Chemical Industry and Engineering Progress ›› 2019, Vol. 38 ›› Issue (06): 2825-2834.DOI: 10.16085/j.issn.1000-6613.2018-1935
• Materials science and technology • Previous Articles Next Articles
Research progress on thermal properties of fatty acid phase change energy storage materials
Qingjun GU( ),Hua FEI(
),Hua FEI( ),Linya WANG,Min FANG,Dahua JIANG
),Linya WANG,Min FANG,Dahua JIANG
- School of Architectural and Surveying & Mapping Engineering,Jiangxi University of Science and Technology,Ganzhou 341000,Jiangxi,China
-
Received:2018-09-26Online:2019-06-05Published:2019-06-05 -
Contact:Hua FEI
脂肪酸相变储能材料热性能研究进展
- 江西理工大学建筑与测绘工程学院,江西 赣州 341000
-
通讯作者:费华 -
作者简介:顾庆军(1993—),男,硕士研究生,研究方向为相变储能材料。E-mail:<email>gqj18896518935@163.com</email>。 -
基金资助:国家自然科学基金(51666004);江西省自然科学基金(20171BAB206041);江西省教育厅项目(GJJ180498);江西理工大学清江青年优秀人才项目(JXUSTQJYX2017003)
CLC Number:
Cite this article
Qingjun GU, Hua FEI, Linya WANG, Min FANG, Dahua JIANG. Research progress on thermal properties of fatty acid phase change energy storage materials[J]. Chemical Industry and Engineering Progress, 2019, 38(06): 2825-2834.
顾庆军, 费华, 王林雅, 方敏, 蒋达华. 脂肪酸相变储能材料热性能研究进展[J]. 化工进展, 2019, 38(06): 2825-2834.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2018-1935
| 相变材料 | 分子量 | 相变温度/℃ | 相变潜热 /J·g-1 | 热导率 /W·m-1·K-1 |
|---|---|---|---|---|
| CA | 172.27 | 30.10 | 152.00 | 0.153 |
| LA | 200.32 | 43.00 | 177.00 | 0.147 |
| MA | 228.37 | 53.70 | 187.00 | 0.150 |
| PA | 256.42 | 62.30 | 186.00 | 0.162 |
| SA | 284.48 | 70.70 | 203.00 | 0.172 |
| 相变材料 | 分子量 | 相变温度/℃ | 相变潜热 /J·g-1 | 热导率 /W·m-1·K-1 |
|---|---|---|---|---|
| CA | 172.27 | 30.10 | 152.00 | 0.153 |
| LA | 200.32 | 43.00 | 177.00 | 0.147 |
| MA | 228.37 | 53.70 | 187.00 | 0.150 |
| PA | 256.42 | 62.30 | 186.00 | 0.162 |
| SA | 284.48 | 70.70 | 203.00 | 0.172 |
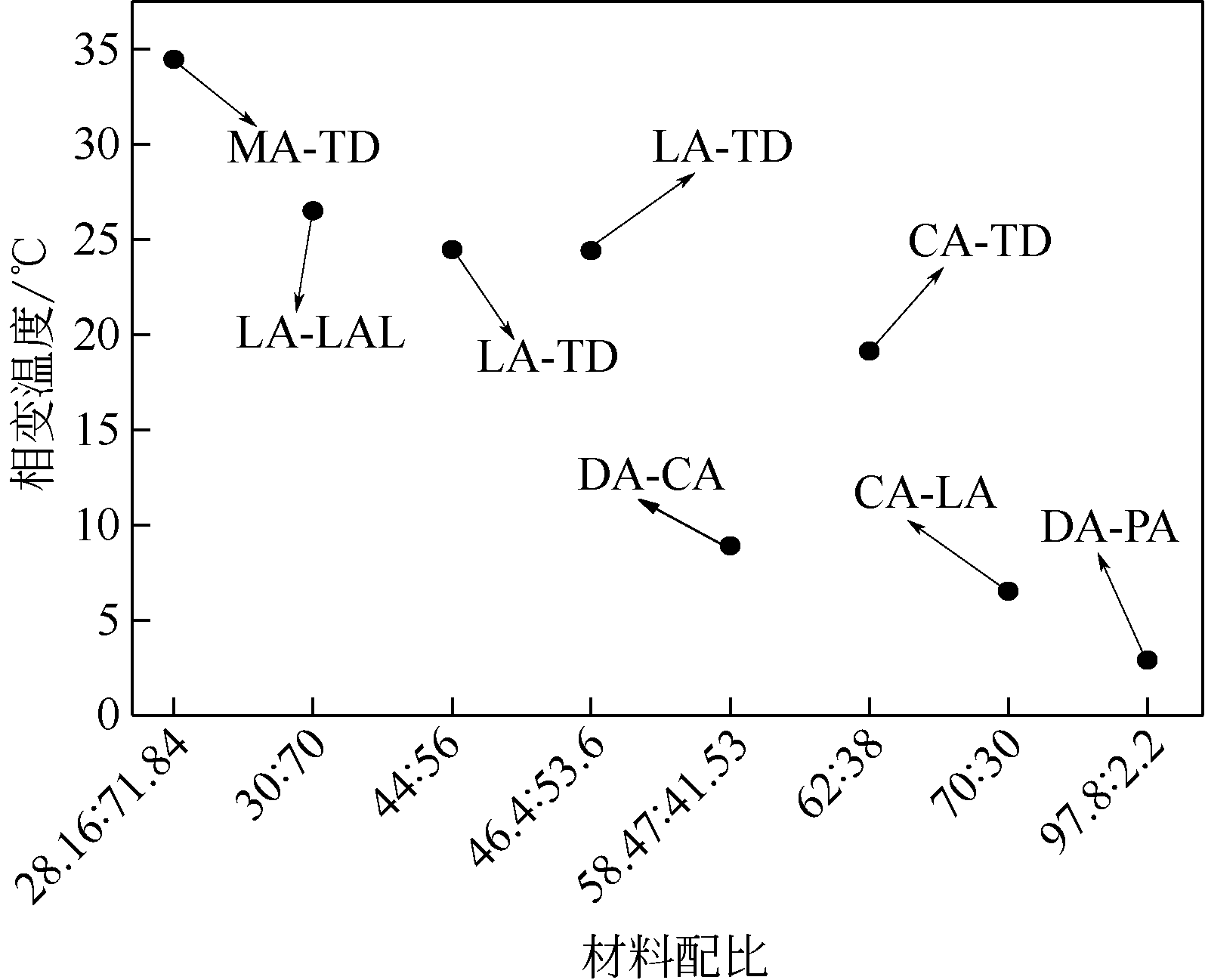
| 单脂肪酸/ 脂肪醇 | 单脂肪酸/脂肪醇相变温度/℃ | 单脂肪酸/脂肪醇 相变焓值/J·g–1 | 二元脂肪酸醇 | 质量配比 | 二元脂肪酸醇 相变温度/℃ | 二元脂肪酸醇 相变焓值/J·g–1 | 文献及年份 |
|---|---|---|---|---|---|---|---|
| LA | 43.40 | 183.23 | LA-TD | 44∶56 | 24.46 | 150.45 | [ |
| TD | 37.40 | 222.93 | |||||
| DA | 6.0 | 205.7 | DA-PA | 97.8∶2.2 | 2.9 | 203.6 | [ |
| PA | 62.1 | 210.2 | |||||
| DA | 23.7 | 220.4 | DA-CA | 58.47∶41.53 | 8.9 | 159 | [ |
| CA | 31.5 | 154 | |||||
| LA | 44.40 | 181.50 | LA-LAL | 30∶70 | 26.50 | 101.22 | [ |
| LAL | 23.10 | 242.15 | |||||
| CA | 30.20 | 142.70 | CA-TD | 62∶38 | 19.13 | 153.40 | [ |
| TD | 37.00 | 207.30 | LA-TD | 46.4∶53.6 | 24.40 | 162.70 | |
| LA | 43.20 | 177.70 | MA-TD | 28.16∶71.84 | 34.45 | 208.00 | |
| MA | 52.10 | 190.00 | |||||
| CA | 15.42 | 150.99 | CA-LA | 70∶30 | 6.52 | 171.06 | [ |
| LA | 22.84 | 207.30 |
| 单脂肪酸/ 脂肪醇 | 单脂肪酸/脂肪醇相变温度/℃ | 单脂肪酸/脂肪醇 相变焓值/J·g–1 | 二元脂肪酸醇 | 质量配比 | 二元脂肪酸醇 相变温度/℃ | 二元脂肪酸醇 相变焓值/J·g–1 | 文献及年份 |
|---|---|---|---|---|---|---|---|
| LA | 43.40 | 183.23 | LA-TD | 44∶56 | 24.46 | 150.45 | [ |
| TD | 37.40 | 222.93 | |||||
| DA | 6.0 | 205.7 | DA-PA | 97.8∶2.2 | 2.9 | 203.6 | [ |
| PA | 62.1 | 210.2 | |||||
| DA | 23.7 | 220.4 | DA-CA | 58.47∶41.53 | 8.9 | 159 | [ |
| CA | 31.5 | 154 | |||||
| LA | 44.40 | 181.50 | LA-LAL | 30∶70 | 26.50 | 101.22 | [ |
| LAL | 23.10 | 242.15 | |||||
| CA | 30.20 | 142.70 | CA-TD | 62∶38 | 19.13 | 153.40 | [ |
| TD | 37.00 | 207.30 | LA-TD | 46.4∶53.6 | 24.40 | 162.70 | |
| LA | 43.20 | 177.70 | MA-TD | 28.16∶71.84 | 34.45 | 208.00 | |
| MA | 52.10 | 190.00 | |||||
| CA | 15.42 | 150.99 | CA-LA | 70∶30 | 6.52 | 171.06 | [ |
| LA | 22.84 | 207.30 |
