化工进展 ›› 2019, Vol. 38 ›› Issue (04): 1903-1912.DOI: 10.16085/j.issn.1000-6613.2018-1353
高附加值萘二酚类中间体的合成与应用进展
李彬1,2,王雪1,姜爽1,2( ),张天永1,3(
),张天永1,3( ),杨靓怡1,王景超1,邵笑1,马骁媛1
),杨靓怡1,王景超1,邵笑1,马骁媛1
- 1. 天津大学化工学院,天津市应用催化科学与工程重点实验室,天津 300354
2. 天津市功能精细化学品技术工程中心,天津 300354
3. 天津化学化工协同创新中心,天津 300072
-
收稿日期:2018-07-02修回日期:2018-11-13出版日期:2019-04-05发布日期:2019-04-05 -
通讯作者:姜爽,张天永 -
作者简介:<named-content content-type="corresp-name">李彬</named-content>(1982—),男,博士,副教授,硕士生导师。|姜爽,讲师,研究方向为精细化学品合成。E-mail:<email>shuangjiang@tju.edu.cn</email>|张天永,教授,博士生导师。E-mail:<email>tyzhang@tju.edu.cn</email> -
基金资助:国家重点研发计划(2017YFB0404701)
Progress in synthesis and application of naphthalenediol intermediates with high value-added
Bin LI1,2,Xue WANG1,Shuang JIANG1,2( ),Tianyong ZHANG1,3(
),Tianyong ZHANG1,3( ),Jingyi YANG1,Jingchao WANG1,Xiao SHAO1,Xiaoyuan MA1
),Jingyi YANG1,Jingchao WANG1,Xiao SHAO1,Xiaoyuan MA1
- 1. Tianjin Key Laboratory of Applied Catalysis Science and Technology, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300354, China
2. Tianjin Engineering Research Center of Functional Fine Chemicals, Tianjin 300354, China
3. Collaborative Innovation Center of Chemical Science and Engineering(Tianjin), Tianjin 300072, China
-
Received:2018-07-02Revised:2018-11-13Online:2019-04-05Published:2019-04-05 -
Contact:Shuang JIANG,Tianyong ZHANG
摘要:
萘二酚类中间体的传统合成工艺具有操作简单和工业化程度高等优点,但存在废酸排放量大、后处理工艺复杂、成本高和环境污染严重等问题,近年来有被绿色合成工艺逐步替代的趋势。本文回顾了以磺化碱熔法和硝化还原法为主的传统合成方法;简述了用微藻类微生物、重组酵母以及多种结构的加氧酶代谢转化的生物转化法和以强酸、生物酶以及有机金属络合物作为催化剂的直接催化羟基化法等新兴绿色合成工艺。着重介绍了1,6-二羟基萘(又称为1,6-萘二酚)的合成工艺进展,特别是在过氧化氢存在下,采用酸催化剂,如用强酸氢氟酸和五氟化锑催化以及用氟锑磺酸、三氟化硼和氢氟酸等直接进行的催化羟基化反应。同时还综述了萘二酚化合物在染料、医药、催化及电池等领域的典型应用情况。最后指出了生物转化法及催化羟基化法制备萘二酚的改进方向是提高反应的收率及萘的转化率,以达到大规模工业化生产的水平;并对萘二酚类中间体应用领域的拓展进行了展望,指出其向精细化、高附加值的医药及材料方向发展的趋势。
中图分类号:
引用本文
李彬, 王雪, 姜爽, 张天永, 杨靓怡, 王景超, 邵笑, 马骁媛. 高附加值萘二酚类中间体的合成与应用进展[J]. 化工进展, 2019, 38(04): 1903-1912.
Bin LI, Xue WANG, Shuang JIANG, Tianyong ZHANG, Jingyi YANG, Jingchao WANG, Xiao SHAO, Xiaoyuan MA. Progress in synthesis and application of naphthalenediol intermediates with high value-added[J]. Chemical Industry and Engineering Progress, 2019, 38(04): 1903-1912.
