化工进展 ›› 2019, Vol. 38 ›› Issue (08): 3838-3851.DOI: 10.16085/j.issn.1000-6613.2018-2025
生物炭基复合材料制备及其对水体特征污染物的吸附性能
- 西安交通大学能源与动力工程学院,陕西 西安710049
-
收稿日期:2018-10-12出版日期:2019-08-05发布日期:2019-08-05 -
通讯作者:王丽 -
作者简介:王靖宜(1995—),女,硕士研究生,研究方向为生物炭改性。E-mail:wangjingyi01@stu.xjtu.edu.cn 。 -
基金资助:陕西省自然科学基础研究计划一般项目(2018JQ4014);陕西省博士后科研项目(2017BSHEDZZ65);中央高校基本科研业务费项目(XJJ20171728);中国博士后科学基金(2016M592798)
Preparation of biochar-based composites and their adsorption performances for characteristic contaminants in wastewater
Jingyi WANG( ),Li WANG(
),Li WANG( ),Wenlong ZHANG,Wei LÜ,Wei YAN,Shanshan LI,Jiangtao FENG
),Wenlong ZHANG,Wei LÜ,Wei YAN,Shanshan LI,Jiangtao FENG
- School of Energy and Power Engineering, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
-
Received:2018-10-12Online:2019-08-05Published:2019-08-05 -
Contact:Li WANG
摘要:
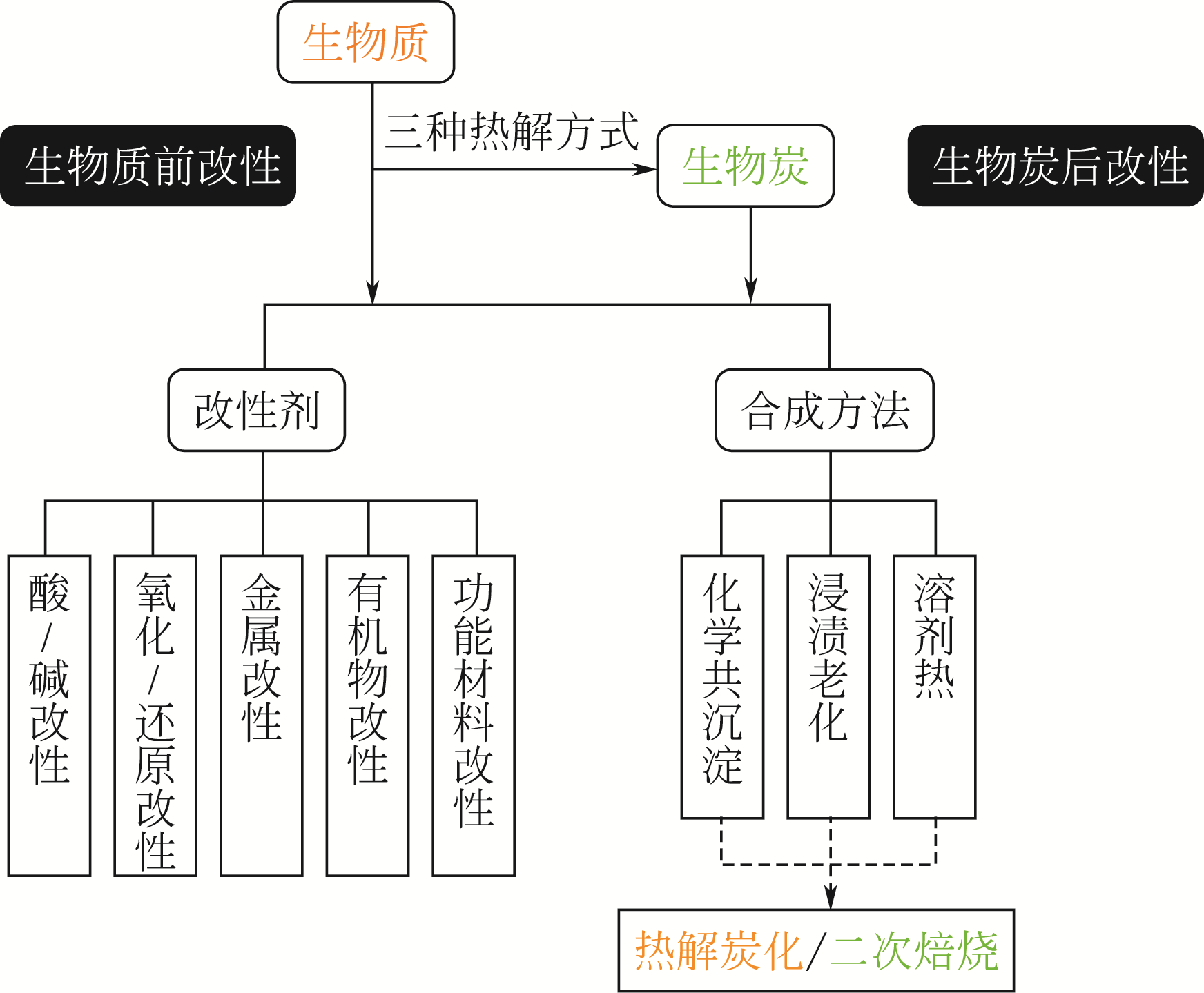
以重金属、药物及个人护理品污染物(PPCPs)和氮磷氟等为代表的水体主要污染物脱除已成为水污染治理研究的重点。吸附法由于其具有操作简易、成本效益高等优势,在水污染治理方面应用广泛。相比于传统吸附材料,生物炭具有原材料丰富、比表面积大、成本低廉等优势,但其表面官能团的丰富度有限,经适当改性能够增加生物炭的吸附活性位,从而提高其吸附性能。本文根据改性剂类型、合成方法及合成的先后顺序,系统阐释了生物炭的改性方法及其复合体的理化特性;结合最新研究报道,在合成方法、吸附性能和机理方面归纳汇总了生物炭基复合材料在水体重金属、PPCPs和其他污染物中的吸附应用,并对生物炭基吸附剂再生与资源化利用的新理念及应用进行了概述,最后展望了生物炭基吸附剂有待深入研究的方面,并提出建设性的意见。
中图分类号:
引用本文
王靖宜,王丽,张文龙,吕伟,延卫,李珊珊,冯江涛. 生物炭基复合材料制备及其对水体特征污染物的吸附性能[J]. 化工进展, 2019, 38(08): 3838-3851.
Jingyi WANG,Li WANG,Wenlong ZHANG,Wei LÜ,Wei YAN,Shanshan LI,Jiangtao FENG. Preparation of biochar-based composites and their adsorption performances for characteristic contaminants in wastewater[J]. Chemical Industry and Engineering Progress, 2019, 38(08): 3838-3851.
