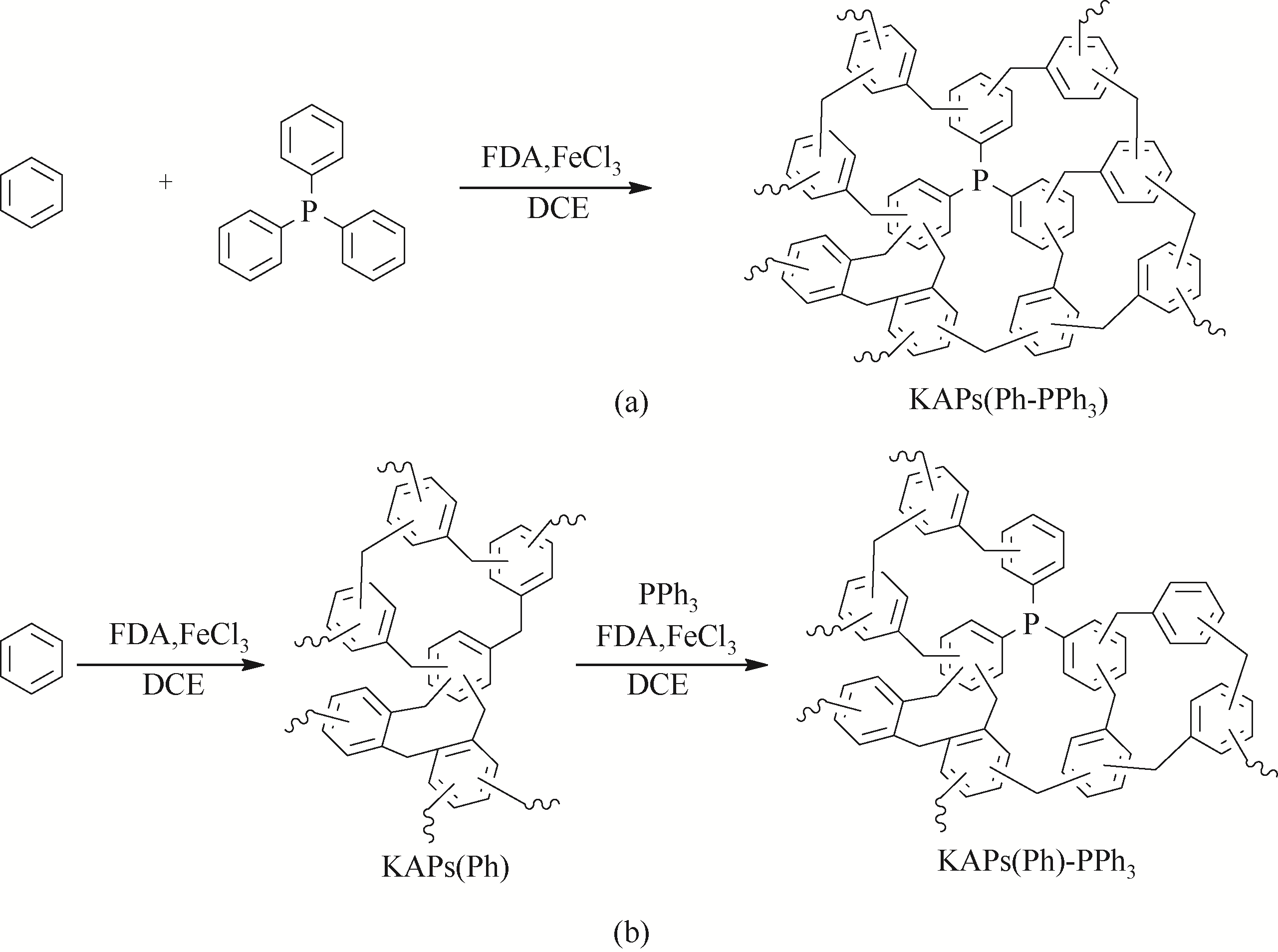
Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (4): 1983-2004.DOI: 10.16085/j.issn.1000-6613.2020-1924
• Special column:Industrial catalysis • Previous Articles Next Articles
Synthesis of phosphorus-containing porous organic polymers and their applications in heterogeneous catalysis
WU Miaojiang1,2( ), SUN Peng1, LI Fuwei1(
), SUN Peng1, LI Fuwei1( )
)
- 1.State Key Laboratory for Oxo Synthesis & Selective Oxidation, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, Gansu, China
2.University of Chinese Academy of Sciences, Beijing 100049, China
-
Received:2020-09-21Online:2021-04-14Published:2021-04-05 -
Contact:LI Fuwei
含磷多孔有机聚合物的合成及其在多相催化中的应用
- 1.中国科学院兰州化学物理研究所羰基合成与选择氧化国家重点实验室,甘肃 兰州 730000
2.中国科学院大学,北京 100049
-
通讯作者:李福伟 -
作者简介:吴淼江(1993—),男,博士研究生,研究方向为绿色催化。E-mail:wumiaojiang16@mails.ucas.ac.cn 。 -
基金资助:国家自然科学基金(21972151);中国科学院“西部之光”交叉团队项目
CLC Number:
Cite this article
WU Miaojiang, SUN Peng, LI Fuwei. Synthesis of phosphorus-containing porous organic polymers and their applications in heterogeneous catalysis[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 1983-2004.
吴淼江, 孙鹏, 李福伟. 含磷多孔有机聚合物的合成及其在多相催化中的应用[J]. 化工进展, 2021, 40(4): 1983-2004.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-1924
| 单体组成① | S/V(S后/V后)② | 孔径③ | 金属 | 催化反应 | 参考文献 |
|---|---|---|---|---|---|
| Heck偶联缩聚 | |||||
a1/b1=1/1 a2/b1=1/1 | 318/0.239 684/0.342 | 0~2nm | 钯 | Suzuki-Miyaura偶联反应 | [ |
| a3/b2=1.0/1.1 | 554/0.959 | 0~10nm | 钯 | 硝基芳烃、α,β-不饱和酮的加氢反应 | [ |
| Sonogashira-Hagihara偶联缩聚 | |||||
a4/b3=1/1 a4/b4=1/1 a4/b5=0.75/1.0 a4/b6=0.75/1.0 | 391/0.56 407/0.36 474/1.00 509/0.88 | 0~5nm 0~5nm 0~5nm 0~5nm | 钌 铱 | β-酮酯化合物的不对称氢化反应 喹哪啶不对称氢化反应 | [ |
| Suzuki-Miyaura偶联缩聚 | |||||
| a5/b7=1/2 | (354/—) | 0~7nm | 钯 | Suzuki-Miyaura偶联反应 | [ |
| a6/b7=1.17/1.76 | 72.4/— | 0~10nm | 无 | 光催化析氢反应 | [ |
| Yamamoto偶联缩聚 | |||||
| a8 | 650/—(114/—) | 0~6nm | 钯 无 | Suzuki偶联反应 环氧化物和CO2生成环碳酸酯的反应 | [ |
a5 a7 | 1284/1.11 1353/1.07 | 0~4nm 0~4nm | 钯 | Suzuki偶联反应 | [ |
| 单体组成① | S/V(S后/V后)② | 孔径③ | 金属 | 催化反应 | 参考文献 |
|---|---|---|---|---|---|
| Heck偶联缩聚 | |||||
a1/b1=1/1 a2/b1=1/1 | 318/0.239 684/0.342 | 0~2nm | 钯 | Suzuki-Miyaura偶联反应 | [ |
| a3/b2=1.0/1.1 | 554/0.959 | 0~10nm | 钯 | 硝基芳烃、α,β-不饱和酮的加氢反应 | [ |
| Sonogashira-Hagihara偶联缩聚 | |||||
a4/b3=1/1 a4/b4=1/1 a4/b5=0.75/1.0 a4/b6=0.75/1.0 | 391/0.56 407/0.36 474/1.00 509/0.88 | 0~5nm 0~5nm 0~5nm 0~5nm | 钌 铱 | β-酮酯化合物的不对称氢化反应 喹哪啶不对称氢化反应 | [ |
| Suzuki-Miyaura偶联缩聚 | |||||
| a5/b7=1/2 | (354/—) | 0~7nm | 钯 | Suzuki-Miyaura偶联反应 | [ |
| a6/b7=1.17/1.76 | 72.4/— | 0~10nm | 无 | 光催化析氢反应 | [ |
| Yamamoto偶联缩聚 | |||||
| a8 | 650/—(114/—) | 0~6nm | 钯 无 | Suzuki偶联反应 环氧化物和CO2生成环碳酸酯的反应 | [ |
a5 a7 | 1284/1.11 1353/1.07 | 0~4nm 0~4nm | 钯 | Suzuki偶联反应 | [ |
| 单体组成① | S/V(S后/V后)② | 孔径③ | 金属 | 催化反应 | 参考文献 |
|---|---|---|---|---|---|
| a9/b8=16.7/25 | 646/— | — | 钯 铑 | 环己酮与异丙醇的氢转移反应 | [ |
| a9/b8=13.0/20.0 | 135/0.04(58/0.009) | — | 钯 | 1,3-丁二烯的调聚反应 | [ |
a9/b8=12.7/20 a10/b8=9.75/20 a11/b8=7.15/20 | 150/— 33/— 42/— | — | 钌 | 甲酸分解反应 | [ |
| a10/b9=1/1 | 469/0.475 | 0~180nm | 钌 | CO2加氢合成N,N-二甲基甲酰胺的反应 | [ |
| 单体组成① | S/V(S后/V后)② | 孔径③ | 金属 | 催化反应 | 参考文献 |
|---|---|---|---|---|---|
| a9/b8=16.7/25 | 646/— | — | 钯 铑 | 环己酮与异丙醇的氢转移反应 | [ |
| a9/b8=13.0/20.0 | 135/0.04(58/0.009) | — | 钯 | 1,3-丁二烯的调聚反应 | [ |
a9/b8=12.7/20 a10/b8=9.75/20 a11/b8=7.15/20 | 150/— 33/— 42/— | — | 钌 | 甲酸分解反应 | [ |
| a10/b9=1/1 | 469/0.475 | 0~180nm | 钌 | CO2加氢合成N,N-二甲基甲酰胺的反应 | [ |
| 单体组成① | S/V(S后/V后)② | 孔径③ | 金属 | 催化反应 | 参考文献 |
|---|---|---|---|---|---|
| a12/b10/c1=1/1/3 | 1036/—(1025/1.5) | 0~100nm | 钯 钯 | Suzuki-Miyaura偶联反应 氯化苄的Suzuki-Miyaura偶联 | [ [ |
| a12/b10/c1=1/1/3 | 993/0.12(916/1.18) | 0~100nm | 钌 | 芳基酮、NH4OAc和DMF的环化反应;重氮二羰基化合物和烯烃的环加成反应 | [ |
a12/b10/c1=1/1/2 a12/b11/c1=1/1/2 a12/b12/c1=3/1/6 a12/b13/c1=3/1/6 | (723/0.92) (524/0.66) (672/0.70) (549/0.84) | 0~20nm | 铑 | 氢甲酰化反应 | [ |
| a13/b12/c1=1/2/8 | 350/0.438 | 0~100nm | 无 | 对硝基苯酚的还原反应 | [ |