| 磺化剂 | 分子式 | 物理状态 | 副反应 | 活泼性 |
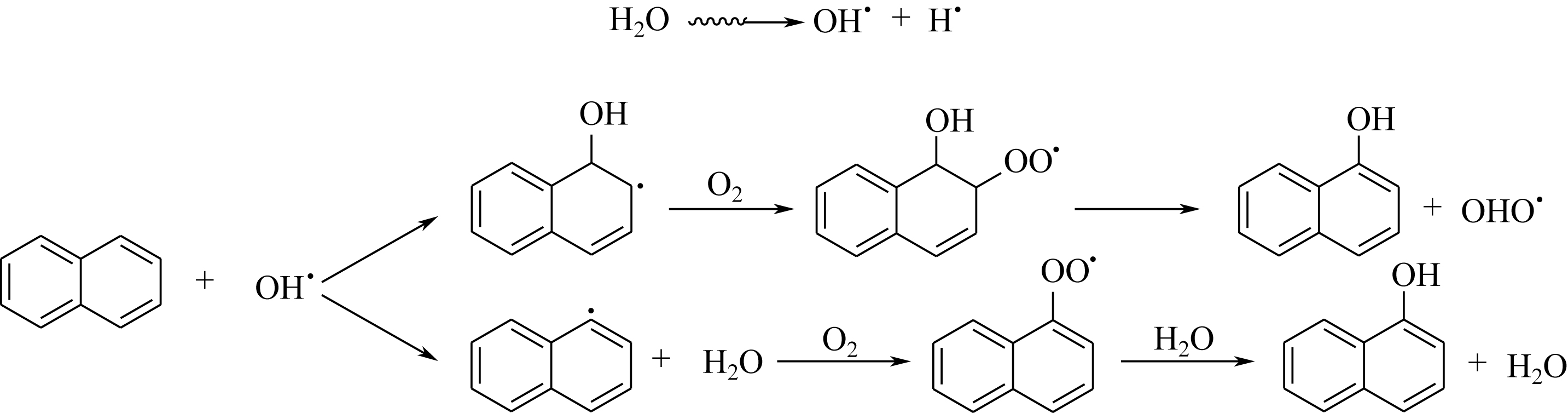
|---|---|---|---|---|
| 三氧化硫 | SO3 | 气态 | 很多 | 活泼 |
| 液态 | 很多 | 非常活泼 | ||
| 氯磺酸 | Cl SO3H | 液态 | 多 | 活泼 |
| 发烟硫酸 | H2SO4 SO3 | 液态 | 较多 | 比较活泼 |
| 硫酸 | H2SO4 | 液态 | 较多 | 比较活泼 |
| 氨基磺酸 | NH2SO3H | 固态 | 较少 | 温和 |
表1 常用磺化剂反应特性的比较
| 磺化剂 | 分子式 | 物理状态 | 副反应 | 活泼性 |
|---|---|---|---|---|
| 三氧化硫 | SO3 | 气态 | 很多 | 活泼 |
| 液态 | 很多 | 非常活泼 | ||
| 氯磺酸 | Cl SO3H | 液态 | 多 | 活泼 |
| 发烟硫酸 | H2SO4 SO3 | 液态 | 较多 | 比较活泼 |
| 硫酸 | H2SO4 | 液态 | 较多 | 比较活泼 |
| 氨基磺酸 | NH2SO3H | 固态 | 较少 | 温和 |
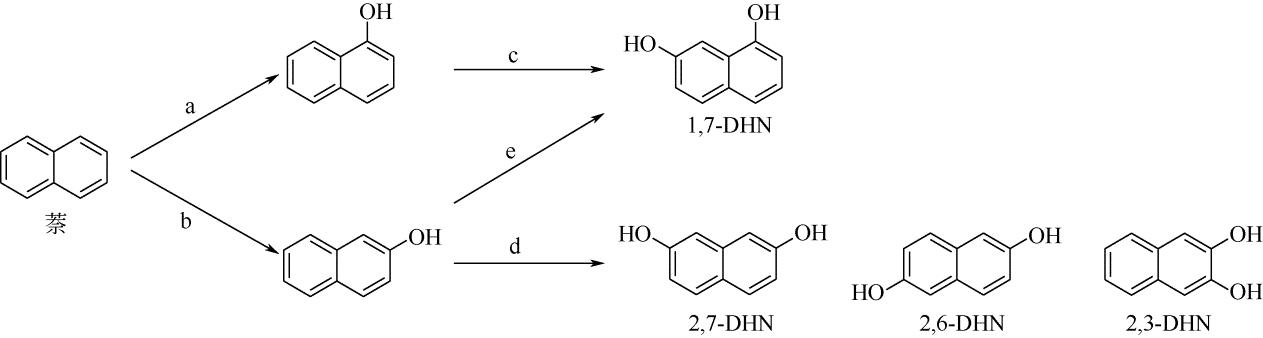
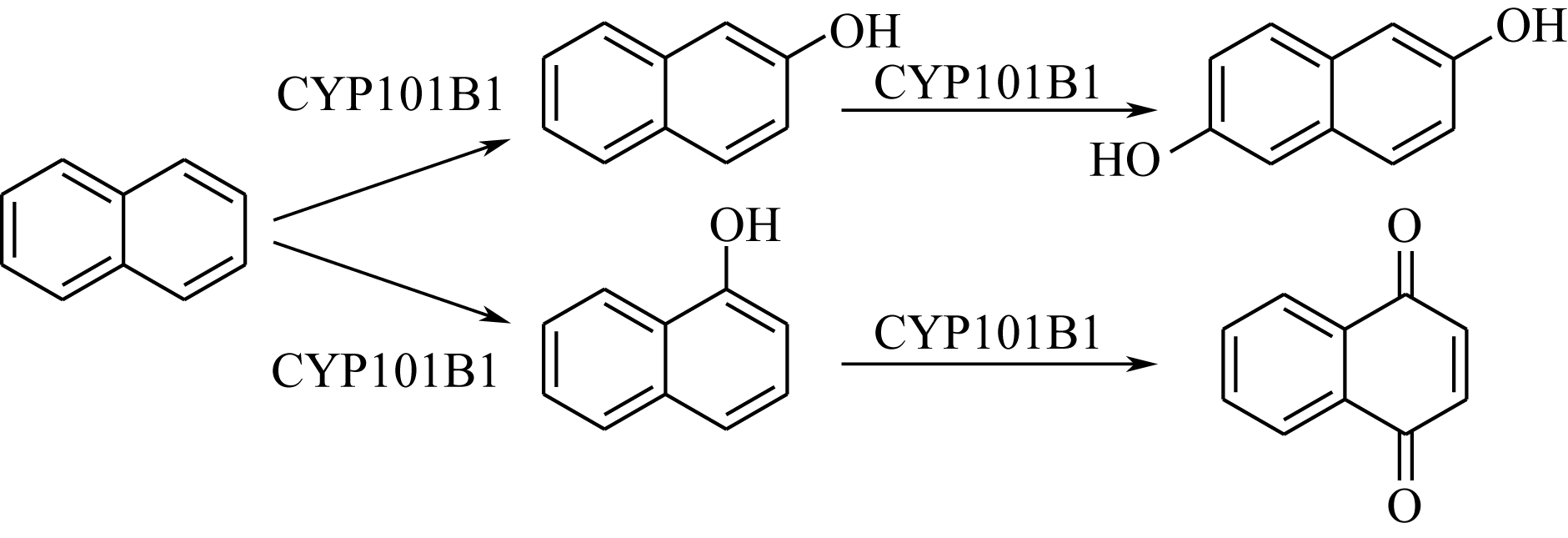
| 酶 | 1-萘酚 选择性/% | 2-萘酚 选择性/% | 反应过程 (与图 |
|---|---|---|---|
| 野生型TOM | 99 | <1 | a、b、c、e |
| TOM TmoA3 V106A | 99 | <1 | a |
| 野生型ToMO | 90 | 10 | b、c、d、e |
| 野生型TpMO | 63 | 37 | d、e |
| 野生型T4MO | 52 | 48 | d、e |
| TmoA I100A | 8 | 92 | b |
| TmoA I100S | 5 | 95 | |
| TmoA I100G | 12 | 88 | |
| TmoA I100L | 83 | 17 | |
| TmoA G103S/A107G | 99 | <1 | a |
表2 不同种加氧酶反应的选择性及适用的反应过程[21,22,23]
| 酶 | 1-萘酚 选择性/% | 2-萘酚 选择性/% | 反应过程 (与图 |
|---|---|---|---|
| 野生型TOM | 99 | <1 | a、b、c、e |
| TOM TmoA3 V106A | 99 | <1 | a |
| 野生型ToMO | 90 | 10 | b、c、d、e |
| 野生型TpMO | 63 | 37 | d、e |
| 野生型T4MO | 52 | 48 | d、e |
| TmoA I100A | 8 | 92 | b |
| TmoA I100S | 5 | 95 | |
| TmoA I100G | 12 | 88 | |
| TmoA I100L | 83 | 17 | |
| TmoA G103S/A107G | 99 | <1 | a |
| 序号 | 取代胺结构 | 产品6结构 | 收率/% |
|---|---|---|---|
| 4 |  | | 79 |
| 5 |  | | 77 |
| 6 |  | | 75 |
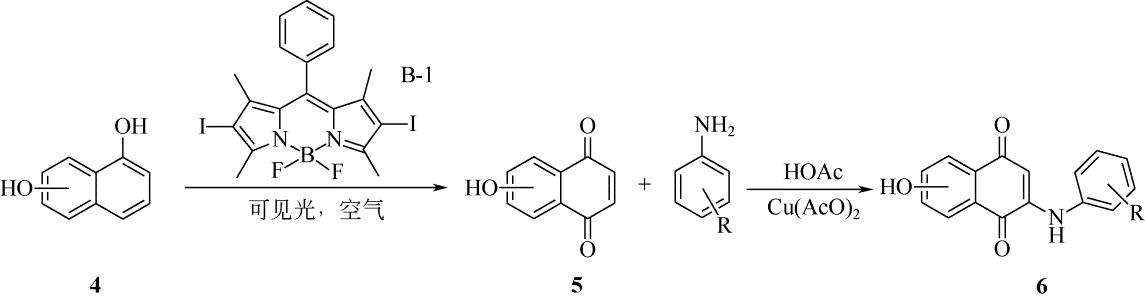
表4 1,4-萘醌与苯胺合成反应
| 序号 | 取代胺结构 | 产品6结构 | 收率/% |
|---|---|---|---|
| 4 |  | | 79 |
| 5 |  | | 77 |
| 6 |  | | 75 |
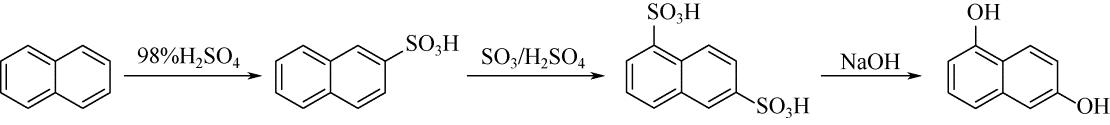
| 1 | 张天永, 杨秋生, 卢洲, 等 . 萘酚的催化合成技术进展[J]. 化工进展, 2009, 28(1): 55-61. |
| ZHANG T Y , YANG Q S , LU Z , et al . Progress of catalytic synthesis of naphthol[J]. Chemical Industry and Engineering Progress, 2009, 28(1): 55-61. | |
| 2 | FISHMAN A , TAO Y , RUI L , et al . Controlling the regiospecific oxidation of aromatics via active site engineering of toluene para-monooxygenase of Ralstonia pickettii PKO1[J]. Journal of Biological Chemistry, 2005, 280(1): 506-514. |