| 物理指标 | 参数范围 | 平均值 | 生物质炭化后含量对比 |
|---|---|---|---|
| 含碳量 | 30%~90% | 64% | 木质>秸秆>壳类>粪便>污泥 |
| 灰分 | 0~40% | 15.52% | 污泥>粪便>秸秆>壳类>木质 |
| 比表面积 | 0~520m2·g-1 | 124.83m2·g-1 | 壳类>秸秆>木质>粪便>污泥 |
| pH | 5~12 | 9.15 | 秸秆>污泥>粪便>木质>壳类 |
| 孔隙度 | 750~1360m2·g-1(大孔隙);51~138m2·g-1(小孔隙) | ||
| 平均密度 | 1.6 g·cm-3 | ||
| 主要元素 | C、O、N、H | ||
表1 生物炭的主要物理特性[27]
| 物理指标 | 参数范围 | 平均值 | 生物质炭化后含量对比 |
|---|---|---|---|
| 含碳量 | 30%~90% | 64% | 木质>秸秆>壳类>粪便>污泥 |
| 灰分 | 0~40% | 15.52% | 污泥>粪便>秸秆>壳类>木质 |
| 比表面积 | 0~520m2·g-1 | 124.83m2·g-1 | 壳类>秸秆>木质>粪便>污泥 |
| pH | 5~12 | 9.15 | 秸秆>污泥>粪便>木质>壳类 |
| 孔隙度 | 750~1360m2·g-1(大孔隙);51~138m2·g-1(小孔隙) | ||
| 平均密度 | 1.6 g·cm-3 | ||
| 主要元素 | C、O、N、H | ||
| 改性剂 | 常用成分举例 | 理化特性优势 | 水体特征污染物 |
|---|---|---|---|
| 酸/碱 | H3PO4,HNO3,HCl,H2SO4;KOH,NaOH,Ca(OH)2,氨水,尿素,碳酸盐,碳酸氢盐等碱式盐 | 表面官能团含量提高,比表面积增大,多孔结构更为显著,材料分散性、热稳定性提高 | 无机物(P,N,F) 有机物(PPCPs,EDCs,POPs,染料) 重金属(Cu,Cd,Pb,Hg,Cr,Sb,As) |
| 氧化剂/还原剂 | H2O2,KMnO4,过硫酸盐;NaBH4,Na2SO3,FeSO4 | ||
| 金属 | MgO,MnO2,La2O3,CeO2;AlOOH,FeOOH,LaOOH;Fe3O4,MgFe2O4,MnFe2O4 | ||
| 有机物 | EDTA(乙二胺四乙酸),EDA(乙二胺),DMF(二甲基甲酰胺),CTAB(十六烷基三甲基溴化铵) SDBS(十二烷基苯磺酸钠),乙二醇,甲醇,表氯醇 | ||
| 功能材料 | 壳聚糖,水凝胶,GO(石墨烯氧化物),RGO(还原石墨烯氧化物),PPy(聚吡咯),CNTs(碳纳米管),g-MoS2 |
表2 生物炭的改性剂活化及其理化特性
| 改性剂 | 常用成分举例 | 理化特性优势 | 水体特征污染物 |
|---|---|---|---|
| 酸/碱 | H3PO4,HNO3,HCl,H2SO4;KOH,NaOH,Ca(OH)2,氨水,尿素,碳酸盐,碳酸氢盐等碱式盐 | 表面官能团含量提高,比表面积增大,多孔结构更为显著,材料分散性、热稳定性提高 | 无机物(P,N,F) 有机物(PPCPs,EDCs,POPs,染料) 重金属(Cu,Cd,Pb,Hg,Cr,Sb,As) |
| 氧化剂/还原剂 | H2O2,KMnO4,过硫酸盐;NaBH4,Na2SO3,FeSO4 | ||
| 金属 | MgO,MnO2,La2O3,CeO2;AlOOH,FeOOH,LaOOH;Fe3O4,MgFe2O4,MnFe2O4 | ||
| 有机物 | EDTA(乙二胺四乙酸),EDA(乙二胺),DMF(二甲基甲酰胺),CTAB(十六烷基三甲基溴化铵) SDBS(十二烷基苯磺酸钠),乙二醇,甲醇,表氯醇 | ||
| 功能材料 | 壳聚糖,水凝胶,GO(石墨烯氧化物),RGO(还原石墨烯氧化物),PPy(聚吡咯),CNTs(碳纳米管),g-MoS2 |
| 改性合成方法 | 炭基吸附剂(生物质原料) | 污染物 | 脱除率(R max)/ 吸附容量(Q m) | 吸附机理预测 | 参考文献 |
|---|---|---|---|---|---|
| 生物炭分别氧化处理(H2O2)、酸处理(HCl)、碱处理(KOH) | nZVI固定化氧化/酸/碱活化改性生物炭(玉米秸秆) | Cr(Ⅵ) | HCl活化较KOH和H2O2活化R max提高13.29% | 氧化还原、原电池作用、表面沉淀 | [ |
| 生物质在Fe(NO3)3、HNO3溶液浸渍后600℃热解,与吡咯单体混合后逐滴加入FeCl3再持续混合(30℃)24h(吡咯原位化学氧化聚合) | 聚吡咯修饰磁性生物炭(MBC/PPy)(玉米芯) | Cr(Ⅵ) | 19.23mgCr·g-1 | 静电引力、离子交换、氧化还原、表面沉淀、螯合作用 | [ |
| 生物质与KHCO3混合后800℃热解,后与Fe2+溶液混合并滴加NaBH4湿化学还原法合成 | nZVI负载亲水性功能炭基复合材料(玉米秸秆) | Pb(Ⅱ) Cu(Ⅱ) Zn(Ⅱ) | 195.1mgPb·g-1 161.9mgCu·g-1 109.7mgZn·g-1 | 共沉淀、表面络合、沉淀、氧化还原 | [ |
| 化学沉淀制备纳米羟基磷灰石,与生物质充分混合后600℃热解 | 纳米羟基磷灰石负载生物炭(nHAP/BC)(玉米秸秆) | Pb(Ⅱ) | 383.75mgPb·g-1 | 溶解-沉淀、络合作用 | [ |
| 生物质与KHCO3混合后热解,经过过硫酸铵氧化后进行水热反应 | 生物炭浸渍α-FeOOH纳米棒的多级孔结构(玉米秸秆) | Cu(Ⅱ) | 71.9%(较BC、α-FeOOH提高29.6%、65.2%) | 表面络合、氧化还原 | [ |
| 生物质与两种盐溶液([Cu2+]/[Al3+]=2)及尿素混合后110℃水热反应12h | Cu/Al-LDHs-BC(剑麻纤维) | Cu(Ⅱ) | 99.6% | 静电引力、离子交换、配体交换 | [ |
| 生物炭与Fe(acac)3180℃混合并焙烧(100℃),后与ZnCl2的乙二醇溶液及硫脲持续混合(180℃) | ZnS纳米颗粒负载磁性生物炭(稻壳) | Pb(Ⅱ) | 367.65mgPb·g-1 (较磁性BC提高10倍) | — | [ |
| 生物炭与Fe3+、Fe2+溶液浸渍后反应釜水热合成 | 磁性生物炭(稻壳) | Pb(Ⅱ) U(Ⅵ) | 129mgPb·g-1 118mgU·g-1 | 外球络合、静电引力;内球络合、表面沉淀 | [ |
| 20%H2O2氧化生物炭后与Fe3+、Fe2+溶液共沉淀(先后加入TSA、EC、IDA等有机溶剂) | 亚氨基二乙酸(IDA)磁性生物炭(棕榈纤维) | Cd(Ⅱ) | 197.96mgCd·g-1 | 表面络合、静电引力、离子交换 | [ |
| 生物质炭化后与Fe3O4纳米颗粒混合(先后加入TSA、EC等有机溶剂)后加入尿素、NaOH60℃油浴加热 | 尿素功能化改性生物炭(棕榈纤维) | Pb(Ⅱ) | 188.18mgpb·g-1 | 表面络合、静电引力、离子交换 | [ |
| 生物质浸渍于KMnO4溶液(超声辐照)后热解 | KMnO4改性功能性生物炭 (山核桃木) | Pb(Ⅱ) Cu(Ⅱ) Cd(Ⅱ) | 153.1mgPb·g-1 34.2mgCu·g-1 28.1mgCd·g-1 | 静电作用、位点识别(表面MnO x 和含氧基团提供) | [ |
| 生物质与盐溶液浸渍、化学共沉淀后干燥热解 | Fe/Zn双金属掺杂生物炭(木屑) | Cu(Ⅱ) 四环素 | 初次、二次、三次再生R max为92.1%、95.7%、89% | 位点识别、桥键作用增强、位点竞争 | [ |
| 生物质微波热解后与Fe2+、Fe3+、LaCl3溶液化学共沉淀 | 镧掺杂磁性生物炭(芦苇) | Sb(Ⅴ) | 18.92mgSb·g-1(分别为Fe-BC、BC的3.90倍、8.52倍) | 内球络合、氢键、静电引力、配体交换 | [ |
| KMnO4加入生物炭(500℃热解)与FeSO4悬浮液中化学共沉(pH10) | 铁酸锰改性纳米生物炭(MnFe2O4-BC)(茶末) | Sb(Ⅲ) Cd(Ⅱ) | 237.53mgSb·g-1 181.49mgCd·g-1 | 羟基去质子化、络合、Sb(III)氧化 | [ |
| 前体H3PO4浸渍后一步法热解(250℃空气氛围) | 表面功能化改性生物炭 (柚子皮) | Ag(Ⅰ) Pb(Ⅱ) | 137.4mgAg·g-1 88.7mgPb·g-1 | 表面络合、静电引力,Ag(I)还原、羟基磷酸铅沉淀 | [ |
| 生物质与盐溶液混合后180℃水热反应10h | 尖晶石锰铁氧体改性生物炭(裙带菜根) | Pb(Ⅱ) Cu(Ⅱ) Cd(Ⅱ) | 307.68mgPb·g-1 211.48mgCu·g-1 188.20mgCd·g-1 | 静电引力、外球络合、边界层扩散、吸热机制 | [ |
| 10%H2O2溶液浸渍氧化生物炭 | H2O2活化生物炭 (牦牛粪便) | Pb(Ⅱ) Cu(Ⅱ) Zn(Ⅱ) Cd(Ⅱ) | 169.57mgPb·g-1 71.39mgCu·g-1 42.21mgZn·g-1 82.95mgCd·g-1 (2.2倍、1.6倍、1.7倍、1.5倍) | 重金属-羧基络合、Pb-碳酸盐/磷酸盐沉淀 | [ |
| 生物炭与CMC化学共沉淀(先后逐滴加入戊二醛,乙酸) | 羧甲基壳聚糖(CMC)涂覆污泥生物炭 | Pb(Ⅱ) Hg(Ⅱ) | 210mgPb·g-1(<60min) 594.17mgHg·g-1 | 表面络合 | [ |
| 生物炭的液相还原改性(NaBH4) | 固定化nZVI污泥生物炭 | Cr(Ⅵ) Pb(Ⅱ) | Cr6+:90%,Pb2+:82% (<30min) | 配体交换、氧化还原、表面沉淀 | [ |
表3 生物炭基材料改性及吸附重金属的应用(热解未特别标注均为N2氛围慢速热解)
| 改性合成方法 | 炭基吸附剂(生物质原料) | 污染物 | 脱除率(R max)/ 吸附容量(Q m) | 吸附机理预测 | 参考文献 |
|---|---|---|---|---|---|
| 生物炭分别氧化处理(H2O2)、酸处理(HCl)、碱处理(KOH) | nZVI固定化氧化/酸/碱活化改性生物炭(玉米秸秆) | Cr(Ⅵ) | HCl活化较KOH和H2O2活化R max提高13.29% | 氧化还原、原电池作用、表面沉淀 | [ |
| 生物质在Fe(NO3)3、HNO3溶液浸渍后600℃热解,与吡咯单体混合后逐滴加入FeCl3再持续混合(30℃)24h(吡咯原位化学氧化聚合) | 聚吡咯修饰磁性生物炭(MBC/PPy)(玉米芯) | Cr(Ⅵ) | 19.23mgCr·g-1 | 静电引力、离子交换、氧化还原、表面沉淀、螯合作用 | [ |
| 生物质与KHCO3混合后800℃热解,后与Fe2+溶液混合并滴加NaBH4湿化学还原法合成 | nZVI负载亲水性功能炭基复合材料(玉米秸秆) | Pb(Ⅱ) Cu(Ⅱ) Zn(Ⅱ) | 195.1mgPb·g-1 161.9mgCu·g-1 109.7mgZn·g-1 | 共沉淀、表面络合、沉淀、氧化还原 | [ |
| 化学沉淀制备纳米羟基磷灰石,与生物质充分混合后600℃热解 | 纳米羟基磷灰石负载生物炭(nHAP/BC)(玉米秸秆) | Pb(Ⅱ) | 383.75mgPb·g-1 | 溶解-沉淀、络合作用 | [ |
| 生物质与KHCO3混合后热解,经过过硫酸铵氧化后进行水热反应 | 生物炭浸渍α-FeOOH纳米棒的多级孔结构(玉米秸秆) | Cu(Ⅱ) | 71.9%(较BC、α-FeOOH提高29.6%、65.2%) | 表面络合、氧化还原 | [ |
| 生物质与两种盐溶液([Cu2+]/[Al3+]=2)及尿素混合后110℃水热反应12h | Cu/Al-LDHs-BC(剑麻纤维) | Cu(Ⅱ) | 99.6% | 静电引力、离子交换、配体交换 | [ |
