化工进展 ›› 2021, Vol. 40 ›› Issue (7): 4036-4046.DOI: 10.16085/j.issn.1000-6613.2020-1551
改性聚吡咯材料去除水中氟离子的性能
冯江涛1( ), 王睎1, 赵旭阳1, 龚向红2, 延卫1(
), 王睎1, 赵旭阳1, 龚向红2, 延卫1( )
)
- 1.西安交通大学能源与动力工程学院环境工程系,陕西 西安 710049
2.义乌市水处理有限责任公司,浙江 义乌 322000
-
收稿日期:2020-08-05修回日期:2020-09-24出版日期:2021-07-06发布日期:2021-07-19 -
通讯作者:延卫 -
作者简介:冯江涛(1981—),男,博士,副教授,研究方向为新型吸附材料。E-mail:fjtes@xjtu.edu.cn 。 -
基金资助:国家自然科学基金面上项目(51978569)
Removal of fluoride from water by modified polypyrrole
FENG Jiangtao1( ), WANG Xi1, ZHAO Xuyang1, GONG Xianghong2, YAN Wei1(
), WANG Xi1, ZHAO Xuyang1, GONG Xianghong2, YAN Wei1( )
)
- 1.Department of Environmental Engineering, School of Energy and Power Engineering, Xi'an Jiaotong University, Xi'an 710049, Shaanxi, China
2.Yiwu Water Treatment Co. , Ltd. , Yiwu 322000, Zhejiang, China
-
Received:2020-08-05Revised:2020-09-24Online:2021-07-06Published:2021-07-19 -
