Chemical Industry and Engineering Progress ›› 2019, Vol. 38 ›› Issue (03): 1297-1307.DOI: 10.16085/j.issn.1000-6613.2018-1114
Previous Articles Next Articles
Application of deep eutectic solvents in the removal of organic sulfur compounds from fuel
Yingna CUI1,2( ),Ming BAO1(
),Ming BAO1( ),Changping LI3(
),Changping LI3( )
)
- 1. State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024, Liaoning, China
2. College of Environment and Chemical Engineering, Dalian University, Dalian 116622, Liaoning, China
3. School of Ecological Environment and Civil Engineering, Dongguan University of Technology, Dongguan 523808, Guangdong, China
-
Received:2018-05-29Revised:2018-10-10Online:2019-03-05Published:2019-03-05 -
Contact:Ming BAO,Changping LI
深共融溶剂在燃油脱除有机硫化物中的应用
- 1. 大连理工大学精细化工重点实验室, 辽宁 大连 116024
2. 大连大学环境与化学工程学院,辽宁 大连 116622
3. 东莞理工学院环境与建筑工程学院,广东 东莞 523000
-
通讯作者:包明,李长平 -
作者简介:崔颖娜 (1980—),女,讲师,研究方向为油品脱硫。E-mail:cuiyingna@dlu.edu.cn 。|包明,教授,研究方向为绿色化学。E-mail:mingbao@dlut.edu.cn |李长平,教授,研究方向为油品脱硫。E-mail:licpbit@hotmail.com -
基金资助:国家自然科学基金(21546007,21133005);大连市高层次人才项目(2016RJ11);大连市青年科技之星项目(2016RQ079)
CLC Number:
Cite this article
Yingna CUI,Ming BAO,Changping LI. Application of deep eutectic solvents in the removal of organic sulfur compounds from fuel[J]. Chemical Industry and Engineering Progress, 2019, 38(03): 1297-1307.
崔颖娜,包明,李长平. 深共融溶剂在燃油脱除有机硫化物中的应用[J]. 化工进展, 2019, 38(03): 1297-1307.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2018-1114
| 序号 | DESs | 萃取条件 | 单次脱硫效果 | 多次脱硫效果 | 回收再循环利用 | 多次重复利用 |
|---|---|---|---|---|---|---|
| 1 | TBAC/PEG[ (摩尔比1∶2) | m DES∶m oil=1∶1、25℃、30min、硫初始浓度1600mg/L | 对BT模拟油,脱硫率为70.44% | 4次萃取后,脱硫率99.48% | 4次回收循环使用后,脱硫率基本不变 | 经过6次重复利用后,深共融溶剂趋于饱和状态,失去萃取能力 |
| 2 | TBAC/PEG/FeCl3 [ (摩尔比4∶1∶0.05) | m DES:m oil=1∶1、25℃、30min、硫初始浓度1600 mg/L | 对DBT模拟油,脱硫率为89.53% | 2次萃取后,脱硫率100% | 5次回收循环使用后,脱硫率基本不变 | 第5次使用后深共融溶剂的萃取率低于50% |
| 3 | [CH3(CH2)3]4PBr/FeCl3 [ | DES质量分数0.525、50℃、115min、硫初始浓度500μg/g | 对DBT和T的模拟油,脱硫率分别为65%和48%,对商业柴油脱硫率为32% | 6次萃取后,商业柴油的硫浓度降至40μg/g 以下 | 5次回收循环使用后,脱硫率基本不变 | — |
| 4 | [CH3(CH2)3]4PBr/ SnCl2 .2H2O[ (摩尔比1∶1) | DESs质量分数0.5、30℃、100min、硫初始浓度500 μg/g | 对DBT和T的模拟油,脱硫率分别为69.57%和47.28% | 5次萃取后,DBT和T的萃取率分别为97%和80% | — | 第2次重复利用时,对模拟油中DBT和T的脱硫率分别降至38%和12% |
| 5 | TBAB/IM[ (摩尔比1∶1) | DES质量分数0.5、30°C、95min、硫初始浓度500μg/g | 对DBT和T的模拟油,脱硫率分别为70%和47%,对商业柴油的脱硫率为47% | 4次萃取后,模拟油中DBT和T的浓度分别降至10μg/g和40μg/g以下,5次萃取后,商业柴油硫浓度降至15μg/g以下 | 5次回收循环使用后,脱硫率基本不变 | — |
| 6 | TBAB/PEG-600[ (摩尔比1∶2) | V DES∶V oil=1∶1、25℃、30min、硫初始浓度500μg/g | 对DBT和T的模拟油,脱硫率分别为82.40%和62.16% | 3次萃取后,对DBT和T萃取率分别为100%和95.15% | 5次回收循环使用后,脱硫率基本不变 | 5次重复利用后,对模拟油中DBT和T的脱硫率分别降至31.72%和9.09% |
| 7 | AlCl3-氯化石蜡-52-甲苯[ | DESs 6%、 293K、10 min、硫初始浓度500μg/g | 对3-MT、BT、DBT模拟油的脱硫率分别为99.81%、99.65%和89.64%,可使真实燃油的硫含量从682.05μg/g降至48.37μg/g | — | — | — |
| 8 | AlCl3/CH3CONH2 [ (摩尔比1.8∶1) | DESs 6%、50℃、 40min、 硫初始浓度1000μg/g | 模拟油中3-MT、BT、DBT 的转化率分别为97.31%、98.21%和 87.39%;在真实燃油中,硫含量可从580μg/g降至17.9μg/g | — | — | — |
| 9 | TBAB/HCOOH[ (摩尔比1∶1) | m DES∶m oi=1∶0.5、30℃、40 min、硫初始浓度500μg/g | 对BT、DBT、T模拟油的脱硫率分别为81.75%、80.47%、72%,对FCC汽油和柴油的脱硫率分别为40.94%和35.27% | 3次萃取后,对BT、DBT、T模拟油的脱 硫率分别为98.32%、98.24%、97.6%;4次萃取后, FCC汽油和柴油的脱硫率分别为83.61%和70.21% | — | 4次重复利用后,对BT、DBT、T模拟油的脱硫率分别为25.09%、24.14%、23.96% |
