化工进展 ›› 2025, Vol. 44 ›› Issue (5): 2694-2704.DOI: 10.16085/j.issn.1000-6613.2024-1965
• 可再生能源利用 • 上一篇
木质素降解酶人工调控的研究进展
- 西安交通大学化学工程与技术学院,陕西 西安 710049
-
收稿日期:2024-12-02修回日期:2025-03-09出版日期:2025-05-25发布日期:2025-05-20 -
通讯作者:费强 -
作者简介:王鑫颖(2000—),女,硕士研究生,研究方向为木质素的酶解与高值转化。E-mail:wangxinying@stu.xjtu.edu.cn。 -
基金资助:国家重点研发计划(2023YFC3403500);陕西省重点研发计划(2024NC-YBXM-226);中国博士后科学基金(2023M732780);陕西省生物合成与医用化工创新团队
Research progress on the artificial regulation of lignin-degrading enzymes
WANG Xinying( ), LI Aipeng, SU Wenrui, FEI Qiang(
), LI Aipeng, SU Wenrui, FEI Qiang( )
)
- School of Chemical Engineering and Technology, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
-
Received:2024-12-02Revised:2025-03-09Online:2025-05-25Published:2025-05-20 -
Contact:FEI Qiang
摘要:
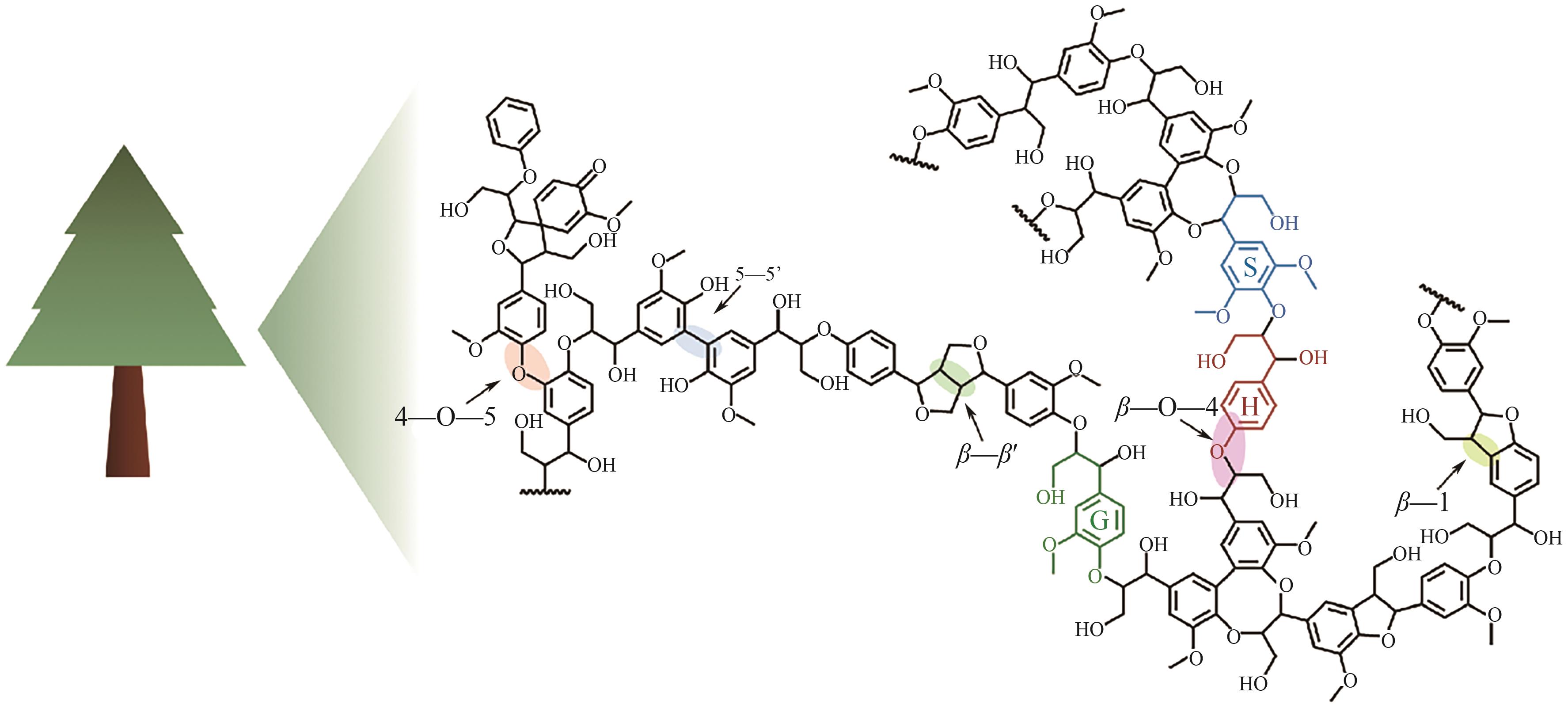
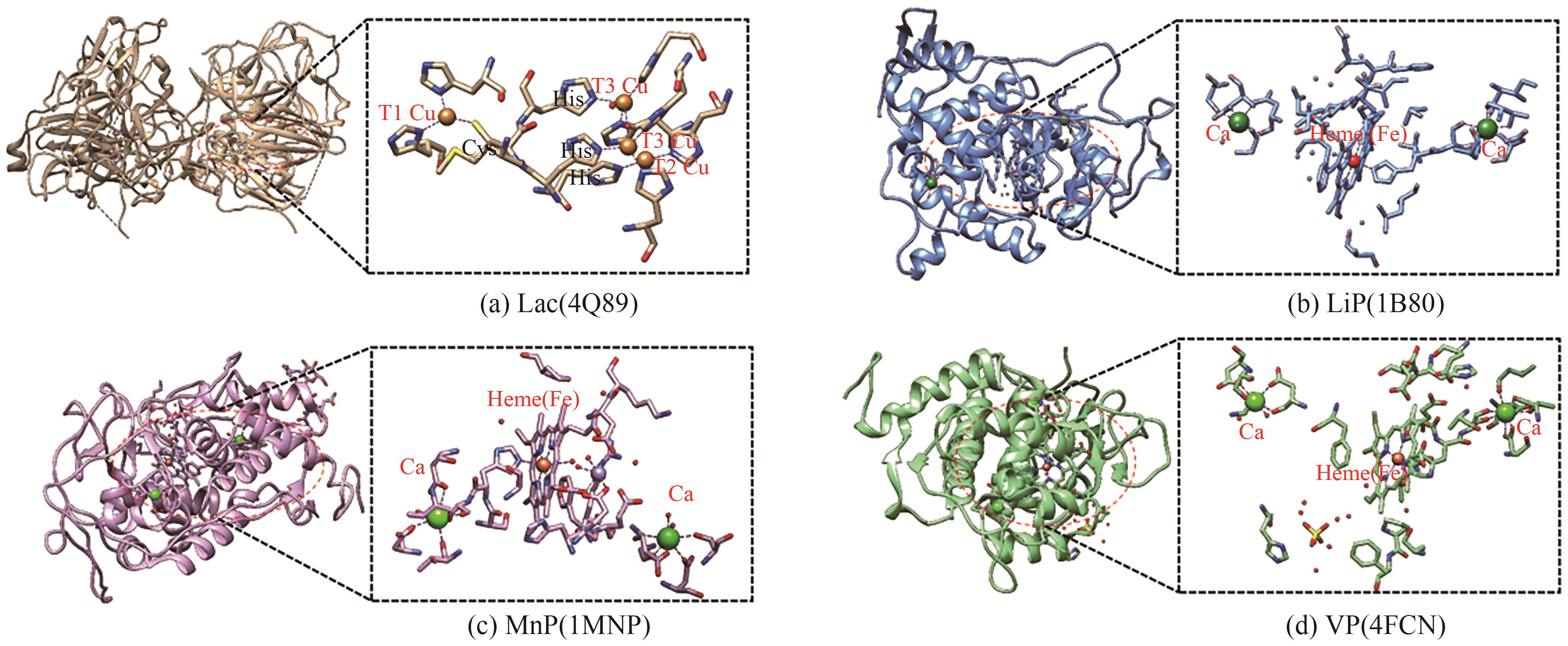
作为可再生生物质的重要组分,木质素是自然界储量最为丰富的芳香族高聚物,是芳香高值化学品合成的潜在绿色原料。然而,木质素的异质性和复杂结构给其降解利用造成了严峻挑战。自然界存在种类繁多、特异性多样的木质素降解酶,使得酶介导的生物降解能够突破木质素顽固性结构的限制,在温和条件下降解木质素。尽管如此,天然木质素降解酶的表达量、催化活性和稳定性等往往不尽人意。近年来,通过蛋白质表达调控、酶分子改造,木质素降解酶的合成和催化性能的人工调控已取得诸多优秀成果。鉴于此,本文首先对重要的木质素降解酶及其催化特性进行了简要介绍;在此基础上,重点总结了木质素降解酶的表达和催化性能强化方面的研究进展,对当前面临的理论和技术挑战进行了深入分析并提出了针对性的应对策略。希望为更高效木质素生物降解体系的开发提供有价值的参考,助力“双碳”目标的实现。
中图分类号:
引用本文
王鑫颖, 李爱朋, 苏文蕊, 费强. 木质素降解酶人工调控的研究进展[J]. 化工进展, 2025, 44(5): 2694-2704.
