| 1 |
MEENA R A A, YUKESH K R, SINDHU J, et al. Trends and resource recovery in biological wastewater treatment system[J]. Bioresource Technology Reports, 2019, 7: 100235.
|
| 2 |
CHIPAKO T L, RANDALL D G. Urine treatment technologies and the importance of pH[J]. Journal of Environmental Chemical Engineering, 2020, 8(1): 103622.
|
| 3 |
LANGERGRABER G, MUELLEGGER E. Ecological sanitation—A way to solve global sanitation problems?[J]. Environment International, 2005, 31(3): 433-444.
|
| 4 |
CARDOSO M, NOLASCO M A, SCHOLZ M. Phosphorus recovery from municipal wastewater treatment: critical review of challenges and opportunities for developing countries[J]. Journal of Environmental Management, 2019, 248: 109268.
|
| 5 |
蒋善庆, 王晓昌, 李超, 等.源分离尿液资源化利用与风险控制技术研究进展[J].安全与环境学报, 2014, 14(5): 174-182.
|
|
JIANG Shanqing, WANG Xiaochang, LI Chao, et al. Review on the resource utilization and risks control technologies of source separated urine[J] Journal of Safety and Environment, 2014, 14(5): 174-182.
|
| 6 |
朱国荣, 谢倍珍, 刘红. 载人深空探测活动中的尿液处理回收技术分析[J]. 深空探测学报, 2018, 5(6): 582-590.
|
|
ZHU Guorong, XIE Beizhen, LIU Hong. Analysis of urine treatment and recovery technology used in manned deep space exploration.[J] Journal of Deep Space Exploration, 2018, 5(6): 582-590.
|
| 7 |
FLANAGAN C P, RANDALL D G. Development of a novel nutrient recovery urinal for on-site fertilizer production[J]. Journal of Environmental Chemical Engineering, 2018, 6(5): 6344-6350.
|
| 8 |