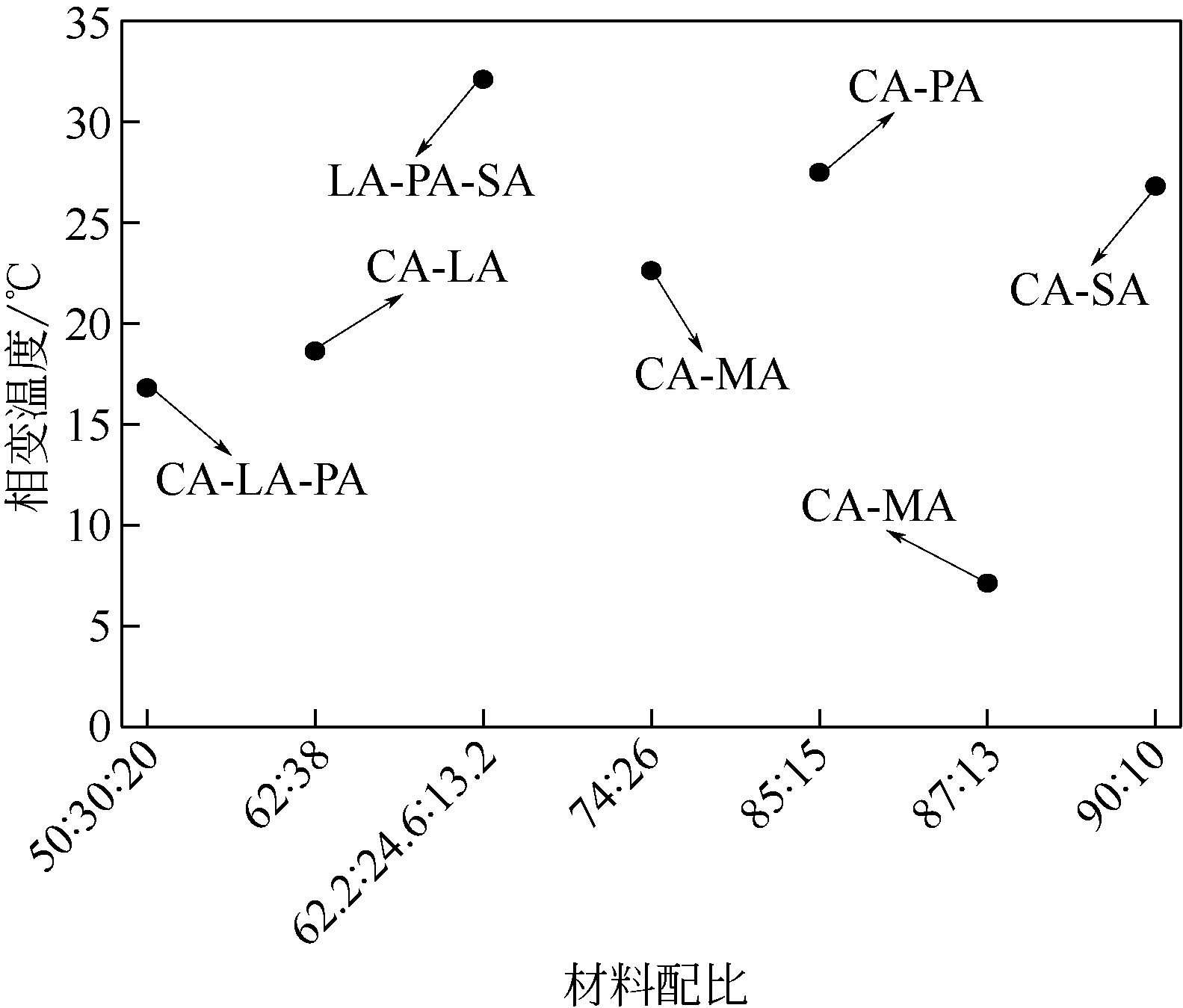
| 单脂肪酸 | 单脂肪酸 相变温度/℃ | 单脂肪酸 相变焓值/J·g–1 | 二元或多元脂肪酸 | 质量配比 | 二元或多元脂酸相变温度/℃ | 二元或多元脂肪酸 相变焓值/J·g–1 | 文献及年份 |
|---|---|---|---|---|---|---|---|
| CA | 32.14 | 156.40 | CA-PA | 85:15 | 27.48 | 151.54 | [ |
| PA | 58.90 | 189.60 | |||||
| CA | 32.14 | 156.40 | CA-MA | 74:26 | 22.61 | 154.83 | [ |
| MA | 53.86 | 190.00 | |||||
| CA | 16 | 146.45 | CA-MA | 87:13 | 7.13 | 146.1 | [ |
| MA | 52.2 | 182.6 | |||||
| CA | 31.49 | 150.80 | CA-LA | 62:38 | 18.63 | 121.00 | [ |
| LA | 43.53 | 172.60 | |||||
| CA | 31.00 | 154.90 | CA-SA | 90:10 | 26.8 | 96.4 | [ |
| SA | 67.53 | 211.60 | |||||
| CA | 30.30 | 152.10 | CA-LA-PA | 50:30:20 | 16.80 | 140.50 | [ |
| LA | 42.80 | 180.20 | |||||
| PA | 61.40 | 220.70 | |||||
| LA | 44.00 | 174.90 | LA-PA-SA | 62.2:24.6:13.2 | 32.10 | 151.60 | [ |
| PA | 62.00 | 198.40 | |||||
| SA | 68.50 | 201.80 |
| 单脂肪酸 | 单脂肪酸 相变温度/℃ | 单脂肪酸 相变焓值/J·g–1 | 二元或多元脂肪酸 | 质量配比 | 二元或多元脂酸相变温度/℃ | 二元或多元脂肪酸 相变焓值/J·g–1 | 文献及年份 |
|---|---|---|---|---|---|---|---|
| CA | 32.14 | 156.40 | CA-PA | 85:15 | 27.48 | 151.54 | [ |
| PA | 58.90 | 189.60 | |||||
| CA | 32.14 | 156.40 | CA-MA | 74:26 | 22.61 | 154.83 | [ |
| MA | 53.86 | 190.00 | |||||
| CA | 16 | 146.45 | CA-MA | 87:13 | 7.13 | 146.1 | [ |
| MA | 52.2 | 182.6 | |||||
| CA | 31.49 | 150.80 | CA-LA | 62:38 | 18.63 | 121.00 | [ |
| LA | 43.53 | 172.60 | |||||
| CA | 31.00 | 154.90 | CA-SA | 90:10 | 26.8 | 96.4 | [ |
| SA | 67.53 | 211.60 | |||||
| CA | 30.30 | 152.10 | CA-LA-PA | 50:30:20 | 16.80 | 140.50 | [ |
| LA | 42.80 | 180.20 | |||||
| PA | 61.40 | 220.70 | |||||
| LA | 44.00 | 174.90 | LA-PA-SA | 62.2:24.6:13.2 | 32.10 | 151.60 | [ |
| PA | 62.00 | 198.40 | |||||
| SA | 68.50 | 201.80 |
| 单脂肪酸/石蜡 | 单脂肪酸/石蜡相变温度/℃ | 单脂肪酸/石蜡 相变焓值/J·g–1 | 脂肪酸-石蜡 二元混合物 | 质量配比 | 脂肪酸-石蜡二元 混合物相变温度/℃ | 脂肪酸-石蜡二元混合物相变焓值/J·g–1 | 文献及年份 |
|---|---|---|---|---|---|---|---|
| CA | 28.5 | 145.5 | CA-PW | 80∶20 | 25.5 | 144.7 | [ |
| PW | 52 | 250 | |||||
| PA | 62.98 | 236.18 | PA-PW | 15∶85 | 44.5 | 210.5 | [ |
| PW | 52.76 | 220.52 | |||||
| SA | 56 | 177.6 | SA-PW | 50∶50 | 50.62 | 171.9 | [ |
| PW | 52.5 | 169.7 |
| 单脂肪酸/石蜡 | 单脂肪酸/石蜡相变温度/℃ | 单脂肪酸/石蜡 相变焓值/J·g–1 | 脂肪酸-石蜡 二元混合物 | 质量配比 | 脂肪酸-石蜡二元 混合物相变温度/℃ | 脂肪酸-石蜡二元混合物相变焓值/J·g–1 | 文献及年份 |
|---|---|---|---|---|---|---|---|
| CA | 28.5 | 145.5 | CA-PW | 80∶20 | 25.5 | 144.7 | [ |
| PW | 52 | 250 | |||||
| PA | 62.98 | 236.18 | PA-PW | 15∶85 | 44.5 | 210.5 | [ |
| PW | 52.76 | 220.52 | |||||
| SA | 56 | 177.6 | SA-PW | 50∶50 | 50.62 | 171.9 | [ |
| PW | 52.5 | 169.7 |
| 相变材料 | 多孔基材 | 质量配比 | 作用 | 参考文献 |
|---|---|---|---|---|
| CA-LA | Dm | 85∶15 | 热导率从0.22W·m-1·K-1增加到0.47W·m-1·K-1 | [ |
| CA | Bn | 40∶60 | 热导率从0.18W·m-1·K-1增加到0.43W·m-1·K-1 | [ |
| CA | Kl | 17.5∶82.5 | 热导率从0.18W·m-1·K-1增加到0.23W·m-1·K-1 | [ |
| CA-LA-PA | EG | 95∶5 | 热导率增加到0.738W·m-1·K-1,约是原材料的4.5倍 | [ |
| LA-PA-SA | EG | 93.75∶6.25 | 融化时间减少了85%,凝固时间减少了83.3% | [ |
| SA-AC | EG | 90∶10 | 热导率从0.336W·m-1·K-1增加到5.909W·m-1·K-1 | [ |
| 相变材料 | 多孔基材 | 质量配比 | 作用 | 参考文献 |
|---|---|---|---|---|
| CA-LA | Dm | 85∶15 | 热导率从0.22W·m-1·K-1增加到0.47W·m-1·K-1 | [ |
| CA | Bn | 40∶60 | 热导率从0.18W·m-1·K-1增加到0.43W·m-1·K-1 | [ |
| CA | Kl | 17.5∶82.5 | 热导率从0.18W·m-1·K-1增加到0.23W·m-1·K-1 | [ |
| CA-LA-PA | EG | 95∶5 | 热导率增加到0.738W·m-1·K-1,约是原材料的4.5倍 | [ |
| LA-PA-SA | EG | 93.75∶6.25 | 融化时间减少了85%,凝固时间减少了83.3% | [ |
| SA-AC | EG | 90∶10 | 热导率从0.336W·m-1·K-1增加到5.909W·m-1·K-1 | [ |
| 相变材料 | 多孔基材 | 质量配比 | 作用 | 参考文献 |
|---|---|---|---|---|