| 3 | 唐培堃 . 精细有机合成化学与工艺学[M]. 天津: 天津大学出版社, 1993. |
| TANG P K . Fine organic synthetic chemistry and technology[M]. Tianjin: Tianjin University Press, 1993. | |
| 4 | 宋志军 . 石油磺酸盐磺化工艺及性能研究[J]. 化工设计通讯, 2018, 44(2): 111. |
| SONG Z J . Study on sulfonation process and properties of petroleum sulfonates[J]. Chemical Engineering Design Communications, 2018, 44(2): 111. | |
| 5 | 周绿山, 明大增, 李志祥, 等 . 三氧化硫制备石油磺酸盐工艺及组成分析方法综述[J]. 化工科技, 2013, 21(2): 61-66. |
| ZHOU L S , MING D Z , LI Z X , et al . Review on the preparation process of petroleum sulfonate by sulfur trioxide and the analytical methods for its composition[J]. Science & Technology in Chemical Industry, 2013, 21(2): 61-66. | |
| 6 | 肖洋, 郭世明, 张祥 . 硅胶硫酸对芳环的磺化[J]. 染料与染色, 2013, 50(5): 47-50. |
| XIAO Y , GUO S M , ZHANG X . Sulfonation of aromatic compound by silica sulfuric acid[J]. Dyestuffs and Coloration, 2013, 50(5): 47-50. | |
| 7 | 肖谷清, 王芳, 危冬梅, 等 . 超高交联树脂的超声辅助磺化[J]. 离子交换与吸附, 2017, 33(3): 236-244. |
| XIAO G Q , WANG F , WEI D M , et al . Ultrasound assisted sulfonation of the high micropore area hyper-cross-linked resin[J]. Ion Exchange and Adsorption, 2017, 33(3): 236-244. | |
| 8 | 刘东 . 萘在浓硫酸中的磺化反应历程研究[J]. 染料与染色, 2017, 54(6): 37-39. |
| LIU D . Study on mechanism of sulfonating naphthalene with concentrated sulfuric acid[J]. Dyestuffs and Coloration, 2017, 54(6): 37-39. | |
| 9 | 陈彦全, 韩梅, 焦凤军, 等 . 微反应器中甲苯液相SO3磺化工艺研究[J]. 化学反应工程与工艺, 2013, 29(3): 253-259. |
| CHEN Y Q , HAN M , JIAO F J , et al . Study on the sulfonation of toluene with SO3 in microreactor[J]. Chemical Reaction Engineering and Technology, 2013, 29(3): 253-259. | |
| 10 | 李树民, 李晨, 蔡公建 . 三氧化硫气体多管膜式磺化器的改进[J]. 日用化学品科学, 2016, 39(8): 28-30. |
| LI S M , LI C , CAI G J . Development of SO3 multitube film sulfonation reactor[J]. Detergent & Cosmetics, 2016, 39(8): 28-30. | |
| 11 | 李维思 . 氨基磺酸催化的缩合反应的研究[D]. 南京:南京工业大学, 2008. |
| LI W S . Study on condensation reaction catalyzed by amino sulfonic acid[D]. Nanjing: Nanjing Tech University, 2008. | |
| 12 | ALEBOYEH H , WALTER S , ALEBOYEH A A , et al . Naphthalenedisulphonic acid formation with 20% olem[J]. Organic Process Research & Development, 1997, 1(6): 411-414. |