| 10 | [BPY]Br/Mal[ (摩尔比1∶1) | m DES∶m oil=1∶2、40℃、30 min、硫初始浓度500μg/g | 对FCC汽油中硫化物的脱硫率为94.14% | — | — | — |
| 11 | TEtA/Pr[ (摩尔比1∶3) | m DES=1.75g、V oil=5mL、30℃、10min、硫初始浓度DBT 500mg/L、BT、4,6-DMDBT、RSH 250mg/L | 对模拟油的脱硫率为51.6% | 4次萃取后,模拟油中的硫的浓度可从500mg/L降至10mg/L | 5次回收循环使用后,脱硫率基本不变 | 5次重复利用后,硫的分配系数K N从2.14降至1.96 |
| 12 | MIM/Pr[ (摩尔比1∶1) | m DES= 1.75g、V oil=5mL、30℃、10min、硫初始浓度DBT 500mg/L、BT、4,6-DMDBT、RSH 250mg/L | 硫的分配系数K N为2.31,脱硫率为53.6% | 5次萃取后,可得到无硫燃油 | 5次回收循环使用后,脱硫率基本不变 | — |
| 13 | [C12DMEA]Cl/FeCl3 [ (摩尔比1∶1) | m DES=1g、V oil=5mL,30oC、10min、硫初始浓度500mg/L | 对DBT的模拟油,脱硫率为52.9% | 5次萃取后,脱硫率可达99.3% | 2次回收循环使用后,脱硫率从52.9%降至52.5% | — |
| 14 | TBAC/EG[ (摩尔比1∶2) | m DES∶m oil =1∶1、30℃、20 min、硫初始浓度1000 μg/g | 对2-MT和BT两种硫化物混合的模拟油的脱硫率为64.3% | 5次萃取后,脱硫率达99.5% | 6次回收循环使用后,脱硫率基本不变 | — |
| 15 | TBAB/Sul[ (1∶7) | m DES:m oil=1∶1、25℃、6h、T在正庚烷中的含量为10% | 单次脱硫率为35.07% | 5次萃取后,脱硫率可达98% | 4次回收循环使用后,脱硫率基本不变 | — |
| 16 | TBPB∶DMF[ (1∶3) | m DES∶m oil =1∶1、30℃、30 min、硫初始浓度500mg/L | 对DBT模拟油的脱硫率为82.1% | 3次萃取后,硫浓度可降至10mg/L以下 | 5次回收循环使用后,脱硫率基本不变 | 5次重复利用后,脱硫率仅为5.4%,基本失去萃取能力 |
| 序号 | DESs | 萃取条件 | 单次脱硫效果 | 多次脱硫效果 | 回收再循环利用 | 多次重复利用 |
|---|---|---|---|---|---|---|
| 1 | TBAC/PEG[ (摩尔比1∶2) | m DES∶m oil=1∶1、25℃、30min、硫初始浓度1600mg/L | 对BT模拟油,脱硫率为70.44% | 4次萃取后,脱硫率99.48% | 4次回收循环使用后,脱硫率基本不变 | 经过6次重复利用后,深共融溶剂趋于饱和状态,失去萃取能力 |
| 2 | TBAC/PEG/FeCl3 [ (摩尔比4∶1∶0.05) | m DES:m oil=1∶1、25℃、30min、硫初始浓度1600 mg/L | 对DBT模拟油,脱硫率为89.53% | 2次萃取后,脱硫率100% | 5次回收循环使用后,脱硫率基本不变 | 第5次使用后深共融溶剂的萃取率低于50% |
| 3 | [CH3(CH2)3]4PBr/FeCl3 [ | DES质量分数0.525、50℃、115min、硫初始浓度500μg/g | 对DBT和T的模拟油,脱硫率分别为65%和48%,对商业柴油脱硫率为32% | 6次萃取后,商业柴油的硫浓度降至40μg/g 以下 | 5次回收循环使用后,脱硫率基本不变 | — |
| 4 | [CH3(CH2)3]4PBr/ SnCl2 .2H2O[ (摩尔比1∶1) | DESs质量分数0.5、30℃、100min、硫初始浓度500 μg/g | 对DBT和T的模拟油,脱硫率分别为69.57%和47.28% | 5次萃取后,DBT和T的萃取率分别为97%和80% | — | 第2次重复利用时,对模拟油中DBT和T的脱硫率分别降至38%和12% |
| 5 | TBAB/IM[ (摩尔比1∶1) | DES质量分数0.5、30°C、95min、硫初始浓度500μg/g | 对DBT和T的模拟油,脱硫率分别为70%和47%,对商业柴油的脱硫率为47% | 4次萃取后,模拟油中DBT和T的浓度分别降至10μg/g和40μg/g以下,5次萃取后,商业柴油硫浓度降至15μg/g以下 | 5次回收循环使用后,脱硫率基本不变 | — |
| 6 | TBAB/PEG-600[ (摩尔比1∶2) | V DES∶V oil=1∶1、25℃、30min、硫初始浓度500μg/g | 对DBT和T的模拟油,脱硫率分别为82.40%和62.16% | 3次萃取后,对DBT和T萃取率分别为100%和95.15% | 5次回收循环使用后,脱硫率基本不变 | 5次重复利用后,对模拟油中DBT和T的脱硫率分别降至31.72%和9.09% |
| 7 | AlCl3-氯化石蜡-52-甲苯[ | DESs 6%、 293K、10 min、硫初始浓度500μg/g | 对3-MT、BT、DBT模拟油的脱硫率分别为99.81%、99.65%和89.64%,可使真实燃油的硫含量从682.05μg/g降至48.37μg/g | — | — | — |
| 8 | AlCl3/CH3CONH2 [ (摩尔比1.8∶1) | DESs 6%、50℃、 40min、 硫初始浓度1000μg/g | 模拟油中3-MT、BT、DBT 的转化率分别为97.31%、98.21%和 87.39%;在真实燃油中,硫含量可从580μg/g降至17.9μg/g | — | — | — |
| 9 | TBAB/HCOOH[ (摩尔比1∶1) | m DES∶m oi=1∶0.5、30℃、40 min、硫初始浓度500μg/g | 对BT、DBT、T模拟油的脱硫率分别为81.75%、80.47%、72%,对FCC汽油和柴油的脱硫率分别为40.94%和35.27% | 3次萃取后,对BT、DBT、T模拟油的脱 硫率分别为98.32%、98.24%、97.6%;4次萃取后, FCC汽油和柴油的脱硫率分别为83.61%和70.21% | — | 4次重复利用后,对BT、DBT、T模拟油的脱硫率分别为25.09%、24.14%、23.96% |
| 10 | [BPY]Br/Mal[ (摩尔比1∶1) | m DES∶m oil=1∶2、40℃、30 min、硫初始浓度500μg/g | 对FCC汽油中硫化物的脱硫率为94.14% | — | — | — |
| 11 | TEtA/Pr[ (摩尔比1∶3) | m DES=1.75g、V oil=5mL、30℃、10min、硫初始浓度DBT 500mg/L、BT、4,6-DMDBT、RSH 250mg/L | 对模拟油的脱硫率为51.6% | 4次萃取后,模拟油中的硫的浓度可从500mg/L降至10mg/L | 5次回收循环使用后,脱硫率基本不变 | 5次重复利用后,硫的分配系数K N从2.14降至1.96 |