| SA | Gr | 95∶5 | 热导率最大提高了63.4% | [ |
| SA | Gr | 95∶5 | 热导率提高了71.7% | [ |
| SA | TiO2 | 30.36∶69.64 | 热导率最大提高了2.5倍 | [ |
| PA | SiO2 | 83.25∶16.75 | 热导率从0.21W·m-1·K-1增加到0.71W·m-1·K-1 | [ |
| PA | AlOOH | 52∶48 | 热导率从0.16W·m-1·K-1增加到0.84W·m-1·K-1 | [ |
| 相变材料 | 多孔基材 | 质量配比 | 作用 | 参考文献 |
|---|---|---|---|---|
| SA | Gr | 95∶5 | 热导率最大提高了63.4% | [ |
| SA | Gr | 95∶5 | 热导率提高了71.7% | [ |
| SA | TiO2 | 30.36∶69.64 | 热导率最大提高了2.5倍 | [ |
| PA | SiO2 | 83.25∶16.75 | 热导率从0.21W·m-1·K-1增加到0.71W·m-1·K-1 | [ |
| PA | AlOOH | 52∶48 | 热导率从0.16W·m-1·K-1增加到0.84W·m-1·K-1 | [ |
| 相变材料 | 质量配比 | 高导材料 | 添加量 | 作用 | 参考文献 |
|---|---|---|---|---|---|
| MA | — | Gr | 0.5%,1%,2% | 热导率分别增加了6.7%,20%,40% | [ |
| CA-MA/VMT | 20:80 | EG | 2% | 热导率从0.12W·m-1·K-1增加到0.22W·m-1·K-1 | [ |
| LA-PA-SA/EP | 55:45 | EG | 2% | 热导率提高了95% | [ |
| CA-MA/PMMA | 72:28 | MG | 2%,5%,7%,10%,15% | 热导率分别增加了10.8%,33.4%,46%,59.5%,89% | [ |
| PA-SA/Bn | 50:50 | EG | 4% | 热导率提高了5.6倍 | [ |
| SF/CA-PA | 68:32 | CNT | 1%,3%,5% | 热导率分别增加了12.9%,32.3%,56.1% | [ |
| SA | — | CNT | 0.1% | 热导率提高了8.3% | [ |
| SA | — | CNT | 40% | 热导率提高了27.9倍 | [ |
| SA | — | CNT | 0.1%,0.5%,1%,3% | 热导率分别增加了4.6%,6.3%,8.6%,11.4% | [ |
| PA | — | CNT | 1% | 热导率提高了35% | [ |
| MA-SA | 54:46 | CNT | 9%,12%,15% | 热导率分别增加了23.2%,49.4%,63.7% | [ |
| MA/HDPE | 70:30 | Al2O3 | 4%,8%,12% | 热导率分别增加了38.7%,71%,94.6% | [ |
| LA-MA-SA/aEV | 57.7:42.3 | Al2O3 | 12% | 热导率提高了37.5% | [ |
| DA-CA | 58.47:41.53 | Al2O3 | 0.1g·L-1 | 热导率提高了14.9% | [ |
| Cu | 0.1g·L-1 | 热导率提高了8.5% | |||
| CA-PA | 79:21 | Al2O3 | 20% | 热导率从0.3038W·m-1·K-1增加到0.5051W·m-1·K-1 | [ |
| CA-CA | 30:70 | Cu | 0.1%,0.2%,0.3%,0.4% | 热导率分别增加了5.1%,5.8%,6.2%,6.6% | [ |
| Al2O3 | 0.1%,0.2%,0.3%,0.4% | 热导率分别增加了4%,8%,11%,13% | |||
| Fe2O3 | 0.1%,0.2%,0.3% | 热导率分别增加了6.6%,9.5%,17.5% | |||
| PA-LA | 80:20 | Ss | — | 几乎没影响 | [ |
| SA-MA | 80:20 | Cu | — | 融化时间减少了22%,凝固时间减少了61% | |
| Gr | — | 融化时间减少了54%,凝固时间减少了71% | |||
| Ss | — | 几乎没影响 | |||
| Cu | — | 融化时间减少了33%,凝固时间减少了65% | |||
| Gr | — | 融化时间减少了33%,凝固时间减少了50% |
| 相变材料 | 质量配比 | 高导材料 | 添加量 | 作用 | 参考文献 |
|---|---|---|---|---|---|
| MA | — | Gr | 0.5%,1%,2% | 热导率分别增加了6.7%,20%,40% | [ |
| CA-MA/VMT | 20:80 | EG | 2% | 热导率从0.12W·m-1·K-1增加到0.22W·m-1·K-1 | [ |
| LA-PA-SA/EP | 55:45 | EG | 2% | 热导率提高了95% | [ |
| CA-MA/PMMA | 72:28 | MG | 2%,5%,7%,10%,15% | 热导率分别增加了10.8%,33.4%,46%,59.5%,89% | [ |
| PA-SA/Bn | 50:50 | EG | 4% | 热导率提高了5.6倍 | [ |
| SF/CA-PA | 68:32 | CNT | 1%,3%,5% | 热导率分别增加了12.9%,32.3%,56.1% | [ |
| SA | — | CNT | 0.1% | 热导率提高了8.3% | [ |
| SA | — | CNT | 40% | 热导率提高了27.9倍 | [ |
| SA | — | CNT | 0.1%,0.5%,1%,3% | 热导率分别增加了4.6%,6.3%,8.6%,11.4% | [ |
| PA | — | CNT | 1% | 热导率提高了35% | [ |
| MA-SA | 54:46 | CNT | 9%,12%,15% | 热导率分别增加了23.2%,49.4%,63.7% | [ |
| MA/HDPE | 70:30 | Al2O3 | 4%,8%,12% | 热导率分别增加了38.7%,71%,94.6% | [ |
| LA-MA-SA/aEV | 57.7:42.3 | Al2O3 | 12% | 热导率提高了37.5% | [ |
| DA-CA | 58.47:41.53 | Al2O3 | 0.1g·L-1 | 热导率提高了14.9% | [ |
| Cu | 0.1g·L-1 | 热导率提高了8.5% | |||
| CA-PA | 79:21 | Al2O3 | 20% | 热导率从0.3038W·m-1·K-1增加到0.5051W·m-1·K-1 | [ |
| CA-CA | 30:70 | Cu | 0.1%,0.2%,0.3%,0.4% | 热导率分别增加了5.1%,5.8%,6.2%,6.6% | [ |
| Al2O3 | 0.1%,0.2%,0.3%,0.4% | 热导率分别增加了4%,8%,11%,13% | |||
| Fe2O3 | 0.1%,0.2%,0.3% | 热导率分别增加了6.6%,9.5%,17.5% | |||
| PA-LA | 80:20 | Ss | — | 几乎没影响 | [ |
| SA-MA | 80:20 | Cu | — | 融化时间减少了22%,凝固时间减少了61% | |
| Gr | — | 融化时间减少了54%,凝固时间减少了71% | |||
| Ss | — | 几乎没影响 | |||
| Cu | — | 融化时间减少了33%,凝固时间减少了65% | |||
| Gr | — | 融化时间减少了33%,凝固时间减少了50% |