LU S, LI H, TAN G, et al. Resource recovery microbial fuel cells for urine-containing wastewater treatment without external energy consumption[J]. Chemical Engineering Journal, 2019, 373: 1072-1080.
|
| 9 |
SOLANKI A, BOYER T H. Physical-chemical interactions between pharmaceuticals and biochar in synthetic and real urine[J]. Chemosphere, 2019, 218: 818-826.
|
| 10 |
VOLPIN F, HEO H, HASAN JOHIR M A, et al. Techno-economic feasibility of recovering phosphorus, nitrogen and water from dilute human urine via forward osmosis[J]. Water Research, 2019, 150: 47-55.
|
| 11 |
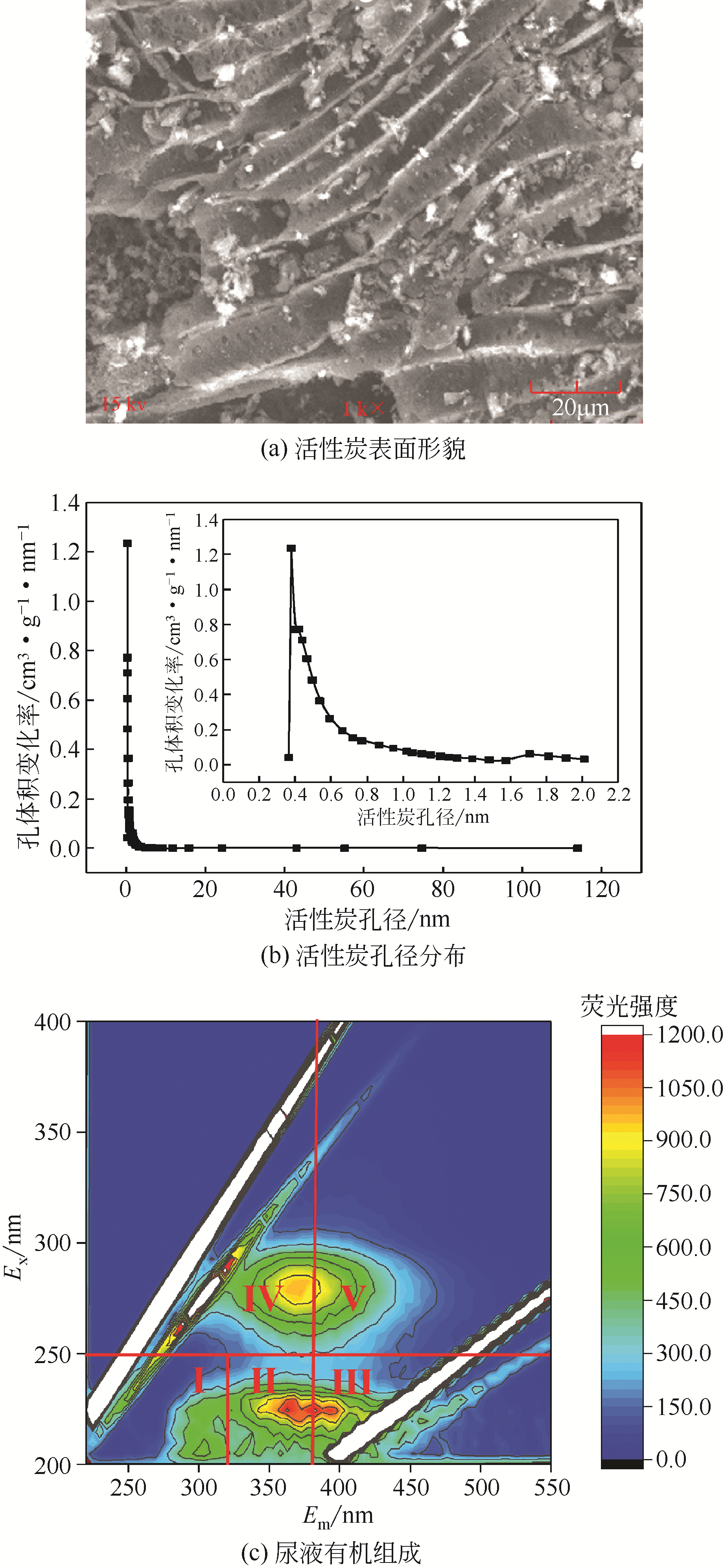
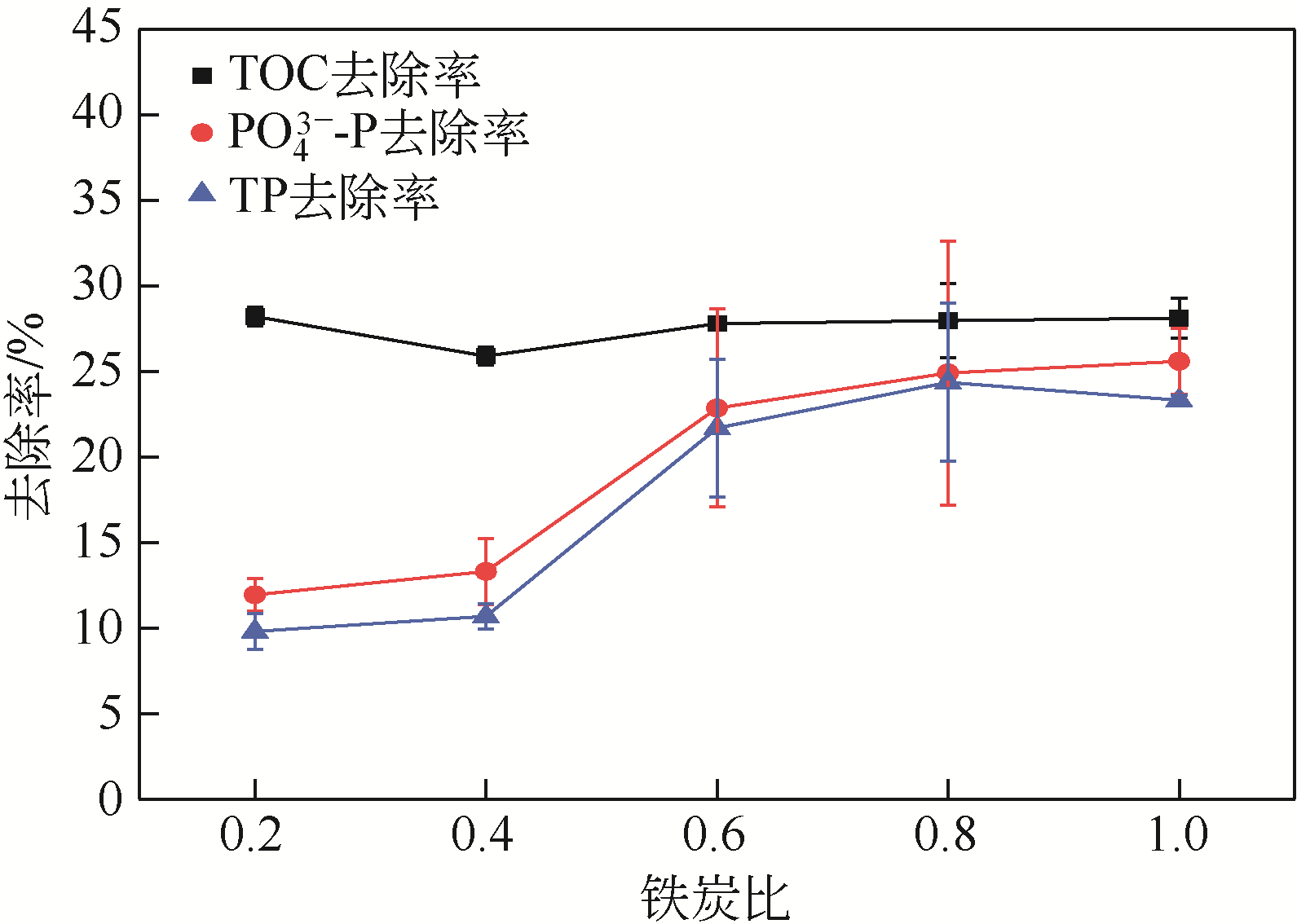
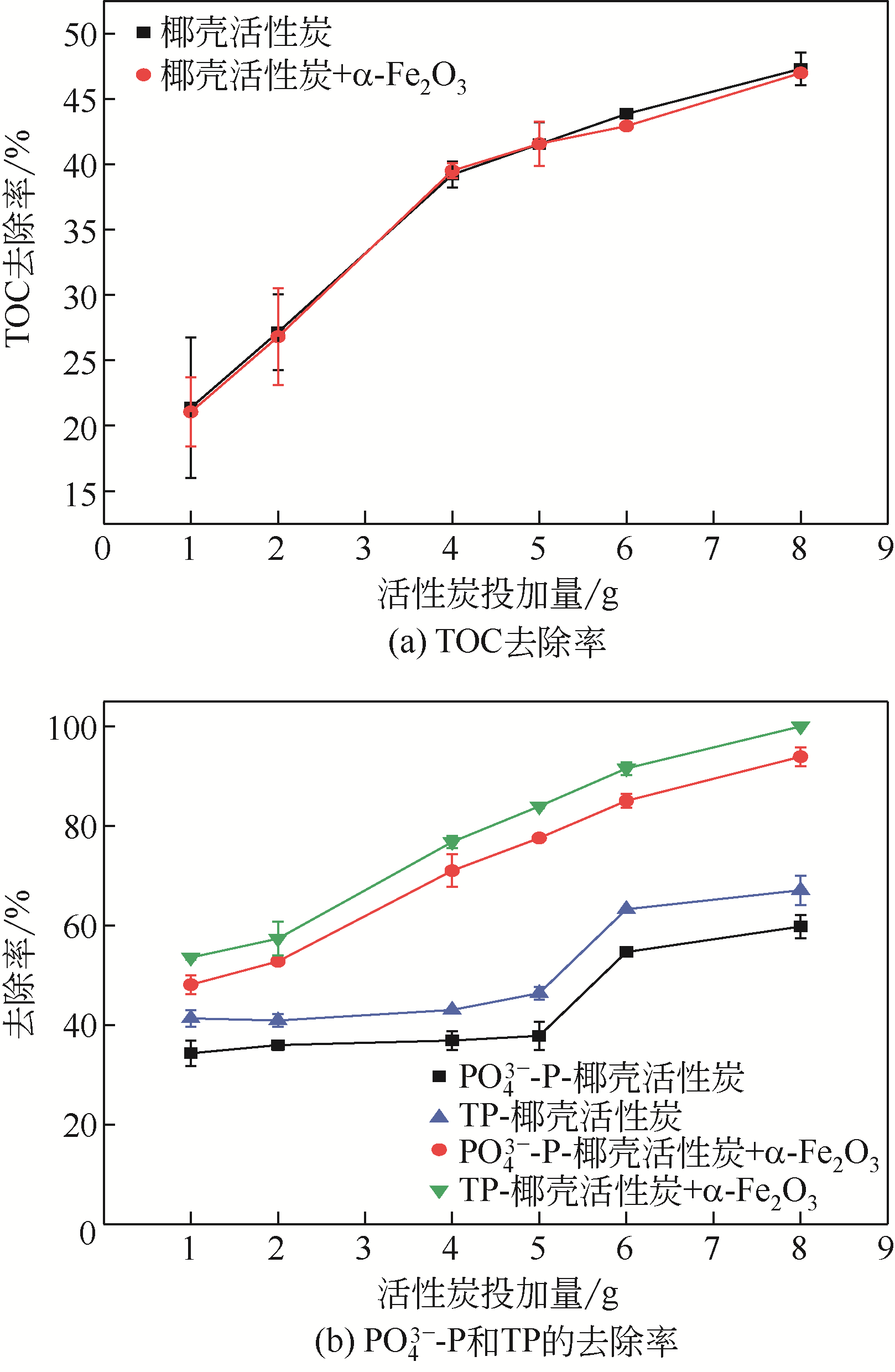
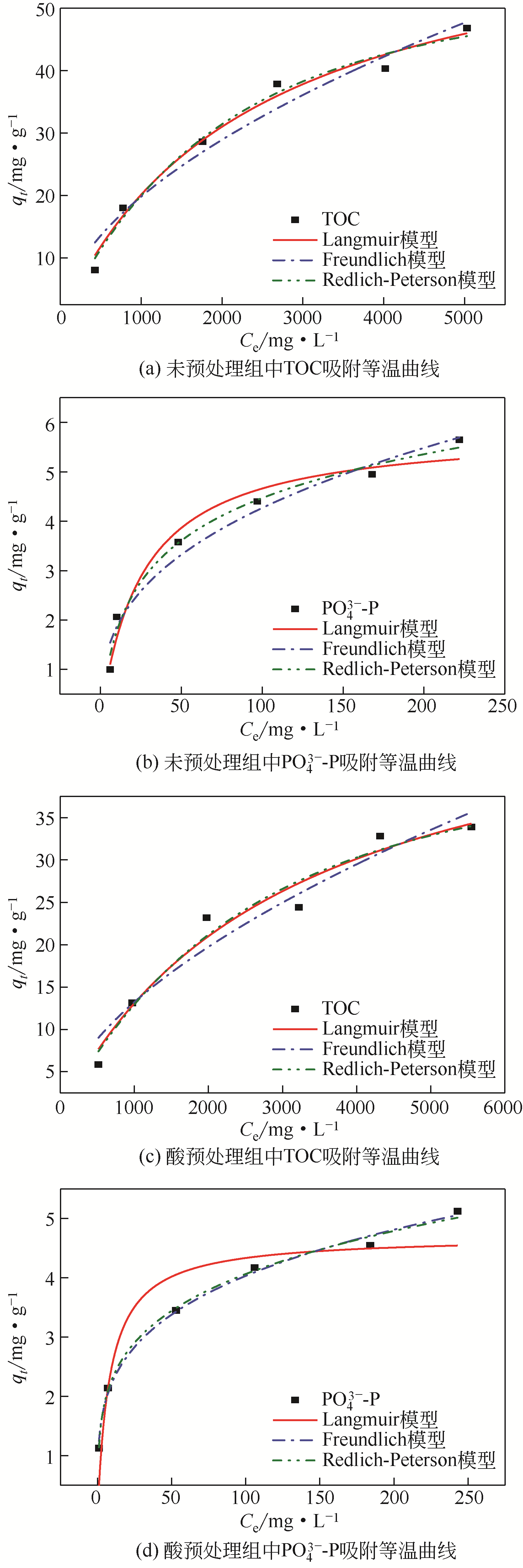
焦赟仪, 郑利兵, 魏源送, 等. 铁氧化物对活性炭处理尿液的影响[J]. 环境工程学报, 2020, 14(12): 3381-3390.
|
|
JIAO Yunyi, ZHENG Libing, WEI Yuansong, et al. Effect of iron oxides on urine treatment by activated carbon[J]. Chinese Journal of Environmental Engineering, 2020, 14(12): 3381-3390
|
| 12 |
GANROT Z, DAVE G, NILSSON E. Recovery of N and P from human urine by freezing, struvite precipitation and adsorption to zeolite and active carbon[J]. Bioresource Technology, 2007, 98(16): 3112-3121.
|
| 13 |
ZHU Z, HUANG C P, ZHU Y, et al. A hierarchical porous adsorbent of nano-α-Fe2O3/Fe3O4 on bamboo biochar (HPA-Fe/C-B) for the removal of phosphate from water[J]. Journal of Water Process Engineering, 2018, 25: 96-104.
|
| 14 |
CHEN W, WESTERHOFF P, LEENHEER J A, et al. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter[J]. Environmental Science & Technology, 2003, 37(24): 5701-5710.