| a14/b15/c1=1/1/3 | 282/—(59/—) | 0~10nm | 钌 | 硝基芳烃的还原 | [ |
a12/c3=2/3 a15/c3=2/3 | 788/—(608/—) 491/—(466/—) | 0~2nm | 金 | 对硝基苯酚的还原反应 | [ |
| a12/b14/c1=1/1/2 | 20/0.06(18/0.05) | — | 银 | 末端炔烃与二氧化碳的羧基化反应 | [ |
| a16/b10/c1=1/20/60 | 702/0.546 | 0~10nm | 钯 | Suzuki-Miyaura偶联反应 芳基卤化物和芳基硼酸酯的自偶联反应 | [ |
| a17/b10/c1=1/40/120 | 810/0.52 | 0~14nm | 钌 | 甲酸制氢反应 | [ |
a18/c2(Solvent) a19/c2(Solvent) a20/c2(Solvent) | 300/0.203 20/0.12 49/0.07 | 2.2nm 1.7nm 5.9nm | 钌 金 | 苯甲醇的胺化反应 炔烃水合反应;苯胺与苯乙炔的氢胺化反应 | [ |
| 单体组成① | S/V(S后/V后)② | 孔径③ | 金属 | 催化反应 | 参考文献 |
|---|---|---|---|---|---|
| a12/b10/c1=1/1/3 | 1036/—(1025/1.5) | 0~100nm | 钯 钯 | Suzuki-Miyaura偶联反应 氯化苄的Suzuki-Miyaura偶联 | [ [ |
| a12/b10/c1=1/1/3 | 993/0.12(916/1.18) | 0~100nm | 钌 | 芳基酮、NH4OAc和DMF的环化反应;重氮二羰基化合物和烯烃的环加成反应 | [ |
a12/b10/c1=1/1/2 a12/b11/c1=1/1/2 a12/b12/c1=3/1/6 a12/b13/c1=3/1/6 | (723/0.92) (524/0.66) (672/0.70) (549/0.84) | 0~20nm | 铑 | 氢甲酰化反应 | [ |
| a13/b12/c1=1/2/8 | 350/0.438 | 0~100nm | 无 | 对硝基苯酚的还原反应 | [ |
| a14/b15/c1=1/1/3 | 282/—(59/—) | 0~10nm | 钌 | 硝基芳烃的还原 | [ |
a12/c3=2/3 a15/c3=2/3 | 788/—(608/—) 491/—(466/—) | 0~2nm | 金 | 对硝基苯酚的还原反应 | [ |
| a12/b14/c1=1/1/2 | 20/0.06(18/0.05) | — | 银 | 末端炔烃与二氧化碳的羧基化反应 | [ |
| a16/b10/c1=1/20/60 | 702/0.546 | 0~10nm | 钯 | Suzuki-Miyaura偶联反应 芳基卤化物和芳基硼酸酯的自偶联反应 | [ |
| a17/b10/c1=1/40/120 | 810/0.52 | 0~14nm | 钌 | 甲酸制氢反应 | [ |
a18/c2(Solvent) a19/c2(Solvent) a20/c2(Solvent) | 300/0.203 20/0.12 49/0.07 | 2.2nm 1.7nm 5.9nm | 钌 金 | 苯甲醇的胺化反应 炔烃水合反应;苯胺与苯乙炔的氢胺化反应 | [ |
| 单体组成① | S/V(S后/V后)② | 孔径③ | 金属 | 催化反应 | 参考文献 |
|---|---|---|---|---|---|
| a21 | 1086/1.70 | 0~100nm | 铑 钌 铑 | 氢甲酰化反应 肉桂醛加氢反应 甲醇羰化反应 | [ [ [ |
| a21 | 1089/2.1 | 0~100nm | 钯 | Suzuki-Miyaura偶联反应 羰化偶联反应 | [ [ |
a21/b18=5/1 a21/b19=5/1 a21/b20=5/1 | — 591/1.03(482/—) — | 0~12nm | 锌 | 环氧化物和CO2生成环碳酸酯的反应 | [ |
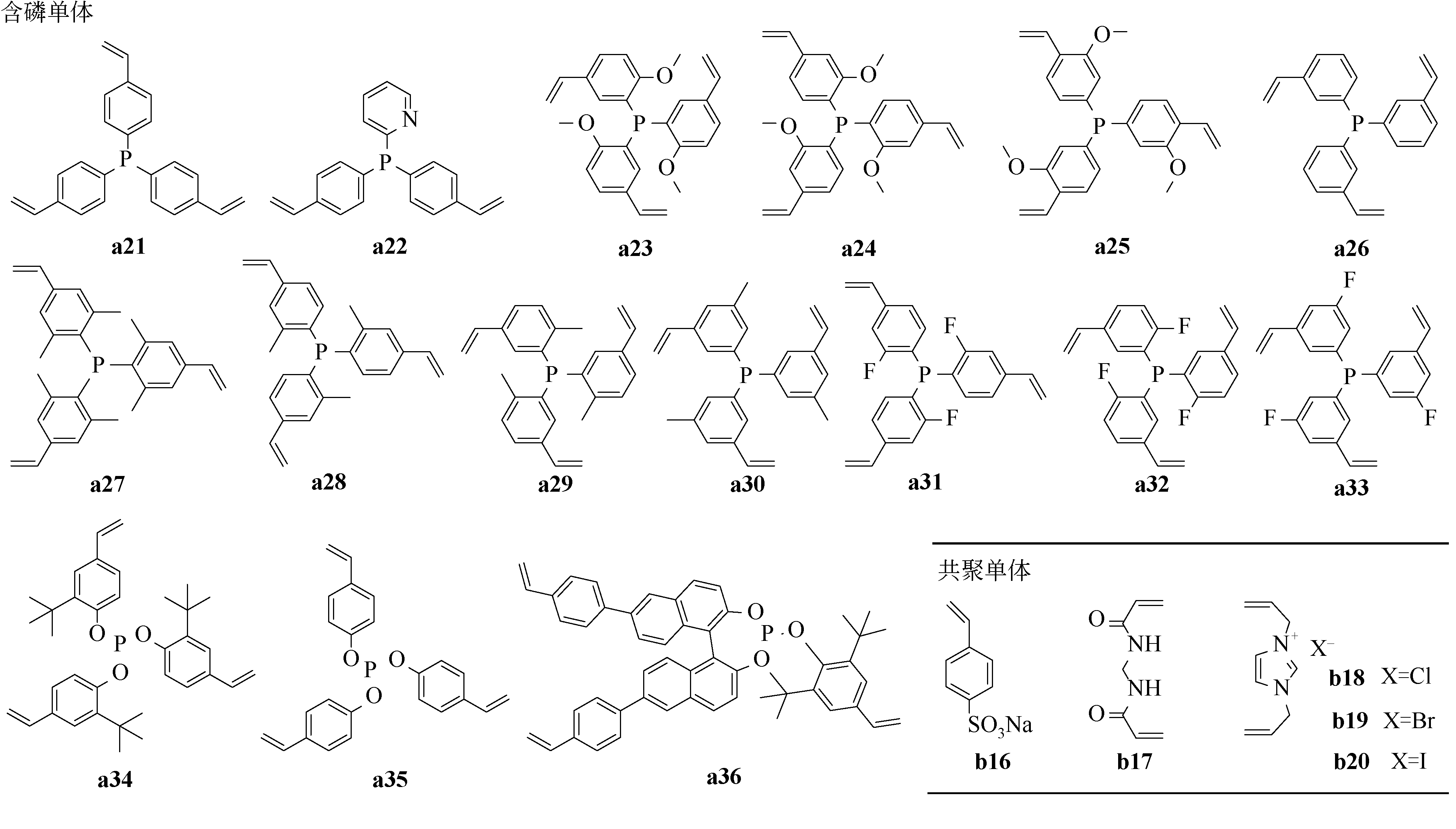
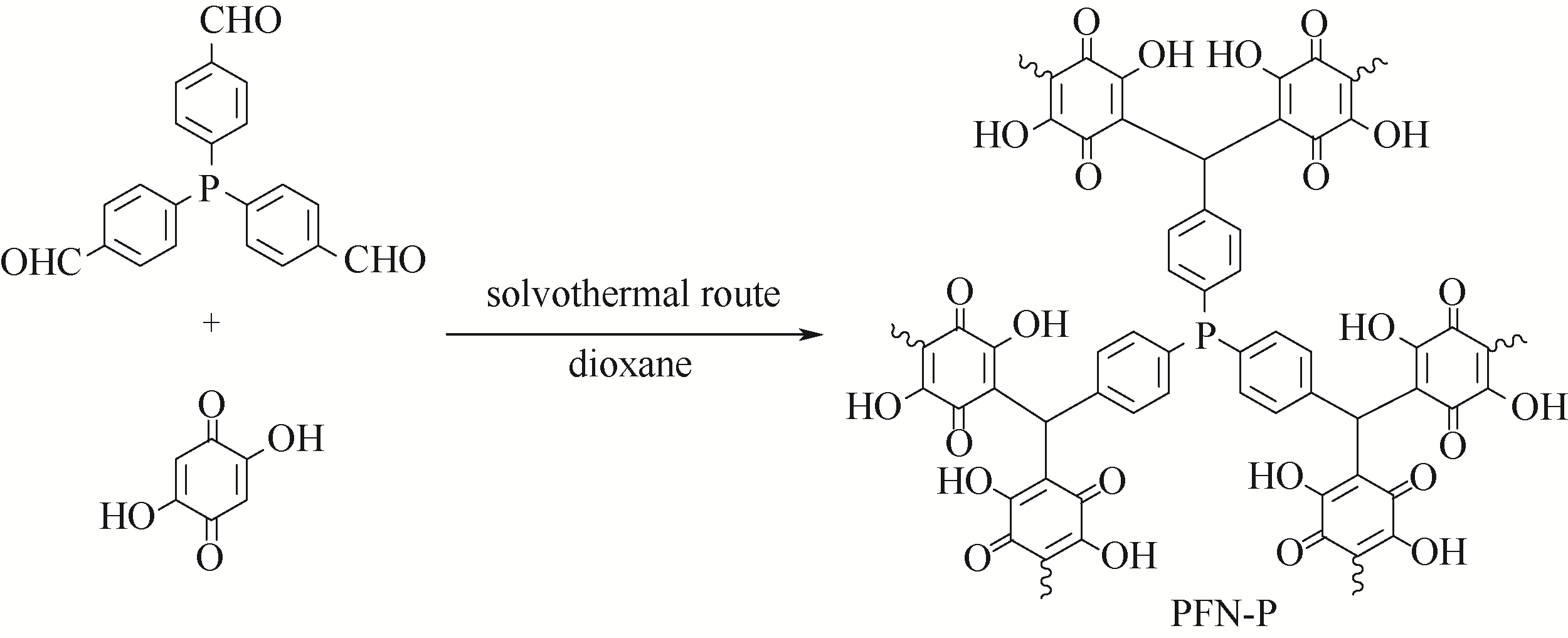
a22 a22/b16=1/2.56 | 475/0.64 44.3/0.133 | 0~1nm 0~10nm | 钯 | 烯烃烷酯基化反应 | [ [ |
| a21/b17=1.9/1 | 767/0.921(706/0.727) | — | 钌 | N-甲酰化反应 | [ |
| a23 | (810/0.759) | 0~10nm | 钯 | 末端炔烃的双锡烷基化 | [ |
a21/b17=1/8.8 a21/b17=1/5.2 a21/b17=1/3.3 a21/b17=1/2.2 a21/b17=1/1.48 a21/b17=1/0.55 | 663/1.68 646/1.53 675/1.29 647/0.95 760/0.93 834/1.36 | 0~20nm | 钯 | Suzuki-Miyaura偶联反应 | [ |
a34 a35 a36 | 643/0.43 811/0.70 523/0.96 | 0~20nm 0~20nm 0~100nm | 铑 | 氢甲酰化反应 | [ [ |
| a21 | 928/—(758/—) | 0~100nm | 金 | 炔烃水合反应;炔丙基酯的重排反应;氧化炔烃偶联反应 | [ |
| a21 | — | — | 铱 | 次磷酰胺和苯甲醇的反应;双胍类化合物和苯甲醇的反应 | [ |
| 单体组成① | S/V(S后/V后)② | 孔径③ | 金属 | 催化反应 | 参考文献 |
|---|---|---|---|---|---|
| a21 | 1086/1.70 | 0~100nm | 铑 钌 铑 | 氢甲酰化反应 肉桂醛加氢反应 甲醇羰化反应 | [ [ [ |
| a21 | 1089/2.1 | 0~100nm | 钯 | Suzuki-Miyaura偶联反应 羰化偶联反应 | [ [ |
a21/b18=5/1 a21/b19=5/1 a21/b20=5/1 | — 591/1.03(482/—) — | 0~12nm | 锌 | 环氧化物和CO2生成环碳酸酯的反应 | [ |
a22 a22/b16=1/2.56 | 475/0.64 44.3/0.133 | 0~1nm 0~10nm | 钯 | 烯烃烷酯基化反应 | [ [ |
| a21/b17=1.9/1 | 767/0.921(706/0.727) | — | 钌 | N-甲酰化反应 | [ |
| a23 | (810/0.759) | 0~10nm | 钯 | 末端炔烃的双锡烷基化 | [ |
a21/b17=1/8.8 a21/b17=1/5.2 a21/b17=1/3.3 a21/b17=1/2.2 a21/b17=1/1.48 a21/b17=1/0.55 | 663/1.68 646/1.53 675/1.29 647/0.95 760/0.93 834/1.36 | 0~20nm | 钯 | Suzuki-Miyaura偶联反应 | [ |
a34 a35 a36 | 643/0.43 811/0.70 523/0.96 | 0~20nm 0~20nm 0~100nm | 铑 | 氢甲酰化反应 | [ [ |
| a21 | 928/—(758/—) | 0~100nm | 金 | 炔烃水合反应;炔丙基酯的重排反应;氧化炔烃偶联反应 | [ |
| a21 | — | — | 铱 | 次磷酰胺和苯甲醇的反应;双胍类化合物和苯甲醇的反应 | [ |
| 单体组成① | S/V(S后/V后)② | 孔径③ | 金属 | 催化反应 | 参考文献 |
|---|---|---|---|---|---|
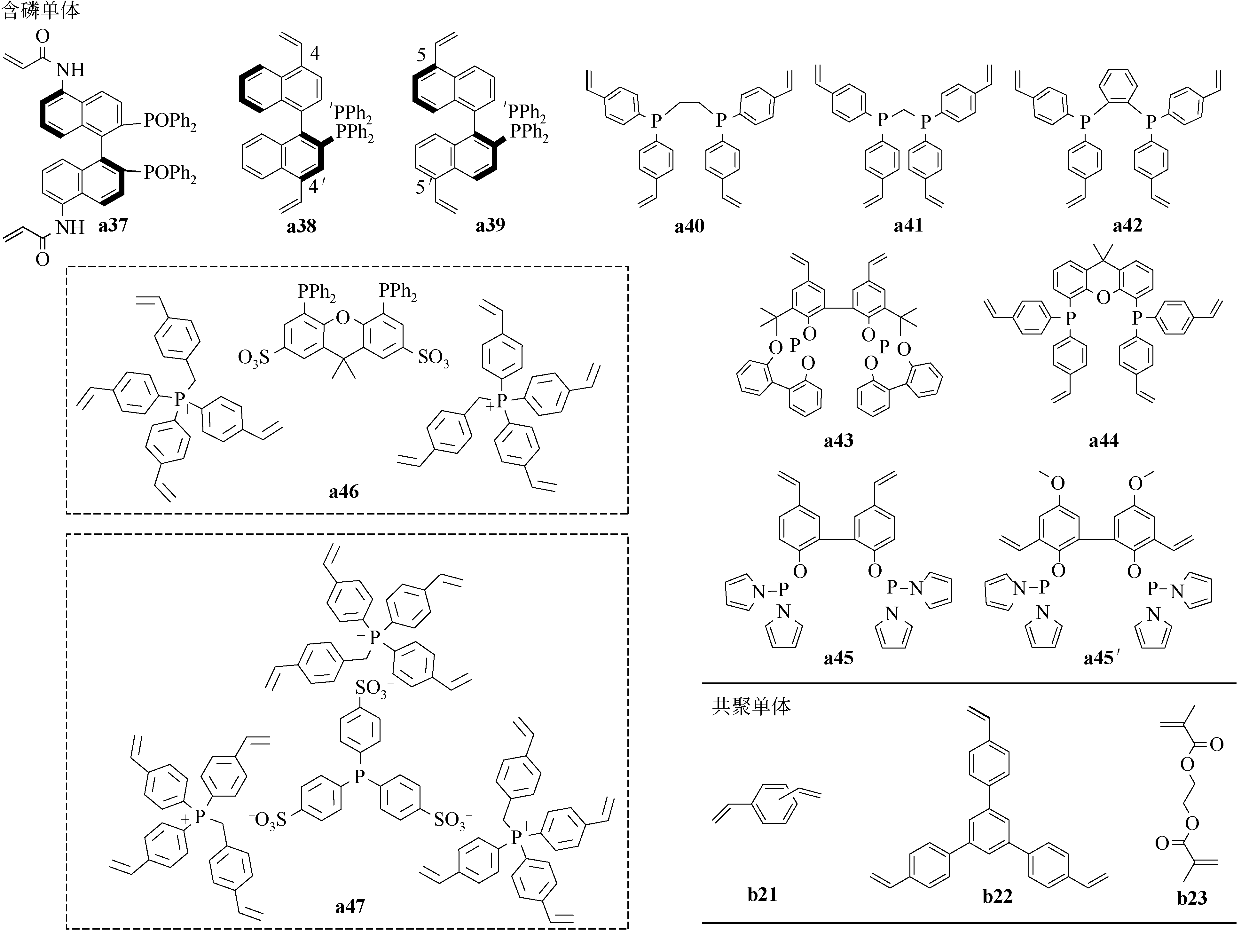
a37/b21=1/8 a37/b21=1/4 a37/b21=1/2 a37/b21=3/4 a37/b21=1/1 | 654/0.78 585/0.63 518/0.42 379/0.21 5/0.0035 | 0~80nm | 钌 | β-酮酯的不对称氢化反应 | [ |
| a38,a39 | (1058~1070/1.19~1.64) | 0~10nm | 钌 | β-酮酯的不对称氢化反应 | [ |
a39/b21=1/9.3 a39/b22=1/3.5 a39/b23=1/10.3 | 697/0.68(490/0.56) 997/1.19 —/— | 0~10nm | 铑 | 氢甲酰化反应 | [ |
a40 a41 a42 | 959/1.15 892/1.07 846/0.81 | 0~100nm | 铑 | 氢甲酰化反应 | [ |
| a41 | 629.0/1.065 | — | 钯 | 由硫代苯甲酰胺和异腈合成噻唑的反应 | [ |
| a43/a34=1/20 | 635/0.72(556/0.68) | 0~10nm | 铑 | 氢甲酰化反应 | [ |