| 方法 | 增强材料 | 优点 | 缺点 |
|---|---|---|---|
| 多孔基材吸附 | 硅藻土、膨润土、高岭土、 | 吸附过程简单,导热增强效果 | 成本较高,降低复合材料的相变潜热 |
| 膨胀石墨等 | 明显,抑制液相泄漏 | ||
| 微胶囊化 | 二氧化硅、二氧化钛、碳酸钙、氧化铝等 | 直接加工成型,使用安全方便, | 成本高,制备工艺复杂,易磨损破裂, |
| 提高导热性能,抑制液相泄漏 | 降低复合材料的相变潜热 | ||
| 添加高导热材料 | 石墨、碳纳米管、金属、 | 操作简单易实现,成本较低,改善导热性能 | 降低复合材料的潜热 |
| 石墨烯等 |
| 方法 | 增强材料 | 优点 | 缺点 |
|---|---|---|---|
| 多孔基材吸附 | 硅藻土、膨润土、高岭土、 | 吸附过程简单,导热增强效果 | 成本较高,降低复合材料的相变潜热 |
| 膨胀石墨等 | 明显,抑制液相泄漏 | ||
| 微胶囊化 | 二氧化硅、二氧化钛、碳酸钙、氧化铝等 | 直接加工成型,使用安全方便, | 成本高,制备工艺复杂,易磨损破裂, |
| 提高导热性能,抑制液相泄漏 | 降低复合材料的相变潜热 | ||
| 添加高导热材料 | 石墨、碳纳米管、金属、 | 操作简单易实现,成本较低,改善导热性能 | 降低复合材料的潜热 |
| 石墨烯等 |
| 添加剂 | 优点 | 缺点 |
|---|---|---|
| 石墨 | 导热增强效果最显著,抑制液相泄漏 | 降低复合材料的相变潜热 |
| 碳纳米管 | 导热增强效果较显著,抑制液相泄漏 | 酸化等处理过程复杂,成本较高,降低相变材料的潜热值及 |
| 液相自然对流作用 | ||
| 金属 | 改善导热性能,过程简单,成本低, | 有可能减小复合材料的相变潜热 |
| 有可能提高复合材料的相变潜热 |
| 添加剂 | 优点 | 缺点 |
|---|---|---|
| 石墨 | 导热增强效果最显著,抑制液相泄漏 | 降低复合材料的相变潜热 |
| 碳纳米管 | 导热增强效果较显著,抑制液相泄漏 | 酸化等处理过程复杂,成本较高,降低相变材料的潜热值及 |
| 液相自然对流作用 | ||
| 金属 | 改善导热性能,过程简单,成本低, | 有可能减小复合材料的相变潜热 |
| 有可能提高复合材料的相变潜热 |
| 1 | KANT K , SHUKLA A , SHARMA A . Ternary mixture of fatty acids as phase change materials for thermal energy storage applications[J]. Energy Reports,2016,2:274-279. |
| 2 | ZHANG W Y , ZHANG X G , HUANG Z H ,et al . Preparation and characterization of capric-palmitic-stearic acid ternary eutectic mixture/expanded vermiculite composites as form-stabilized thermal energy storage materials[J]. Journal of Materials Science & Technology,2018,34(2):379-386. |
| 3 | YUAN Y P , ZHANG N , TAO W Q ,et al . Fatty acids as phase change materials: a review[J].Renewable and Sustainable Energy Reviews,2014,29(7):482-498. |
| 4 | HASAN A , SAYIGH A A . Some fatty acids as phase change thermal energy storage materials[J]. Renewable Energy,1994,4(1):69-76. |
| 5 | SHARMA A , TYAGI V V , CHEN C R ,et al . Review on thermal energy storage with phase change materials and applications[J].Renewable and Sustainable Energy Reviews,2009,13(2):318-345. |
| 6 | 张天驰,俞海云,冒爱琴,等 . 有机相变储能材料导热增强方法研究进展[J].过程工程学报,2017,17(1):201-208. |
| ZHANG Tianchi , YU Haiyun , MAO Aiqin ,et al .Research advances in organic phase change materials for technology of thermal enhancement[J]. The Chinese Journal of Process Engineering,2017,17(1):201-208. | |
| 7 | 苑坤杰,张正国,方晓明,等 .水合无机盐及其复合相变储热材料的研究进展[J].化工进展,2016,35(6):1820-1826. |
| YUAN Kunjie , ZHANG Zhengguo , FANG Xiaoming ,et al .Research progress of inorganic hydrated salts and their phase change heat storage composites[J].Chemical Industry and Engineering Progress,2016,35(6):1820-1826. | |
| 8 | ROZANNA D , CHUAH T G , SALMIAH A ,et al . Fatty acids as phase change materials (PCMs) for thermal energy storage: a review[J]. International Journal of Green Energy,2005,1(4):495-513. |
| 9 | FELDMAN D , SHAPIRO M M , BANU D ,et al .Fatty acids and their mixtures as phase change materials for thermal energy storage[J]. Solar Energy Materials,1989,18(3):201-216. |
| 10 | SARI A , KAYGUSUZ K . Some fatty acids used for latent heat storage: thermal stability and corrosion of metals with respect to thermal cycling[J]. Renewable Energy,2003,28(6):939-948. |
| 11 | 李琳,琚诚兰,戴浩,等 .脂肪酸类相变储能建筑材料的研究及应用[J].现代化工,2015,35(8):46-49. |
| LI Lin , JU Chenglan , DAI Hao ,et al . Research and application of fatty acids phase change building materials[J].Modern Chemical Industry,2015,35(8):46-49. | |
| 12 | 蔡伟,孙志高,马鸿凯,等 . 月桂酸-十四醇二元复合相变材料的相变特性[J].太阳能学报,2017,38(9):2493-2497. |