| 12 | MIM/Pr[ (摩尔比1∶1) | m DES= 1.75g、V oil=5mL、30℃、10min、硫初始浓度DBT 500mg/L、BT、4,6-DMDBT、RSH 250mg/L | 硫的分配系数K N为2.31,脱硫率为53.6% | 5次萃取后,可得到无硫燃油 | 5次回收循环使用后,脱硫率基本不变 | — |
| 13 | [C12DMEA]Cl/FeCl3 [ (摩尔比1∶1) | m DES=1g、V oil=5mL,30oC、10min、硫初始浓度500mg/L | 对DBT的模拟油,脱硫率为52.9% | 5次萃取后,脱硫率可达99.3% | 2次回收循环使用后,脱硫率从52.9%降至52.5% | — |
| 14 | TBAC/EG[ (摩尔比1∶2) | m DES∶m oil =1∶1、30℃、20 min、硫初始浓度1000 μg/g | 对2-MT和BT两种硫化物混合的模拟油的脱硫率为64.3% | 5次萃取后,脱硫率达99.5% | 6次回收循环使用后,脱硫率基本不变 | — |
| 15 | TBAB/Sul[ (1∶7) | m DES:m oil=1∶1、25℃、6h、T在正庚烷中的含量为10% | 单次脱硫率为35.07% | 5次萃取后,脱硫率可达98% | 4次回收循环使用后,脱硫率基本不变 | — |
| 16 | TBPB∶DMF[ (1∶3) | m DES∶m oil =1∶1、30℃、30 min、硫初始浓度500mg/L | 对DBT模拟油的脱硫率为82.1% | 3次萃取后,硫浓度可降至10mg/L以下 | 5次回收循环使用后,脱硫率基本不变 | 5次重复利用后,脱硫率仅为5.4%,基本失去萃取能力 |
| 序号 | DESs | 氧化剂 | 萃取条件 | 脱硫效果 | 回收再循环利用 |
|---|---|---|---|---|---|
| 1 | ChCl/p-TsOH[ (摩尔比1∶2) | H2O2 | m DES∶m oil∶m | 对BT模拟油,脱硫率99.99%,对真实燃油的脱硫率为97.25% | 4次回收循环使用后,对BT模拟油脱硫仍可达98.87% |
| 2 | TBAC/OXA[ (摩尔比1∶2) | H2O2 | V DES=2mL、V oil=10mL、n(H2O2)∶n(DBT) = 5、 50℃、3h、硫初始浓度500mg/L | 对DBT模拟油,脱硫率为91% | 4次回收循环使用后,脱硫率基本不变 |
| 3 | L-Pro/p-TsOH[ (摩尔比1∶2) | H2O2 | V DES=2mL、V oil=10mL、n(H2O2)∶n(DBT) = 5、 60℃、2h、硫初始浓度500mg/L | 对DBT模拟油,脱硫率为99% | 6次回收循环使用后,脱硫率基本不变 |
| 4 | ChCl/CH3COOH[ (摩尔比1∶2) | O2 | V DES=3mL、V oil=15mL、V (aldehyde)=250μL、V (air) =5mL/min、30°C、3h、250W高压汞灯、硫初始浓度500mg/L | 对模拟油中BT、DBT、4,6-DMDBT的最高脱硫率分别为100%,98.6%和68.6% | —① |
| 5 | [HMIM]Cl/H2C2O4 [ | H2O2 | V DES=1.25mL、V oil=5mL、 n(H2O2)∶n(S)=12、40℃、140min、硫初始浓度500mg/L | 对DBT模拟油,脱硫率为92.2%。 | 7次回收循环使用后,脱硫率基本不变 |
| 6 | ChCl/H2C2O4 [ (摩尔比1∶1) | H2O2 | V DES = 1mL、V oil = 5mL、V H2O2=0.3mL、40℃、160min、硫质量浓度为500mg/L | 对DBT模拟油,脱硫率为93. 4% | 5 次回收循环使用后,脱硫率基本不变 |
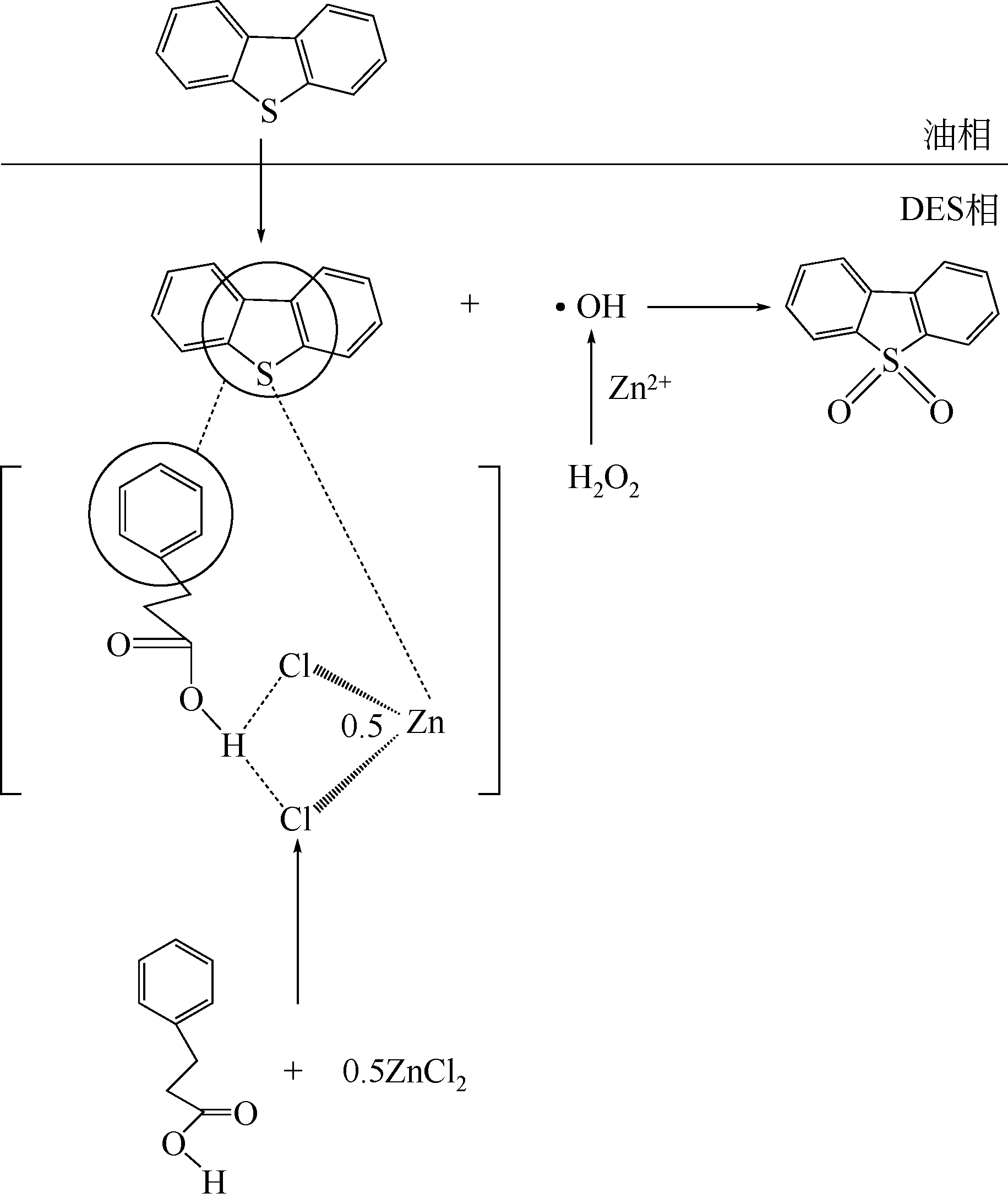
| 7 | CH3CONH2/ZnCl2 [ (摩尔比1∶0.4) | H2O2 | V oil =5mL、DES/oil=1∶3(V/V)、n(H2O2)∶n(S)=8、70℃、200min、硫质量分数为500μg/g | 对DBT模拟油,脱硫率为87.12% | 5次回收循环使用后,脱硫率基本不变 |
| 8 | TEAC/TFA[ (摩尔比1∶1) | H2O2 | V DES=1.25mL、V oil=5mL、n(H2O2)∶n(S)=6、40℃、140min、硫质量分数为500μg/g | 对DBT、BT和T的脱硫率分别为95.4%,56.2%和23.4% | 5次回收循环使用后,脱硫率基本不变 |
| 9 | [C4Pyr]Cl/ /p-TsOH[ (摩尔比1∶0.2) | H2O2 | V DES= 1.00mL、V oil= 5mL、n(H2O2)∶n(DBT)= 6、50℃、硫质量分数为500μg/g | 对DBT、4,6-DMDBT和BT的脱硫率分别为98.2%、96.0%、40.2% | 5次回收循环使用后,对DBT模拟油的脱硫率为95.1% |
| 10 | C6H5CH2CH2COOH/ZnCl2 [ (摩尔比1∶0.5) | H2O2 | V oil =5mL、DES∶oil=0.25∶1(V/V)、n(H2O2)/n(S)=6、50℃、80min、硫质量分数为500μg/g | 对DBT、BT、4,6-DMDBT的模拟油的脱硫率分别为99.23%,96.12%和98.4% | 5次回收循环使用后,脱硫率基本不变 |
| 11 | Pr/ZnCl2 [ (摩尔比1∶0.5) | H2O2 | V oil = 5mL、DES/oil=0.15∶1(V/V)、n(H2O2)/n(S)=4、30℃、180min、硫的初始浓度500mg/L | 对DBT、4,6-DMDBT的模拟油和汽油的脱硫率分别为99.42%,98.80%和66.67% | 5次回收循环使用后,对DBT模拟油脱硫率为96.31% |
| 12 | ChCl/2PEG[ (摩尔比1∶2) | H2O2 | 磷钨酸(HPW)为催化剂、n(HPW)=0.0052mmol、V oil=5mL、V DES=0.5 ml、n(H2O2)/n(S)=3、50℃、3h、硫的初始浓度500mg/L | 对DBT模拟油的脱硫率为99.1% | 4次回收循环使用后,脱硫率基本不变 |
| 13 | ChCl/2Ac[ (摩尔比1∶2) | H2O2 | [PSTEtA]3PW12O40为催化剂、V oil=5mL、m(catalyst)= 0.025g、n(H2O2)/n(S)=3、40℃、2.5h | 对DBT模拟油的脱硫率为可达100% | — |
| 14 | ChCl/Gl[ (摩尔比1∶2) | H2O2 | 光催化剂Cu-Fe/TiO2 2.0% 、V DES∶V Oxidizedoil=1∶5、n(H2O2)/n(S)=4、室温、20min、硫初始浓度为100mg/L | 对DBTO2的脱硫率为97.06%,2次萃取后,DBTO2的浓度降至0 | 3次回收循环使用后,对DBTO2的脱硫率为88.34% |