| 13 | 王永灿, 宋东明, 张永辉 . 萘磺酸的合成研究[J]. 染料与染色, 2001, 38(2): 37-39. |
| WANG Y C , SONG D M , ZHANG Y H . A study on the synthesis of naphthalene sulfonic acids[J]. Dyestuffs and Coloration, 2001, 38(2): 37-39. | |
| 14 | 李霞, 孙利方, 曹波 . 1,6-二羟基萘的制备研究[J]. 辽宁丝绸, 2007, 14(2): 9-10. |
| LI X , SUN L F , CAO B . Study on preparation of 1,6-dihydroxynaphthalene[J]. Liaoning Tussah Silk, 2007, 14(2): 9-10. | |
| 15 | Yoji SHIMIZU . Purification of anhydrous 1,6-dihydroxynaphthalene:JP 2005-380868[P]. 2005-12-07. |
| 16 | 张水鹤 . 浅谈萘磺化衍生物的绿色生产和可持续发展[J]. 染料与染色, 2016, 53(3): 27-28, 31. |
| ZHANG S H . Eco-friendly production and sustainable development of sulfonatives of naphthalene[J]. Dyestuffs and Coloration, 2016, 53(3): 27-28, 31. | |
| 17 | PIKUS J D , STUDTS J M , MCCLAY K , et al . Changes in the regiospecificity of aromatic hydroxylation produced by active site engineering in the diiron enzyme toluene 4-monooxygenase[J]. Biochemistry, 1997, 36(31): 9283-9289. |
| 18 | RUI L , KWON Y M , FISHMAN A , et al . Saturation mutagenesis of toluene ortho-monooxygenase of Burkholderia cepacia G4 for enhanced 1-naphthol synthesis and chloroform degradation[J]. Applied & Environmental Microbiology, 2004, 70(6): 3246-3252. |
| 19 | TAO Y , BENTLEY W E , WOOD T K . Regiospecific oxidation of naphthalene and fluorene by toluene monooxygenases and engineered toluene 4-monooxygenases of Pseudomonas mendocina KR1[J]. Biotechnology & Bioengineering, 2010, 90(1): 85-94. |
| 20 | VARDAR G , RYU K, WOOD T K . Protein engineering of toluene-o-xylene monooxygenase from Pseudomonas stutzeri OX1 for oxidizing nitrobenzene to 3-nitrocatechol, 4-nitrocatechol, and nitrohydroquinone[J]. Journal of Biotechnology, 2005, 115(2): 145-156. |
| 21 | CANADA K A , SACHIYO I , HOJAE S , et al . Directed evolution of toluene ortho-monooxygenase for enhanced 1-naphthol synthesis and chlorinated ethene degradation[J]. Journal of Bacteriology, 2002, 184(2): 344-349. |
| 22 | GIBSON D T , PARALES R E . Aromatic hydrocarbon dioxygenases in environmental biotechnology[J]. Environmental Biotechnology, 2000, 11(3): 236-243. |