| 生物炭与Fe(acac)3180℃混合并焙烧(100℃),后与ZnCl2的乙二醇溶液及硫脲持续混合(180℃) | ZnS纳米颗粒负载磁性生物炭(稻壳) | Pb(Ⅱ) | 367.65mgPb·g-1 (较磁性BC提高10倍) | — | [ |
| 生物炭与Fe3+、Fe2+溶液浸渍后反应釜水热合成 | 磁性生物炭(稻壳) | Pb(Ⅱ) U(Ⅵ) | 129mgPb·g-1 118mgU·g-1 | 外球络合、静电引力;内球络合、表面沉淀 | [ |
| 20%H2O2氧化生物炭后与Fe3+、Fe2+溶液共沉淀(先后加入TSA、EC、IDA等有机溶剂) | 亚氨基二乙酸(IDA)磁性生物炭(棕榈纤维) | Cd(Ⅱ) | 197.96mgCd·g-1 | 表面络合、静电引力、离子交换 | [ |
| 生物质炭化后与Fe3O4纳米颗粒混合(先后加入TSA、EC等有机溶剂)后加入尿素、NaOH60℃油浴加热 | 尿素功能化改性生物炭(棕榈纤维) | Pb(Ⅱ) | 188.18mgpb·g-1 | 表面络合、静电引力、离子交换 | [ |
| 生物质浸渍于KMnO4溶液(超声辐照)后热解 | KMnO4改性功能性生物炭 (山核桃木) | Pb(Ⅱ) Cu(Ⅱ) Cd(Ⅱ) | 153.1mgPb·g-1 34.2mgCu·g-1 28.1mgCd·g-1 | 静电作用、位点识别(表面MnO x 和含氧基团提供) | [ |
| 生物质与盐溶液浸渍、化学共沉淀后干燥热解 | Fe/Zn双金属掺杂生物炭(木屑) | Cu(Ⅱ) 四环素 | 初次、二次、三次再生R max为92.1%、95.7%、89% | 位点识别、桥键作用增强、位点竞争 | [ |
| 生物质微波热解后与Fe2+、Fe3+、LaCl3溶液化学共沉淀 | 镧掺杂磁性生物炭(芦苇) | Sb(Ⅴ) | 18.92mgSb·g-1(分别为Fe-BC、BC的3.90倍、8.52倍) | 内球络合、氢键、静电引力、配体交换 | [ |
| KMnO4加入生物炭(500℃热解)与FeSO4悬浮液中化学共沉(pH10) | 铁酸锰改性纳米生物炭(MnFe2O4-BC)(茶末) | Sb(Ⅲ) Cd(Ⅱ) | 237.53mgSb·g-1 181.49mgCd·g-1 | 羟基去质子化、络合、Sb(III)氧化 | [ |
| 前体H3PO4浸渍后一步法热解(250℃空气氛围) | 表面功能化改性生物炭 (柚子皮) | Ag(Ⅰ) Pb(Ⅱ) | 137.4mgAg·g-1 88.7mgPb·g-1 | 表面络合、静电引力,Ag(I)还原、羟基磷酸铅沉淀 | [ |
| 生物质与盐溶液混合后180℃水热反应10h | 尖晶石锰铁氧体改性生物炭(裙带菜根) | Pb(Ⅱ) Cu(Ⅱ) Cd(Ⅱ) | 307.68mgPb·g-1 211.48mgCu·g-1 188.20mgCd·g-1 | 静电引力、外球络合、边界层扩散、吸热机制 | [ |
| 10%H2O2溶液浸渍氧化生物炭 | H2O2活化生物炭 (牦牛粪便) | Pb(Ⅱ) Cu(Ⅱ) Zn(Ⅱ) Cd(Ⅱ) | 169.57mgPb·g-1 71.39mgCu·g-1 42.21mgZn·g-1 82.95mgCd·g-1 (2.2倍、1.6倍、1.7倍、1.5倍) | 重金属-羧基络合、Pb-碳酸盐/磷酸盐沉淀 | [ |
| 生物炭与CMC化学共沉淀(先后逐滴加入戊二醛,乙酸) | 羧甲基壳聚糖(CMC)涂覆污泥生物炭 | Pb(Ⅱ) Hg(Ⅱ) | 210mgPb·g-1(<60min) 594.17mgHg·g-1 | 表面络合 | [ |
| 生物炭的液相还原改性(NaBH4) | 固定化nZVI污泥生物炭 | Cr(Ⅵ) Pb(Ⅱ) | Cr6+:90%,Pb2+:82% (<30min) | 配体交换、氧化还原、表面沉淀 | [ |
| 改性合成方法 | 炭基吸附剂(生物质原料) | PPCPs类型 | 脱除率(R max)/ 吸附容量(Q m) | 吸附机理 | 参考 文献 |
|---|---|---|---|---|---|
| 生物炭与铁氧体(40∶1)球磨法物理混合 | 超细磁性生物炭 (椰子、松子、核桃壳) | 卡马西平(CBZ) 四环素(TC) | 62.7mgCBZ·g-1; 94.2mgTC·g-1 | — | [ |
| 生物炭(450~500℃快速热解) 碱处理后,甲醇溶液浸渍活化 | 甲醇改性生物炭(稻壳) | 四环素 | 较BC提高45.6% | n-π EDA相互作用、氢键 | [ |
| 生物炭与PEG10000加入MoS2纳米片合成溶液中,整体于反应釜180℃水热合成24h | g-MoS2修饰生物炭基纳米材料(稻秸) | 盐酸四环素 | 249.45mg·g-1 | 孔隙充填、静电引力、氢键、π-π共轭 | [ |
| 分别用高低浓度(0.1mol·L-1, 0.075mol·L-1)的CeCl3溶液与生物质浸渍混合后600℃热解 | 多孔纳米氧化铈负载生物炭(松木片) | 左氧氟沙星 | 73.0mg·g-1(0.1mol·L-1 WHC) 14.2mg·g-1(0.075 mol·L-1 WLC) (WHC,WLC分别较原生物炭提高9.46倍和1.84倍) | 氢键、孔隙充填(WLC)、表面络合(WHC) | [ |
| 生物炭(300℃热解15min得到)于4mol·L-1 NaOH室温浸渍并干燥后, 整体二次热解(800℃,2h) | NaOH碱活化生物炭 (去皮火炬松木) | 四环素 | 274.8mg·g-1 | π-π EDA相互作用、氢键 | [ |
| 生物炭置于水中(通N2),先后加入Fe(NO3)3, NaOH浸渍老化60h | 针铁矿纳米颗粒改性生物炭(棕榈木) | 泰乐菌素 | 5.39mg·g-1 | 疏水作用、静电引力、氢键、阳离子交换、π-π EDA相互作用 | [ |
| 生物炭(400℃热解2h得到)于H3PO4溶液浸渍后整体600℃二次热解2h | 功能化改性生物炭(桉木) | 磺胺甲嘧啶(SMT) 磺胺甲恶唑(SMX) 磺胺噻唑(STZ) 氯霉素(CP) | STZ>SMX>CP>SMT (pH4.0~4.25) | 氢键(磺胺类)、电荷辅助氢键(CAHB, 对CP)、π-π作用 | [ |
| 生物质浸渍于含十二烷基苯磺酸钠(SDBS)分散剂的碳纳米管(CNT)悬浮液, 干燥后600℃热解 | CNT-生物炭复合物(CNT-SDBS-HC,CNT-SDBS-BC) 山核桃木(HC)/甘蔗残渣(BC) | 磺胺吡啶(SPY),Pb(II) | R max SPY86%,Pb71%(CNT-SDBS-HC) R max SPY56%,Pb53%(CNT-SDBS-BC) | π-π相互作用、疏水作用(SPY);表面络合、阳离子交换(Pb) | [ |
| 加入0.4g 1,3,6,8-芘四碳酸四钠盐(PySA) 后,生物质与GO悬液充分混合老化后600℃热解1h | 石墨烯氧化物(GO)包覆生物炭纳米复合物(竹屑) | 磺胺甲嘧啶 | Q m是BC的2.14倍 | π-π相互作用、孔隙充填、阳离子交换、氢键、静电引力 | [ |
| 生物炭加入壳聚糖的乙酸悬液, 充分搅拌后滴加NaOH并老化24h | 壳聚糖/生物炭水凝胶珠 (柚子皮) | 环丙沙星 | >76mg·g-1 (Q 0 160mg·L-1) | π-π相互作用、氢键、疏水作用 | [ |
| H3PO4浸渍生物炭 | H3PO4改性稻秆(RCA)/ 畜肥(SCA)生物炭 | 四环素 | RCA 552.0mg·g-1; SCA 365.4mg·g-1 | 氢键、π-π相互作用、静电引力 | [ |
表4 生物炭基材料改性及吸附PPCPs的应用 (热解未特别标注均为N2氛围慢速热解)
| 改性合成方法 | 炭基吸附剂(生物质原料) | PPCPs类型 | 脱除率(R max)/ 吸附容量(Q m) | 吸附机理 | 参考 文献 |
|---|---|---|---|---|---|
| 生物炭与铁氧体(40∶1)球磨法物理混合 | 超细磁性生物炭 (椰子、松子、核桃壳) | 卡马西平(CBZ) 四环素(TC) | 62.7mgCBZ·g-1; 94.2mgTC·g-1 | — | [ |
| 生物炭(450~500℃快速热解) 碱处理后,甲醇溶液浸渍活化 | 甲醇改性生物炭(稻壳) | 四环素 | 较BC提高45.6% | n-π EDA相互作用、氢键 | [ |
| 生物炭与PEG10000加入MoS2纳米片合成溶液中,整体于反应釜180℃水热合成24h | g-MoS2修饰生物炭基纳米材料(稻秸) | 盐酸四环素 | 249.45mg·g-1 | 孔隙充填、静电引力、氢键、π-π共轭 | [ |
| 分别用高低浓度(0.1mol·L-1, 0.075mol·L-1)的CeCl3溶液与生物质浸渍混合后600℃热解 | 多孔纳米氧化铈负载生物炭(松木片) | 左氧氟沙星 | 73.0mg·g-1(0.1mol·L-1 WHC) 14.2mg·g-1(0.075 mol·L-1 WLC) (WHC,WLC分别较原生物炭提高9.46倍和1.84倍) | 氢键、孔隙充填(WLC)、表面络合(WHC) | [ |
| 生物炭(300℃热解15min得到)于4mol·L-1 NaOH室温浸渍并干燥后, 整体二次热解(800℃,2h) | NaOH碱活化生物炭 (去皮火炬松木) | 四环素 | 274.8mg·g-1 | π-π EDA相互作用、氢键 | [ |
| 生物炭置于水中(通N2),先后加入Fe(NO3)3, NaOH浸渍老化60h | 针铁矿纳米颗粒改性生物炭(棕榈木) | 泰乐菌素 | 5.39mg·g-1 | 疏水作用、静电引力、氢键、阳离子交换、π-π EDA相互作用 | [ |
| 生物炭(400℃热解2h得到)于H3PO4溶液浸渍后整体600℃二次热解2h | 功能化改性生物炭(桉木) | 磺胺甲嘧啶(SMT) 磺胺甲恶唑(SMX) 磺胺噻唑(STZ) 氯霉素(CP) | STZ>SMX>CP>SMT (pH4.0~4.25) | 氢键(磺胺类)、电荷辅助氢键(CAHB, 对CP)、π-π作用 | [ |
| 生物质浸渍于含十二烷基苯磺酸钠(SDBS)分散剂的碳纳米管(CNT)悬浮液, 干燥后600℃热解 | CNT-生物炭复合物(CNT-SDBS-HC,CNT-SDBS-BC) 山核桃木(HC)/甘蔗残渣(BC) | 磺胺吡啶(SPY),Pb(II) | R max SPY86%,Pb71%(CNT-SDBS-HC) R max SPY56%,Pb53%(CNT-SDBS-BC) | π-π相互作用、疏水作用(SPY);表面络合、阳离子交换(Pb) | [ |
| 加入0.4g 1,3,6,8-芘四碳酸四钠盐(PySA) 后,生物质与GO悬液充分混合老化后600℃热解1h | 石墨烯氧化物(GO)包覆生物炭纳米复合物(竹屑) | 磺胺甲嘧啶 | Q m是BC的2.14倍 | π-π相互作用、孔隙充填、阳离子交换、氢键、静电引力 | [ |
| 生物炭加入壳聚糖的乙酸悬液, 充分搅拌后滴加NaOH并老化24h | 壳聚糖/生物炭水凝胶珠 (柚子皮) | 环丙沙星 | >76mg·g-1 (Q 0 160mg·L-1) | π-π相互作用、氢键、疏水作用 | [ |
| H3PO4浸渍生物炭 | H3PO4改性稻秆(RCA)/ 畜肥(SCA)生物炭 | 四环素 | RCA 552.0mg·g-1; SCA 365.4mg·g-1 | 氢键、π-π相互作用、静电引力 | [ |
| 改性合成方法 | 炭基吸附剂(生物质原料) | 污染物 | 脱除率(R max)/ 吸附容量(Q m) | 吸附机理 | 参考 文献 |
|---|---|---|---|---|---|
| 两种改性方式: 生物质(1)或生物炭(2)与盐溶液浸渍老化, 管式炉600℃煅烧1h | 二元多类型(Zn/Al,Mg/Al,Ni/Fe)水滑石LDH组装生物炭复合体(玉米秸秆) | P | 152.1mg·g-1 (B-Zn/Al-LDH)(1) | 层间阴离子交换、表面络合 | [ |