Contact:YAN Wei
摘要:
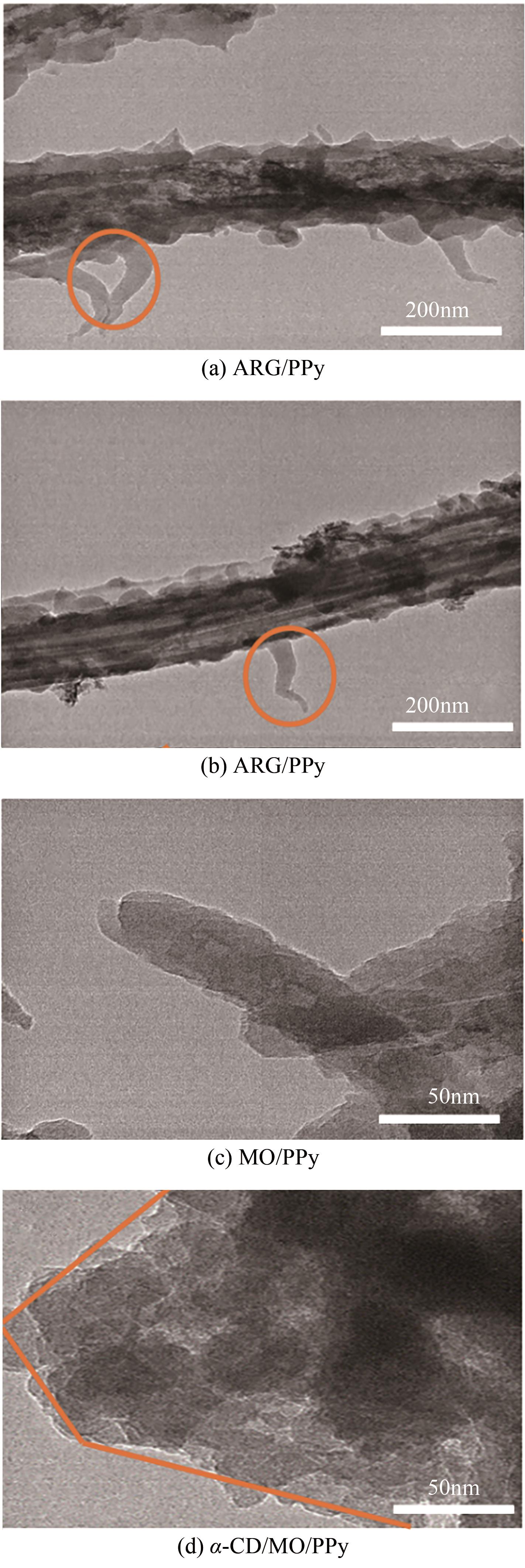
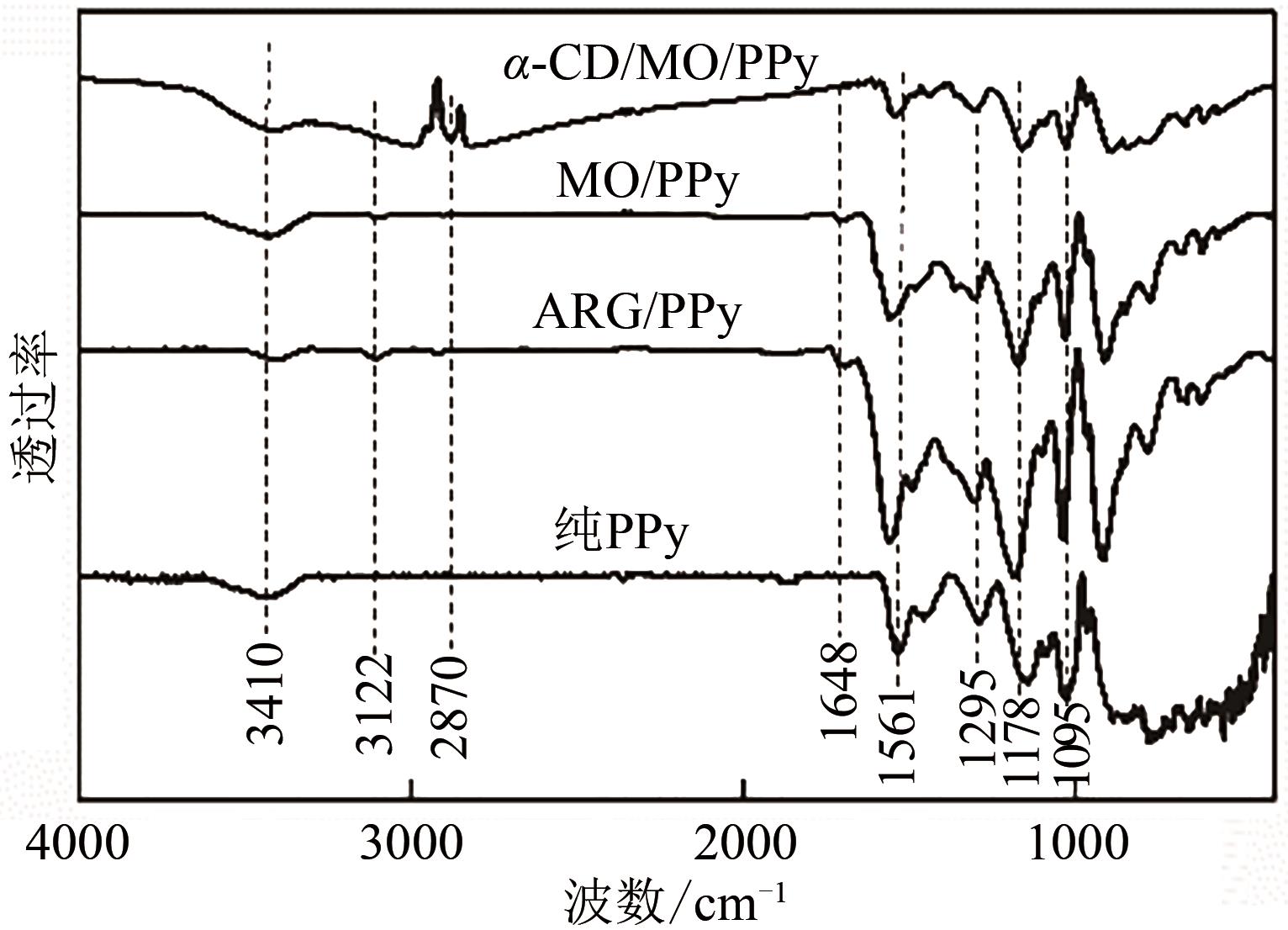
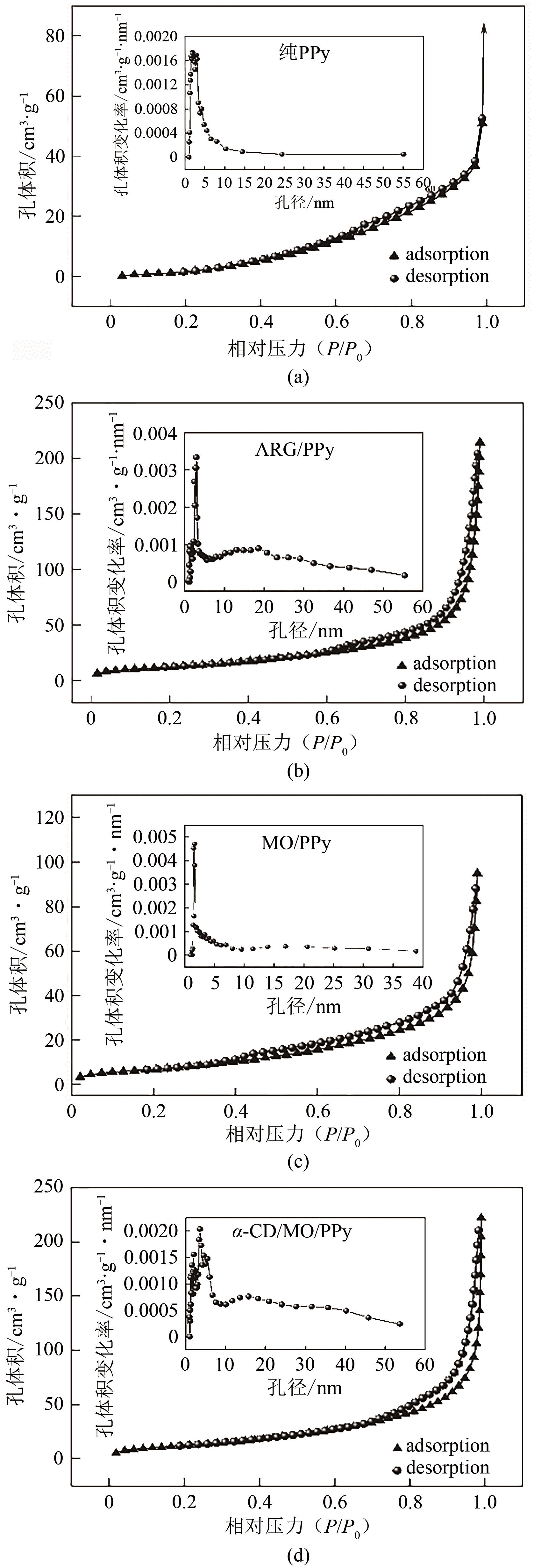
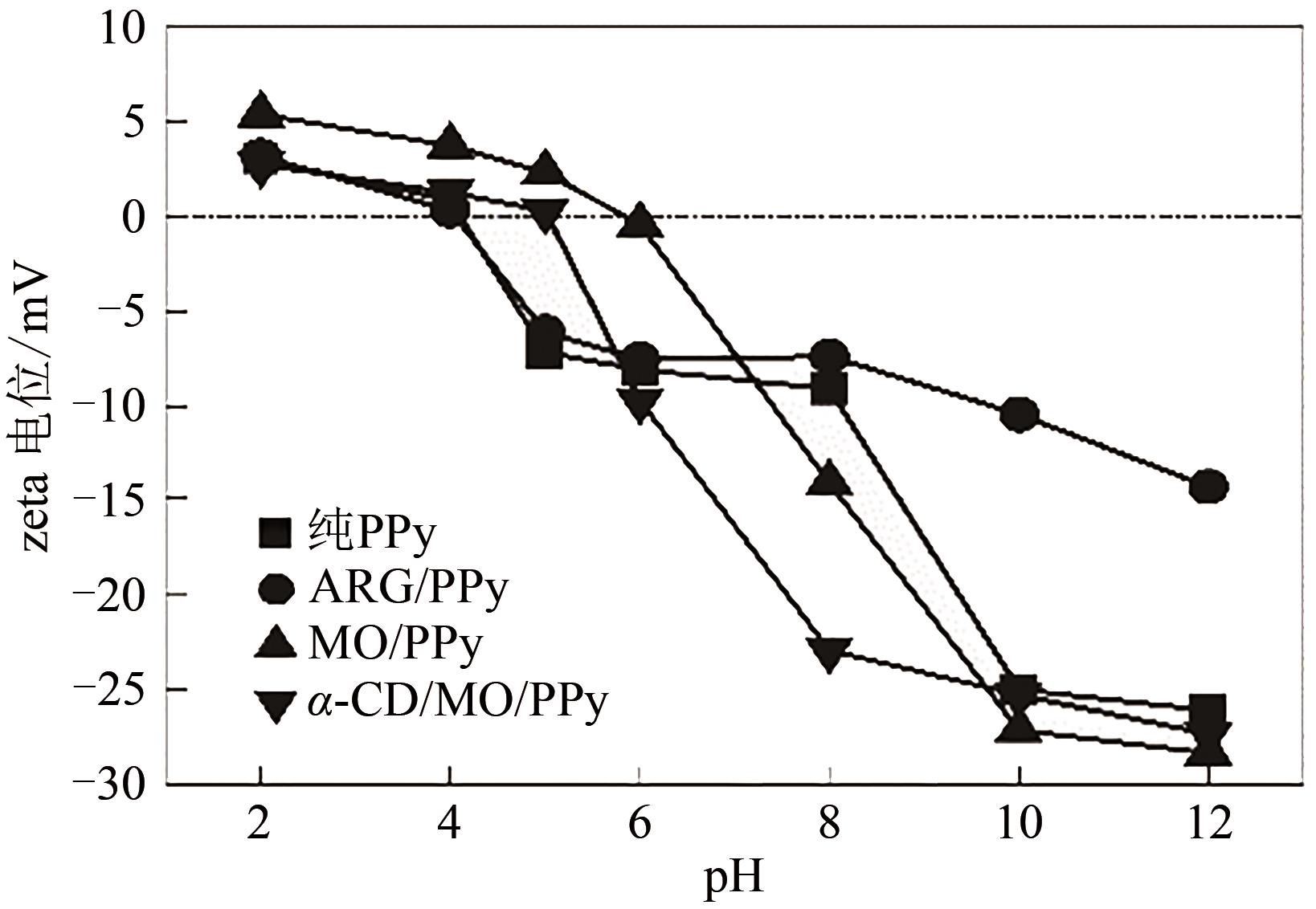
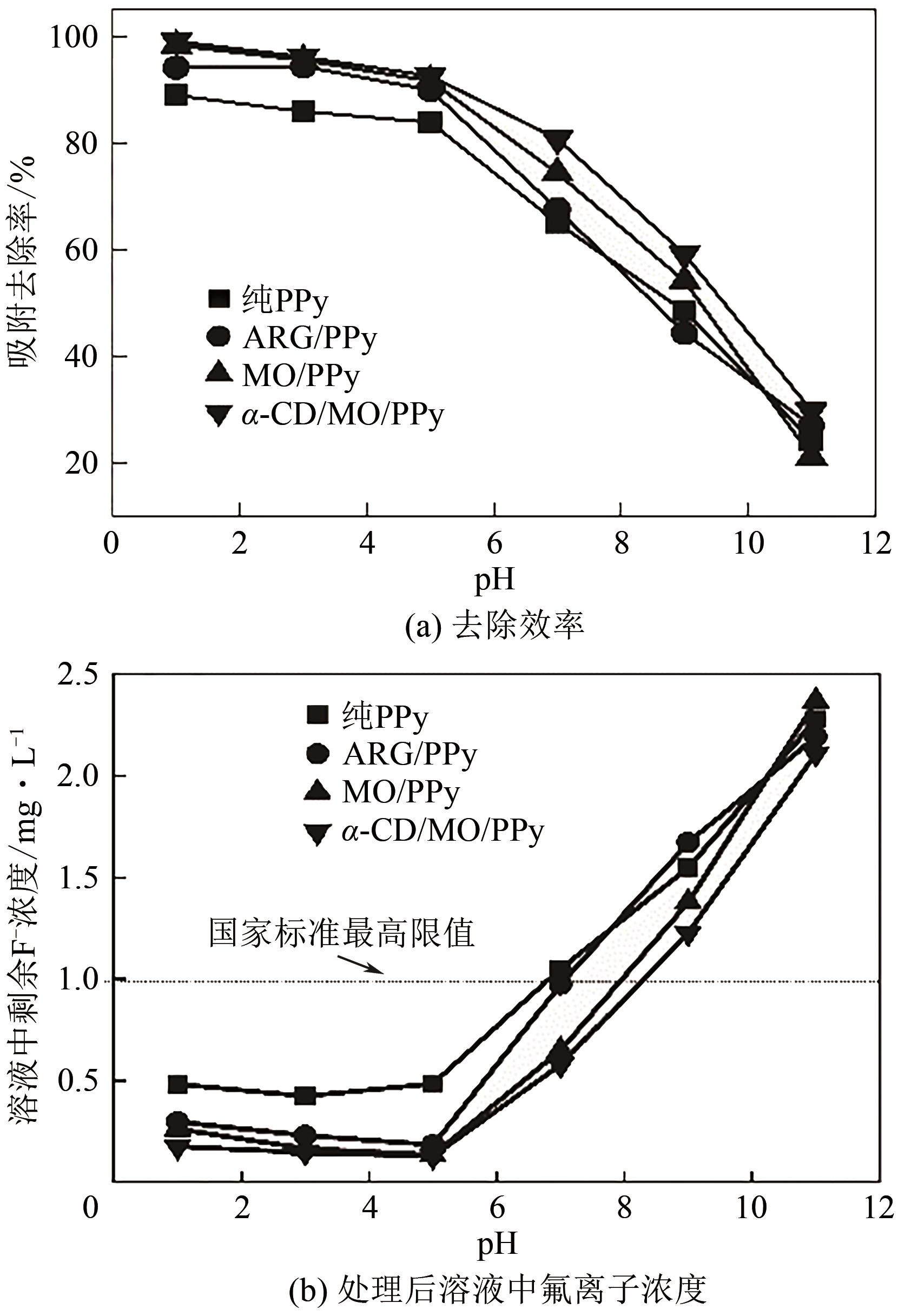
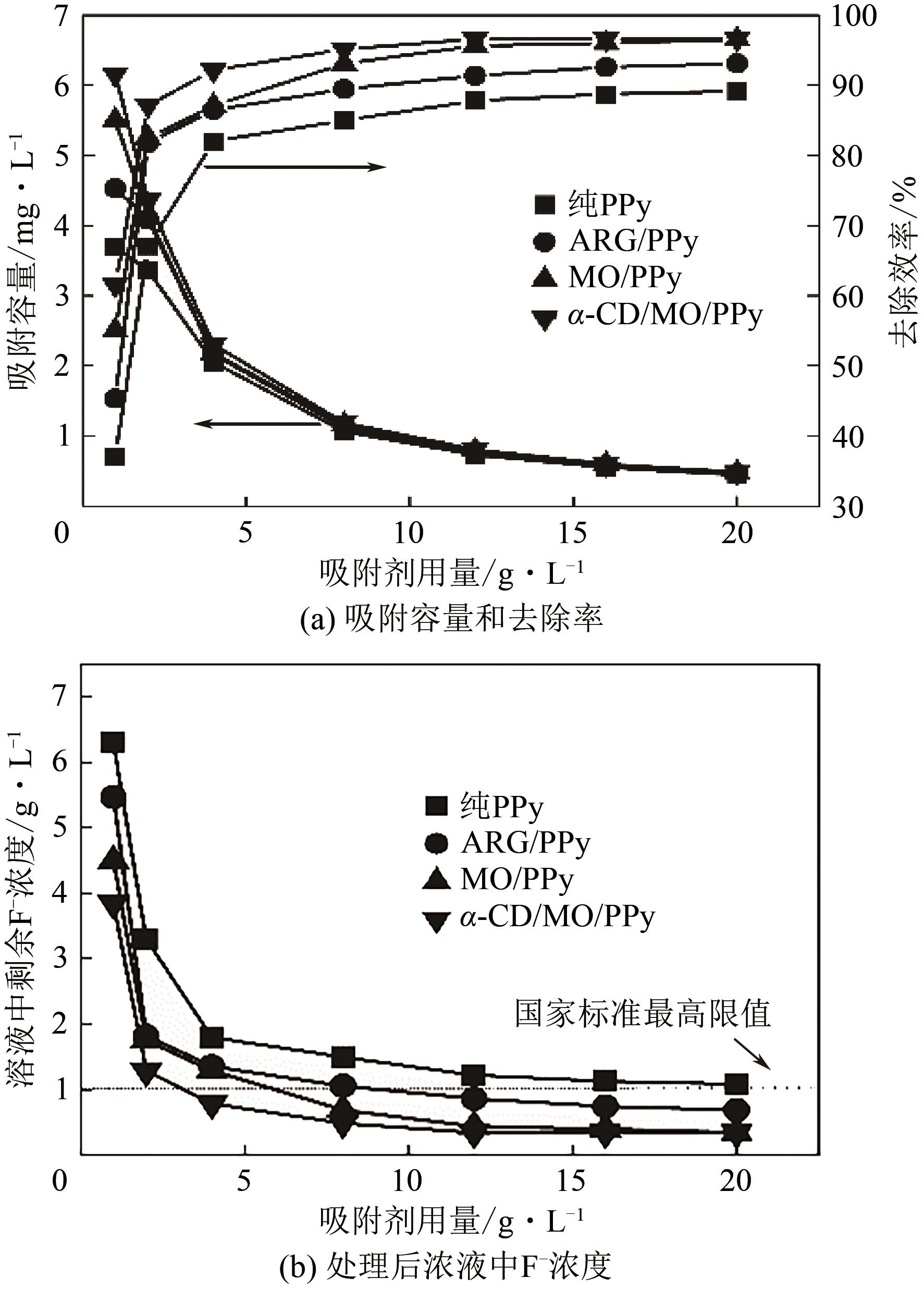
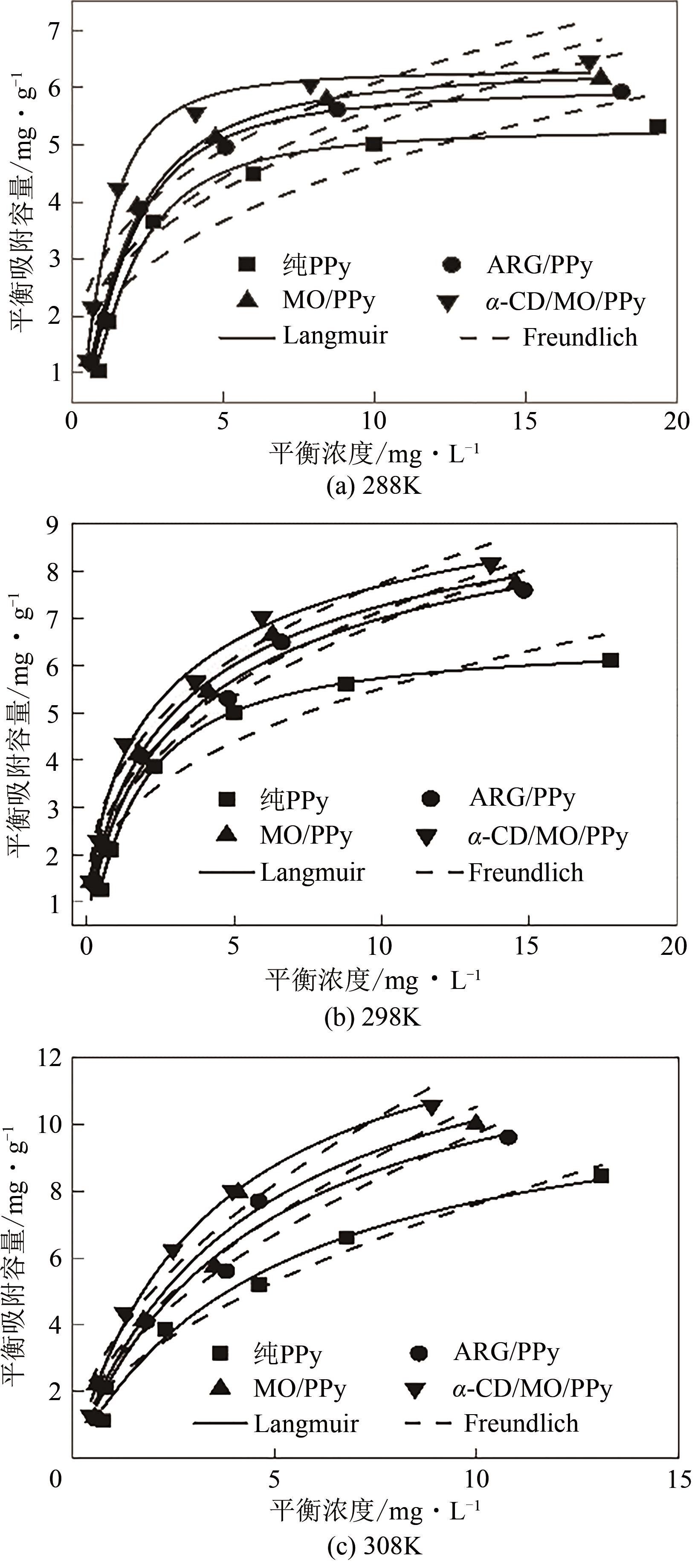
通过控制合成条件和在聚合过程中分别引入酸性红G(ARG)、甲基橙(MO)、α-环糊精(α-CD)和甲基橙等掺杂剂合成了表面带有毛刺的纤维、不同直径纳米纤维编织成的微米片状特殊微形貌的改性聚吡咯(PPy)材料。通过场发射扫描电子显微镜(SEM)、透射电子显微镜(TEM)、傅里叶变换红外光谱分析(FTIR)、氮气吸脱附分析(BET)、zeta电位分析等方法对其物理化学性能进行了表征,并将其应用于水体低浓度氟离子的吸附去除,比较了改性聚吡咯材料与传统方法合成的颗粒状聚吡咯对氟离子吸附的性能。结果表明:改性的特殊微形貌PPy的比表面积比传统颗粒状PPy提高了7~10倍;在常温和pH=5的条件下,F-初始浓度在3~5mg/L,投加量为2g/L时,改性的特殊微形貌PPy可在10min之内达到吸附平衡,并将F-的浓度降低到国家《生活引用水卫生标准》(GB 5749—2006)规定的1mg/L以下,说明特殊微形貌的控制对PPy吸附F-具有很大影响。另外,该吸附行为是自发的放热过程,Langmiur模型可以很好地拟合该吸附过程,PPy对F-的去除主要涉及离子交换作用。
中图分类号:
引用本文
冯江涛, 王睎, 赵旭阳, 龚向红, 延卫. 改性聚吡咯材料去除水中氟离子的性能[J]. 化工进展, 2021, 40(7): 4036-4046.