| 23 | TAO Y , BENTLEY W E , WOOD T K . Phenol and 2-naphthol production by toluene 4-monooxygenases using an aqueous/dioctyl phthalate system[J]. Applied Microbiology & Biotechnology, 2005, 68(5): 614-621. |
| 24 | OPPENHEIM S F , STUDTS J M , FOX B G, et al . Aromatic hydroxylation catalyzed by toluene 4-monooxygenase in organic solvent/aqueous buffer mixtures[J]. Applied Biochemistry & Biotechnology, 2001, 90(3): 187-197. |
| 25 | 王晓, 张天永, 姜爽, 等 . 含铁催化剂催化苯直接羟基化制备苯酚的研究进展[J]. 化工进展, 2015, 34(2): 381-388, 446. |
| WANG X , ZHANG T Y , JAING S , et al . Progress of direct hydroxylation of benzene to phenol using iron-containing catalyst[J]. Chemical Industry and Engineering Progress, 2015, 34(2): 381-388, 446. | |
| 26 | WANG X , ZHANG T Y , YANG Q S , et al . Synthesis and characterization of bio-inspired diiron complexes and their catalytic activity for direct hydroxylation of aromatic compounds[J]. European Journal of Inorganic Chemistry, 2015, 2015(5): 817-825. |
| 27 | 王晓 . 含铁催化剂直接催化芳香化合物羟基化的研究[D]. 天津: 天津大学, 2014. |
| WANG X . Research on the direct hydroxylation of aromatic compounds catalyzed by theiron-containing catalysts[D]. Tianjin:Tianjin University, 2014. | |
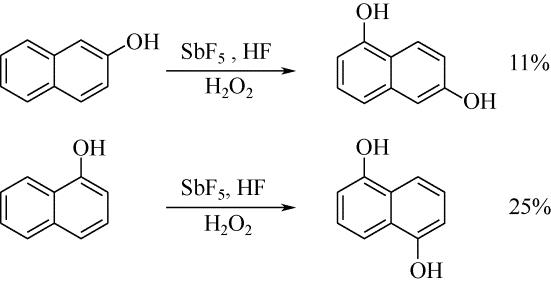
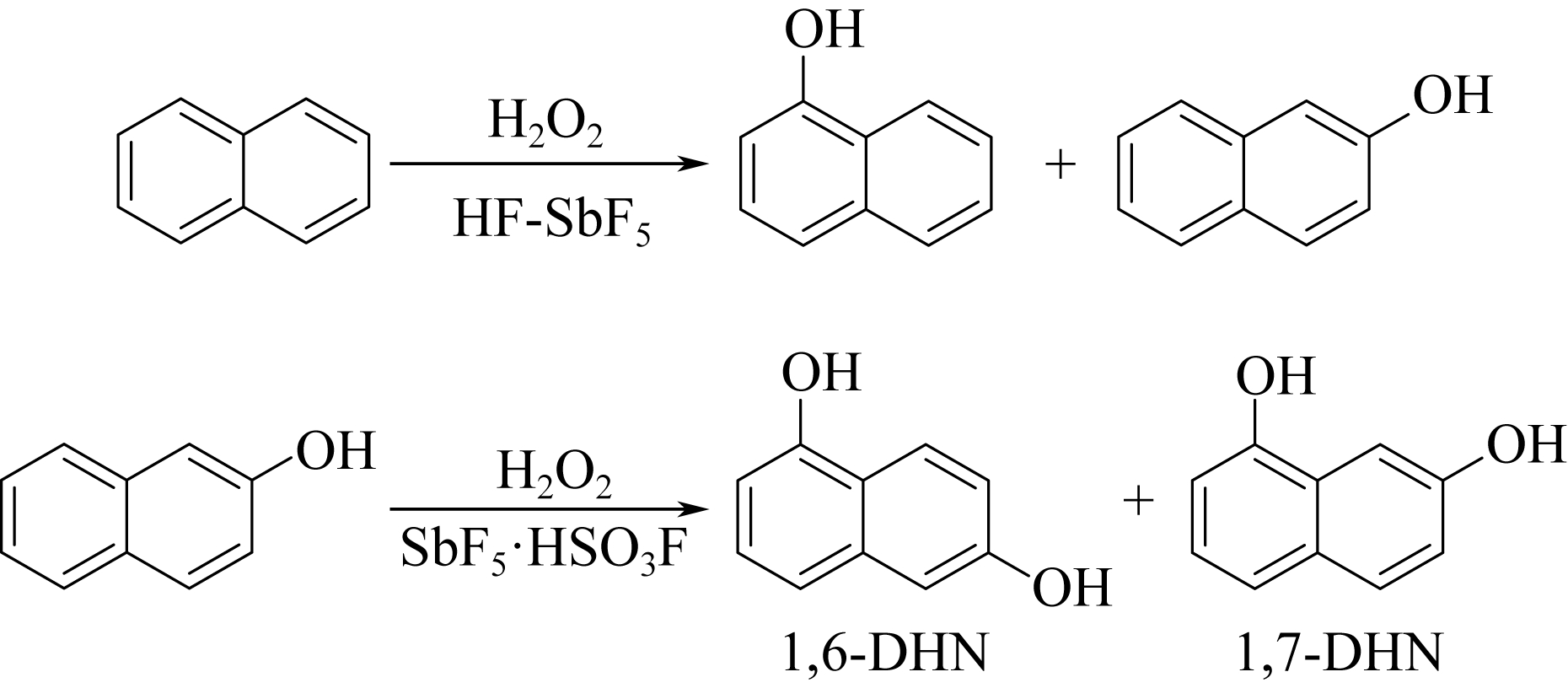
| 28 | JACQUESY J C , JOUANNETAUD M P , MORELLET G . Hydroxylation of naphthols in a superacid medium[J]. Tetrahedron Letters, 1983, 24(30): 3099-3102. |
| 29 | OLAH G A , KEUMI T , LECOQ J C , et al . Oxyfunctionalization of hydrocarbons part 17: acid-dependent high regioselectivity hydroxylation of naphthalene with hydrogen peroxide giving 1- or 2-naphthol[J]. Journal of Organic Chemistry, 1991,56(21): 6148-6151. |
| 30 | REDDY M P , BALAKRISHNAN I . Homolytic hydroxylation of naphthalene in oxygenated aqueous solutions by gamma radiolysis at higher temperatures[J]. Journal of Physical Chemistry, 1968, 72(13): 4609-4613. |
| 31 | 张天永, 史慧娴, 卢洲, 等 . Photo-Fenton和Photo-Fenon-like试剂对萘羟基化反应的影响[J]. 精细石油化工, 2010, 27(2): 24-27. |