| 生物炭与Fe、La盐溶液共沉淀 | La(OH)3改性磁性生物炭(菠萝皮) | P | 101.16mg·g-1 (BC的28倍) | 沉淀、静电引力、配体交换、 内球络合 | [ |
| 加入Mg2+、Fe3+溶液后调pH约10,60℃充分混合4h (共沉淀)后干燥并800℃热解1h | MgFe2O4立方尖晶石改性生物炭纳米复合物(裙带菜根) | P | 80.4%;163.02mgP·g-1 (BC、MgFe2O4的13.5倍和3.7倍) | 内球络合 | [ |
| 生物质与Fe2+、Fe3+和LaCl3或CeCl3溶液混合,滴加氨水共沉淀 | 镧/铈掺杂磁性生物炭(芦苇) | P | La/Fe-BC: 20.5mg·g-1 (Ce/Fe-BC、Fe-BC的1.6倍、2.9倍) | 内球络合、静电引力 | [ |
| 生物炭与La(NO3)3化学共沉淀 | 镧改性生物炭(柚子皮) | F | 19.86mgF·g-1 | 静电引力、离子交换 | [ |
| 生物质与MgCl2溶液混合后热解 | MgO纳米片复合生物炭 (甜菜,甘蔗渣,杨木,松木,花生壳) | P NO3 --N | 835mgN·g-1(甜菜); 95mgN·g-1(花生壳); (BC的11倍) | — | [ |
| 生物炭(闪速热解)与FeCl3浸渍后二次热解 | α-Fe2O3/Fe3O4磁性生物炭(道格拉斯冷杉) | NO3 --N F | 15mgN·g-1; 9mgF·g-1 | 静电引力、氢键 | [ |
| 生物质与LaCl3溶液充分混合并干燥后多温度下热解(300℃、400℃、500℃、600℃)0.5h | 镧改性生物炭(橡树锯末) | NH4 +-N NO3 --N P | 10.1mgN·g-1, 100.0mgN·g-1, 142.7mgN·g-1 (2.9倍、12.2倍、5.5倍) | 路易斯酸碱作用 | [ |
| 生物质与不同浓度的镁盐浸渍老化后,于550℃焙烧1h | MgO浸渍生物炭 (甘蔗残渣) | P NH4 +-N 腐殖酸盐(HM) | 20%Mg最佳: 398mgP·g-1 22mgN·g-1 247mgHM·g-1 | 鸟粪石沉淀、静电引力、π-π相互作用 | [ |
| 生物质磷酸液活化后,分别与乙酸铵(AA) 和氯化铵(AC)溶液浸渍后微波热解(450℃,15min) | 乙酸铵活化氮掺杂生物炭 (PAB-AA/PAB-AC) (芦苇) | 酸性红18 | BC的2.41倍、2.18倍 | π-π相互作用、孔隙填充、路易斯酸碱作用、静电引力、氢键 | [ |
| ZnCl2与CMC和生物炭混合为悬液后被NaBH4还原(湿化学法) | 羧甲基纤维素(CMC)固定化 纳米ZnO/生物炭复合物 (nZORc/BC) (竹子) | 亚甲基蓝(MB) | 17.01g·kg-1 (不含CMC的20倍) | 静电引力、离子交换 | [ |
| 生物质与镁铝盐溶液(2∶1,3∶1,4∶1)混合后, 滴加NaOH (pH 10)化学共沉淀 | Mg/Al-LDH/BC复合物(牛骨) | 亚甲基蓝(MB) | 406.47mgMB·g-1 | — | [ |
| CaCl2浸渍生物质后热解 | 钙盐改性生物炭 (山核桃坚果壳) | 酸性蓝74, 活性蓝4 | BC的3倍以上 | 静电引力 | [ |
| 生物炭(400~500℃热解0.5h)KOH碱活化并干燥后683℃再热解53min | 城市固废活性生物炭 (60%纸质, 25%园林垃圾, 15%纺织品) | 对乙酰氨基酚(APAP) 亚甲基蓝(MB) | R max MB99.9%, R max APAP63.7% | 静电引力、表面络合 | [ |
| 生物质于酸液中水热炭化(200℃,3h)/生物质先于碱液中水热炭化(200℃,3h),再与上述酸液混合二次水热炭化(酸辅助/两步水热反应) | 水热炭化生物炭(竹屑) | 刚果红(CR) 2-萘酚(NP) | 90.51mgCR·g-1 72.93mgNP·g-1 | 疏水作用、表面络合 | [ |
| 蒙脱石与稻壳悬液混合(1∶1)180℃水热炭化16h,产物甲醇浸渍,再混入不同浓度KOH碱液活化 | KOH改性蒙脱石/生物炭纳米复合物(稻壳) | 17β-雌二醇(E2) 17α-乙炔基雌二醇(EE2) | 138mgE2·g-1 69mgEE2·g-1 (KOH质量比1%时) | 疏水作用、π-π相互作用、静电引力、氢键 | [ |
| 分别在400℃、600℃、800℃热解后与Fe3+、Fe2+溶液共沉淀 (超声) | 磁性生物炭纳米颗粒(甘蔗渣) | 17β-雌二醇 | 50.24mg·g-1 (400℃) 41.71mg·g-1 (800℃) 34.06mg·g-1 (800℃) | 疏水作用、π-π相互作用 | [ |
| 生物炭(400℃热解)浸渍于H3PO4并600℃热解活化2h | H3PO4活化生物炭 (蓝桉树木屑) | 雌酮(E1) 17β-雌二醇(E2) 雌三醇(E3) 17α-乙炔基雌二醇(EE2) 双酚A(BPA) 4-叔丁基苯酚(4tBP) | R max 94%~100% E1>E2≥EE2>BPA>4tBP>E3 | 电荷辅助氢键(CAHB)、氢键、π-π相互作用 | [ |
| 生物质与GO(改进Hummers法制备)悬液充分混合后600℃热解1h | 生物炭基还原石墨烯氧化物(RGO)复合材料 (玉米秸秆) | 阿特拉津Pb(Ⅱ) | 26.10mgPb·g-1 67.55mg·g-1(atrazine) | 孔隙填充、静电引力、沉淀、π-π相互作用 | [ |
表5 生物炭基材料改性及吸附其他污染物的应用(热解未特别标注均为N2氛围慢速热解)
| 改性合成方法 | 炭基吸附剂(生物质原料) | 污染物 | 脱除率(R max)/ 吸附容量(Q m) | 吸附机理 | 参考 文献 |
|---|---|---|---|---|---|
| 两种改性方式: 生物质(1)或生物炭(2)与盐溶液浸渍老化, 管式炉600℃煅烧1h | 二元多类型(Zn/Al,Mg/Al,Ni/Fe)水滑石LDH组装生物炭复合体(玉米秸秆) | P | 152.1mg·g-1 (B-Zn/Al-LDH)(1) | 层间阴离子交换、表面络合 | [ |
| 生物炭与Fe、La盐溶液共沉淀 | La(OH)3改性磁性生物炭(菠萝皮) | P | 101.16mg·g-1 (BC的28倍) | 沉淀、静电引力、配体交换、 内球络合 | [ |
| 加入Mg2+、Fe3+溶液后调pH约10,60℃充分混合4h (共沉淀)后干燥并800℃热解1h | MgFe2O4立方尖晶石改性生物炭纳米复合物(裙带菜根) | P | 80.4%;163.02mgP·g-1 (BC、MgFe2O4的13.5倍和3.7倍) | 内球络合 | [ |
| 生物质与Fe2+、Fe3+和LaCl3或CeCl3溶液混合,滴加氨水共沉淀 | 镧/铈掺杂磁性生物炭(芦苇) | P | La/Fe-BC: 20.5mg·g-1 (Ce/Fe-BC、Fe-BC的1.6倍、2.9倍) | 内球络合、静电引力 | [ |
| 生物炭与La(NO3)3化学共沉淀 | 镧改性生物炭(柚子皮) | F | 19.86mgF·g-1 | 静电引力、离子交换 | [ |
| 生物质与MgCl2溶液混合后热解 | MgO纳米片复合生物炭 (甜菜,甘蔗渣,杨木,松木,花生壳) | P NO3 --N | 835mgN·g-1(甜菜); 95mgN·g-1(花生壳); (BC的11倍) | — | [ |
| 生物炭(闪速热解)与FeCl3浸渍后二次热解 | α-Fe2O3/Fe3O4磁性生物炭(道格拉斯冷杉) | NO3 --N F | 15mgN·g-1; 9mgF·g-1 | 静电引力、氢键 | [ |
| 生物质与LaCl3溶液充分混合并干燥后多温度下热解(300℃、400℃、500℃、600℃)0.5h | 镧改性生物炭(橡树锯末) | NH4 +-N NO3 --N P | 10.1mgN·g-1, 100.0mgN·g-1, 142.7mgN·g-1 (2.9倍、12.2倍、5.5倍) | 路易斯酸碱作用 | [ |
| 生物质与不同浓度的镁盐浸渍老化后,于550℃焙烧1h | MgO浸渍生物炭 (甘蔗残渣) | P NH4 +-N 腐殖酸盐(HM) | 20%Mg最佳: 398mgP·g-1 22mgN·g-1 247mgHM·g-1 | 鸟粪石沉淀、静电引力、π-π相互作用 | [ |
| 生物质磷酸液活化后,分别与乙酸铵(AA) 和氯化铵(AC)溶液浸渍后微波热解(450℃,15min) | 乙酸铵活化氮掺杂生物炭 (PAB-AA/PAB-AC) (芦苇) | 酸性红18 | BC的2.41倍、2.18倍 | π-π相互作用、孔隙填充、路易斯酸碱作用、静电引力、氢键 | [ |
| ZnCl2与CMC和生物炭混合为悬液后被NaBH4还原(湿化学法) | 羧甲基纤维素(CMC)固定化 纳米ZnO/生物炭复合物 (nZORc/BC) (竹子) | 亚甲基蓝(MB) | 17.01g·kg-1 (不含CMC的20倍) | 静电引力、离子交换 | [ |
| 生物质与镁铝盐溶液(2∶1,3∶1,4∶1)混合后, 滴加NaOH (pH 10)化学共沉淀 | Mg/Al-LDH/BC复合物(牛骨) | 亚甲基蓝(MB) | 406.47mgMB·g-1 | — | [ |
| CaCl2浸渍生物质后热解 | 钙盐改性生物炭 (山核桃坚果壳) | 酸性蓝74, 活性蓝4 | BC的3倍以上 | 静电引力 | [ |
| 生物炭(400~500℃热解0.5h)KOH碱活化并干燥后683℃再热解53min | 城市固废活性生物炭 (60%纸质, 25%园林垃圾, 15%纺织品) | 对乙酰氨基酚(APAP) 亚甲基蓝(MB) | R max MB99.9%, R max APAP63.7% | 静电引力、表面络合 | [ |
| 生物质于酸液中水热炭化(200℃,3h)/生物质先于碱液中水热炭化(200℃,3h),再与上述酸液混合二次水热炭化(酸辅助/两步水热反应) | 水热炭化生物炭(竹屑) | 刚果红(CR) 2-萘酚(NP) | 90.51mgCR·g-1 72.93mgNP·g-1 | 疏水作用、表面络合 | [ |
| 蒙脱石与稻壳悬液混合(1∶1)180℃水热炭化16h,产物甲醇浸渍,再混入不同浓度KOH碱液活化 | KOH改性蒙脱石/生物炭纳米复合物(稻壳) | 17β-雌二醇(E2) 17α-乙炔基雌二醇(EE2) | 138mgE2·g-1 69mgEE2·g-1 (KOH质量比1%时) | 疏水作用、π-π相互作用、静电引力、氢键 | [ |
| 分别在400℃、600℃、800℃热解后与Fe3+、Fe2+溶液共沉淀 (超声) | 磁性生物炭纳米颗粒(甘蔗渣) | 17β-雌二醇 | 50.24mg·g-1 (400℃) 41.71mg·g-1 (800℃) 34.06mg·g-1 (800℃) | 疏水作用、π-π相互作用 | [ |
| 生物炭(400℃热解)浸渍于H3PO4并600℃热解活化2h | H3PO4活化生物炭 (蓝桉树木屑) | 雌酮(E1) 17β-雌二醇(E2) 雌三醇(E3) 17α-乙炔基雌二醇(EE2) 双酚A(BPA) 4-叔丁基苯酚(4tBP) | R max 94%~100% E1>E2≥EE2>BPA>4tBP>E3 | 电荷辅助氢键(CAHB)、氢键、π-π相互作用 | [ |
| 生物质与GO(改进Hummers法制备)悬液充分混合后600℃热解1h | 生物炭基还原石墨烯氧化物(RGO)复合材料 (玉米秸秆) | 阿特拉津Pb(Ⅱ) | 26.10mgPb·g-1 67.55mg·g-1(atrazine) | 孔隙填充、静电引力、沉淀、π-π相互作用 | [ |