| CAI Wei , SUN Zhigao , Hongkai MA ,et al .Phase transition properties of LA-TD binary composite phase change materials[J].Acta Energiae Solaris Sinica,2017,38(9):2493-2497. | |
| 13 | 周孙希,章学来,刘升,等 . 癸醇-棕榈酸/膨胀石墨低温复合相变材料的制备与性能[J].化工学报,2019,70(1):290-297. |
| ZHOU Sunxi , ZHANG Xuelai , LIU Sheng ,et al .Preparation and properties of decyl alcohol-palmitic acid/expanded graphite low temperature composite phase change material[J].CIESC Journal,2019,70(1):290-297. | |
| 14 | 黄艳,章学来 . 十二醇-癸酸-纳米粒子复合相变材料传热性能[J].化工学报,2016,67(6):2271-2276. |
| HUANG Yan , ZHANG Xuelai . Heat transfer property of lauryl alcohol-capric acid-nanoparticle composite phase change materials[J].CIESC Journal,2016,67(6):2271-2276. | |
| 15 | 仇影,吴其胜,黎水平,等 . 二元有机/煤系高岭土复合相变储能材料的制备及其热性能[J].材料科学与工程学报,2013,31(2):268-272. |
| CHOU Ying, WU Qisheng , LI Shuiping ,et al .Preparation and thermal properties of binary organic/Kaolin composites as shape-stabilized phase change material for thermal energy storage[J].Journal of Materials Science and Engineering,2013,31(2):268-272. | |
| 16 | HUANG J , LU S , KONG X ,et al . Form-stable phase change materials based on eutectic mixture of tetradecanol and fatty acids for building energy storage: preparation and performance analysis[J]. Materials,2013,6(10):4758-4775. |
| 17 | ZUO J , LI W Z , WENG L D .Thermal performance of caprylic acid/1-dodecanol eutectic mixture as phase change material (PCM)[J].Energy & Buildings,2011,43(1):207-210. |
| 18 | SARI A , BICER A , AL-AHMED A ,et al .Silica fume/capric acid-palmitic acid composite phase change material doped with CNTs for thermal energy storage[J]. Solar Energy Materials and Solar Cells,2018,179:353-361. |
| 19 | SARI A , BICER A , KARAIPEKLI A ,et al .Preparation, characterization and thermal regulation performance of cement based-composite phase change material[J]. Solar Energy Materials and Solar Cells,2018,174:523-529. |
| 20 | 李玉洋,章学来,徐笑锋,等 .正辛酸-肉豆蔻酸低温相变材料的制备和循环性能[J].化工进展,2018,37(2):689-693. |
| LI Yuyang , ZHANG Xuelai , XU Xiaofeng ,et al .Preparation and cyclic properties of low temperature phase change materials of n-caprylic acid and myristic acid[J].Chemical Industry and Engineering Progress,2018,37(2):689-693. | |
| 21 | ZHANG Z L , YUAN Y P , ZHANG N ,et al .Experimental investigation on thermophysical properties of capric acidelauric acid phase change slurries for thermal storage system[J].Energy,2015,90:359-368. |
| 22 | 樊铁林,陈蜜蜜,檀星,等 .脂肪酸/废加气块定形相变储能集料制备及性能[J].化工进展,2017,36(3):996-1002. |
| FAN Tielin , CHEN Mimi , TAN Xing ,et al .Preparation and properties of shape-stabilized phase change aggregate from fatty acid and waste autoclaved aerated concrete[J].Chemical Industry and Engineering Progress,2017,36(3):996-1002. | |
| 23 | 孟新,张焕芝,赵梓名,等 . 三元脂肪酸/膨胀石墨复合相变材料的制备、包覆定形及热性能[J].高等学校化学学报,2012,33(3):526-530. |
| MENG Xin , ZHANG Huanzhi , ZHAO Ziming ,et al . Preparation encapsulation and thermal properties of fatty acid / expanded graphite composites as shape-stabilized phase change materials[J].Chemical Journal of Chinese Universities,2012,33(3):526-530. | |
| 24 | ZHANG N , YUAN Y P , YUAN Y G ,et al .Lauric-palmitic-stearic acid/expanded perlite composite as form-stable phase change material: preparation and thermal properties[J].Energy and Buildings,2014,82:505-511. |
| 25 | GUO Z H , JIA Y K , WANG X . Research on the capric acid/paraffin and sepiolite combination as phase change energy storage materials[C]//7th National Conference on Functional Materials and Applications.Chongqing:Function Materials,2010:1927-1931. |