| 15 | CoCl2/ChCl/2PEG-200[ (摩尔比1∶1∶2) | KHSO5 | m oil =6g、m oil=2g、PMS溶液0.9g、20℃、60min、硫初始浓度为500mg/L | 对DBT模拟油的脱硫率接近100% | 7次回收循环使用后,脱硫率略有降低,降低5% |
| 16 | ChCl/p-TsOH[ (摩尔比1∶2) | H2O2 | m DES∶m oil =1∶1、m(H2O2)∶m(DBT)=10∶1、50℃、60min、硫初始浓度为6000μg/g | 对DBT模拟油的脱硫率为99.1%,对真实燃油的脱硫率可达67.7% | — |
| 序号 | DESs | 氧化剂 | 萃取条件 | 脱硫效果 | 回收再循环利用 |
|---|---|---|---|---|---|
| 1 | ChCl/p-TsOH[ (摩尔比1∶2) | H2O2 | m DES∶m oil∶m | 对BT模拟油,脱硫率99.99%,对真实燃油的脱硫率为97.25% | 4次回收循环使用后,对BT模拟油脱硫仍可达98.87% |
| 2 | TBAC/OXA[ (摩尔比1∶2) | H2O2 | V DES=2mL、V oil=10mL、n(H2O2)∶n(DBT) = 5、 50℃、3h、硫初始浓度500mg/L | 对DBT模拟油,脱硫率为91% | 4次回收循环使用后,脱硫率基本不变 |
| 3 | L-Pro/p-TsOH[ (摩尔比1∶2) | H2O2 | V DES=2mL、V oil=10mL、n(H2O2)∶n(DBT) = 5、 60℃、2h、硫初始浓度500mg/L | 对DBT模拟油,脱硫率为99% | 6次回收循环使用后,脱硫率基本不变 |
| 4 | ChCl/CH3COOH[ (摩尔比1∶2) | O2 | V DES=3mL、V oil=15mL、V (aldehyde)=250μL、V (air) =5mL/min、30°C、3h、250W高压汞灯、硫初始浓度500mg/L | 对模拟油中BT、DBT、4,6-DMDBT的最高脱硫率分别为100%,98.6%和68.6% | —① |
| 5 | [HMIM]Cl/H2C2O4 [ | H2O2 | V DES=1.25mL、V oil=5mL、 n(H2O2)∶n(S)=12、40℃、140min、硫初始浓度500mg/L | 对DBT模拟油,脱硫率为92.2%。 | 7次回收循环使用后,脱硫率基本不变 |
| 6 | ChCl/H2C2O4 [ (摩尔比1∶1) | H2O2 | V DES = 1mL、V oil = 5mL、V H2O2=0.3mL、40℃、160min、硫质量浓度为500mg/L | 对DBT模拟油,脱硫率为93. 4% | 5 次回收循环使用后,脱硫率基本不变 |
| 7 | CH3CONH2/ZnCl2 [ (摩尔比1∶0.4) | H2O2 | V oil =5mL、DES/oil=1∶3(V/V)、n(H2O2)∶n(S)=8、70℃、200min、硫质量分数为500μg/g | 对DBT模拟油,脱硫率为87.12% | 5次回收循环使用后,脱硫率基本不变 |
| 8 | TEAC/TFA[ (摩尔比1∶1) | H2O2 | V DES=1.25mL、V oil=5mL、n(H2O2)∶n(S)=6、40℃、140min、硫质量分数为500μg/g | 对DBT、BT和T的脱硫率分别为95.4%,56.2%和23.4% | 5次回收循环使用后,脱硫率基本不变 |
| 9 | [C4Pyr]Cl/ /p-TsOH[ (摩尔比1∶0.2) | H2O2 | V DES= 1.00mL、V oil= 5mL、n(H2O2)∶n(DBT)= 6、50℃、硫质量分数为500μg/g | 对DBT、4,6-DMDBT和BT的脱硫率分别为98.2%、96.0%、40.2% | 5次回收循环使用后,对DBT模拟油的脱硫率为95.1% |
| 10 | C6H5CH2CH2COOH/ZnCl2 [ (摩尔比1∶0.5) | H2O2 | V oil =5mL、DES∶oil=0.25∶1(V/V)、n(H2O2)/n(S)=6、50℃、80min、硫质量分数为500μg/g | 对DBT、BT、4,6-DMDBT的模拟油的脱硫率分别为99.23%,96.12%和98.4% | 5次回收循环使用后,脱硫率基本不变 |
| 11 | Pr/ZnCl2 [ (摩尔比1∶0.5) | H2O2 | V oil = 5mL、DES/oil=0.15∶1(V/V)、n(H2O2)/n(S)=4、30℃、180min、硫的初始浓度500mg/L | 对DBT、4,6-DMDBT的模拟油和汽油的脱硫率分别为99.42%,98.80%和66.67% | 5次回收循环使用后,对DBT模拟油脱硫率为96.31% |
| 12 | ChCl/2PEG[ (摩尔比1∶2) | H2O2 | 磷钨酸(HPW)为催化剂、n(HPW)=0.0052mmol、V oil=5mL、V DES=0.5 ml、n(H2O2)/n(S)=3、50℃、3h、硫的初始浓度500mg/L | 对DBT模拟油的脱硫率为99.1% | 4次回收循环使用后,脱硫率基本不变 |
| 13 | ChCl/2Ac[ (摩尔比1∶2) | H2O2 | [PSTEtA]3PW12O40为催化剂、V oil=5mL、m(catalyst)= 0.025g、n(H2O2)/n(S)=3、40℃、2.5h | 对DBT模拟油的脱硫率为可达100% | — |
| 14 | ChCl/Gl[ (摩尔比1∶2) | H2O2 | 光催化剂Cu-Fe/TiO2 2.0% 、V DES∶V Oxidizedoil=1∶5、n(H2O2)/n(S)=4、室温、20min、硫初始浓度为100mg/L | 对DBTO2的脱硫率为97.06%,2次萃取后,DBTO2的浓度降至0 | 3次回收循环使用后,对DBTO2的脱硫率为88.34% |
| 15 | CoCl2/ChCl/2PEG-200[ (摩尔比1∶1∶2) | KHSO5 | m oil =6g、m oil=2g、PMS溶液0.9g、20℃、60min、硫初始浓度为500mg/L | 对DBT模拟油的脱硫率接近100% | 7次回收循环使用后,脱硫率略有降低,降低5% |
| 16 | ChCl/p-TsOH[ (摩尔比1∶2) | H2O2 | m DES∶m oil =1∶1、m(H2O2)∶m(DBT)=10∶1、50℃、60min、硫初始浓度为6000μg/g | 对DBT模拟油的脱硫率为99.1%,对真实燃油的脱硫率可达67.7% | — |
| 1 | 孙志娟, 余谟鑫, 张心亚, 等 . 油品中噻吩类硫化物脱除技术研究进展[J]. 化工进展, 2005, 24(9): 1002-1005. |
| SUN Z J , YU M X , ZHANG X Y , et al . Technology of desulfurization of thiophene type sulfide in oil[J]. Chemical Industry and Engineering Progress, 2005, 24(9): 1002-1005. | |