| 1 | NURCHI V M , VILLAESCUSA I . Sorption of toxic metal ions by solid sorbents: a predictive speciation approach based on complex formation constants in aqueous solution[J]. Coordination Chemistry Reviews, 2012, 256(1): 212-221. |
| 2 | 赵迎新, 王亚舒, 季民, 等 . 吸附法去除水中药品及个人护理品(PPCPs)研究进展[J]. 工业水处理, 2017(6): 1-5. |
| ZHAO Y X , WANG Y S , JI M , et al . Research progress in the removal of pharmaceutical and personal care products (PPCPs) from water by adsorption method[J]. Industrial Water Treatment, 2017(6): 1-5. | |
| 3 | 刘桂芳, 闫红梅, 高远, 等 . 碳材料吸附水中PPCPs的研究进展[J]. 工业水处理, 2015(10): 6-11. |
| LIU G F , YAN H M , GAO Y , et al . Advances in the adsorption of PPCPs in water by carbon materials[J]. Industrial Water Treatment, 2015(10): 6-11. | |
| 4 | ABDELMELEK S BEN , GREAVES J , ISHIDA K P , et al . Removal of pharmaceutical and personal care products from reverse osmosis retentate using advanced oxidation processes[J]. Environmental Science & Technology, 2011, 45(8): 3665-3671. |
| 5 | MESTRE A S , PIRES J , NOGUEIRA J M F , et al . Activated carbons for the adsorption of ibuprofen[J]. Carbon, 2007, 45(10): 1979-1988. |
| 6 | YU Z R , PELDSZUS S , HUCK M . Adsorption characteristics of selected pharmaceuticals and an endocrine disrupting compound—naproxen, carbamazepine and nonylphenol—on activated carbon[J]. Water Research, 2008, 42(12): 2873-2882. |
| 7 | OLLER I , MALATO S , SANCHEZ-PEREZ J A . Combination of advanced oxidation processes and biological treatments for wastewater decontamination—A review[J]. Science of the Total Environment, 2011, 409(20): 4141-4166. |
| 8 | NOROUZIAN R S , LAKOURAJ M . Preparation and heavy metal ion adsorption behavior of novel supermagnetic nanocomposite based on thiacalix[4]arene and polyaniline: conductivity, isotherm and kinetic study[J]. Synthetic Metals, 2015, 203: 135-148. |
| 9 | ALTMANN J , RUHL A S , ZIETZSCHMANN F , et al . Direct comparison of ozonation and adsorption onto powdered activated carbon for micropollutant removal in advanced wastewater treatment[J]. Water Research, 2014, 55: 185-193. |
| 10 | 王怀臣, 冯雷雨, 陈银广 . 废物资源化制备生物质炭及其应用的研究进展[J]. 化工进展, 2012, 31(4): 907-914. |
| WANG H C , FENG L Y , CHEN Y G . Advances in biochar production from wastes and its applications[J]. Chemical Industry and Engineering Progress, 2012, 31(4): 907-914. | |
| 11 | YI S Z , SUN Y Y , HU X , et al . Porous nano-cerium oxide wood chip biochar composites for aqueous levofloxacin removal and sorption mechanism insights[J]. Environmental Science and Pollution Research, 2018, 25(26): 25629-25637. |
| 12 | TAKAYA C A , FLETCHER L A , SINGH S , et al . Recovery of phosphate with chemically modified biochars[J]. Journal of Environmental Chemical Engineering, 2016, 4(1): 1156-1165. |
| 13 | WANG L , WANG J Y , WANG Z X , et al . Enhanced antimonate [Sb(Ⅴ)] removal from aqueous solution by La-doped magnetic biochars[J]. Chemical Engineering Journal, 2018, 354: 623-632. |
| 14 | JUNG K W , LEE S, LEE Y J . Synthesis of novel magnesium ferrite (MgFe2O4)/biochar magnetic composites and its adsorption behavior for phosphate in aqueous solutions[J]. Bioresource Technology, 2017, 245: 751-759. |
| 15 | WANG S J , GUO W , GAO F , et al . Lead and uranium sorptive removal from aqueous solution using magnetic and nonmagnetic fast pyrolysis rice husk biochars[J]. RSC Advances, 2018, 8(24): 13205-13217. |
| 16 | ZHANG M , GAO B , YAO Y , et al . Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions[J]. Chemical Engineering Journal, 2012, 210: 26-32. |
| 17 | QIU H , LIANG C , YU J H , et al . Preferable phosphate sequestration by nano-La(Ⅲ) (hydr)oxides modified wheat straw with excellent properties in regeneration[J]. Chemical Engineering Journal, 2017, 315: 345-354. |
| 18 | LI R H , WANG J J , ZHOU B Y , et al . Recovery of phosphate from aqueous solution by magnesium oxide decorated magnetic biochar and its potential as phosphate-based fertilizer substitute[J]. Bioresource Technology, 2016, 215: 209-214. |
| 19 | FRANCISKI M A , PERES E C , GODINHO M , et al . Development of CO2 activated biochar from solid wastes of a beer industry and its application for methylene blue adsorption[J]. Waste Management, 2018, 78: 630-638. |
| 20 | KOŁODYŃSKA D , WNĘTRZAK R , LEAHY J J , et al . Kinetic and adsorptive characterization of biochar in metal ions removal[J]. Chemical Engineering Journal, 2012, 197: 295-305. |
| 21 | WANG Y , LIU R H . H2O2 treatment enhanced the heavy metals removal by manure biochar in aqueous solutions[J]. Science of the Total Environment, 2018, 628/629: 1139-1148. |
| 22 | DAI L C , ZHU W K , HE L , et al . Calcium-rich biochar from crab shell: an unexpected super adsorbent for dye removal[J]. Bioresource Technology, 2018, 267: 510-516. |
| 23 | DIAO Z H , DU J J , JIANG D , et al . Insights into the simultaneous removal of Cr6+ and Pb2+ by a novel sewage sludge-derived biochar immobilized nanoscale zero valent iron: coexistence effect and mechanism[J]. Science of the Total Environment, 2018, 642: 505-515. |
| 24 | SCHMIDT M W I , NOACK A G . Black carbon in soils and sediments: analysis, distribution, implications, and current challenges[J]. Global Biogeochemical Cycles, 2000, 14(3): 777-793. |