WANG Xinying, LI Aipeng, SU Wenrui, FEI Qiang. Research progress on the artificial regulation of lignin-degrading enzymes[J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2694-2704.
| 宿主 | 酶 | 基因来源 | 菌株 | 载体 | 温度/℃ | 表达形式 | 酶活 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 大肠杆菌表达系统 | Lac | Bacillus vallismortis fmb-103 | E. coli BL21(DE3) | pET-28a | 30 | 可溶性表达 | 1545.6U/L | [ |
| Bacillus amyloliquefaciens | E. coli BL21(DE3) | pET-20b(+) | 25 | 可溶性表达 | 20255U/L | [ | ||
| Sordaria macrospora k-hell | E. coli BL21(DE3) | pET-30a | 16 | 可溶性表达 | 239U/L | [ | ||
| LiP | Irpex Lacteus | E. coli BL21(DE3) | pET-21a | — | 部分可溶性表达 | 11.2U/mg | [ | |
| P. chrysosporium | E. coli BL21(DE3) | pET-21a | 37 | 包涵体 | 171.86 U/mg | [ | ||
| MnP | Geobacillus sp. ID17 | E. coli BL21 | pD441-SR | 23 | 包涵体 | 4383U/mg | [ | |
| Ceriporiopsis subvermispora | E. coli BL21 | pCold1 | 15 | 可溶性表达 | — | [ | ||
| P. chrysosporium | E. coli Shuffle T7 | pET23b | 30 | 部分可溶性表达 | 0.445U/mg | [ | ||
| VP | P. eryngii | E. coli BL21(DE3) | pET-32a(+) | 16 | 部分可溶性表达 | 7.2U/mg | [ | |
| P. eryngii | E. coli W3110 | pFLAG1 | 37 | 包涵体 | — | [ | ||
| Physisporinus vitreus | E. coli BL21 (DE3) | pET-28a(+) | 37 | 包涵体 | 23.1U/mg | [ | ||
| 酵母表达系统 | Lac | Streptomyces coelicolor | P. pastoris | pGAPZαA | 30 | 可溶性表达 | 1200U/L | [ |
| Agrocybe pediades | S. cerevisiae | pJRoC30 | 28 | 可溶性表达 | 781U/L | [ | ||
| Basidiomycete PM1 | P. pastoris | pPICZαA | 25 | 可溶性表达 | 3220U/L | [ | ||
| LiP | P. chrysosporium | P. pastoris | pJ901 | 30 | 可溶性表达 | 4480U/L | [ | |
| P. chrysosporium | S. cerevisiae | pYES2 | 30 | 可溶性表达 | 367U/L | [ | ||
| P. chrysosporium | P. pastoris | pPICZα | 30 | 可溶性表达 | 15U/L | [ | ||
| MnP | Peniophora incarnata | S. cerevisiae | pESC-URA | 30 | 可溶性表达 | 3580U/L | [ | |
| Ganoderma lucidum | P. pastoris | pAO815 | 28 | 可溶性表达 | 524.61U/L | [ | ||
| Moniliophthora roreri | P. pastoris | pPICZα(A) | 30 | 可溶性表达 | 3659.5U/L | [ | ||
| VP | P. eryngii | S. cerevisiae | pJRoC30 | 30 | 可溶性表达 | 15500U/L | [ | |
| P. eryngii | P. pastoris | pPICZαA | 15 | 可溶性表达 | 3.3U/L | [ | ||
| 其他表达系统 | Lac | Trametes sp. AH28-2 | Trichoderma reesei | pGTCL | 28 | 可溶性表达 | 3.62 IU/mL | [ |
| Phanerochaete flavidoalba | A. niger | pAN52-4 | 30 | 可溶性表达 | 2500U/L | [ | ||
| Aspergillus AF2 | Aspergillus flavus | — | 30 | 可溶性表达 | 79.57U/mL | [ | ||
| LiP | Phanerodontia chrysosporium | Cyberlindnera jadinii | pBR322 | 30 | 可溶性表达 | 68.52U/L | [ | |
| Thermothelomycesthermophiles M77 | Aspergillus nidulans A733 | pEXPYR | 37 | 可溶性表达 | 1645mU/L | [ | ||
| MnP | Dichomitus squalens | P. chrysosporium | pUDGM2 | 37 | 可溶性表达 | 600U/L | [ | |
| Pleurotus ostreatus | Lentinula edodes | pGhL1 | 20 | 可溶性表达 | 30U/L | [ | ||
| VP | P. eryngii | Aspergillus nidulans | PAN7-1 | 28 | 可溶性表达 | 466U/L | [ | |
| P. eryngii | Emericella nidulans | — | 28 | 可溶性表达 | 165U/L | [ | ||
| Pleurotus sapidus | Hansenula polymorpha | pFPMT121 | 24 | 可溶性表达 | 450mU/mg | [ |
表1 木质素降解酶在不同菌株中的表达情况
| 宿主 | 酶 | 基因来源 | 菌株 | 载体 | 温度/℃ | 表达形式 | 酶活 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 大肠杆菌表达系统 | Lac | Bacillus vallismortis fmb-103 | E. coli BL21(DE3) | pET-28a | 30 | 可溶性表达 | 1545.6U/L | [ |
| Bacillus amyloliquefaciens | E. coli BL21(DE3) | pET-20b(+) | 25 | 可溶性表达 | 20255U/L | [ | ||
| Sordaria macrospora k-hell | E. coli BL21(DE3) | pET-30a | 16 | 可溶性表达 | 239U/L | [ | ||