FENG Jiangtao, WANG Xi, ZHAO Xuyang, GONG Xianghong, YAN Wei. Removal of fluoride from water by modified polypyrrole[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 4036-4046.
| 样品名称 | 碳原子 /% | 氮原子 /% | 氧原子 /% | 氯原子 /% |
|---|---|---|---|---|
| 纯PPy | 50.55 | 46.44 | — | 3.01 |
| ARG/PPy | 57.50 | 28.40 | 10.79 | 3.31 |
| MO/PPy | 50.44 | 31.23 | 14.68 | 3.65 |
| α-CD/MO/PPy | 55.33 | 30.56 | 10.16 | 3.95 |
表1 不同形貌PPy材料的EDS分析(质量分数)
| 样品名称 | 碳原子 /% | 氮原子 /% | 氧原子 /% | 氯原子 /% |
|---|---|---|---|---|
| 纯PPy | 50.55 | 46.44 | — | 3.01 |
| ARG/PPy | 57.50 | 28.40 | 10.79 | 3.31 |
| MO/PPy | 50.44 | 31.23 | 14.68 | 3.65 |
| α-CD/MO/PPy | 55.33 | 30.56 | 10.16 | 3.95 |
| 样品名称 | SBET/m2·g-1 | 总孔容V/cm3·g-1 | 平均孔径R/nm |
|---|---|---|---|
| 纯PPy | 6.35 | 0.140 | 9.49 |
| ARG/PPy | 45.36 | 0.333 | 11.87 |
| MO/PPy | 62.10 | 0.481 | 10.75 |
| α-CD/MO/PPy | 65.10 | 0.403 | 12.75 |
表2 合成的PPy材料的比表面积、孔容和孔径值
| 样品名称 | SBET/m2·g-1 | 总孔容V/cm3·g-1 | 平均孔径R/nm |
|---|---|---|---|
| 纯PPy | 6.35 | 0.140 | 9.49 |
| ARG/PPy | 45.36 | 0.333 | 11.87 |
| MO/PPy | 62.10 | 0.481 | 10.75 |
| α-CD/MO/PPy | 65.10 | 0.403 | 12.75 |
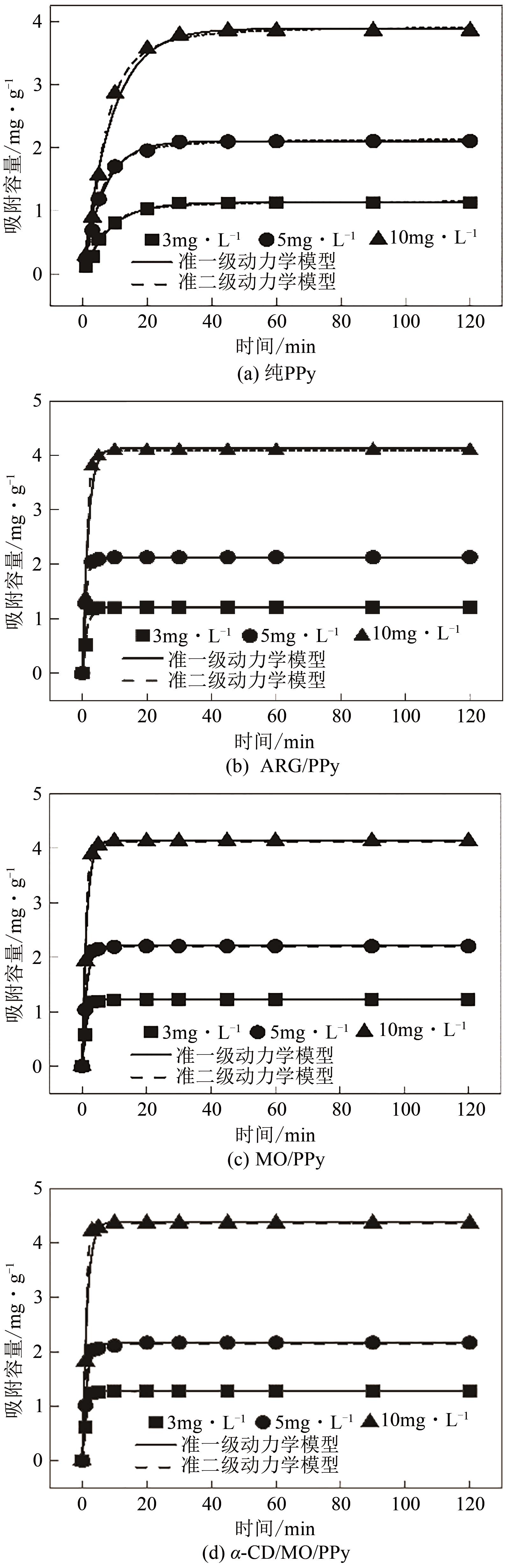
| 样品 | C0 | qe,e | 准一级动力学 模型参数 | 准二级动力学 模型参数 | |||||
|---|---|---|---|---|---|---|---|---|---|
| k1 | qe,c | R2 | qe,c | k2 | R2 | ||||
| 纯PPy | 3 | 1.125 | 0.121 | 1.126 | 0.9964 | 1.126 | 0.063 | 0.9993 | |
| 5 | 2.107 | 0.153 | 2.109 | 0.9946 | 2.109 | 0.056 | 0.9971 | ||
| 10 | 3.852 | 0.113 | 3.865 | 0.9909 | 3.865 | 0.037 | 0.9978 | ||
| ARG/PPy | 3 | 1.205 | 0.769 | 1.205 | 0.9846 | 1.205 | 0.702 | 0.9999 | |
| 5 | 2.128 | 0.526 | 2.129 | 0.9994 | 2.129 | 0.646 | 0.9998 | ||
| 10 | 4.089 | 0.514 | 4.092 | 0.9792 | 4.092 | 0.578 | 0.9996 | ||
| MO/PPy | 3 | 1.227 | 0.727 | 1.223 | 0.9918 | 1.223 | 0.891 | 0.9980 | |
| 5 | 2.205 | 0.726 | 2.206 | 0.9927 | 2.206 | 0.797 | 0.9991 | ||
| 10 | 4.124 | 0.710 | 4.854 | 0.9941 | 4.854 | 0.675 | 0.9999 | ||
| α-CD/MO/PPy | 3 | 1.275 | 0.753 | 1.275 | 0.9922 | 1.275 | 0.941 | 0.9999 | |
| 5 | 2.268 | 0.704 | 2.258 | 0.9940 | 2.258 | 0.890 | 0.9984 | ||
| 10 | 4.357 | 0.675 | 4.354 | 0.9948 | 4.354 | 0.722 | 0.9993 | ||
表3 4种PPy材料吸附F-的动力学拟合参数
| 样品 | C0 | qe,e | 准一级动力学 模型参数 | 准二级动力学 模型参数 | |||||
|---|---|---|---|---|---|---|---|---|---|
| k1 | qe,c | R2 | qe,c | k2 | R2 | ||||
| 纯PPy | 3 | 1.125 | 0.121 | 1.126 | 0.9964 | 1.126 | 0.063 | 0.9993 | |
| 5 | 2.107 | 0.153 | 2.109 | 0.9946 | 2.109 | 0.056 | 0.9971 | ||
| 10 | 3.852 | 0.113 | 3.865 | 0.9909 | 3.865 | 0.037 | 0.9978 | ||
| ARG/PPy | 3 | 1.205 | 0.769 | 1.205 | 0.9846 | 1.205 | 0.702 | 0.9999 | |
| 5 | 2.128 | 0.526 | 2.129 | 0.9994 | 2.129 | 0.646 | 0.9998 | ||
| 10 | 4.089 | 0.514 | 4.092 | 0.9792 | 4.092 | 0.578 | 0.9996 | ||
| MO/PPy | 3 | 1.227 | 0.727 | 1.223 | 0.9918 | 1.223 | 0.891 | 0.9980 | |
| 5 | 2.205 | 0.726 | 2.206 | 0.9927 | 2.206 | 0.797 | 0.9991 | ||
| 10 | 4.124 | 0.710 | 4.854 | 0.9941 | 4.854 | 0.675 | 0.9999 | ||
| α-CD/MO/PPy | 3 | 1.275 | 0.753 | 1.275 | 0.9922 | 1.275 | 0.941 | 0.9999 | |
| 5 | 2.268 | 0.704 | 2.258 | 0.9940 | 2.258 | 0.890 | 0.9984 | ||
| 10 | 4.357 | 0.675 | 4.354 | 0.9948 | 4.354 | 0.722 | 0.9993 | ||
| 吸附剂 | T/K | Langmuir模型 | Freundlich模型 | |||||
|---|---|---|---|---|---|---|---|---|
| Qm,c | KL | R2 | KF | 1/n | R2 | |||
| 纯PPy | 288 | 5.282 | 0.041 | 0.9918 | 2.086 | 0.350 | 0.8131 | |
| 298 | 6.554 | 0.168 | 0.9946 | 2.575 | 0.330 | 0.8920 | ||
| 308 | 11.536 | 0.200 | 0.9818 | 2.677 | 0.524 | 0.9648 | ||