| 25 | CORNELISSEN G , KUKULSKA Z , KALAITZIDIS S , et al . Relations between environmental black carbon sorption and geochemical sorbent characteristics[J]. Environmental Science & Technology, 2004, 38(13): 3632-40. |
| 26 | 杨广西 . 生物炭的化学改性及其对铜的吸附研究[D]. 合肥: 中国科学技术大学, 2014. |
| YANG G X . Chemical modification of biochar and its adsorption toward copper(Ⅱ) [D]. Hefei: University of Science and Technology of China, 2014. | |
| 27 | 袁帅, 赵立欣, 孟海波, 等 . 生物炭主要类型、理化性质及其研究展望[J]. 植物营养与肥料学报, 2016, 22(5): 1402-1417. |
| YUAN S , ZHAO L X , MENG H B , et al . The main types of biochar and their properties and expectative researches[J]. Journal of Plant Nutrition and Fertilizer, 2016, 22(5): 1402-1417. | |
| 28 | OK Y S, CHANG S X , GAO B , et al . SMART biochar technology—A shifting paradigm towards advanced materials and healthcare research[J]. Environmental Technology & Innovation, 2015, 4: 206-209. |
| 29 | FANG J , ZHAN L , OK Y S, et al . Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass[J]. Journal of Industrial and Engineering Chemistry, 2018, 57: 15-21. |
| 30 | AHMED M B , ZHOU J L , NGO H H, et al . Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater[J]. Bioresource Technology, 2016, 214: 836-851. |
| 31 | CHEN T W , LUO L , DENG S H , et al . Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure[J]. Bioresource Technology, 2018, 267: 431-437. |
| 32 | PENG H B , GAO P , CHU G , et al . Enhanced adsorption of Cu(Ⅱ) and Cd(Ⅱ) by phosphoric acid-modified biochars[J]. Environmental Pollution, 2017, 229: 846-853. |
| 33 | JIN J , LI S W , PENG X Q , et al . HNO3 modified biochars for uranium (Ⅵ) removal from aqueous solution[J]. Bioresource Technology, 2018, 256: 247-253. |
| 34 | RAJAPAKSHA A U , CHEN S S , TSANG D C W , et al . Engineered/designer biochar for contaminant removal/immobilization from soil and water: potential and implication of biochar modification[J]. Chemosphere, 2016, 148: 276-291. |
| 35 | JANG H M , YOO S, CHOI Y K , et al . Adsorption isotherm, kinetic modeling and mechanism of tetracycline on Pinus taeda-derived activated biochar[J]. Bioresource Technology, 2018, 259: 24-31. |
| 36 | YANG F , ZHANG S S , LI H P , et al . Corn straw-derived biochar impregnated with alpha-FeOOH nanorods for highly effective copper removal[J]. Chemical Engineering Journal, 2018, 348: 191-201. |
| 37 | ZHOU X H , ZHOU J J , LIU Y C , et al . Preparation of iminodiacetic acid-modified magnetic biochar by carbonization, magnetization and functional modification for Cd(Ⅱ) removal in water[J]. Fuel, 2018, 233: 469-479. |
| 38 | WANG S S , GAO B , LI Y C , et al . Adsorptive removal of arsenate from aqueous solutions by biochar supported zero-valent iron nanocomposite: batch and continuous flow tests[J]. Journal of Hazardous Materials, 2017, 322: 172-181. |
| 39 | YANG F , ZHANG S S , SUN Y Q , et al . Fabrication and characterization of hydrophilic corn stalk biochar-supported nanoscale zero-valent iron composites for efficient metal removal[J]. Bioresource Technology, 2018, 265: 490-497. |
| 40 | ZHANG M , GAO B . Removal of arsenic, methylene blue, and phosphate by biochar/AlOOH nanocomposite[J]. Chemical Engineering Journal, 2013, 226: 286-292. |
| 41 | YIN Q Q , REN H P , WANG R K , et al . Evaluation of nitrate and phosphate adsorption on Al-modified biochar: influence of Al content[J]. Science of the Total Environment, 2018, 631/632: 895-903. |
| 42 | ZHOU Y Y , LIU X C , XIANG Y J , et al . Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: adsorption mechanism and modelling[J]. Bioresource Technology, 2017, 245: 266-273. |
| 43 | LI R H , WANG J J , ZHOU B Y , et al . Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios[J]. Science of the Total Environment, 2016, 559: 121-129. |
| 44 | HU F P , WANG M , PENG X M , et al . High-efficient adsorption of phosphates from water by hierarchical CuAl/biomass carbon fiber layered double hydroxide[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 555: 314-323. |
| 45 | XUE L H , GAO B , WAN Y S , et al . High efficiency and selectivity of MgFe-LDH modified wheat-straw biochar in the removal of nitrate from aqueous solutions[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 63: 312-317. |
| 46 | REDDY D . H K, YUN Y S. Spinel ferrite magnetic adsorbents: alternative future materials for water purification?[J]. Coordination Chemistry Reviews, 2016, 315: 90-111. |
| 47 | WANG Y Y , JI H Y , LU H H , et al . Simultaneous removal of Sb(Ⅲ) and Cd(Ⅱ) in water by adsorption onto a MnFe2O4 biochar nanocomposite[J]. RSC Advances, 2018, 8(6): 3264-3273. |
| 48 | WANG L , WANG J Y , HE C , et al . Development of rare earth element doped magnetic biochars with enhanced phosphate adsorption performance[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 561: 236-243. |
| 49 | TAN X F , LIU Y G , GU Y L , et al . Biochar-based nano-composites for the decontamination of wastewater: a review[J]. Bioresource Technology, 2016, 212: 318-333. |
| 50 | ZAIDI N A H M , LIM L B L, USMAN A . Enhancing adsorption of Pb(Ⅱ) from aqueous solution by NaOH and EDTA modified Artocarpus odoratissimus leaves[J]. Journal of Environmental Chemical Engineering, 2018, 6(6): 7172-7184. |