| LiP | Irpex Lacteus | E. coli BL21(DE3) | pET-21a | — | 部分可溶性表达 | 11.2U/mg | [ | |
| P. chrysosporium | E. coli BL21(DE3) | pET-21a | 37 | 包涵体 | 171.86 U/mg | [ | ||
| MnP | Geobacillus sp. ID17 | E. coli BL21 | pD441-SR | 23 | 包涵体 | 4383U/mg | [ | |
| Ceriporiopsis subvermispora | E. coli BL21 | pCold1 | 15 | 可溶性表达 | — | [ | ||
| P. chrysosporium | E. coli Shuffle T7 | pET23b | 30 | 部分可溶性表达 | 0.445U/mg | [ | ||
| VP | P. eryngii | E. coli BL21(DE3) | pET-32a(+) | 16 | 部分可溶性表达 | 7.2U/mg | [ | |
| P. eryngii | E. coli W3110 | pFLAG1 | 37 | 包涵体 | — | [ | ||
| Physisporinus vitreus | E. coli BL21 (DE3) | pET-28a(+) | 37 | 包涵体 | 23.1U/mg | [ | ||
| 酵母表达系统 | Lac | Streptomyces coelicolor | P. pastoris | pGAPZαA | 30 | 可溶性表达 | 1200U/L | [ |
| Agrocybe pediades | S. cerevisiae | pJRoC30 | 28 | 可溶性表达 | 781U/L | [ | ||
| Basidiomycete PM1 | P. pastoris | pPICZαA | 25 | 可溶性表达 | 3220U/L | [ | ||
| LiP | P. chrysosporium | P. pastoris | pJ901 | 30 | 可溶性表达 | 4480U/L | [ | |
| P. chrysosporium | S. cerevisiae | pYES2 | 30 | 可溶性表达 | 367U/L | [ | ||
| P. chrysosporium | P. pastoris | pPICZα | 30 | 可溶性表达 | 15U/L | [ | ||
| MnP | Peniophora incarnata | S. cerevisiae | pESC-URA | 30 | 可溶性表达 | 3580U/L | [ | |
| Ganoderma lucidum | P. pastoris | pAO815 | 28 | 可溶性表达 | 524.61U/L | [ | ||
| Moniliophthora roreri | P. pastoris | pPICZα(A) | 30 | 可溶性表达 | 3659.5U/L | [ | ||
| VP | P. eryngii | S. cerevisiae | pJRoC30 | 30 | 可溶性表达 | 15500U/L | [ | |
| P. eryngii | P. pastoris | pPICZαA | 15 | 可溶性表达 | 3.3U/L | [ | ||
| 其他表达系统 | Lac | Trametes sp. AH28-2 | Trichoderma reesei | pGTCL | 28 | 可溶性表达 | 3.62 IU/mL | [ |
| Phanerochaete flavidoalba | A. niger | pAN52-4 | 30 | 可溶性表达 | 2500U/L | [ | ||
| Aspergillus AF2 | Aspergillus flavus | — | 30 | 可溶性表达 | 79.57U/mL | [ | ||
| LiP | Phanerodontia chrysosporium | Cyberlindnera jadinii | pBR322 | 30 | 可溶性表达 | 68.52U/L | [ | |
| Thermothelomycesthermophiles M77 | Aspergillus nidulans A733 | pEXPYR | 37 | 可溶性表达 | 1645mU/L | [ | ||
| MnP | Dichomitus squalens | P. chrysosporium | pUDGM2 | 37 | 可溶性表达 | 600U/L | [ | |
| Pleurotus ostreatus | Lentinula edodes | pGhL1 | 20 | 可溶性表达 | 30U/L | [ | ||
| VP | P. eryngii | Aspergillus nidulans | PAN7-1 | 28 | 可溶性表达 | 466U/L | [ | |
| P. eryngii | Emericella nidulans | — | 28 | 可溶性表达 | 165U/L | [ | ||
| Pleurotus sapidus | Hansenula polymorpha | pFPMT121 | 24 | 可溶性表达 | 450mU/mg | [ |
| 酶 | 来源 | 表达宿主 | 改造策略 | 结果 | 参考文献 |
|---|---|---|---|---|---|
| Lac | Thermus thermophilus | E. coli Rosetta(DE3) | 理性设计 | E170Y相较于野生酶显示出更高的活性 | [ |
| Basidiomycete PM1 | S. cerevisiae | 计算机辅助 | kcat增加2倍;pH稳定性提高 | [ | |
| Bacillus subtilis | E. coli Tuner | 定向进化 | 氧化还原电位增加 | [ | |
| Agrocybe pediades, | S. cerevisiae | 定向进化 | 中性pH下酶活提升,pH稳定性提升 | [ | |
| Bacillus subtilis | E. coli BL21 | 理性设计 | 漆酶在有机溶剂中的稳定性及活性提升 | [ | |
| Bacillus safensis | E. coli BL21 (DE3) | 计算机辅助 | 借助分子动力学模拟(MD)、HotSpot Wizard和DEZYME 工具获得的突变酶比活性提高1.59倍 | [ | |
| LiP | P. chrysosporium | E. coli BL21 (DE2) pLysS | 计算机辅助 | 通过半经验量子力学方法SQM计算确定LiP与VA结合的 关键残基(Glu168和Glu250) | [ |
| P. chrysosporium | E. coli | 理性设计 | 热稳定性、比活性和kcat/KM提升 | [ | |
| P. chrysosporium | E. coli | 理性设计 | 催化性能提升,kcat和kcat/KM值分别增加21.1倍和4.9倍 | [ | |
| P. chrysosporium | E. coli BL21 | 计算机辅助 | 稳定性及酸性pH条件下更高的活性 | [ | |
| VP | P. eryngii | S. cerevisiae | 定向进化 | 温度及pH稳定性提升;H2O2的KM提高15倍 | [ |
| P. eryngii | E. coli BL21(DE3) | 理性设计 | 过氧化氢稳定性增强 | [ | |
| P. eryngii | E. coli W3110 | 理性设计 | H2O2存在下的稳定性提高了11.7倍 | [ | |