| ZHANG T Y , SHI H X , LU Z , et al . Hydroxylation of naphthalene by Photo-Fenton and Photo-Fenton-like reaction[J]. Speciality Petrochemicals, 2010, 27(2): 24-27. | |
| 32 | HALL E A , SARKAR M R , BELL S G . The selective oxidation of substituted aromatic hydrocarbons and the observation of uncoupling via redox cycling during naphthalene oxidation by the CYP101B1 system[J]. Catalysis Science & Technology, 2017, 7(7): 1537-1548. |
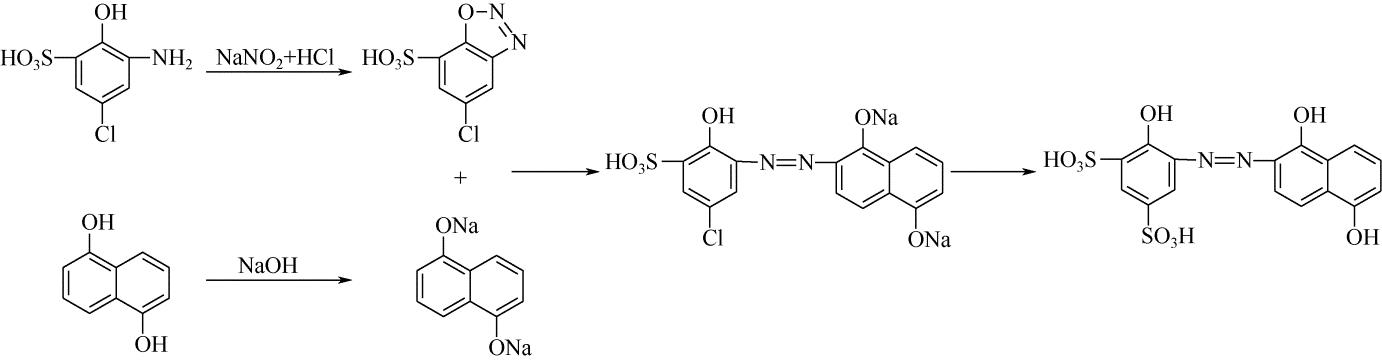
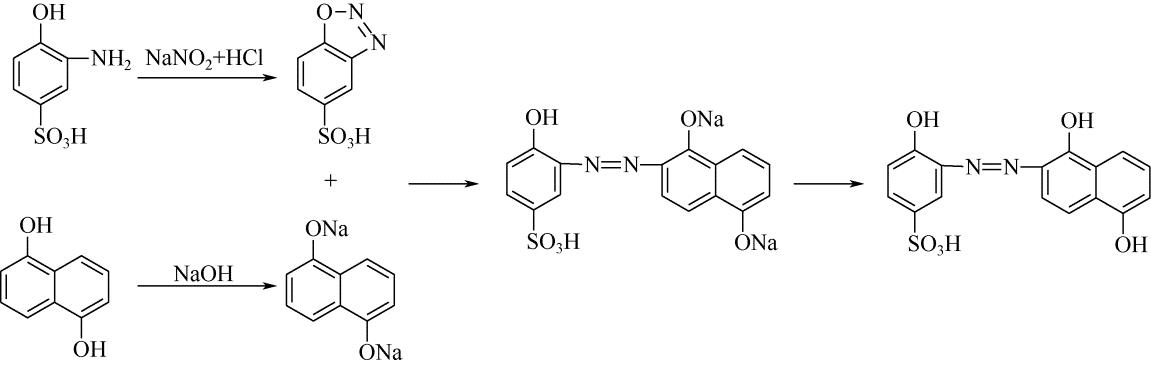
| 33 | 王瑾, 贺业凯, 高淑华 . 酸性媒介黑P2B染料合成工艺[J]. 辽宁化工, 2009, 38(9): 613-616. |
| WANG J , HE Y K , GAO S H . Synthesis process of acid mordant black P2B dye[J]. Liaoning Chemical Industry, 2009, 38(9): 613-616. | |
| 34 | 贺业凯, 王瑾, 高淑华 . 酸性媒介黑PV染料合成工艺改进[J]. 辽宁化工, 2009, 38(10): 709-710. |
| HE Y K , WANG J , GAO S H . Improvement of production technology of acid mordant black PV dye[J]. Liaoning Chemical Industry, 2009, 38(10): 709-710. | |
| 35 | 殷辉, 李美真 . 氯化稀土在酸性媒介染色中的应用研究[J]. 染整技术, 2010, 32(10): 32-34. |
| YIN H , LI M Z . Study on the application of rare earth chloride in acid medium dyeing[J]. Textile Dyeing and Finishing Journal, 2010, 32(10): 32-34. | |
| 36 | LIU J , YU L T , WU C , et al . New nanoconfined galvanic replacement synthesis of hollow Sb@C Yolk-Shell spheres constituting a stable anode for high-rate Li/Na-ion batteries[J]. Nano Letters, 2017, 17(3): 2034-2042. |
| 37 | ZHANG X , LIU T , ZHANG S F , et al . Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly(vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes[J]. Journal of the American Chemical Society, 2017, 139(39): 13779-13785. |
| 38 | 喻王李, 陈俊, 周文佩, 等 . 含偶氮苯共轭微孔聚合物的制备及其锂离子电池性能研究[J]. 化学与生物工程, 2018, 35(5): 20-25. |
| YU W L , CHEN J , ZHOU W P , et al . Preparation of azobenzene-based conjugated microporous polymers and performance of lithium ion battery[J]. Chemistry & Bioengineering, 2018, 35(5): 20-25. | |
| 39 | 王莉, 何向明, 蒲薇华, 等 . 金属锂二次电池研究进展[J]. 化学进展, 2006, 18(5): 641-647. |
| WANG L , HE X M , PU W H , et al . Progress in rechargeable lithium metal batteries[J]. Progress in Chemistry, 2006, 18(5): 641-647. | |