| 2 | 宋红艳, 何静, 李春喜 . 燃料油深度脱硫的技术策略及研究进展[J]. 石油化工, 2015, 44(3): 279-286. |
| SONG H Y , HE J , LI C X . Technical strategies and recent advances for deep desulfurization of duel oils[J]. Petrochemical Technology, 2015, 44(3): 279-286. | |
| 3 | 王宏伟, 贺振富, 田辉平 . FCC汽油非临氢脱硫技术进展[J]. 化工进展, 2005, 24(11): 1216-1224. |
| WANG H W , HE Z F , TIAN H P . Progress in desulfurization of FCC gasoline without hydrogenation[J]. Chemical Industry and Engineering Progress, 2005, 24(11): 1216-1224. | |
| 4 | 郑凯元, 曲凤娇, 陈英杰. 等 . 非加氢脱硫技术研究进展及其在原油预脱硫中的应用展望[J]. 化工进展, 2013, 32(12): 2859-2866. |
| ZHENG K Y , QU F J , CHEN Y J , et al . Progress of non-hydrodesulfurization technologies of producing clean fuels and pre-desulfurization of crude oil[J]. Chemical Industry and Engineering Progress, 2013, 32(12): 2859-2866. | |
| 5 | 项小燕, 林金清 . 离子液体萃取燃料油脱硫技术的研究进展[J]. 化工进展, 2007, 26(12): 1681-1685. |
| XIANG X Y , LIN J Q . Progress of extractive desulfurization technology with ionic liquids[J]. Chemical Industry and Engineering Progress, 2007, 26(12): 1681-1685. | |
| 6 | PAIVA A , CRAVEIRO R , AROSO I , et al . Natural deep eutectic solvents-solvents for the 21st century[J]. ACS Sustainable Chemistry Engineering, 2014, 2:1063-1071. |
| 7 | HOU Y C , YAO C F , WU W Z . Deep eutectic solvents:green solvents for separation applications[J]. Acta Physico-Chimica Sinica, 2018, 34(8): 873-885. |
| 8 | LI C P , LI D , ZOU S S , et al . Extraction desulfurization process of fuels with ammonium-based deep eutectic solvents[J]. Green Chemistry, 2013, 15:2793-2799. |
| 9 | LI C P , ZHANG J J , LI Z , et al . Extraction desulfurization of fuels with ‘metal ions’ based deep eutectic solvents (MDESs)[J]. Green Chemistry, 2016, 18:3789-3795. |
| 10 | GANO Z S , MJALLI F S , AL-WAHAIBI T , et al . Extractive desulfurization of liquid fuel with FeCl3-based deep eutectic solvents:Experimental design and optimization by central-composite design[J]. Chemical Engineering and Processing: Process Intensification, 2015, 93: 10-20. |
| 11 | GANO Z S , MJALLI F S , AL-WAHAIBI T , et al . The novel application of hydrated metal halide (SnCl2·2H2O) -based deep eutectic solvent for the extractive desulfurization of liquid fuels[J]. International Journal of Chemical Engineering and Applications, 2015, 6(5): 367-371. |
| 12 | GANO Z S , MJALLI F S , AL-WAHAIBI T , et al . Desulfurization of liquid fuel via extraction with imidazole-containing deep eutectic solvent[J]. Green Processing and Synthesis, 2017, 6(5): 511-521. |
| 13 | RAHMA W S A , MJALLI F S , AL-WAHAIBI T , et al . Polymeric-based deep eutectic solvents for effective extractive desulfurization of liquid fuel at ambient conditions[J]. Chemical Engineering Research and Design, 2017, 120:271-283. |
| 14 | TANG X D , ZHANG Y F , LI J J , et al . Deep extractive desulfurization with arenium ion deep eutectic solvents[J]. Industrial & Engineering Chemistry Research, 2015, 54:4625-4632. |
| 15 | TANG X D , ZHANG Y F , LI J J , et al . Alkylation of thiophenic compounds catalyzed by deep eutectic solvents[J]. Catalysis Communications, 2015, 70:40-43. |