| P. eryngii | E. coli W3110 | 理性设计 | pH稳定性提升 | [ | |
| P. eryngii | E. coli BL21(DE3) | 理性设计 | pH及热稳定性提升 | [ | |
| P. eryngii | S. cerevisiae | 计算机辅助 | pH稳定性、热稳定性及底物特异性提升 | [ |
表2 木质素降解酶的改造
| 酶 | 来源 | 表达宿主 | 改造策略 | 结果 | 参考文献 |
|---|---|---|---|---|---|
| Lac | Thermus thermophilus | E. coli Rosetta(DE3) | 理性设计 | E170Y相较于野生酶显示出更高的活性 | [ |
| Basidiomycete PM1 | S. cerevisiae | 计算机辅助 | kcat增加2倍;pH稳定性提高 | [ | |
| Bacillus subtilis | E. coli Tuner | 定向进化 | 氧化还原电位增加 | [ | |
| Agrocybe pediades, | S. cerevisiae | 定向进化 | 中性pH下酶活提升,pH稳定性提升 | [ | |
| Bacillus subtilis | E. coli BL21 | 理性设计 | 漆酶在有机溶剂中的稳定性及活性提升 | [ | |
| Bacillus safensis | E. coli BL21 (DE3) | 计算机辅助 | 借助分子动力学模拟(MD)、HotSpot Wizard和DEZYME 工具获得的突变酶比活性提高1.59倍 | [ | |
| LiP | P. chrysosporium | E. coli BL21 (DE2) pLysS | 计算机辅助 | 通过半经验量子力学方法SQM计算确定LiP与VA结合的 关键残基(Glu168和Glu250) | [ |
| P. chrysosporium | E. coli | 理性设计 | 热稳定性、比活性和kcat/KM提升 | [ | |
| P. chrysosporium | E. coli | 理性设计 | 催化性能提升,kcat和kcat/KM值分别增加21.1倍和4.9倍 | [ | |
| P. chrysosporium | E. coli BL21 | 计算机辅助 | 稳定性及酸性pH条件下更高的活性 | [ | |
| VP | P. eryngii | S. cerevisiae | 定向进化 | 温度及pH稳定性提升;H2O2的KM提高15倍 | [ |
| P. eryngii | E. coli BL21(DE3) | 理性设计 | 过氧化氢稳定性增强 | [ | |
| P. eryngii | E. coli W3110 | 理性设计 | H2O2存在下的稳定性提高了11.7倍 | [ | |
| P. eryngii | E. coli W3110 | 理性设计 | pH稳定性提升 | [ | |
| P. eryngii | E. coli BL21(DE3) | 理性设计 | pH及热稳定性提升 | [ | |
| P. eryngii | S. cerevisiae | 计算机辅助 | pH稳定性、热稳定性及底物特异性提升 | [ |
| 41 | YANG Li-Hua, QIAO Bin, XU Qiu-Man, et al. Biodegradation of sulfonamide antibiotics through the heterologous expression of laccases from bacteria and investigation of their potential degradation pathways[J]. Journal of Hazardous Materials, 2021, 416: 125815. |
| 42 | KIISKINEN Laura-Leena, SALOHEIMO Markku. Molecular cloning and expression in Saccharomyces cerevisiae of a laccase gene from the ascomycete Melanocarpus albomyces [J]. Applied and Environmental Microbiology, 2004, 70(1): 137-144. |
| 43 | Pablo AZA, MOLPECERES Gonzalo, DE SALAS Felipe, et al. Design of an improved universal signal peptide based on the α-factor mating secretion signal for enzyme production in yeast[J]. Cellular and Molecular Life Sciences, 2021, 78(7): 3691-3707. |
| 44 | 刘启, 钱芷兰, 宋丽丽, 等. 巴斯德毕赤酵母底盘细胞的工程化改造及应用[J]. 合成生物学, 2022, 3(6): 1150-1173. |
| LIU Qi, QIAN Zhilan, SONG Lili, et al. Rewiring and application of Pichia pastoris chassis cell[J]. Synthetic Biology Journal, 2022, 3(6): 1150-1173. | |
| 45 | STEVENS Joseph Craig, RODGERS David W, DUMON Claire, et al. Characterization and enzyme engineering of a hyperthermophilic laccase toward improving its activity in ionic liquid[J]. Frontiers in Energy Research, 2020, 8: 158. |
| 46 | SANTIAGO Gerard, DE SALAS Felipe, Fátima LUCAS M, et al. Computer-aided laccase engineering: Toward biological oxidation of arylamines[J]. ACS Catalysis, 2016, 6(8): 5415-5423. |
| 47 | Vânia BRISSOS, PEREIRA Luciana, MUNTEANU Florentina-Daniela, et al. Expression system of CotA-laccase for directed evolution and high-throughput screenings for the oxidation of high-redox potential dyes[J]. Biotechnology Journal, 2009, 4(4): 558-563. |
| 48 | RASEKH Behnam, KHAJEH Khosro, RANJBAR Bijan, et al. Protein engineering of laccase to enhance its activity and stability in the presence of organic solvents[J]. Engineering in Life Sciences, 2014, 14(4): 442-448. |