| ARG/PPy | 288 | 6.010 | 0.044 | 0.9904 | 2.418 | 0.346 | 0.8167 | |
| 298 | 10.906 | 0.150 | 0.9883 | 2.931 | 0.373 | 0.9560 | ||
| 308 | 13.983 | 0.221 | 0.9874 | 2.941 | 0.540 | 0.9298 | ||
| MO/PPy | 288 | 6.359 | 0.090 | 0.9915 | 2.523 | 0.348 | 0.8330 | |
| 298 | 10.940 | 0.198 | 0.9854 | 3.172 | 0.353 | 0.9587 | ||
| 308 | 14.443 | 0.233 | 0.9814 | 3.241 | 0.540 | 0.9263 | ||
| α-CD/MO/PPy | 288 | 6.361 | 0.122 | 0.9922 | 2.823 | 0.308 | 0.8045 | |
| 298 | 11.594 | 0.175 | 0.9871 | 3.432 | 0.328 | 0.9660 | ||
| 308 | 14.713 | 0.295 | 0.9922 | 3.497 | 0.530 | 0.9418 | ||
表4 4种PPy材料吸附F-的Langmuir和Freundlich等温线方程拟合参数
| 吸附剂 | T/K | Langmuir模型 | Freundlich模型 | |||||
|---|---|---|---|---|---|---|---|---|
| Qm,c | KL | R2 | KF | 1/n | R2 | |||
| 纯PPy | 288 | 5.282 | 0.041 | 0.9918 | 2.086 | 0.350 | 0.8131 | |
| 298 | 6.554 | 0.168 | 0.9946 | 2.575 | 0.330 | 0.8920 | ||
| 308 | 11.536 | 0.200 | 0.9818 | 2.677 | 0.524 | 0.9648 | ||
| ARG/PPy | 288 | 6.010 | 0.044 | 0.9904 | 2.418 | 0.346 | 0.8167 | |
| 298 | 10.906 | 0.150 | 0.9883 | 2.931 | 0.373 | 0.9560 | ||
| 308 | 13.983 | 0.221 | 0.9874 | 2.941 | 0.540 | 0.9298 | ||
| MO/PPy | 288 | 6.359 | 0.090 | 0.9915 | 2.523 | 0.348 | 0.8330 | |
| 298 | 10.940 | 0.198 | 0.9854 | 3.172 | 0.353 | 0.9587 | ||
| 308 | 14.443 | 0.233 | 0.9814 | 3.241 | 0.540 | 0.9263 | ||
| α-CD/MO/PPy | 288 | 6.361 | 0.122 | 0.9922 | 2.823 | 0.308 | 0.8045 | |
| 298 | 11.594 | 0.175 | 0.9871 | 3.432 | 0.328 | 0.9660 | ||
| 308 | 14.713 | 0.295 | 0.9922 | 3.497 | 0.530 | 0.9418 | ||
| 吸附剂 | 吸附容量/mg·g-1 | 吸附平衡 时间/min | 参考文献 |
|---|---|---|---|
| 聚苯胺(翠绿亚胺碱) | 1.82 | 5 | [ |
| 氯掺杂聚吡咯 | 6.37 | 10 | [ |
| 聚吡咯接枝花生壳生物炭 | 17.15 | 360 | [ |
| PPy/Fe3O4 | 17.6~22.3 | 20 | [ |
| 聚吡咯包覆水合氧化锡 | 26.16~28.99 | 30 | [ |
| PPy/TiO2 | 33.18 | 30 | [ |
| 聚吡咯接枝柚皮生物炭 | 18.52 | 360 | [ |
| 镧改性介孔氧化铝 | 26.45 | 360 | [ |
| β-环糊精改性掺铈水合氧化铁 | 53.30 | 120 | [ |
| ARG/PPy | 10.906 | 10 | 本研究 |
| MO/PPy | 10.940 | 10 | 本研究 |
| α-CD/MO/PPy | 11.594 | 10 | 本研究 |
表5 本研究所得改性聚吡咯与文献报道的材料对氟离子的吸附性能比较
| 吸附剂 | 吸附容量/mg·g-1 | 吸附平衡 时间/min | 参考文献 |
|---|---|---|---|
| 聚苯胺(翠绿亚胺碱) | 1.82 | 5 | [ |
| 氯掺杂聚吡咯 | 6.37 | 10 | [ |
| 聚吡咯接枝花生壳生物炭 | 17.15 | 360 | [ |
| PPy/Fe3O4 | 17.6~22.3 | 20 | [ |
| 聚吡咯包覆水合氧化锡 | 26.16~28.99 | 30 | [ |
| PPy/TiO2 | 33.18 | 30 | [ |
| 聚吡咯接枝柚皮生物炭 | 18.52 | 360 | [ |
| 镧改性介孔氧化铝 | 26.45 | 360 | [ |
| β-环糊精改性掺铈水合氧化铁 | 53.30 | 120 | [ |
| ARG/PPy | 10.906 | 10 | 本研究 |
| MO/PPy | 10.940 | 10 | 本研究 |
| α-CD/MO/PPy | 11.594 | 10 | 本研究 |
| 吸附剂 | ?G/kJ·mol-1 | ?H/kJ·mol-1 | ?S/kJ·mol-1·K-1 | ||
|---|---|---|---|---|---|
| 288K | 298K | 308K | |||
| 纯PPy | -14.38 | -14.96 | -16.57 | 8.561 | 0.087 |
| ARG/PPy | -14.82 | -15.97 | -17.39 | 14.732 | 0.110 |
| MO/PPy | -15.02 | -16.06 | -17.69 | 6.683 | 0.084 |
| α-CD/MO/PPy | -15.19 | -16.36 | -18.13 | 11.902 | 0.105 |
表6 4种PPy材料吸附F-的热力学参数
| 吸附剂 | ?G/kJ·mol-1 | ?H/kJ·mol-1 | ?S/kJ·mol-1·K-1 | ||
|---|---|---|---|---|---|
| 288K | 298K | 308K | |||
| 纯PPy | -14.38 | -14.96 | -16.57 | 8.561 | 0.087 |
| ARG/PPy | -14.82 | -15.97 | -17.39 | 14.732 | 0.110 |
| MO/PPy | -15.02 | -16.06 | -17.69 | 6.683 | 0.084 |
| α-CD/MO/PPy | -15.19 | -16.36 | -18.13 | 11.902 | 0.105 |
| 1 | 雷绍民, 郭振华. 氟污染的危害及含氟废水处理技术研究进展[J]. 金属矿山, 2012, 430(4): 152-155. |
| LEI Shaomin, GUO Zhenhua. Hazards of fluoride pollution and technical research progress of treating fluoride-containing wastewater [J]. Metal Mine, 2012, 430(4): 152-155. | |
