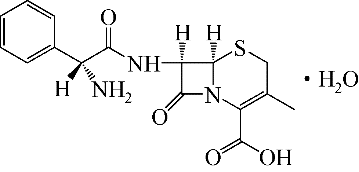
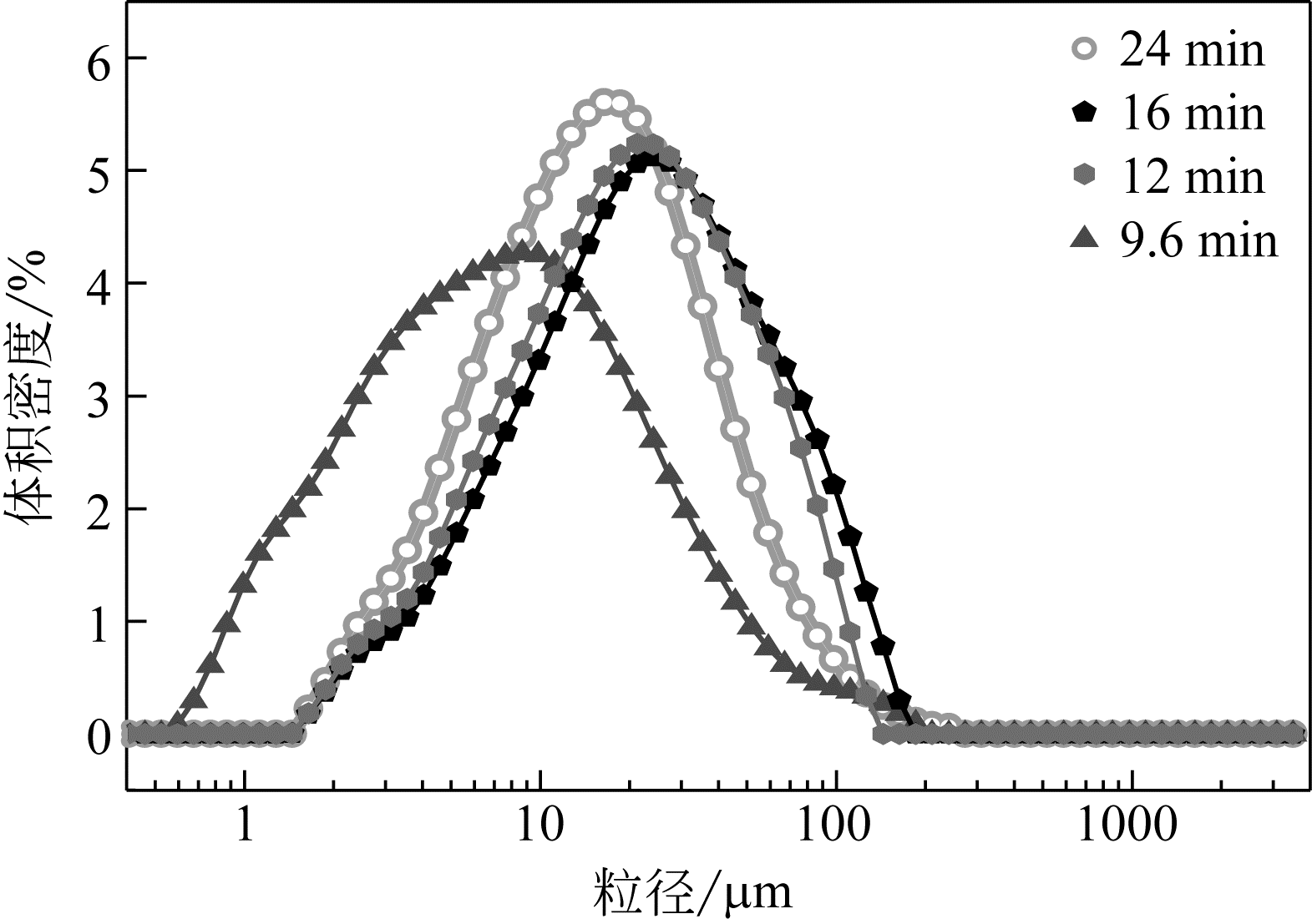
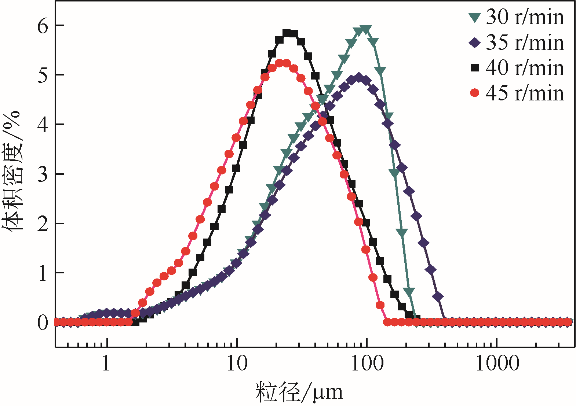
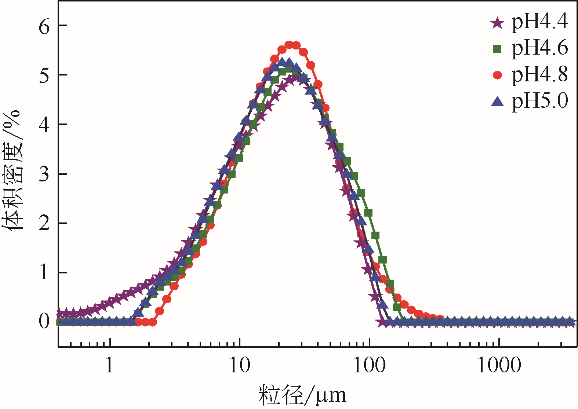
| 1 |
DIAGOJ, LUDESCHERJ. Bet a lactam production: US 5719276[P]. 1998-02-17
|
| 2 |
顾勤兰, 王泽, 尤启冬. 一步结晶法合成头孢氨苄[J]. 中国药师, 2006, 9(1): 3-4.