a43/a21=1/9.2 a43/a21=1/45.5 a43/a21=1/22.7 a43/a21=1/11.5 a43/a21=1/7.6 a43/a21=1/6.1 | 1088.0/2.07(985.3/1.94) 1195/2.62 1201/2.79 1271/2.72 1589/3.82 907.3/1.72 | 0~20nm | 铑 | 氢甲酰化反应 | [ |
a44 a44/a21=1/5 | 561/0.648(506/0.596) 1022/1.24 | 0~8nm 0~10nm | 钯 铑 | 醛脱羰反应 氢甲酰化反应 | [ [ |
| a45/a21=1/9 | (423.6/0.41) | — | 铑 | 氢甲酰化反应 | [ |
| a45' | 140/0.4 | 0~100nm | 铑 | 氢甲酰化反应 | [ |
a46 a47 | 290/0.69 547/0.56(536/—) | 0~30nm 0~100nm | 铑 | 氢甲酰化反应 | [ |
| 单体组成① | S/V(S后/V后)② | 孔径③ | 金属 | 催化反应 | 参考文献 |
|---|---|---|---|---|---|
a37/b21=1/8 a37/b21=1/4 a37/b21=1/2 a37/b21=3/4 a37/b21=1/1 | 654/0.78 585/0.63 518/0.42 379/0.21 5/0.0035 | 0~80nm | 钌 | β-酮酯的不对称氢化反应 | [ |
| a38,a39 | (1058~1070/1.19~1.64) | 0~10nm | 钌 | β-酮酯的不对称氢化反应 | [ |
a39/b21=1/9.3 a39/b22=1/3.5 a39/b23=1/10.3 | 697/0.68(490/0.56) 997/1.19 —/— | 0~10nm | 铑 | 氢甲酰化反应 | [ |
a40 a41 a42 | 959/1.15 892/1.07 846/0.81 | 0~100nm | 铑 | 氢甲酰化反应 | [ |
| a41 | 629.0/1.065 | — | 钯 | 由硫代苯甲酰胺和异腈合成噻唑的反应 | [ |
| a43/a34=1/20 | 635/0.72(556/0.68) | 0~10nm | 铑 | 氢甲酰化反应 | [ |
a43/a21=1/9.2 a43/a21=1/45.5 a43/a21=1/22.7 a43/a21=1/11.5 a43/a21=1/7.6 a43/a21=1/6.1 | 1088.0/2.07(985.3/1.94) 1195/2.62 1201/2.79 1271/2.72 1589/3.82 907.3/1.72 | 0~20nm | 铑 | 氢甲酰化反应 | [ |
a44 a44/a21=1/5 | 561/0.648(506/0.596) 1022/1.24 | 0~8nm 0~10nm | 钯 铑 | 醛脱羰反应 氢甲酰化反应 | [ [ |
| a45/a21=1/9 | (423.6/0.41) | — | 铑 | 氢甲酰化反应 | [ |
| a45' | 140/0.4 | 0~100nm | 铑 | 氢甲酰化反应 | [ |
a46 a47 | 290/0.69 547/0.56(536/—) | 0~30nm 0~100nm | 铑 | 氢甲酰化反应 | [ |
| 单体组成① | S/V(S后/V后)② | 孔径③ | 金属 | 催化反应 | 参考文献 |
|---|---|---|---|---|---|
a48 a49 a48/a21=1/1 a48/a21=1/2 a48/a21=1/3 a48/a21=1/4 a48/a21=1/5 | 824.1/1.03 462.9/0.54 730.1/0.71 859.9/0.85 1115/1.05 1097/1.14 982.2/1.00 | 0~6nm | 钇、钴、铑、铱、钌、锌 | 环氧化物和CO2生成环碳酸酯的反应 | [ |
a48 a50 a51 | 625/0.40 758/0.59 402/0.24 | — | 无 | 环氧化物和CO2生成环碳酸酯的反应 | [ |
| a48/b24=1/74 | 588/0.55 | 0~10nm | 镁 | 环氧化物和CO2生成环碳酸酯的反应 | [ |
a52 a53 a54 a55 | 314/0.3 440/0.52 420/0.48 394/0.62 | 0~16nm 0~19nm 0~19nm 0~20nm | 无 | 环氧化物和CO2生成环碳酸酯的反应 | [ |
| a50 | 758/0.59 | — | 钨 | 环辛烯的环氧化反应 二苯并噻吩的氧化反应 | [ |
| a51 | 507/—(424/—) | 0~7nm | 铑 | 甲醇的羰化反应 | [ |
| 单体组成① | S/V(S后/V后)② | 孔径③ | 金属 | 催化反应 | 参考文献 |
|---|---|---|---|---|---|
a48 a49 a48/a21=1/1 a48/a21=1/2 a48/a21=1/3 a48/a21=1/4 a48/a21=1/5 | 824.1/1.03 462.9/0.54 730.1/0.71 859.9/0.85 1115/1.05 1097/1.14 982.2/1.00 | 0~6nm | 钇、钴、铑、铱、钌、锌 | 环氧化物和CO2生成环碳酸酯的反应 | [ |
a48 a50 a51 | 625/0.40 758/0.59 402/0.24 | — | 无 | 环氧化物和CO2生成环碳酸酯的反应 | [ |
| a48/b24=1/74 | 588/0.55 | 0~10nm | 镁 | 环氧化物和CO2生成环碳酸酯的反应 | [ |
a52 a53 a54 a55 | 314/0.3 440/0.52 420/0.48 394/0.62 | 0~16nm 0~19nm 0~19nm 0~20nm | 无 | 环氧化物和CO2生成环碳酸酯的反应 | [ |
| a50 | 758/0.59 | — | 钨 | 环辛烯的环氧化反应 二苯并噻吩的氧化反应 | [ |
| a51 | 507/—(424/—) | 0~7nm | 铑 | 甲醇的羰化反应 | [ |
| 单体组成① | S/V(S后/V后)② | 孔径③ | 金属 | 催化反应 | 参考文献 |
|---|---|---|---|---|---|
| Aldimine缩合 | |||||
| a56/b25=2/3 | 818/— | 0~2nm | 钯 铂 金 金-钯 | Suzuki-Miyaura偶联反应 硝基苯酚的还原反应 1-溴-4-硝基苯的还原反应 偶联加氢串联反应 | [ |
a56/b25=2/3 a56/b26=2/3 | 903/0.69 2387/4.22 | 0~25nm | 铑 | 氢甲酰化反应 | [ |
| Scholl缩聚 | |||||
| a12/b13=2/3 | (651/—) | 0~2nm | 钯 | Suzuki-Miyaura偶联反应 | [ |
| Phenolic缩聚 | |||||
| a56/b27=1/3 | 775/0.72(231/0.34) | 0~50nm | 钯 | Suzuki-Miyaura偶联反应 | [ |
| 单体组成① | S/V(S后/V后)② | 孔径③ | 金属 | 催化反应 | 参考文献 |
|---|---|---|---|---|---|
| Aldimine缩合 | |||||
| a56/b25=2/3 | 818/— | 0~2nm | 钯 铂 金 金-钯 | Suzuki-Miyaura偶联反应 硝基苯酚的还原反应 1-溴-4-硝基苯的还原反应 偶联加氢串联反应 | [ |
a56/b25=2/3 a56/b26=2/3 | 903/0.69 2387/4.22 | 0~25nm | 铑 | 氢甲酰化反应 | [ |
| Scholl缩聚 | |||||
| a12/b13=2/3 | (651/—) | 0~2nm | 钯 | Suzuki-Miyaura偶联反应 | [ |
| Phenolic缩聚 | |||||
| a56/b27=1/3 | 775/0.72(231/0.34) | 0~50nm | 钯 | Suzuki-Miyaura偶联反应 | [ |
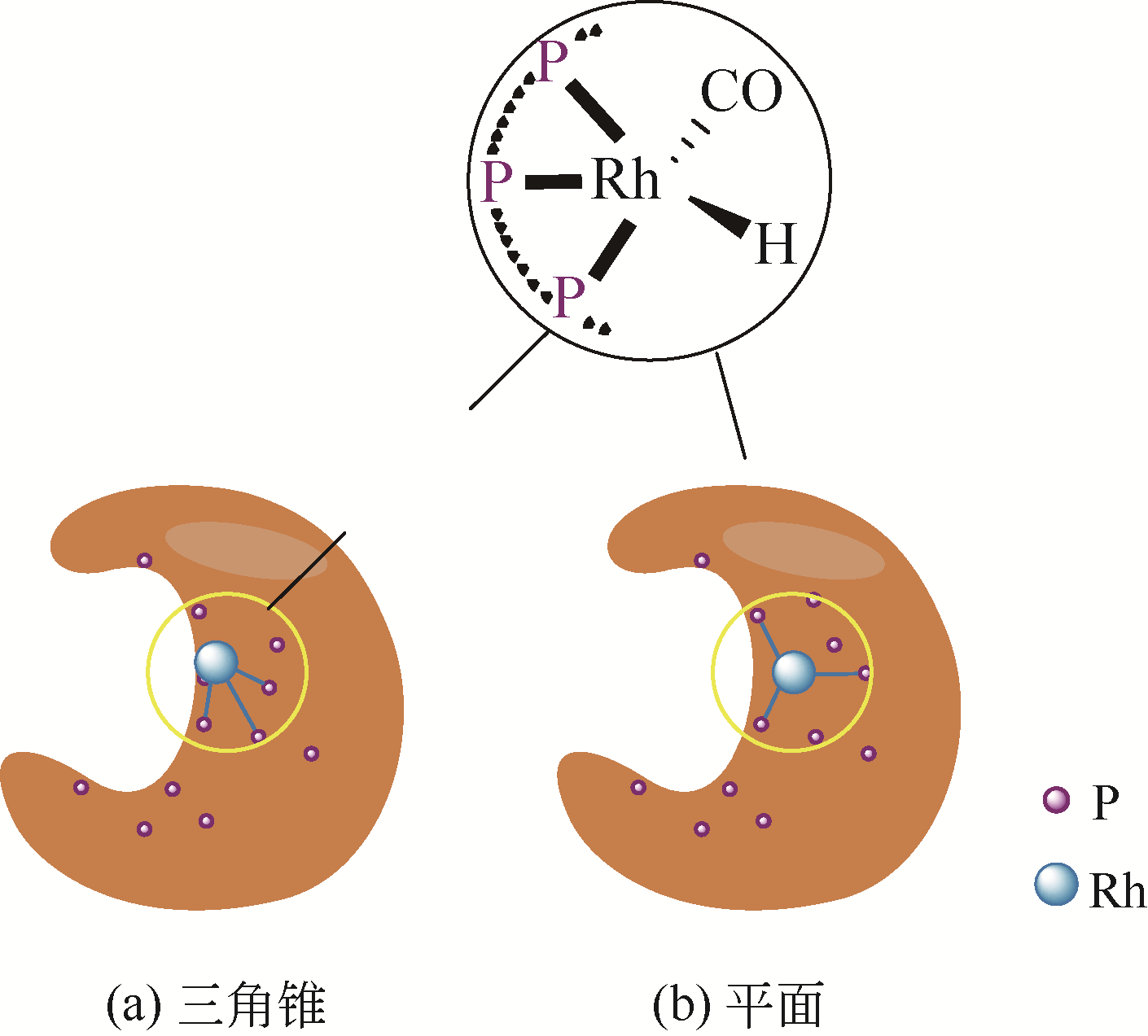
| 1 | ORPEN A G, CONNELLY N G. Structural evidence for the participation of P-X σ* orbitals in metal-PX3 bonding[J]. Journal of the Chemical Society, Chemical Communications, 1985(19): 1310-1311. |