| 26 | 尹徐影,王继芬,关礼辉,等 .棕榈酸/石蜡复合相变储能材料[J].上海第二工业大学学报,2014,31(4):295-300. |
| YIN Xuying , WANG Jifen , GUAN Lihui ,et al . Properties of palmitic acid and paraffin wax composite phase change material[J].Journal of Shanghai Second Polytechnic University,2014,31(4):295-300. | |
| 27 | 刘楠 .石蜡-硬脂酸/膨胀石墨蓄放热特性实验研究[D].天津:天津商业大学,2017. |
| LIU Nan .Experimental study on heat storage and release characteristics of paraffin-stearic acid/expanded graphite[D]. Tianjin:Tianjin University of Commerce,2017. | |
| 28 | 黄雪,崔英德,张步宁,等 .脂肪酸类相变材料传热及液相渗漏的研究进展[J].化工进展,2014,33(10):2676-2680. |
| HUANG Xue , CUI Yingde , ZHANG Buning ,et al . Review on heat transfer and liquid phase leakage of fatty acids phase change materials[J]. Chemical Industry and Engineering Progress,2014,33(10):2676-2680. | |
| 29 | 张毅,张菁燕,黄斌,等 . 脂肪酸相变材料热导率测试及相变传热过程的数值模拟[J].功能材料,2012,14(43):1950-1954. |
| ZHANG Yi , ZHANG Jingyan , HUANG Bin ,et al .Experiment test of thermal conductivity coefficient and heat transfer simulation analysis of fatty acids phase change materials[J].Function Materials,2012,14(43):1950-1954. | |
| 30 | SARI A .Thermal energy storage characteristics of bentonite-based composite PCMs with enhanced thermal conductivity as novel thermal storage building materials[J]. Energy Conversion and Management,2016,117:132-141. |
| 31 | SARI A .Fabrication and thermal characterization of Kaolin-based composite phase change materials for latent heat storage in buildings[J]. Energy and Buildings,2015,96 :193-200. |
| 32 | ZHANG Y P , DING J H , WANG X ,et al . Influence of additives on thermal conductivity of shape-stabilized phase change material[J].Solar Energy Materials and Solar Cells,2006,90(11):1692-1702. |
| 33 | 袁亚光,袁艳平,张楠,等 .月桂酸-棕榈酸-硬脂酸/膨胀石墨复合相变材料的制备及性能[J].化工学报,2014,65(s2):286-292. |
| YUAN Yaguang , YUAN Yanping , ZHANG Nan ,et al .Preparation and properties of lauric-palmitic-stearic acid eutectic mixture/expanded graphite composite phase change material for energy storage[J].CIESC Journal,2014,65(s2):286-292. | |
| 34 | 张素凌,方玉堂, 汪双凤,等 .SA-AC/膨胀石墨的制备及性能研究[J].工程热物理学报,2017,38(12):2691-2696. |
| ZHANG Suling , FANG Yutang , WANG Shuangfeng ,et al . The preparation and study on the properties of SA-AC/expanded graphite composite[J].Journal of Engineering Thermophysics,2017,38(12):2691-2696. | |
| 35 | DAO T D, JEONG H M .Novel stearic acid/graphene core-shell composite microcapsule as a phase change material exhibiting high shape stability and performance[J]. Solar Energy Materials and Solar Cells,2015,137:227-234. |
| 36 | DAO T D, JEONG H M .A pickering emulsion route to a stearic acid/graphene core-shell composite phase change material[J]. Carbon,2016,99:49-57. |
| 37 | LATIBARI S T , MEHRALI M , MEHRALI M ,et al .Facile synthesis and thermal performances of stearic acid/titania core/shell nanocapsules by solegel method[J]. Energy,2015,85:635-644. |
| 38 | LATIBARI S T , MEHRALI M , MEHRALI M ,et al .Synthesis, characterization and thermal properties of nanoencapsulated phase change materials via solegel method[J]. Energy,2013,61:664-672. |
| 39 | PAN L , TAO Q H , ZHANG S D ,et al .Preparation, characterization and thermal properties of micro-encapsulated phase change materials[J]. Solar Energy Materials and Solar Cells,2012,98:66-70. |
| 40 | KLETT J W , ERVIN V J , EDIE D D .Finite-element modeling of heat transfer in carbon/carbon composites[J].Composites Science and Technology,1999,59 (4):593-607. |