| 16 | LI J J , XIAO H , TANG X D , et al . Green carboxylic acid-based deep eutectic solvents as solvents for extractive desulfurization[J]. Energy Fuels, 2016, 30(7): 5411-5418. |
| 17 | LI J J , ZHOU M , TANG X D , et al . Deep desulfurization of FCC gasoline by extraction with dicarboxylic acid-based deep eutectic solvents[J]. Petroleum Science and Technology, 2017, 35(19): 1903-1909. |
| 18 | WANG X , JIANG W , ZHU W S , et al . A simple and cost-effective extractive desulfurization process with novel deep eutectic solvents[J]. RSC Advances, 2016, 6:30345-30352. |
| 19 | JIANG W , LI H P , WANG C , et al . Synthesis of ionic-liquid-based deep eutectic solvents for extractive desulfurization of fuel[J]. Energy Fuels, 2016, 30:8164-8170. |
| 20 | JIANG W , DONG L , LIU W , et al . Biodegradable choline-like deep eutectic solvents for extractive desulfurization of fuel[J]. Chemical Engineering and Processing: Process Intensification, 2017, 115:34-38. |
| 21 | SHU C H , SUNT H . Extractive desulfurisation of gasoline with tetrabutyl ammonium chloride-based deep eutectic solvents[J]. Separation Science and Technology, 2016, 51:1336-1343. |
| 22 | HADJ-KALI M K , MULYONO S , HIZADDIN H F , et al . Removal of thiophene from mixtures with n-heptane by selective extraction using deep eutectic solvents[J]. Industrial & Engineering Chemistry Research, 2016, 55(30): 8415-8523. |
| 23 | CHENG H Y , LIU C Y , ZHANG J J , et al . Screening deep eutectic solvents for extractive desulfurization of fuel based on COSMO-RS model[J]. Chemical Engineering & Processing:Process Intensification, 2018, 125: 246-252. |
| 24 | WARRAG S E E , RODRIGUEZ N R , NASHEF I M , et al . Separation of thiophene from aliphatic hydrocarbons using tetrahexylammonium-based deep eutectic solvents as extracting agents[J]. Journal of Chemical & Engineering Data, 2017, 62(9): 2911-2919. |
| 25 | WARRAG S E E , ADEYEMI I , RODRIGUEZ N R , et al . Effect of the type of ammonium salt on the extractive desulfurization of fuels using deep eutectic solvents[J]. Journal of Chemical & Engineering Data, 2018, 63(4): 1088-1095. |
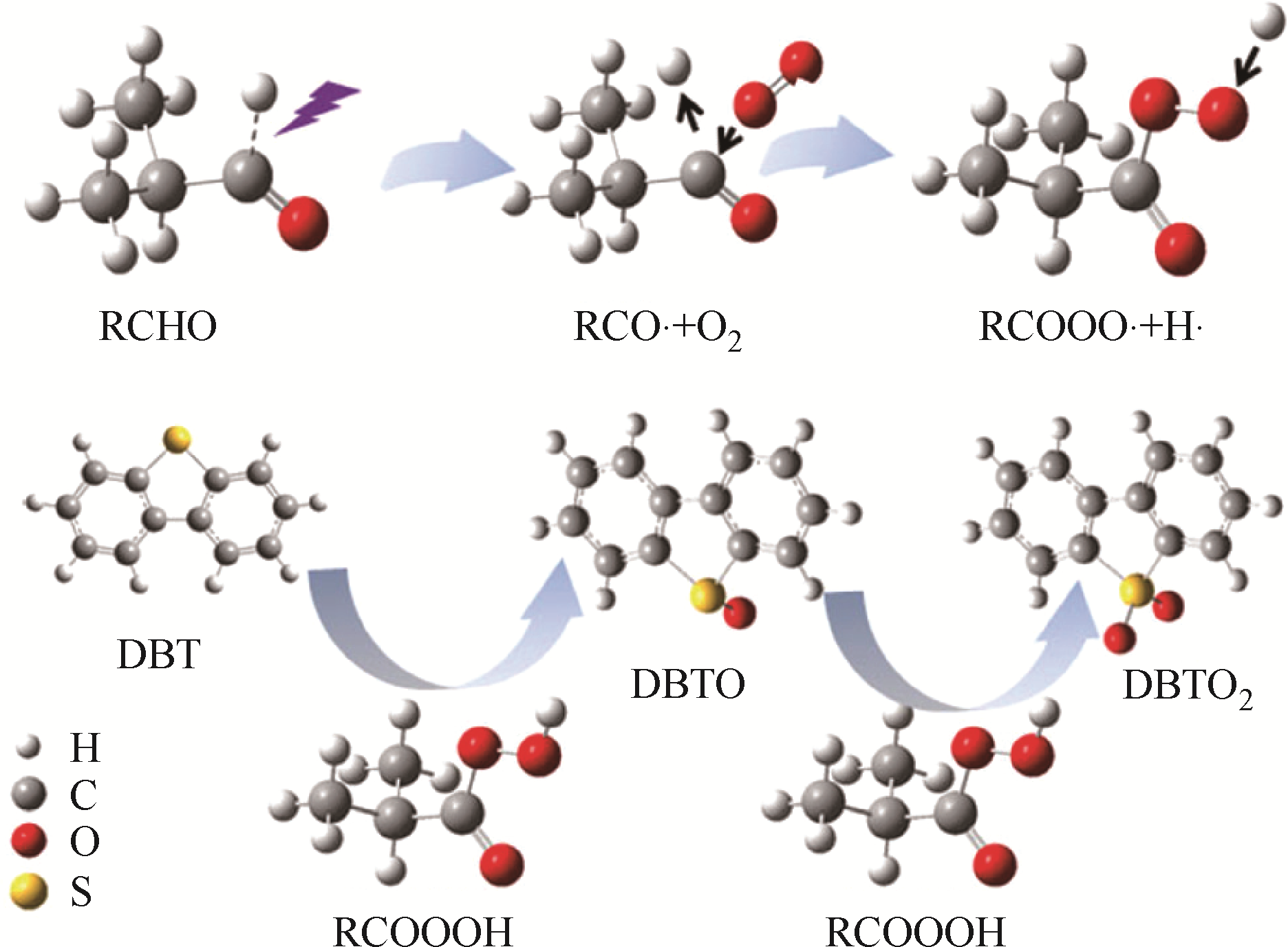
| 26 | YIN J M , WANG J P , LI Z , et al . Deep desulfurization of fuels based on oxidation/extraction process with acidic deep eutectic solvents[J]. Green Chemistry, 2015, 17:4552-4559. |
| 27 | LÜ H Y , LI C P , DENG C L , et al . Deep catalytic oxidative desulfurization (ODS) of dibenzothiophene (DBT) with oxalate-based deep eutectic solvents (DESs)[J]. Chemical Communications, 2015, 51:10703-10706. |
| 28 | HAO L W , WANG M R , SHAN W J , et al . L-proline-based deep eutectic solvents (DESs) for deep catalytic oxidative desulfurization (ODS) of diesel[J]. Journal of Hazardous Materials, 2017, 339:216-222. |
| 29 | ZHU W S , WANG C , LI H P , et al . One-pot extraction combined with metal-free photochemical aerobic oxidative desulfurization in deep eutectic solvent[J]. Green Chemistry, 2015, 17:2464-2472. |
| 30 | 侯良培, 赵荣祥, 李秀萍, 等 . 甲基咪唑盐酸盐/草酸型低共熔溶剂的制备及其在模拟油中氧化脱硫中的应[J]. 化工学报, 2016, 67(9): 3972-3980. |
