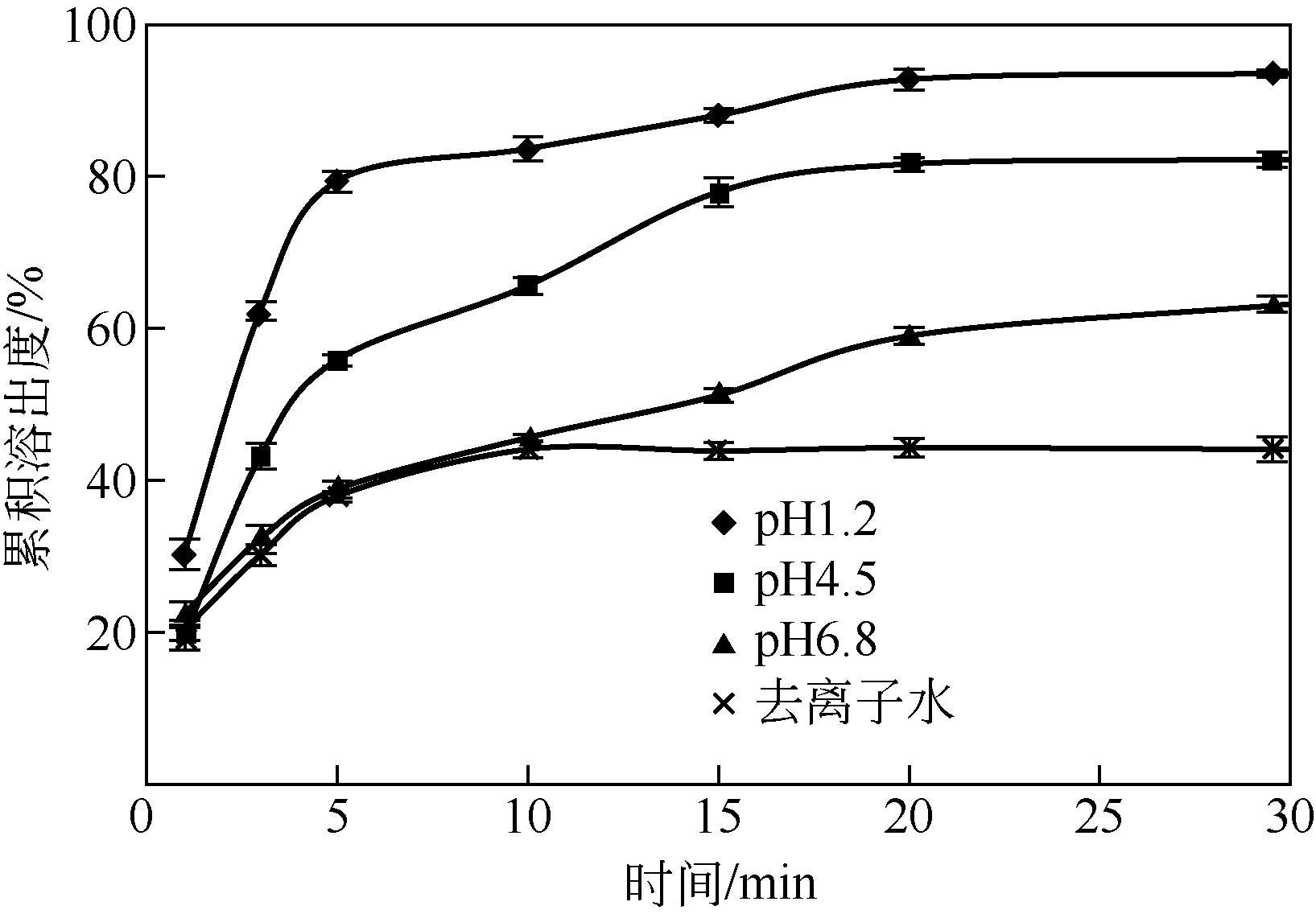
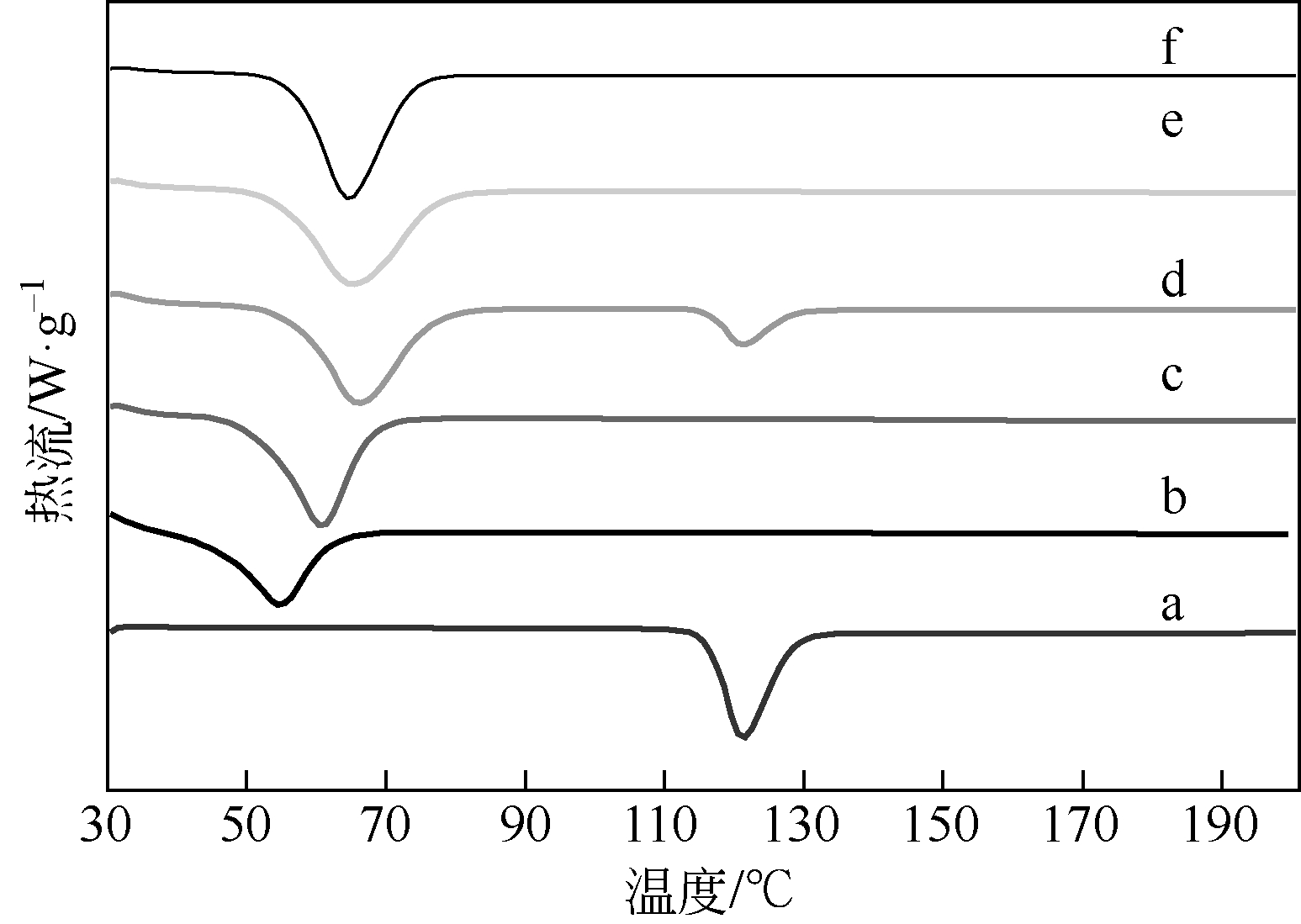
| 1 |
赵连友, 孙宁玲, 孙英贤, 等 . α/β受体阻滞剂在高血压治疗中应用的中国专家共识[J]. 中国实用内科杂志, 2016, 36(12): 1050-1054.
|
|
ZHAO Lianyou , SUN Ningling , SUN Yingxian , et al . Chinese expert consensus on the application of α/β receptor blocking agent in the treatment of hypertension[J]. Chinese Journal of Practical Internal Medicine, 2016, 36(12): 1050-1054.
|
| 2 |
BENET L Z . The role of BCS ( biopharmaceutics classification system)and BDDCS ( biopharmaceutics drug disposition classification system)in drug development[J]. J. Pharm. Sci., 2013, 102(1): 34-42.
|
| 3 |
赵悦清, 周仕海, 柳文洁, 等 . 口服固体制剂溶出方法建立和验证的技术探讨[J]. 药学学报, 2018, 53(2): 202−209.
|
|
ZHAO Yueqing , ZHOU Shihai , LIU Wenjie , et al . Discussion of development and validation of dissolution methods for solid oral dosage forms[J]. Acta Pharmaceutica Sinica, 2018, 53(2): 202-209.
|
| 4 |
李帅, 龚前飞, 廖萍, 等 . 仿制药一致性评价中溶出度试验方法的探讨[J]. 上海医药, 2018, 39(3): 24-28.
|
|
LI Shuai , GONG Qianfei , LIAO Ping , et al . The discussion of dissolution test methods in consistency evaluation for generics[J]. Shanghai Medical & Pharmaceutical Journal, 2018, 39(3): 24-28.