| 2 | DHARMARATNE R W. Fluoride in drinking water and diet: the causative factor of chronic kidney diseases in the North Central Province of Sri Lanka [J]. Environmental Health and Preventive Medicine, 2015, 20(4): 237-242. |
| 3 | HUANG Haiming, LIU Jiahui, ZHANG Peng, et al. Investigation on the simultaneous removal of fluoride, ammonia nitrogen and phosphate from semiconductor wastewater using chemical precipitation [J]. Chemical Engineering Journal, 2017, 307: 696-706. |
| 4 | 李兴扬, 王茹, 张旭, 等. 乳状液膜分离富集氟离子的应用研究[J]. 化工进展, 2014, 33(9): 2304-2308, 2320. |
| LI Xingyang, WANG Ru, ZHANG Xu, et al. Separation and preconcentration of fluoride by emulsion liquid membrane system [J]. Chemical Industry and Engineering Progress, 2014, 33(9): 2304-2308, 2320. | |
| 5 | ZHANG Qingrui, VOLISETTY S, CAO Yiping, et al. Selective and efficient removal of fluoride from water: in situ engineered amyloid fibril/ZrO2 hybrid membranes [J]. Angewandte Chemie International Edition, 2019, 58(18): 6012-6016. |
| 6 | GRZEGORZEK M, MAJEWSKA-NOWAK K, AHMED A E. Removal of fluoride from multicomponent water solutions with the use of monovalent selective ion-exchange membranes [J]. Science of the Total Environment, 2020, 722: 137681. |
| 7 | BHATNAGAR A, KUMAR E, SILLANPAA M. Fluoride removal from water by adsorption—A review [J]. Chemical Engineering Journal, 2011, 171(3): 811-840. |
| 8 | TANG Dandan, ZHANG Gaoke. Efficient removal of fluoride by hierarchical Ce-Fe bimetal oxides adsorbent: thermodynamics, kinetics and mechanism [J]. Chemical Engineering Journal, 2016, 283: 721-729. |
| 9 | CASCALES J J L, OTERO T F. Molecular dynamic simulation of the hydration and diffusion of chloride ions from bulk water to polypyrrole matrix [J]. Journal of Chemical Physics, 2004, 120(4): 1951-1957. |
| 10 | VERNITSKAYA T V, EFIMOV O N. Polypyrrole: a conducting polymer; its synthesis, properties and applications [J]. Russian Chemical Reviews, 1997, 66(5): 489-505. |
| 11 | KARTHIKEYAN M, SATHEESHKUMAR K K, ELANGO K P. Removal of fluoride ions from aqueous solution by conducting polypyrrole [J]. Journal of Hazardous Materials, 2009, 167(1/2/3): 300-305. |
| 12 | LI Chunlu, CHEN Nan, ZHAO Yanan, et al. Polypyrrole-grafted peanut shell biological carbon as a potential sorbent for fluoride removal: sorption capability and mechanism [J]. Chemosphere, 2016, 163: 81-89. |
| 13 | FENG Jiangtao, YAN Wei, ZHU Jinwei. Synthesis of novel hexagonal micro-sheet polypyrrole and micro-sheet polypyrrole with grooves in the presence of alpha-cyclodextrin/Acid Red G inclusion compounds [J]. Synthetic Metals, 2010, 160(9/10): 939-945. |
| 14 | FENG Jiangtao, LI Jingjing, Wei LYU, et al. Synthesis of polypyrrole nano-fibers with hierarchical structure and its adsorption property of Acid Red G from aqueous solution [J]. Synthetic Metals, 2014, 191: 66-73. |
| 15 | DAI Tingyang, LU Yun. Water-soluble methyl orange fibrils as versatile templates for the fabrication of conducting polymer microtubules [J]. . Macromolecular Rapid Communications, 2007, 28(5): 629-633. |
| 16 | OMASTOVA M, TRCHOVA M, KOVAROVA J, et al. Synthesis and structural study of polypyrroles prepared in the presence of surfactants [J]. Synthetic Metals, 2003, 138(3): 447-455. |
| 17 | LEOFANTI G, PADOVAN M, TOZZOLA G, et al. Surface area and pore texture of catalysts [J]. Catalysis Today, 1998, 41(1/2/3): 207-219. |
| 18 | ZHANG X, BAI Renbi, TONG Yen Wah. Selective adsorption behaviors of proteins on polypyrrole-based adsorbents [J]. Separation and Purification Technology, 2006, 52(1): 161-169. |