| 2 | VOUGIOUKALAKIS G C, GRUBBS R H. Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts[J]. Chemical Reviews, 2010, 110(3): 1746-1787. |
| 3 | CHI Yun, CHOU Pitai. Transition-metal phosphors with cyclometalating ligands: fundamentals and applications[J]. Chemical Society Reviews, 2010, 39(2): 638-655. |
| 4 | GAO Ke, YOSHIKAI N. Low-valent cobalt catalysis: new opportunities for C—H functionalization[J]. Accounts of Chemical Research, 2014, 47(4): 1208-1219. |
| 5 | KOBAYASHI S, ISHITANI H. Catalytic enantioselective addition to imines[J]. Chemical Reviews, 1999, 99(5): 1069-1094. |
| 6 | GÖTTKER-SCHNETMANN I, WHITE PR, BROOKHART M. Iridium bis(phosphinite) p-XPCP pincer complexes: highly active catalysts for the transfer dehydrogenation of alkanes[J]. Journal of the American Chemical Society, 2004, 126(6): 1804-1811. |
| 7 | TAMAO K, SUMITANI K, KUMADA M. Selective carbon-carbon bond formation by cross-coupling of Grignard reagents with organic halides. Catalysis by nickel-phosphine complexes[J]. Journal of the American Chemical Society, 1972, 94(12): 4374-4376. |
| 8 | MIYAURA N, SUZUKI A. Palladium-catalyzed cross-coupling reactions of organoboron compounds[J]. Chemical Reviews, 1995, 95(7): 2457-2483. |
| 9 | LOW J J, GODDARD W A. Theoretical studies of oxidative addition and reductive elimination. 3. Carbon-hydrogen and carbon-carbon reductive coupling from palladium and platinum bis(phosphine) complexes[J]. Journal of the American Chemical Society, 1986, 108(20): 6115-6128. |
| 10 | LEADBEATER N E, MARCO M. Preparation of polymer-supported ligands and metal complexes for use in catalysis[J]. Chemical Reviews, 2002, 102(10): 3217-3273. |
| 11 | REETZ M T, LOHMER G, SCHWICKARDI R. Systhesis and catalytic activity of dendritic diphosphane metal complexes[J]. Angewandte Chemie International Edition, 1997, 36(1314): 1526-1529. |
| 12 | MCMORN P, HUTCHINGS G J. Heterogeneous enantioselective catalysts: strategies for the immobilisation of homogeneous catalysts[J]. Chemical Society Reviews, 2004, 33(2): 108-122. |
| 13 | GAWANDE M B, BRANCO P S, VARMA R S. Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies[J]. Chemical Society Reviews, 2013, 42(8): 3371-3393. |
| 14 | WU Dingcai, XU Fei, SUN Bin, et al. Design and preparation of porous polymers[J]. Chemical Reviews, 2012, 112(7): 3959-4015. |
| 15 | SUN Qi, DAI Zhifeng, MENG Xiangju, et al. Porous polymer catalysts with hierarchical structures[J]. Chemical Society Reviews, 2015, 44(17): 6018-6034. |
| 16 | SUN Qi, DAI Zhifeng, MENG Xiangju, et al. Task-specific design of porous polymer heterogeneous catalysts beyond homogeneous count[J]. ACS Catalysis, 2015, 5(8): 4556-4567. |
| 17 | SUN Qi, XIAO Fengshou. Exploration of porous organic polymers as a platform for biomimetic catalysis[J]. Acta Chimica Sinica, 2020, 78(9): 827-832. |
| 18 | DONG Ke, SUN Qi, MENG Xiangju, et al. Strategies for the design of porous polymers as efficient heterogeneous catalysts: from co-polymerization to self-polymerization[J]. Catalysis Science & Technology, 2017, 7(5): 1028-1039. |
| 19 | ZHANG Yugen, RIDUAN Si N. Functional porous organic polymers for heterogeneous catalysis[J]. Chemical Society Reviews, 2012, 41(6): 2083-2094. |
| 20 | 乙烯多相氢甲酰化及其加氢制正丙醇工业装置开车成功[EB/OL]. , 2020-09-14. |
| 21 | WANG Wenlong, CUI Lifeng, SUN Peng, et al. Reusable N-heterocyclic carbene complex catalysts and beyond: a perspective on recycling strategies[J]. Chemical Reviews, 2018, 118(19): 9843-9929. |
| 22 | Hui LYU, WANG Wenlong, LI Fuwei. Porous organic polymers with built-in N-heterocyclic carbenes: selective and efficient heterogeneous catalyst for the reductive N-formylation of amines with CO2[J]. Chemistry: a European Journal, 2018, 24(62): 16588-16594. |
| 23 | WANG Wenlong, ZHAO Liyuan, Hui LYU, et al. Modular “click” preparation of bifunctional polymeric heterometallic catalysts[J]. Angewandte Chemie International Edition, 2016, 55(27): 7665-7670. |