| HOU L P , ZHAO R X , LI X P , et al . Preparation of methylimidazole hydrochloride/oxalic acid type deep eutectic solvent and its application in oxidative desulfurization of model oil[J]. CIESC Journal, 2016, 67(9): 3972-3980. | |
| 31 | 毛春峰, 赵荣祥, 李秀萍 . 氯化胆碱/草酸低共熔溶剂高效脱除模拟油中硫[J]. 化学工程, 2017, 45(5): 6-10. |
| MAO C F , ZHAO R X , LI X P . High removal rate of sulfur in model oil by ChCl /H2C2O4 deep eutectic solvents[J]. Chemical Engineering (China), 2017, 45(5): 6-10. | |
| 32 | 毛春峰, 赵荣祥, 李秀萍, 等 . CH3CONH2/ZnCl2低共熔溶剂脱除模拟油中的硫化物[J]. 辽宁石油化工大学学报, 2017, 37(5):1-7. |
| MAO C F , ZHAO R X , LI X P , et al . Removal of sulfides from simulated oil by CH3CONH2/ZnCl2 deep eutectic solvent[J]. Journal of | |
| Liaoning Shihua University, 2017, 37(5): 1-7. | |
| 33 | 侯良培, 赵荣祥, 李秀萍, 酸性低共熔溶剂四乙基氯化铵/三氟乙酸高效催化氧化脱除模拟油中的硫化物 [J]. 化工学报, 2017, 68(4): 1614-1620. |
| HOU L P, ZHAO R X, LI X P, Highly efficient catalytic oxidation desulfurization from model oil based on acid deep eutectic solvents tetraethylammonium chloride/trifluoroacetic acid[J]. CIESC Journal, 2017, 68(4): 1614-1620. | |
| 34 | 侯良培, 赵荣祥, 李秀萍 . [C4Pyr]Cl/nTsOH 型低共熔溶剂的制备及其氧化脱除模拟油中的二苯并噻吩[J]. 石油化工, 2017, 46(7): 888-895. |
| HOU L P , ZHAO R X , LI X P . Preparation of C4Pyr]Cl/nTsOH deep eutectic solvents and its application in oxidative removal of dibenzothiophene from model oil[J]. Petrochemical Technology,2017, 46(7): 888-895. | |
| 35 | MAO C F , ZHAO R X , LI X P . Phenylpropanoic acid-based DESs as efficient extractants and catalysts for the removal of sulfur compounds from oil[J]. Fuel, 2017, 189:400-407 |
| 36 | MAO C F , ZHAO R X , LI X P . Propionic acid-based deep eutectic solvents:synthesis and ultra-deep oxidative desulfurization activity[J]. RSC Advances, 2017, 7:42590-42596. |
| 37 | LIU W , JIANG W , ZHU W Q , et al . Oxidative desulfurization of fuels promoted by choline chloride-baseddeep eutectic solvents[J]. Journal of Molecular Catalysis A: Chemical, 2016, 424:261-268. |
| 38 | JIANG W , DONG L , LIU W , et al . Designing multifunctional SO3H-based polyoxometalate catalysts for oxidative desulfurization in acid deep eutectic solvent[J]. RSC Advances, 2017, 7:55318-55325. |
| 39 | ZAID H F M , KAIT C F , MUTALI M I A . Extractive deep desulfurization of diesel using choline chloride-glycerol eutectic-based ionic liquid as a green solvent[J]. Fuel, 2017, 192:10-17. |
| 40 | XU H , ZGANG D D , WU F M , et al . Deep desulfurization of fuels with cobalt chloride-choline chloride/polyethylene glycol metal deep eutectic solvents[J]. Fuel, 2018, 225:104-110. |
| 41 | LI Z H , LIU D , MEN Z W, et al . Insight into effective denitrification and desulfurization of liquid fuel with deep eutectic solvents:an innovative evaluation criterion to filtrate extractants using the compatibility index[J]. Green Chemistry, 2018, 20: 3112-3120. |
| 42 | TANG L , LUO G Q , KANG L H , et al . A novel [Bmim]PW/HMS catalyst with high catalytic performance for the oxidative desulfurization process[J]. Korean Journal of Chemical Engineering, 2013, 30(2): 314-320. |
| 43 | OTSUKI S , NONAKA T , TAKASHIMA N , et al . Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction[J]. Energy Fuels, 2000, 14(6): 1232-1239. |