| 41 | INCE S , SEKI Y , EZAN M A ,et al .Thermal properties of myristic acid/graphite nanoplates composite phase change materials[J]. Renewable Energy, 2015,75(75):243-248. |
| 42 | KARAIPEKLI A , SARI A .Capric-myristic acid/vermiculite composite as form-stable phase change material for thermal energy storage[J].Solar Energy,2009,83(3):323-332. |
| 43 | MENG D , WANG L J . Characterization and thermal conductivity of modified graphite/fatty acid eutectic/PMMA form-stable phase change material[J].Journal of Wuhan University of Technology:Mater.Sci.Ed., 2013,28(3):586-591. |
| 44 | HUANG X , ALVA G , LIU L K ,et al .Preparation, characterization and thermal properties of fatty acid eutectics/bentonite/expanded graphite composites as novel form-stable thermal energy storage materials[J]. Solar Energy Materials and Solar Cells,2017,166:157-166. |
| 45 | CHOI D H ,LEE J, HONG H ,et al .Thermal conductivity and heat transfer performance enhancement of phase change materials (PCM) containing carbon additives for heat storage application[J]. International Journal of Refrigeration,2014,42:112-120. |
| 46 | ZHANG Q , LIU J .Sebacic acid/CNT sponge phase change material with excellent thermal conductivity and photo-thermal performance[J]. Solar Energy Materials and Solar Cells,2018,179:217-222. |
| 47 | LI T X ,LEE J H, WANG R Z ,et al .Enhancement of heat transfer for thermal energy storage application using stearic acid nanocomposite with multi-walled carbon nanotubes[J]. Energy,2013,55(1):752-761. |
| 48 | 王继芬,谢华清,辛忠,等 . 酸化碳纳米管棕搁酸复合相变储能材料的研究[J].工程热物理学报,2010,31(8):1389-1391. |
| WANG Jifen , XIE Huaqing , XIN Zhong ,et al .Experimental study on palmitic acid composites containing carbon manotubes by acid treatment[J].Journal of Engineering Thermophysics,2010,31(8):1389-1391. | |
| 49 | TANG Y J , ALVA G , HUANG X ,et al .Thermal properties and morphologies of MA-SA eutectics/CNTs as composite PCMs in thermal energy storage[J]. Energy and Buildings,2016,127:603-610. |
| 50 | TANG Y J , SU D , HUANG X ,et al . Synthesis and thermal properties of the MA/HDPE composites with nano-additives as form-stable PCM with improved thermal conductivity[J]. Applied Energy,2016,180:116-129. |
| 51 | WEI H T , LI X Q .Preparation and characterization of a lauric-myristic-stearic acid/Al2O3-loaded expanded vermiculite composite phase change material with enhanced thermal conductivity[J]. Solar Energy Materials and Solar Cells,2017,166 :1-8. |
| 52 | 包艳华,王庭慰 . 脂肪酸低熔混合物的制备及强化传热研究[J].现代化工,2011,31(3):43-45. |
| BAO Yanhua , WANG Tingwei .Preparation and heat transfer enhancement performance of eutectic mixture of fatty acids as phase change material[J].Modern Chemical Industry,2011,31(3):43-45. | |
| 53 | 陈裕丰,章学来,丁锦宏,等 . 癸酸/辛酸-纳米复合相变材料的热物性实验研究[J].制冷学报,2017,38(5):41-45. |
| CHEN Yufeng , ZHANG Xuelai , DING Jinhong ,et al .Experimental research on thermophysical properties of capric/caprylic acid nanocomposite phase change material[J].Journal of Refrigeration,2017,38(5):41-45. | |
| 54 | MAZMAN M , CABEZA L F , MEHLING H ,et al .Heat transfer enhancement of fatty acids when used as PCMs in thermal energy storage[J].International Journal of Energy Research,2008,32(2):135-143. |
| [1] | HUANG Yue, ZHAO Lixin, YAO Zonglu, YU Jiadong, LI Zaixing, SHEN Ruixia, AN Kemeng, HUANG Yali. Research progress in directed bioconversion of lactic acid and acetic acid from wood lignocellulosic wastes [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2691-2701. |