| 24 | WANG Wenlong, ZHENG Anmin, ZHAO Peiqing, et al. Au-NHC@porous organic polymers: synthetic control and its catalytic application in alkyne hydration reactions[J]. ACS Catalysis, 2013, 4(1): 321-327. |
| 25 | YUE Chengtao, WANG Wenlong, LI Fuwei. Building N-heterocyclic carbene into triazine-linked polymer for multiple CO2 utilization[J]. ChemSusChem, 2020, 13(22): 5996-6004. |
| 26 | XU Yanhong, JIN Shangbin, XU Hong, et al. Conjugated microporous polymers: design, synthesis and application[J]. Chemical Society Reviews, 2013, 42(20): 8012-8031. |
| 27 | JIANG Xiaoyu, ZHAO Wuxue, WANG Wei, et al. One-pot approach to Pd-loaded porous polymers with properties tunable by the oxidation state of the phosphorus core[J]. Polymer Chemistry, 2015, 6(35): 6351-6357. |
| 28 | DING Zongcang, LI Cunyao, CHEN Junjia, et al. Palladium/phosphorus-doped porous organic polymer as recyclable chemoselective and efficient hydrogenation catalyst under ambient conditions[J]. Advanced Synthesis & Catalysis, 2017, 359(13): 2280-2287. |
| 29 | WANG Xu, LU Shengmei, LI Jun, et al. Conjugated microporous polymers with chiral BINAP ligand built-in as efficient catalysts for asymmetric hydrogenation[J]. Catalysis Science & Technology, 2015, 5(5): 2585-2589. |
| 30 | CHEN Jian, ZHAGN Ju, ZHU Dajian, et al. Novel polymer-supported phosphine palladium catalyst: one-pot synthesis from and application in Suzuki-Miyaura coupling reaction[J]. Journal of Porous Materials, 2016, 24(4): 847-853. |
| 31 | HUANG Jieyang, TARABEK J, KULKARNI R, et al. A π-conjugated, covalent phosphinine framework[J]. Chemistry, 2019, 25(53): 12342-12348. |
| 32 | ZHANG Qiang, ZHANG Suobo, LI Shenghai. Novel functional organic network containing quaternary phosphonium and tertiary phosphorus[J]. Macromolecules, 2012, 45(7): 2981-2988. |
| 33 | ZHANG Qiang, YANG Yanqin, ZHANG Suobo. Novel functionalized microporous organic networks based on triphenylphosphine[J]. Chemistry, 2013, 19(30): 10024-10029. |
| 34 | ROSE M, BOHLMANN W, SABO M, et al. Element-organic frameworks with high permanent porosity[J]. Chemical Communications, 2008 (21): 2462-2464. |
| 35 | FRITSCH J, DRACHE F, NICKERL G, et al. Porous phosphorus-based element organic frameworks: a new platform for transition metal catalysts immobilization[J]. Microporous and Mesoporous Materials, 2013, 172: 167-173. |
| 36 | HAUSOUL P J C, EGGENHUISEN T M, NAND D, et al. Development of a 4, 4ʹ-biphenyl/phosphine-based COF for the heterogeneous Pd-catalysed telomerisation of 1,3-butadiene[J]. Catalysis Science & Technology, 2013, 3(10): 2571-2579. |
| 37 | HAUSOUL P J C, BROICHER C, VEGLIANTE R, et al. Solid molecular phosphine catalysts for formic acid decomposition in the biorefinery[J]. Angewandte Chemie International Edition, 2016, 55(18): 5597-5601. |
| 38 | GUNASEKAR G H, PADMANABAN S, PARK Kwangho, et al. An efficient and practical system for the synthesis of N,N-dimethylformamide by CO2 hydrogenation using a heterogeneous Ru catalyst: from batch to continuous flow[J]. ChemSusChem, 2020, 13(7): 1735-1739. |
| 39 | SCHMID L, CANONICA A, BAIKER A. Ruthenium-catalysed formylation of amines with dense carbon dioxide as C1-source[J]. Applied Catalysis A: General, 2003, 255(1): 23-33. |
| 40 | KRÖCHER O, KÖPPEL R A, BAIKER A. Highly active ruthenium complexes with bidentate phosphine ligands for the solvent-free catalytic synthesis of N,N-dimethylformamide and methyl formate[J]. Chemical Communications, 1997(5): 453-454. |
| 41 | LI Buyi, GUAN Zhenhong, WANG Wei, et al. Highly dispersed Pd catalyst locked in knitting aryl network polymers for Suzuki-Miyaura coupling reactions of aryl chlorides in aqueous media[J]. Advanced Materials, 2012, 24(25): 3390-3395. |
| 42 | GUAN Zhenhong, LI Buyi, Guoliang HAI, et al. A highly efficient catalyst for Suzuki-Miyaura coupling reaction of benzyl chloride under mild conditions[J]. RSC Advances, 2014, 4(69): 36437-36443. |