|
| 5 |
赵许杰 . 茯苓皮总三萜滴丸的制备及研究[D]. 济南: 山东中医药大学, 2014.
|
|
ZHAO Xujie . Study on preparation and pharmacy properties of poria triterpenoids dropping pills[D]. Jinan: Shandong University of Traditional Chinese Medicine, 2014.
|
| 6 |
国家药典委员会 . 中华人民共和国药典:四部[S]. 2015年版. 北京:中国医药科技出版社, 2015: 121-122.
|
|
Chinese Pharmacopoeia Commission Chinese pharmacopoeia: the fourth part[S]. 2015 Edition. Beijing: China medical technology press, 2015: 121-122.
|
| 7 |
付丽娜 . 难溶性药物体外溶出评价及溶出度方法的建立[D]. 杭州: 浙江工业大学, 2014.
|
|
FU Lina . Study on in vitro dissolution and establishment of the dissolution methods of poorly souble drugs[D]. Hangzhou: Zhejiang University of Technology, 2014.
|
| 8 |
王丽葵, 陈中亚, 王威, 等 . 国内外溶出度测定及评价方法的异同点[J]. 中国医药工业杂志, 2015, 46(12): 521-523.
|
|
WANG Likui , CHEN Zhongya , WANG Wei , et al . Similarities and differences of dissolution testing and evaluating methods at home and abroad[J]. Chinese Journal of Pharmaceuticals, 2015, 46(12): 521-523.
|
 ),Shengjuan SHAO,Huifang WANG
),Shengjuan SHAO,Huifang WANG