| 51 | WANG W Y , YUE Q Y , XU X , et al . Optimized conditions in preparation of giant reed quaternary amino anion exchanger for phosphate removal[J]. Chemical Engineering Journal, 2010, 157(1): 161-167. |
| 52 | WANG L , CHEN Z Z , WEN H , et al . Microwave assisted modification of activated carbons by organic acid ammoniums activation for enhanced adsorption of Acid Red 18[J]. Powder Technology, 2018, 323: 230-237. |
| 53 | INYANG M , GAO B , ZIMMERMAN A , et al . Sorption and cosorption of lead and sulfapyridine on carbon nanotube-modified biochars[J]. Environmental Science and Pollution Research, 2015, 22(3): 1868-1876. |
| 54 | JING X R , WANG Y Y , LIU W J , et al . Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar[J]. Chemical Engineering Journal, 2014, 248: 168-174. |
| 55 | AFZAL M Z , SUN X F , LIU J , et al . Enhancement of ciprofloxacin sorption on chitosan/biochar hydrogel beads[J]. Science of the Total Environment, 2018, 639: 560-569. |
| 56 | ZENG Z T , YE S J , WU H P , et al . Research on the sustainable efficacy of g-MoS2 decorated biochar nanocomposites for removing tetracycline hydrochloride from antibiotic-polluted aqueous solution[J]. Science of the Total Environment, 2019, 648: 206-217. |
| 57 | HUANG D L , WANG X , ZHANG C , et al . Sorptive removal of ionizable antibiotic sulfamethazine from aqueous solution by graphene oxide-coated biochar nanocomposites: influencing factors and mechanism[J]. Chemosphere, 2017, 186: 414-421. |
| 58 | ZHANG Y , CAO B , ZHAO L L , et al . Biochar-supported reduced graphene oxide composite for adsorption and coadsorption of atrazine and lead ions[J]. Applied Surface Science, 2018, 427: 147-155. |
| 59 | YAN L L , KONG L , QU Z , et al . Magnetic biochar decorated with ZnS nanocrytals for Pb(Ⅱ) removal[J]. ACS Sustainable Chemistry & Engineering, 2015, 3(1): 125-132. |
| 60 | DONG H R , DENG J M , XIE Y K , et al . Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(Ⅵ) removal from aqueous solution[J]. Journal of Hazardous Materials, 2017, 332: 79-86. |
| 61 | YANG Y Q , CHEN N , FENG C P , et al . Chromium removal using a magnetic corncob biochar/polypyrrole composite by adsorption combined with reduction: reaction pathway and contribution degree[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2018, 556: 201-209. |
| 62 | 张连科, 王洋, 王维大, 等 . 生物炭负载纳米羟基磷灰石复合材料的制备及对铅离子的吸附特性[J]. 化工进展, 2018, 37(9): 3492-3501. |
| ZHANG L K , WANG Y , WANG W D , et al . The preparation of biochar-supported nano-hydroxyapatite and its adsorption of Pb2+ [J]. Chemical Industry and Engineering Progress, 2018, 37(9): 3492-3501. | |
| 63 | WANG T , LI C , WANG C Q , et al . Biochar/MnAl-LDH composites for Cu(Ⅱ) removal from aqueous solution[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 538: 443-450. |
| 64 | ZHOU X H , LIU Y C , ZHOU J J , et al . Efficient removal of lead from aqueous solution by urea-functionalized magnetic biochar: preparation, characterization and mechanism study[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 91: 457-467. |
| 65 | WANG H Y , GAO B , WANG S S , et al . Removal of Pb(Ⅱ), Cu(Ⅱ), and Cd(Ⅱ) from aqueous solutions by biochar derived from KMnO4 treated hickory wood[J]. Bioresource Technology, 2015, 197: 356-362. |
| 66 | ZHAO T , YAO Y , LI D R , et al . Facile low-temperature one-step synthesis of pomelo peel biochar under air atmosphere and its adsorption behaviors for Ag(Ⅰ) and Pb(Ⅱ)[J]. Science of the Total Environment, 2018, 640: 73-79. |
| 67 | JUNG K W , LEE S Y, LEE Y J . Facile one-pot hydrothermal synthesis of cubic spinel-type manganese ferrite/biochar composites for environmental remediation of heavy metals from aqueous solutions[J]. Bioresource Technology, 2018, 261: 1-9. |
| 68 | IFTHIKAR J , JIAO X , NGAMBIA A , et al . Facile one-pot synthesis of sustainable carboxymethyl chitosan-sewage sludge biochar for effective heavy metal chelation and regeneration[J]. Bioresource Technology, 2018, 262: 22-31. |
| 69 | LIAN F , SUN B B , SONG Z G , et al . Physicochemical properties of herb-residue biochar and its sorption to ionizable antibiotic sulfamethoxazole[J]. Chemical Engineering Journal, 2014, 248: 128-134. |
| 70 | SHAN D N , DENG S B , ZHAO T N , et al . Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling[J]. Journal of Hazardous Materials, 2016, 305: 156-163. |
| 71 | GUO X T , DONG H , YANG C , et al . Application of goethite modified biochar for tylosin removal from aqueous solution[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2016, 502: 81-88. |
| 72 | AHMED M B , ZHOU J L , NGO H H, et al . Competitive sorption affinity of sulfonamides and chloramphenicol antibiotics toward functionalized biochar for water and wastewater treatment[J]. Bioresource Technology, 2017, 238: 306-312. |
| 73 | INYANG M , GAO B , ZIMMERMAN A , et al . Sorption and cosorption of lead and sulfapyridine on carbon nanotube-modified biochars[J]. Environmental Science and Pollution Research, 2015, 22(3): 1868-1876. |
| 74 | HAN D , LIU X , ZHANG G , et al . Effects of cationic surfactant on pentachlorophenol sorption by sediment, active carbon and biochar [J]. Fresenius Environmental Bulletin, 2013, 22(4): 1280-1286. |