| 43 | JIA Zhifang, WANG Kewei, TAN Bien, et al. Ruthenium complexes immobilized on functionalized knitted hypercrosslinked polymers as efficient and recyclable catalysts for organic transformations[J]. Advanced Synthesis & Catalysis, 2017, 359(1): 78-88. |
| 44 | JIANG Miao, DING Yunjie, YAN Li, et al. Rh catalysts supported on knitting aryl network polymers for the hydroformylation of higher olefins[J]. Chinese Journal of Catalysis, 2014, 35(9): 1456-1464. |
| 45 | TANG Cheng, ZOU Zhijuan, FU Yufang, et al. Highly dispersed DPPF locked in knitting hyper-crosslinked polymers as efficient and recyclable catalyst[J]. Chemistry Select, 2018, 3(21): 5987-5992. |
| 46 | CAI Xinyi, NIE Junqi, YANG Guichun, et al. Phosphorus-rich network polymer supported ruthenium nanoparticles for nitroarene reduction[J]. Materials Letters, 2019, 240: 80-83. |
| 47 | YANG Yuting, WANG Tienan, JING Xiaofei, et al. Phosphine-based porous aromatic frameworks for gold nanoparticle immobilization with superior catalytic activities[J]. Journal of Materials Chemistry A, 2019, 7(16): 10004-10009. |
| 48 | WU Zhilian, LIU Qinggang, YANG Xiaofeng, et al. Knitting aryl network polymers-incorporated Ag nanoparticles: a mild and efficient catalyst for the fixation of CO2 as carboxylic acid[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(11): 9634-9639. |
| 49 | WANG Xinbo, MIN Shixiong, DAS S K, et al. Spatially isolated palladium in porous organic polymers by direct knitting for versatile organic transformations[J]. Journal of Catalysis, 2017, 355: 101-109. |
| 50 | WANG Xinbo, Pei Ling Eleanar ANG, GUAN Chao, et al. Single-site Ruthenium pincer complex knitted into porous organic polymers for dehydrogenation of formic acid[J]. ChemSusChem, 2018, 11(20): 3591-3598. |
| 51 | GUAN Chao, PAN Yupeng, Pei Ling Eleanar ANG, et al. Conversion of CO2 from air into formate using amines and phosphorus-nitrogen PN3P-Ru(II) pincer complexes[J]. Green Chemistry, 2018, 20(18): 4201-4205. |
| 52 | VALVERDE-GONZALEZ A, MARCHAL G, MAYA E M, et al. A step forward in solvent knitting strategies: ruthenium and gold phosphine complex polymerization results in effective heterogenized catalysts[J]. Catalysis Science & Technology, 2019, 9(17): 4552-4560. |
| 53 | SHERRINGTON D C. Preparation, structure and morphology of polymer supports[J]. Chemical Communications, 1998 (21): 2275-2286. |
| 54 | IWAI T, HARADA T, HARA K, et al. Threefold cross-linked polystyrene-triphenylphosphane hybrids: mono-P-ligating behavior and catalytic applications for aryl chloride cross-coupling and C(sp3)-H borylation[J]. Angewandte Chemie International Edition, 2013, 52(47): 12322-12326. |
| 55 | ZHANG Yonglai, WEI Shu, LIU Fujian, et al. Superhydrophobic nanoporous polymers as efficient adsorbents for organic compounds[J]. Nano Today, 2009, 4(2): 135-142. |
| 56 | HOOD D M, JOHNSON R A, CARPENTER A E, et al. Highly active cationic cobalt(II) hydroformylation catalysts[J]. Science, 2020, 367(6477): 542-548. |
| 57 | JIANG Miao, YAN Li, DING Yunjie, et al. Ultrastable 3V-PPh3 polymers supported single Rh sites for fixed-bed hydroformylation of olefins[J]. Journal of Molecular Catalysis A: Chemical, 2015, 404-405: 211-217. |
| 58 | SUN Qi, JIANG Miao, SHEN Zhenju, et al. Porous organic ligands (POLs) for synthesizing highly efficient heterogeneous catalysts[J]. Chemical Communications, 2014, 50(80): 11844-11847. |
| 59 | JIANG Miao, YAN Li, SUN Xueping, et al. Effect of different synthetic routes on the performance of propylene hydroformylation over 3V-PPh3 polymer supported Rh catalysts[J]. Reaction Kinetics Mechanisms and Catalysis, 2015, 116(1): 223-234. |
| 60 | SUN Qi, DAI Zhifeng, MENG Xiangju, et al. Enhancement of hydroformylation performance via increasing the phosphine ligand concentration in porous organic polymer catalysts[J]. Catalysis Today, 2017, 298: 40-45. |
| 61 | ZHOU Yunbing, LI Cunyao, LIN Min, et al. A polymer-bound monodentate-P-ligated Palladium complex as a recyclable catalyst for the Suzuki-Miyaura coupling reaction of aryl chlorides[J]. Advanced Synthesis & Catalysis, 2015, 357(11): 2503-2508. |