| [2] | XU Yuzhen, JIANG Dahua, LIU Jingtao, CHEN Pu. Preparation and properties of fly ash based phase change energy storage materials [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2595-2605. |
| [3] | LI Dongxian, WANG Jia, JIANG Jianchun. Producing aliphatic acids via pressurized hydrolysis of soapstock assisted by ultrasound [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 409-416. |
| [4] | YANG Yukai, XIA Yongpeng, XU Fen, SUN Lixian, GUAN Yanxun, LIAO Lumin, LI Yaying, ZHOU Tianhao, LAO Jianhao, WANG Yu, WANG Yingjing. Research progress of erythritol phase change materials for thermal storage [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4357-4366. |
| [5] | YANG Jin, YIN Yonggao, CHEN Wanhe, WANG Jingyuan, CHEN Jiufa. Preparation and performance optimization of phase change cold storage materials with sodium sulfate hydrate salt [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5977-5985. |
| [6] | LIU Chang, CHEN Yanjun, ZHANG Chaocan. Low temperature phase change materials for subzero applications [J]. Chemical Industry and Engineering Progress, 2022, 41(1): 286-299. |
| [7] | KUAI Zihan, YAN Ting, WU Shaofei, ZHOU Yuxiang, PAN Weiguo. Fabrication and heat storage properties of stearyl alcohol/expanded graphite composite phase change materials [J]. Chemical Industry and Engineering Progress, 2021, 40(S1): 301-310. |
| [8] | MA Mingyan, ZHAI Yuling, XUAN Zihao, ZHOU Shuguang, LI Zhixiang. Stability and thermal performance of ternary hybrid nanofluids [J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4179-4186. |
| [9] | XU Zhong, HOU Jing, WU Enhui, LI Jun, HUANG Ping, TANG Yalan. Effect of graphite on latent heat and conductivity of activated carbon/fatty acid composite phase change materials [J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3878-3891. |
| [10] | XIAO Xiangzhe, CHEN Siyuan, TENG Jun, DONG Shanyan, LIAN Junfeng, ZHU Yichun. A new interpretation of anaerobic hydrolysis: rapid hydrolysis and slow hydrolysis [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1586-1593. |
| [11] | Tingyu WU, Chengjun WANG, Xue WANG, Xiuyu QI, Wei WEI, Chengdong ZHENG. Skin-whitening ingredients and effects of Açaí berry (Euterpe oleracea): a review [J]. Chemical Industry and Engineering Progress, 2020, 39(S2): 285-290. |
| [12] | Shebao LIN, Dongyang SHI, Ailing FENG, Chunxiao LIU, Qiang XU, Lei ZHAO, Jiawei GUO, Wei WANG. Optical refrative index and band gap of B2O3-K2O-Sb2O3 system glasses [J]. Chemical Industry and Engineering Progress, 2020, 39(5): 1882-1887. |
| [13] | Xuming YAN, Xue HUANG, Ruizhao YANG, Guangzhu FENG. A new type of unsaturated polyester resin with reactive multi-walled carbon nanotube and C36 dimer fatty acid: preparation and property [J]. Chemical Industry and Engineering Progress, 2020, 39(5): 1888-1896. |
| [14] | Xiaohua LI,Zichun YANG,Kunfeng LI,Shuang ZHAO,Zhifang FEI,Zhen ZHANG. Preparation and characterization of transparent and compressible methylsilsesquioxane aerogels using MTES as precursor [J]. Chemical Industry and Engineering Progress, 2020, 39(3): 1115-1121. |
| [15] | Jianhong DENG, Hua FEI, Linya WANG, Qingjun GU. Preparation and properties of capric acid-paraffin/expanded graphite form-stable phase change materials [J]. Chemical Industry and Engineering Progress, 2020, 39(11): 4537-4543. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||