| [1] | MA Yi, CAO Shiwei, WANG Jiajun, LIN Liqun, XING Yan, CAO Tengliang, LU Feng, ZHAO Zhenlun, ZHANG Zhijun. Research progress in recovery of spent cathode materials for lithium-ion batteries using deep eutectic solvents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 219-232. |
| [2] | CHEN Kuangyin, LI Ruilan, TONG Yang, SHEN Jianhua. Structure design of gas diffusion layer in proton exchange membrane fuel cell [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 246-259. |
| [3] | XU Jiaheng, LI Yongsheng, LUO Chunhuan, SU Qingquan. Optimization of methanol steam reforming process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 41-46. |
| [4] | LAI Shini, JIANG Lixia, LI Jun, HUANG Hongyu, KOBAYASHI Noriyuki. Research progress of ammonia blended fossil fuel [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4603-4615. |
| [5] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [6] | MA Zhejie, ZHANG Wenli, ZHAO Xuankai, LI Ping. Progress on the influence of oxygen mass transfer resistance in PEMFC cathode catalyst layer [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2860-2873. |
| [7] | LI Dongxian, WANG Jia, JIANG Jianchun. Producing biofuels from soapstock via pyrolysis and subsequent catalytic vapor-phase hydrotreating process [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2874-2883. |
| [8] | JIANG Bolong, CUI Yanyan, SHI Shunjie, CHANG Jiacheng, JIANG Nan, TAN Weiqiang. Synthesis of transition metal Co3O4/ZnO-ZIF oxygen reduction catalyst by Co/Zn-ZIF template method and its electricity generation performance [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3066-3076. |
| [9] | FAN Siqiang, PENG Shaozhong, PENG Chong, HU Yongkang. Research progress in high value-added utilization technology of waste plastics [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 1020-1027. |
| [10] | CHEN Hao, ZHANG Chuanhao, YU Feng, FAN Binbin, LI Ruifeng. Catalytic performance of zeolite Y in oligomerization of isobutyl alcohol [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 794-802. |
| [11] | YU Haiqiang, GUO Quanzhong, DU Keqin, WANG Chuan. Application of pulse electrodeposition PbO2 coating on stainless steel bipolar plate of PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 917-924. |
| [12] | XUE Machen, YANG Bolun, XIA Chungu, ZHU Gangli. Progress in heterogeneous catalyst for ethanol upgrading to higher (C6+) alcohols [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 194-203. |
| [13] | SHEN Tianxu, SHEN Laihong. Investigation of multi-fuel chemical looping combustion in a 3kW interconnected fluidized bed reactors [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 138-147. |
| [14] | FANG Kejing, XIONG Zuhong, LU Min, LI Tao, CHEN Yong. Research progress in preparation, thermal conversion characteristics and application of refuse derived fuel [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 132-140. |
| [15] | KOU Jiawei, CHENG Shuyan, CHENG Fangqin. Research advance of hydrotalcite-based catalysts in photocatalytic reduction of carbon dioxide [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 190-198. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||