| 40 | MORITA Y , NISHIDA S , MURATA T , et al . Organic tailored batteries materials using stable open-shell molecules with degenerate frontier orbitals[J]. Nature Materials, 2011, 10(12): 947-951. |
| 41 | 陈朝逸 . 萘酐类聚酰亚胺正极材料的制备与电化学性能研究[D]. 上海: 东华大学, 2017. |
| CHEN Chaoyi . Preparation and electrochemical properties of naphthalene-based polyimide as cathode materials[D]. Shanghai:Donghua University, 2017. | |
| 42 | NOMURA M . Secondary battery:JP2015-49522[P].2015-03-12. |
| 43 | ITOH S , TAKI M , NAKAO H , et al . Aliphatic hydroxylation by a bis(μ-oxo)dinickel(Ⅲ) complex[J]. Angewandte Chemie International Edition, 2000, 39(2): 389-400. |
| 44 | AKITA M , FUJISAWA K , HIKICHI S , et al . Chemistry of μ-η 2: η 2-peroxo dimetal complexes:a bioinorganic model triggers a new trend of transition metal peroxo species[J]. Research on Chemical Intermediates, 1998, 24(3): 291-307. |
| 45 | PUZARI A , BARUAH J B . Copper(Ⅱ)-catalyzed reactions of activated aromatics[J]. Journal of Organic Chemistry, 2000, 65(8): 2344-2349. |
| 46 | HANDIQUE J G , BARUAH J B . Oxidative oligomerisation of 2-naphthol by cis-bis(glycinato)copper(Ⅱ)[J]. Journal of Molecular Catalysis A: Chemical, 2001, 66(2): 225-231. |
| 47 | HANDIQUE J G , BARUAH J B . The oligomer of 1,6-naphthalenediol as a support in oxidative reactions of cis-bisglycinato copper(Ⅱ) and hydrogenperoxide[J]. Journal of Molecular Catalysis A: Chemical, 2001, 172(1): 19-23. |
| 48 | 高嫒嫒, 安忠维 . 稠环衍生物类液晶的合成进展[J]. 液晶与显示, 2006, 21(2): 149-157. |
| GAO Y Y , AN Z W . Development in synthesis of condensed ring derivative liquid crystal[J]. Chinese Journal of Liquid Crystals and Displays, 2006, 21(2): 149-157. | |
| 49 | 郑敏燕, 魏永生, 耿薇, 等 . 萘二酚酯香蕉型及棒状液晶的合成与液晶性[J]. 咸阳师范学院学报, 2015, 30(2): 53-56. |
| ZHENG M Y , WEI Y S , GENG W , et al . The synthesis of banana-shaped and rod-like liquid crystal of naphthdiol ester[J]. Journal of Xianyang Normal University, 2015, 30(2): 53-56. | |
| 50 | 张腊腊, 王丽朋, 武芸, 等 . 溴代烷基连接的香蕉型液晶化合物的合成[J]. 陇东学院学报, 2017, 28(1): 34-37. |
| ZHANG L L , WANG L P , WU Y , et al . Synthesis of a type of banana liquid crystal compound with alkyl bromide[J]. Journal of Longdong University, 2017, 28(1): 34-37. | |