| 49 | YANG Wenhua, MA Xiangyang, SUN Hui, et al. Simultaneous enhancement of activity and stability of Bacillus safensis-derived laccase and its application in lignocellulose saccharification[J]. Bioresource Technology, 2025, 418: 131983. |
| 50 | ROMERO Jefferson O, Elena FERNÁNDEZ-FUEYO, Fabián AVILA-SALAS, et al. Binding and catalytic mechanisms of veratryl alcohol oxidation by lignin peroxidase: A theoretical and experimental study[J]. Computational and Structural Biotechnology Journal, 2019, 17: 1066-1074. |
| 51 | SEMBA Yasuyuki, ISHIDA Manabu, YOKOBORI Shin-ichi, et al. Ancestral amino acid substitution improves the thermal stability of recombinant lignin-peroxidase from white-rot fungi, Phanerochaete chrysosporium strain UAMH 3641[J]. Protein Engineering, Design & Selection, 2015, 28(7): 221-230. |
| 52 | PHAM Le Thanh Mai, KIM Su Jin, KIM Yong Hwan. Improvement of catalytic performance of lignin peroxidase for the enhanced degradation of lignocellulose biomass based on the imbedded electron-relay in long-range electron transfer route[J]. Biotechnology for Biofuels, 2016, 9: 247. |
| 53 | PHAM Le Thanh Mai, SEO Hogyun, KIM Kyung-Jin, et al. In silico-designed lignin peroxidase from Phanerochaete chrysosporium shows enhanced acid stability for depolymerization of lignin[J]. Biotechnology for Biofuels, 2018, 11: 325. |
| 54 | BAO Xue, HUANG Xuenian, LU Xuefeng, et al. Improvement of hydrogen peroxide stability of Pleurotus eryngii versatile ligninolytic peroxidase by rational protein engineering[J]. Enzyme and Microbial Technology, 2014, 54: 51-58. |
| 55 | Verónica SÁEZ-JIMÉNEZ, ACEBES Sandra, GUALLAR Victor, et al. Improving the oxidative stability of a high redox potential fungal peroxidase by rational design[J]. PLoS One, 2015, 10(4): e0124750. |
| 56 | Verónica SÁEZ-JIMÉNEZ, Elena FERNÁNDEZ-FUEYO, MEDRANO Francisco Javier, et al. Improving the pH-stability of versatile peroxidase by comparative structural analysis with a naturally-stable manganese peroxidase[J]. PLoS One, 2015, 10(10): e0140984. |
| 57 | GAO Yu, LI Jianjun, ZHENG Lanyan, et al. Rational design of Pleurotus eryngii versatile ligninolytic peroxidase for enhanced pH and thermal stability through structure-based protein engineering[J]. Protein Engineering, Design & Selection, 2017, 30(11): 743-751. |
| 58 | Shiran BARBER-ZUCKER, MINDEL Vladimir, Eva GARCIA-RUIZ, et al. Stable and functionally diverse versatile peroxidases designed directly from sequences[J]. Journal of the American Chemical Society, 2022, 144(8): 3564-3571. |
| 59 | FESTA Giovanna, AUTORE Flavia, FRATERNALI Franca, et al. Development of new laccases by directed evolution: Functional and computational analyses[J]. Proteins, 2008, 72(1): 25-34. |
| 60 | GUPTA Nirupama, FARINAS Edgardo T. Narrowing laccase substrate specificity using active site saturation mutagenesis[J]. Combinatorial Chemistry & High Throughput Screening, 2009, 12(3): 269-274. |
| 61 | Tadas JAKOČIŪNAS, PEDERSEN Lasse E, Alicia V LIS, et al. CasPER, a method for directed evolution in genomic contexts using mutagenesis and CRISPR/Cas9[J]. Metabolic Engineering, 2018, 48: 288-296. |
| 62 | David RODRÍGUEZ-ESCRIBANO, DE SALAS Felipe, PARDO Isabel, et al. High-throughput screening assay for laccase engineering toward lignosulfonate valorization[J]. International Journal of Molecular Sciences, 2017, 18(8): 1793. |