| 62 | CHEN Xingkun, ZHU Hejun, SONG Xiangen, et al. Ru-PPh3@porous organic polymer: efficient and stable catalyst for the trickle bed regioselective hydrogenation of cinnamaldehyde[J]. Reaction Kinetics Mechanisms and Catalysis, 2017, 120(2): 637-649. |
| 63 | REN Zhou, Yuan LYU, FENG Siquan, et al. A highly efficient single site Rh-POL-PPh3 catalyst for heterogeneous methanol carbonylation[J]. Molecular Catalysis, 2017, 442: 83-88. |
| 64 | WAN Yali, SONG Fangxiang, YE Tao, et al. Carbonylative Suzuki coupling and alkoxycarbonylation of aryl halides using palladium supported on phosphorus-doped porous organic polymer as an active and robust catalyst[J]. Applied Organometallic Chemistry, 2019, 33(2): e4714. |
| 65 | WANG Wenlong, LI Cunyao, YAN Li, et al. Ionic liquid/Zn-PPh3 integrated porous organic polymers featuring multifunctional sites: highly active heterogeneous catalyst for cooperative conversion of CO2 to cyclic carbonates[J]. ACS Catalysis, 2016, 6(9): 6091-6100. |
| 66 | CORNILS B, KUNTZ E G. Introducing TPPTS and related ligands for industrial biphasic processes[J]. Journal of Organometallic Chemistry, 1995, 502(1/2): 177-186. |
| 67 | CHEN Xingkun, ZHU Hejun, WANG Tao, et al. The 2V-P,N polymer supported palladium catalyst for methoxycarbonylation of acetylene[J]. Journal of Molecular Catalysis A: Chemical, 2016, 414: 37-46. |
| 68 | CHEN Xingkun, ZHU Hejun, WANG Wenlong, et al. Multifunctional single-site catalysts for alkoxycarbonylation of terminal alkynes[J]. ChemSusChem, 2016, 9(17): 2451-2459. |
| 69 | WANG Guoqing, JIANG Miao, JI Guangjun, et al. Bifunctional heterogeneous Ru/POP catalyst embedded with alkali for the N-formylation of amine and CO2 porous ligand creates new reaction route: bifunctional single-atom palladium catalyst for selective distannylation of terminal alkynes[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(14): 5576-5583. |
| 70 | HUANG Wenyong, WANG Guoqing, LI Wenhao, et al. Porous ligand creates new reaction route: bifunctional single-atom palladium catalyst for selective distannylation of terminal alkynes[J]. Chem, 2020, 6(9): 2300-2313. |
| 71 | TANG Yongquan, DONG Ke, WANG Sai, et al. Boosting the hydrolytic stability of phosphite ligand in hydroformylation by the construction of superhydrophobic porous framework[J]. Molecular Catalysis, 2019, 474: 110408. |
| 72 | LEI Yizhu, CHEN Zaifei, LI Guangxing. Palladium/phosphorus-functionalized porous organic polymer with tunable surface wettability for water-mediated Suzuki-Miyaura coupling reaction[J]. RSC Advances, 2019, 9(63): 36600-36607. |
| 73 | SUN Qi, AGUILA B, VERMA G, et al. Superhydrophobicity: constructing homogeneous catalysts into superhydrophobic porous frameworks to protect them from hydrolytic degradation[J]. Chem, 2016, 1(4): 628-639. |
| 74 | CAI Rong, YE Xiaohan, SUN Qi, et al. Anchoring triazole-gold(Ⅰ) complex into porous organic polymer to boost the stability and reactivity of gold(Ⅰ) catalyst[J]. ACS Catalysis, 2017, 7(2): 1087-1092. |
| 75 | YAO Wei, DUAN Zhengchao, ZHANG Yilin, et al. Iridium supported on phosphorus-doped porous organic polymers: active and recyclable catalyst for acceptorless dehydrogenation and borrowing hydrogen reaction[J]. Advanced Synthesis & Catalysis, 2019, 361(24): 5695-5703. |
| 76 | YANG Ji, LIU Jiawang, NEUMANN H, et al. Direct synthesis of adipic acid esters via palladium-catalyzed carbonylation of 1,3-dienes[J]. Science, 2019, 366(6472): 1514-1517. |
| 77 |
LIU Jiawang, HAN Zhaobin, WANG Xiaoming, et al. Highly regio- and enantioselective alkoxycarbonylative amination of terminal allenes catalyzed by a spiroketal-based diphosphine/Pd( ) complex[J]. Journal of the American Chemical Society, 2015, 137(49): 15346-15349. ) complex[J]. Journal of the American Chemical Society, 2015, 137(49): 15346-15349.