| 51 | 陈年, 雷霞, 成琼辉, 等 . 5-氨基酮戊酸光动力疗法治疗27例皮肤肿瘤疗效观察[J]. 实用皮肤病学杂志, 2017, 10(1): 1-3. |
| CHEN N , LEI X , CHENG Q H , et al . Efficacy of ALA-PDT in the treatment of cutaneous tumors[J]. Journal of Practical Dermatology, 2017, 10(1): 1-3. | |
| 52 | GONG D , ZHU X , TIAN Y , et al . A phenylselenium-substituted BODIPY fluorescent turn-off probe for fluorescence imaging of hydrogen sulfide in living cells[J]. Analytical Chemistry, 2017, 89(1-3): 1801-1807. |
| 53 | SONA K , JARMILA S , PETR D , et al . A synthetic approach for the rapid preparation of BODIPY conjugates and their use in imaging of cellular drug uptake and distribution[J]. Chemistry a European Journal, 2018, 24(19): 4957-4966. |
| 54 | LIU W , YAO J , ZHAN C . A novel BODIPY-based low-band-gap small-molecule acceptor for efficient non-fullerene polymer solar cells[J]. Chinese Journal of Chemistry, 2017, 35(12): 1813-1823. |
| 55 | CHAPRAN M , ANGIONI E , FINDLAY N J , et al . An ambipolar BODIPY derivative for a white exciplex OLED and cholesteric liquid crystal laser toward multifunctional devices[J]. ACS Applied Materials & Interfaces, 2017, 9(5): 4750-4757. |
| 56 | CHEN X F , MOHAMED E , KEI O, et al . Assemblies of boron dipyrromethene/porphyrin, phthalocyanine, and C60 moieties as artificial models of photosynthesis:synthesis, supramolecular interactions, and photophysical studies[J]. Chemistry: A European Journal, 2018, 24(15): 3862-3872. |
| 57 | 程探宇 . 氟硼二吡咯(BODIPY)荧光探针的设计、合成与应用研究[D]. 上海: 华东理工大学, 2011. |
| CHENG T Y . Boron dipyrromethene (BODIPY) based fluorescent probes for metal ion:desigh, synthesis and application[D]. Shanghai:East China University of Science and Technology, 2011. | |
| 58 | HUANG L , ZHAO J , GUO S , et al . Bodipy derivatives as organic triplet photosensitizers for aerobic photoorganocatalytic oxidative coupling of amines and photooxidation of dihydroxylnaphthalenes[J]. Journal of Organic Chemistry, 2013, 78(11): 5627-5637. |
| [1] | 李会静1,于炜婷2,戴小敏1,刘袖洞1,马小军2. 微生物转化生产2-苯乙醇的原位产物转移技术研究进展[J]. 化工进展, 2012, 31(03): 633-638. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||