| 63 | CRIBARI Mario A, UNGER Maxwell J, UNARTA Ilona C, et al. Ultrahigh-throughput directed evolution of polymer-degrading enzymes using yeast display[J]. Journal of the American Chemical Society, 2023, 145(50): 27380-27389. |
| 1 | 舒日洋, 徐莹, 张琦, 等. 木质素催化解聚的研究进展[J]. 化工学报, 2016, 67(11): 4523-4532. |
| SHU Riyang, XU Ying, ZHANG Qi, et al. Progress in catalytic depolymerization of lignin[J]. CIESC Journal, 2016, 67(11): 4523-4532. | |
| 2 | CHOPRA Navleen Kaur, SONDHI Sonica. Cloning, expression and characterization of laccase from Bacillus licheniformis NS2324 in E. coli application in dye decolorization[J]. International Journal of Biological Macromolecules, 2022, 206: 1003-1011. |
| 3 | WENG Caihong, PENG Xiaowei, HAN Yejun. Depolymerization and conversion of lignin to value-added bioproducts by microbial and enzymatic catalysis[J]. Biotechnology for Biofuels, 2021, 14(1): 84. |
| 4 | LI Ye, CIRINO Patrick C. Recent advances in engineering proteins for biocatalysis[J]. Biotechnology and Bioengineering, 2014, 111(7): 1273-1287. |
| 5 | WEISS Renate, GUEBITZ Georg M, PELLIS Alessandro, et al. Harnessing the power of enzymes for tailoring and valorizing lignin[J]. Trends in Biotechnology, 2020, 38(11): 1215-1231. |
| 6 | BIAN Luyao, ZHENG Meixia, CHANG Tingting, et al. Degradation of Aflatoxin B1 by recombinant laccase extracellular produced from Escherichia coli [J]. Ecotoxicology and Environmental Safety, 2022, 244: 114062. |
| 7 | WANG Jiayi, YU Shuyu, LI Xiaoyan, et al. High-level expression of Bacillus amyloliquefaciens laccase and construction of its chimeric variant with improved stability by domain substitution[J]. Bioprocess and Biosystems Engineering, 2020, 43(3): 403-411. |
| 8 | YANG Xiaorong, GU Chenguang, LIN Ying. A novel fungal laccase from Sordaria macrospora k-hell: Expression, characterization, and application for lignin degradation[J]. Bioprocess and Biosystems Engineering, 2020, 43(7): 1133-1139. |
| 9 | LI Shouzhi, CHEN Yanzhen, LIANG Xiaoyu, et al. Expanding the application of lignin peroxidases from Irpex Lacteus in Lignin degradation by exploring the effects of small molecules on enzyme activity[J]. E3S Web of Conferences, 2023, 393: 02036. |
| 10 | LI Shouzhi, HE Lu, SHI Na, et al. Preparing the pure lignin peroxidase and exploring the effects of chemicals on the activity[J]. Preparative Biochemistry & Biotechnology, 2024, 54(5): 660-667. |
| 11 | Rodrigo CORTÉS-ANTIQUERA, MÁRQUEZ Sebastián L, ESPINA Giannina, et al. Recombinant expression and characterization of a new laccase, bioinformatically identified, from the Antarctic thermophilic bacterium Geobacillus sp. ID17[J]. Extremophiles, 2023, 27(2): 18. |
| 12 | LIN Meng-I, NAGATA Takashi, KATAHIRA Masato. High yield production of fungal manganese peroxidases by E. coli through soluble expression, and examination of the activities[J]. Protein Expression and Purification, 2018, 145: 45-52. |
| 13 | ALFI Almasul, ZHU Bo, Jasmina DAMNJANOVIĆ, et al. Production of active manganese peroxidase in Escherichia coli by co-expression of chaperones and in vitro maturation by ATP-dependent chaperone release[J]. Journal of Bioscience and Bioengineering, 2019, 128(3): 290-295. |
| 14 | BAO Xue, LIU Aiqiu, LU Xuefeng, et al. Direct over-expression, characterization and H2O2 stability study of active Pleurotus eryngii versatile peroxidase in Escherichia coli [J]. Biotechnology Letters, 2012, 34(8): 1537-1543. |