|
| 15 |
高连敬, 杜尔登, 崔旭峰, 等. 三维荧光结合荧光区域积分法评估净水厂有机物去除效果[J]. 给水排水, 2012, 48(10): 51-56.
|
|
GAO Lianjing, DU Erdeng, CUI Xufeng, et al. The effect of organics removal in water purification plant was evaluated by 3D fluorescence combined with fluorescence area integration[J].Water & Wastewater Engineering, 2012, 48(10): 51-56.
|
| 16 |
郑利兵. 新型疏水膜制备及磁-膜耦合工艺处理高浓高盐废水研究——以脱硫废水为例[D]. 北京: 中国科学院大学, 2018.
|
|
ZHENG Libing. Development of hydrophobic membrane and intergrated magnetic-membrane separation technology for high strength wastewater with high salinity—A case study of desulfurization wastewater[D]. Beijing: University of Chinese Academy of Sciences, 2018.
|
| 17 |
VILELA P B, MATIAS C A, DALALIBERA A, et al. Polyacrylic acid-based and chitosan-based hydrogels for adsorption of cadmium: equilibrium isotherm, kinetic and thermodynamic studies[J]. Journal of Environmental Chemical Engineering, 2019, 7(5): 103327.
|
| 18 |
吴光前, 吴冠男, 张齐生, 等. 竹炭吸附对硝基苯胺的过程与机理研究[J]. 环境工程学报, 2017, 11(1): 251-259.
|
|
WU Guangqian, WU Guannan, ZHANG Qisheng, et al. Study on adsorption process and mechanism of p-nitroaniline on bamboo charcoal[J]. Chinese Journal of Environmental Engineering, 2017, 11(1): 251-259.
|
| 19 |
蒋善庆, 王晓昌. 黄土颗粒吸附水解尿液中磷酸盐特性及机制[J]. 化工进展, 2017, 36(7): 2667-2675.
|
|
JIANG Shanqing, WANG Xiaochang. Performances and mechanisms of phosphate adsorption from hydrolysis urine onto Chinese loess soil[J]. Chemical Industry and Engineering Progress, 2017, 36(7): 2667-2675.
|
| 20 |
XU K, LIN F, DOU X, et al. Recovery of ammonium and phosphate from urine as value-added fertilizer using wood waste biochar loaded with magnesium oxides[J]. Journal of Cleaner Production, 2018, 187: 205-214.
|
| 21 |
HUANG X. Intersection of isotherms for phosphate adsorption on hematite[J]. Journal of Colloid and Interface Science, 2004, 271(2): 296-307.
|
| 22 |
汪家权, 沈燕华, 马玉萍.巢湖流域岩源磷释放的动力学研究[J].环境科学学报, 2010, 30(5): 979-984.
|
|
WANG Jiaquan, SHEN Yanhua, MA Yuping. Release kinetics of rock phosphorus in the Chaohu lake area[J]. Acta Scientiae Circumstantiae, 2010, 30(5): 979-984.
|
| 23 |
SINGLA J, VERMA A, SANGAL V K. Applications of doped mixed metal oxide anode for the electro-oxidation treatment and mineralization of urine metabolite, uric acid[J]. Journal of Water Process Engineering, 2019, 32: 100944.
|
| 24 |
LIBING Z, YUNYI J, HUI Z, et al. Insight into the magnetic lime coagulation-membrane distillation process for desulfurization wastewater treatment: from pollutant removal feature to membrane fouling[J]. Journal of Hazardous Materials, 2020, 391: 122202.
|
| 25 |
UDERT K M, LARSEN T A, BIEBOW M, et al. Urea hydrolysis and precipitation dynamics in a urine-collecting system[J]. Water Research, 2003, 37(11): 2571-2582.
|
 ), 周书葵3, 张良长4, 艾为党4, 康赛1,2, 李晨璐1,2, 郑利兵1,2(
), 周书葵3, 张良长4, 艾为党4, 康赛1,2, 李晨璐1,2, 郑利兵1,2( ), 魏源送1,2
), 魏源送1,2
 ), ZHOU Shukui3, ZHANG Liangchang4, AI Weidang4, KANG Sai1,2, LI Chenlu1,2, ZHENG Libing1,2(
), ZHOU Shukui3, ZHANG Liangchang4, AI Weidang4, KANG Sai1,2, LI Chenlu1,2, ZHENG Libing1,2( ), WEI Yuansong1,2
), WEI Yuansong1,2