| 19 | YU Yang, YU Ling, CHEN J P. Adsorption of fluoride by Fe-Mg-La triple-metal composite: adsorbent preparation, illustration of performance and study of mechanisms [J]. Chemical Engineering Journal, 2015, 262: 839-846. |
| 20 | ESKANDARPOUR A, ONYANGO M S, OCHIENG A, et al. Removal of fluoride ions from aqueous solution at low pH using schwertmannite [J]. Journal of Hazardous Materials, 2008, 152(2): 571-579. |
| 21 | SIMONIN J P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics [J]. Chemical Engineering Journal, 2016, 300: 254-263. |
| 22 | HAMED M M, RIZK S E, NAYL A A. Adsorption kinetics and modeling of gadolinium and cobalt ions sorption by an ion-exchange resin [J]. Particulate Science & Technology, 2016, 34(6): 716-724. |
| 23 | OMRAEI M, ESFANDIAN H, KATAL R, et al. Study of the removal of Zn(Ⅱ) from aqueous solution using polypyrrole nanocomposite [J]. Desalination, 2011, 271(1/2/3): 248-256. |
| 24 | KARTHIK R, MEENAKSHI S. Chemical modification of chitin with polypyrrole for the uptake of Pb(Ⅱ) and Cd(Ⅱ) ions [J]. International Journal of Biological Macromolecules, 2015, 78: 157-164. |
| 25 | KARTHIKEYAN M, SATHEESHKUMAR K K, ELANGO K P. Defluoridation of water via doping of polyanilines [J]. Journal of Hazardous Materials, 2009, 163(2/3): 1026-1032. |
| 26 | BHAUMIK M, LESWIFI T Y, MAITY A, et al. Removal of fluoride from aqueous solution by polypyrrole-Fe3O4 magnetic nanocomposite [J]. Journal of Hazardous Materials, 2011, 186(1): 150-159. |
| 27 | PARASHAR K, BALLAV N, BEBNATH S, et al. Rapid and efficient removal of fluoride ions from aqueous solution using a polypyrrole coated hydrous tin oxide nanocomposite [J]. Journal of Colloid and Interface Science, 2016, 476: 103-118. |
| 28 | CHEN Jie, SHU Chiajung, WANG Ning, et al. Adsorbent synthesis of polypyrrole/TiO2 for effective fluoride removal from aqueous solution for drinking water purification: adsorbent characterization and adsorption mechanism [J]. Journal of Colloid and Interface Science, 2017, 495: 44-52. |
| 29 | WANG Jianguo, CHEN Nan, LI Miao, et al. Efficient removal of fluoride using polypyrrole-modified biochar derived from slow pyrolysis of pomelo peel: sorption capacity and mechanism [J]. Journal of Polymers and the Environment, 2018, 26: 1559-1572. |
| 30 | HE Yuxuan, ZHANG Liming, AN Xiao, et al. Enhanced fluoride removal from water by rare earth (La and Ce) modified alumina: adsorption isotherms, kinetics, thermodynamics and mechanism [J]. Science of the Total Environment, 2019, 688: 184-198. |
| 31 | MIKHOPADHYAY M, NASKAR A, GHOSH U C, et al. One-pot synthesis of β-cyclodextrin amended mesoporous cerium(Ⅳ) incorporated ferric oxide surface towards the evaluation of fluoride removal efficiency from contaminated water for point of use [J]. Journal of Hazardous Materials, 2011, 384: 121235. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [7] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [8] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [9] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [10] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [11] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [12] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| [13] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [14] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [15] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||