|
| 78 | SUN Qi, MENG Xiangju, LIU Xiao, et al. Mesoporous cross-linked polymer copolymerized with chiral BINAP ligand coordinated to a ruthenium species as an efficient heterogeneous catalyst for asymmetric hydrogenation[J]. Chemical Communications, 2012, 48(85): 10505-10507. |
| 79 | WANG Tao, WANG Wenlong, Yuan LYU, et al. Porous Rh/BINAP polymers as efficient heterogeneous catalysts for asymmetric hydroformylation of styrene: enhanced enantioselectivity realized by flexible chiral nanopockets[J]. Chinese Journal of Catalysis, 2017, 38(4): 691-698. |
| 80 | WANG Tao, Yuan LYU, XIONG Kai, et al. Chiral BINAP-based hierarchical porous polymers as platforms for efficient heterogeneous asymmetric catalysis[J]. Chinese Journal of Catalysis, 2017, 38(5): 890-898. |
| 81 | SUN Qi, DAI Zhifeng, LIU Xiaolong, et al. Highly efficient heterogeneous hydroformylation over Rh-metalated porous organic polymers: synergistic effect of high ligand concentration and flexible framework[J]. Journal of the American Chemical Society, 2015, 137(15): 5204-5209. |
| 82 | TONG Wei, LI Wenhao, HE Yan, et al. Palladium-metalated porous organic polymers as recyclable catalysts for the chemioselective synthesis of thiazoles from thiobenzamides and isonitriles[J]. Organic Letters, 2018, 20(8): 2494-2498. |
| 83 | WANG Yuqing, YAN Li, LI Cunyao, et al. Heterogeneous Rh/CPOL-BP&P(OPh)3 catalysts for hydroformylation of 1-butene: the formation and evolution of the active species[J]. Journal of Catalysis, 2018, 368: 197-206. |
| 84 | WANG Yuqing, YAN Li, LI Cunyao, et al. Highly efficient porous organic copolymer supported Rh catalysts for heterogeneous hydroformylation of butenes[J]. Applied Catalysis A: General, 2018, 551: 98-105. |
| 85 | LI Cunyao, YAN Li, LU Lanlu, et al. Single atom dispersed Rh-biphephos&PPh3@porous organic copolymers: highly efficient catalysts for continuous fixed-bed hydroformylation of propene[J]. Green Chemistry, 2016, 18(10): 2995-3005. |
| 86 | LI Cunyao, XIONG Kai, YAN Li, et al. Designing highly efficient Rh/CPOL-bp&PPh3 heterogenous catalysts for hydroformylation of internal and terminal olefins[J]. Catalysis Science & Technology, 2016, 6(7): 2143-2149. |
| 87 | LI Wenhao, LI Cunyao, LI Yan, et al. Palladium-metalated porous organic polymers as recyclable catalysts for chemoselective decarbonylation of aldehydes[J]. Chemical Communications, 2018, 54(61): 8446-8449. |
| 88 | LI Cunyao, SUN Keju, WANG Wenlong, et al. Xantphos doped Rh/POPs-PPh3 catalyst for highly selective long-chain olefins hydroformylation: chemical and DFT insights into Rh location and the roles of xantphos and PPh3[J]. Journal of Catalysis, 2017, 353: 123-132. |
| 89 | JIA Xiaofei, LIANG Zuyu, CHEN Jianbin, et al. Porous organic polymer supported rhodium as a reusable heterogeneous catalyst for hydroformylation of olefins[J]. Organic Letters, 2019, 21(7): 2147-2150. |
| 90 | DONG Ke, SUN Qi, TANG Yongquan, et al. Bio-inspired creation of heterogeneous reaction vessels via polymerization of supramolecular ion pair[J]. Nature Communications, 2019, 10: 3059. |
| 91 | WANG Zhaozhan, YANG Yong. Rh-catalyzed highly regioselective hydroformylation to linear aldehydes by employing porous organic polymer as a ligand[J]. RSC Advances, 2020, 10(49): 29263-29267. |
| 92 | BÖRNER A, FRANKE R. Hydroformylation: fundamentals, processes, and applications in organic synthesis[M]. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co., KGaA, 2016. |
| 93 | LI Cunyao, WANG Wenlong, YAN Li, et al. Phosphonium salt and ZnX2-PPh3 integrated hierarchical POPs: tailorable synthesis and highly efficient cooperative catalysis in CO2 utilization[J]. Journal of Materials Chemistry A, 2016, 4(41): 16017-16027. |
| 94 | SUN Qi, JIN Yingyin, AGUILA B, et al. Porous ionic polymers as a robust and efficient platform for capture and chemical fixation of atmospheric CO2[J]. ChemSusChem, 2017, 10(6): 1160-1165. |
| 95 | WANG Wenlong, WANG Yuqing, LI Cunyao, et al. State-of-the-art multifunctional heterogeneous POP catalyst for cooperative transformation of CO2 to cyclic carbonates[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(6): 4523-4528. |
| 96 | HU Kewei, TANG Yongquan, CUI Jia, et al. Location matters: cooperativity of catalytic partners in porous organic polymers for enhanced CO2 transformation[J]. Chemical Communications, 2019, 55(62): 9180-9183. |
| 97 | SUN Qi, MA Shengqian, DAI Zhifeng, et al. A hierarchical porous ionic organic polymer as a new platform for heterogeneous phase transfer catalysis[J]. Journal of Materials Chemistry A, 2015, 3(47): 23871-23875. |
| 98 | REN Zhou, Yuan LYU, SONG Xiangen, et al. Dual-ionically bound single-site rhodium on porous ionic polymer rivals commercial methanol carbonylation catalysts[J]. Advanced Materials, 2019, 31(50): e1904976. |
| 99 | DIERCKS C S, YAGHI O M. The atom, the molecule, and the covalent organic framework[J]. Science, 2017, 355(6328): eaal1585. |
| 100 | TAO Rao, SHEN Xiran, HU Yiming, et al. Phosphine-based covalent organic framework for the controlled synthesis of broad-scope ultrafine nanoparticles[J]. Small, 2020, 16(8): 2070042. |
| 101 | LIU Yubing, DIKHTIARENKO A, XU Naizhang, et al. Triphenylphosphine-based covalent organic frameworks and heterogeneous Rh-P-COFs catalysts[J]. Chemistry:a European Journal, 2020, 26(53): 12134-12139. |
| 102 | LI Buyi, GUAN Zhenhong, YANG Xinjia, et al. Multifunctional microporous organic polymers[J]. Journal of Materials Chemistry A, 2014, 2(30): 11930-11939. |
| 103 | DENG Gaoyang, WANG Zhonggang. Hierarchical porous phenolic resin and its supported Pd-catalyst for Suzuki-Miyaura reactions in water medium[J]. Macromolecular Rapid Communications, 2018, 39(3): 1700618. |
| 104 | NYULASZI L, VESZPREMI T, REFFY J, et al. Electronic structure and aromaticity of azaphospholes[J]. Journal of the American Chemical Society, 1992, 114(23): 9080-9084. |
| 105 | MÜLLER C, BROECKX L E E, DE KROM I,et al. Developments in the coordination chemistry of phosphinines[J]. European Journal of Inorganic Chemistry, 2013, 2013(2): 187-202. |
| 106 | YANG Zhenzhen, CHEN Hao, LI Bo, et al. Topotactic synthesis of phosphabenzene-functionalized porous organic polymers: efficient ligands in CO2 conversion[J]. Angewandte Chemie: International Edition, 2019, 58(39): 13763-13767. |
| 107 | XU Yang, WANG Tianqi, HE Zidong, et al. Synthesis of triphenylphosphine-based microporous organic nanotube framework supported Pd catalysts with excellent catalytic activity[J]. Polymer Chemistry, 2016, 7(48): 7408-7415. |
| 108 | XU Yang, WANG Tianqi, HE Zidong, et al. Honeycomb-like bicontinuous P-doped porous polymers from hyper-cross-linking of diblock copolymers for heterogeneous catalysis[J]. Macromolecules, 2017, 50(24): 9626-9635. |
| 109 | XU Yang, WANG Tianqi, HE Zidong, et al. Organic ligands incorporated hypercrosslinked microporous organic nanotube frameworks for accelerating mass transfer in efficient heterogeneous catalysis[J]. Applied Catalysis A: General, 2017, 541: 112-119. |
| 110 | LIU Ying, ZHANG Li, GAO Shengguang, et al. Hollow porous organic nanospheres for anchoring Pd(PPh3)4 through a co-hyper-crosslinking mediated self-assembly strategy[J]. New Journal of Chemistry, 2020, 44(16): 6661-6666. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [6] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [7] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [8] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [9] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [10] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [11] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [12] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [13] | XIANG Yang, HUANG Xun, WEI Zidong. Recent progresses in the activity and selectivity improvement of electrocatalytic organic synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4005-4014. |
| [14] | WANG Yaogang, HAN Zishan, GAO Jiachen, WANG Xinyu, LI Siqi, YANG Quanhong, WENG Zhe. Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4043-4057. |
| [15] | LIU Yi, FANG Qiang, ZHONG Dazhong, ZHAO Qiang, LI Jinping. Cu facets regulation of Ag/Cu coupled catalysts for electrocatalytic reduction of carbon dioxide [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4136-4142. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||