|
|
GUQ L, WANGZ,YOUQ D. Synthesis of cefalexin by one step crystallization[J].China Pharmacist, 2006, 9(1): 3-4.
|
| 3 |
LAANV D J M. Mutant penicillin G acylases:US8541199 [P]. 2013-09-24
|
| 4 |
JOSEPHB W H, JOSEPHV D T W J, MARINUSD R. Process for the preparation of an antibiotic:WO022610[P]. 1997-06-26
|
| 5 |
LIUW J, MA C Y, WANGX Z. Novel impinging jet and continuous crystallizer design for rapid reactive crystallization of pharmaceuticals [J]. Procedia Engineering , 2015,102 :499-507.
|
| 6 |
KOZIKA, MATYNIAA, PIOTROWSKIK. Continuous reaction crystallization of struvite from diluted aqueous solutions of phosphate (Ⅴ) ions in DT MSMPR crystallizer[J]. Procedia Engineering, 2012 , 42 (5) :313-322.
|
| 7 |
王静康, 卫宏远, 张元谋. 工业结晶分离技术研究新进展[J]. 化工学报, 1993, 44(5): 565-574.
|
|
WANGJ K,WEIH Y,ZHANGY M. Studes on unsteady-state behavior of continuous crystallization processes[J]. Journal of Chemical Industry and Engineering(China),1993, 44(5): 565-574.
|
| 8 |
尹秋响,王静康. MSMPR连续结晶器的多定态[J]. 化工学报, 1997, 48(6): 692-698.
|
|
YINQ X,WANGJ K. Multiplicity in continuous MSMPR crystallization[J]. Journal of Chemical Industry and Engineering(China),1997, 48(6): 692-698.
|
| 9 |
FURUTAM, MUKAIK, CorkD, et al. Continuous crystallization using a sonicated tubular system for controlling particle size in an API manufacturing process[J].Chemical Engineering and Processing , 2016 , 102 :210-218.
|
| 10 |
CYNTHIAA, CHALLENER. Considering continuous crystallization[J].Pharmaceutical Technology, 2015, 39(5): 38-40.
|
| 11 |
WANGY G, XIAOY, CHENGX, et al. An FPGA-based real-time maximum likelihood 3D position estimation for a continuous crystal PET detector[J]. Ieee Transactions on Nuclear Science, 2016, 63(1): 37-43.
|
| 12 |
THOMASL, LORENZOM. On the nucleation and crystallization of nanoparticles in continuous-flow nonthermal plasma reactors[J]. Journal of Vacuum Science & Technology B, 2014, 32(6): 1-8.
|
| 13 |
CHOUC H, CHANW S, LEE I C, et al. High-performance single-crystal like strained silicon nanowire thin-film transistors via continuous wave laser crystallization[J]. IEEE Electron Device Letters, 2015,36(4): 348-350.
|
| 14 |
DENGYUEC, DHANANJAYS, KAMALESHK, et al.Continuous preparation of polymer coated drug crystals by solid hollow fiber membrane-based cooling crystallization[J]. International Journal of Pharmaceutics, 2016, 499: 395-402.
|
| 15 |
ZHANGD J, XUS J, DUS C, et al. Progress of pharmaceutical continuous crystallization[J]. Engineering, 2017, 3: 354-364.
|
| 16 |
林兰兰. 头孢氨苄一水合物反应结晶过程研究[D]. 天津: 天津大学, 2017.
|
|
LINL L. A study on reactive crystallization process of cefalexin monohydra[D].Tianjin: Tianjin University, 2017.
|
 ),Junbo GONG1(
),Junbo GONG1( ),Hua SUN2(
),Hua SUN2( ),Zhigang DUAN3,Weiguo HU3
),Zhigang DUAN3,Weiguo HU3
 ),龚俊波1(
),龚俊波1( ),孙华2(
),孙华2( ),段志钢3,胡卫国3
),段志钢3,胡卫国3