| 75 | YANG F , ZHANG S S , SUN Y Q , et al . Assembling biochar with various layered double hydroxides for enhancement of phosphorus recovery[J]. Journal of Hazardous Materials, 2019, 365: 665-673. |
| 76 | LIAO T W , LI T , SU X D , et al . La(OH)3-modified magnetic pineapple biochar as novel adsorbents for efficient phosphate removal[J]. Bioresource Technology, 2018, 263: 207-213. |
| 77 | WANG J G , CHEN N , FENG C P , et al . Performance and mechanism of fluoride adsorption from groundwater by lanthanum-modified pomelo peel biochar[J]. Environmental Science and Pollution Research, 2018, 25(16): 15326-15335. |
| 78 | DEWAGE N B , LIYANAGE A S , PITTMAN C U , et al . Fast nitrate and fluoride adsorption and magnetic separation from water on alpha-Fe2O3 and Fe3O4 dispersed on Douglas fir biochar[J]. Bioresource Technology, 2018, 263: 258-265. |
| 79 | WANG Z H , GUO H Y , SHEN F , et al . Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4 +), nitrate (NO3 -), and phosphate (PO4 3-)[J]. Chemosphere, 2015, 119: 646-653. |
| 80 | LI R H , WANG J J , ZHOU B Y , et al . Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment[J]. Journal of Cleaner Production, 2017, 147: 96-107. |
| 81 | WANG L , YAN W , HE C , et al . Microwave-assisted preparation of nitrogen-doped biochars by ammonium acetate activation for adsorption of Acid Red 18[J]. Applied Surface Science, 2018, 433: 222-231. |
| 82 | WANG S S , ZHOU Y X , HAN S W , et al . Carboxymethyl cellulose stabilized ZnO/biochar nanocomposites: enhanced adsorption and inhibited photocatalytic degradation of Methylene Blue[J]. Chemosphere, 2018, 197: 20-25. |
| 83 | MEILI L , LINS P V , ZANTA C L P S , et al . MgAl-LDH/Biochar composites for Methylene Blue removal by adsorption[J]. Applied Clay Science, 2019, 168: 11-20. |
| 84 | AGUAYO-VILLARREAL I A , HERNANDEZ-MONTOYA V , RANGEL-VAZQUEZ N A , et al . Determination of QSAR properties of textile dyes and their adsorption on novel carbonaceous adsorbents[J]. Journal of Molecular Liquids, 2014, 196: 326-333. |
| 85 | SUMALINOG D A G , CAPAREDA S C , LUNA M D G DE . Evaluation of the effectiveness and mechanisms of acetaminophen and methylene blue dye adsorption on activated biochar derived from municipal solid wastes[J]. Journal of Environmental Management, 2018, 210: 255-262. |
| 86 | LI Y , MEAS A , SHAN S D , et al . Hydrochars from bamboo sawdust through acid assisted and two-stage hydrothermal carbonization for removal of two organics from aqueous solution[J]. Bioresource Technology, 2018, 261: 257-264. |
| 87 | TIAN S R , LIU Y G , LIU S B , et al . Hydrothermal synthesis of montmorillonite/hydrochar nanocomposites and application for 17 beta-estradiol and 17 alpha-ethynylestradiol removal[J]. RSC Advances, 2018, 8(8): 4273-4283. |
| 88 | DONG X W , HE L Z , HU H , et al . Removal of 17 beta-estradiol by using highly adsorptive magnetic biochar nanoparticles from aqueous solution[J]. Chemical Engineering Journal, 2018, 352: 371-379. |
| 89 | AHMED M B , ZHOU J L , NGO H H, et al . Sorptive removal of phenolic endocrine disruptors by functionalized biochar: competitive interaction mechanism, removal efficacy and application in wastewater[J]. Chemical Engineering Journal, 2018, 335: 801-811. |
| 90 | FENG Y F , LU H Y , LIU Y , et al . Nano-cerium oxide functionalized biochar for phosphate retention: preparation, optimization and rice paddy application[J]. Chemosphere, 2017, 185: 816-825. |
| 91 | XU K N , LIN F Y , DOU X M , et al . Recovery of ammonium and phosphate from urine as value-added fertilizer using wood waste biochar loaded with magnesium oxides[J]. Journal of Cleaner Production, 2018, 187: 205-214. |
| 92 | YAO Y , GAO B , CHEN J J , et al . Engineered biochar reclaiming phosphate from aqueous solutions: mechanisms and potential application as a slow-release fertilizer[J]. Environmental Science & Technology, 2013, 47(15): 8700-8708. |
| 93 | LI R H , WANG J J , ZHANG Z Q , et al . Recovery of phosphate and dissolved organic matter from aqueous solution using a novel CaO-MgO hybrid carbon composite and its feasibility in phosphorus recycling[J]. Science of the Total Environment, 2018, 642: 526-536. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 王家庆, 宋广伟, 李强, 郭帅成, DAI Qingli. 橡胶混凝土界面改性方法及性能提升路径[J]. 化工进展, 2023, 42(S1): 328-343. |
| [3] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [4] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [5] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [6] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [7] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [8] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [9] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [10] | 王谨航, 何勇, 史伶俐, 龙臻, 梁德青. 气体水合物阻聚剂研究进展[J]. 化工进展, 2023, 42(9): 4587-4602. |
| [11] | 朱杰, 金晶, 丁正浩, 杨会盼, 侯封校. 化学链气化中准东煤灰对CaSO4载氧体改性及其作用机理[J]. 化工进展, 2023, 42(9): 4628-4635. |
| [12] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [13] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [14] | 宋伟涛, 宋慧平, 范朕连, 樊飙, 薛芳斌. 粉煤灰在防腐涂料中的研究进展[J]. 化工进展, 2023, 42(9): 4894-4904. |
| [15] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||