| 15 | PÉREZ-BOADA M, DOYLE W A, RUIZ-DUEÑAS F J, et al. Expression of Pleurotus eryngii versatile peroxidase in Escherichia coli and optimisation of in vitro folding[J]. Enzyme and Microbial Technology, 2002, 30(4): 518-524. |
| 16 | LIU Jiashu, ZHANG Shu, SHI Qipeng, et al. Highly efficient oxidation of synthetic and natural lignin-related compounds by Physisporinus vitreus versatile peroxidase[J]. International Biodeterioration & Biodegradation, 2019, 136: 41-48. |
| 17 | YADAV Deepti, RANJAN Bibhuti, MCHUNU Nokuthula, et al. Enhancing the expression of recombinant small laccase in Pichia pastoris by a double promoter system and application in antibiotics degradation[J]. Folia Microbiologica, 2021, 66(6): 917-930. |
| 18 | Pablo AZA, MOLPECERES Gonzalo, RUIZ-DUEÑAS Francisco Javier, et al. Heterologous expression, engineering and characterization of a novel laccase of Agrocybe pediades with promising properties as biocatalyst[J]. Journal of Fungi, 2021, 7(5): 359. |
| 19 | MATE Diana M, David GONZALEZ-PEREZ, KITTL Roman, et al. Functional expression of a blood tolerant laccase in Pichia pastoris [J]. BMC Biotechnology, 2013, 13: 38. |
| 20 | MAJEKE B M, GARCÍA-APARICIO M, BIKO O D, et al. Synergistic codon optimization and bioreactor cultivation toward enhanced secretion of fungal lignin peroxidase in Pichia pastoris: Enzymatic valorization of technical (industrial) lignins[J]. Enzyme and Microbial Technology, 2020, 139: 109593. |
| 21 | 肖建龙, 张斯童, 孙晓仲, 等. 高效组成型分泌表达木素过氧化物酶酿酒酵母工程菌的构建[J]. 微生物学报, 2020, 60(5): 951-962. |
| XIAO Jianlong, ZHANG Sitong, SUN Xiaozhong, et al. Construction of an engineered Saccharomyces cerevisiae for lignin peroxidase production[J]. Acta Microbiologica Sinica, 2020, 60(5): 951-962. | |
| 22 | WANG Wei, WEN Xianghua. Expression of lignin peroxidase H2 from Phanerochaete chrysosporium by multi-copy recombinant Pichia strain[J]. Journal of Environmental Sciences, 2009, 21(2): 218-222. |
| 23 | LEE Aslan Hwanhwi, KANG Chang-Min, LEE Young Min, et al. Heterologous expression of a new manganese-dependent peroxidase gene from Peniophora incarnata KUC8836 and its ability to remove anthracene in Saccharomyces cerevisiae [J]. Journal of Bioscience and Bioengineering, 2016, 122(6): 716-721. |
| 24 | XU Hui, GUO Mengyuan, GAO Yanhua, et al. Expression and characteristics of manganese peroxidase from Ganoderma lucidum in Pichia pastoris and its application in the degradation of four dyes and phenol[J]. BMC Biotechnology, 2017, 17(1): 19. |
| 25 | WANG Shuai, WANG Xiaolu, PENTTINEN Leena, et al. Patulin detoxification by recombinant manganese peroxidase from Moniliophthora roreri expressed by Pichia pastoris [J]. Toxins, 2022, 14(7): 440. |
| 26 | Eva GARCIA-RUIZ, David GONZALEZ-PEREZ, RUIZ-DUEÑAS Francisco J, et al. Directed evolution of a temperature-, peroxide- and alkaline pH-tolerant versatile peroxidase[J]. Biochemical Journal, 2012, 441(1): 487-498. |
| 27 | BRONIKOWSKI Agathe, KOSCHORRECK Katja, URLACHER Vlada B. Redesign of a new manganese peroxidase highly expressed in Pichia pastoris towards a lignin-degrading versatile peroxidase[J]. ChemBioChem, 2018, 19(23): 2481-2489. |
| 28 | ZHANG Jiwei, QU Yinbo, XIAO Peng, et al. Improved biomass saccharification by Trichoderma reesei through heterologous expression of lacA gene from Trametes sp. AH28-2[J]. Journal of Bioscience and Bioengineering, 2012, 113(6): 697-703. |
| 29 | BENGHAZI Lamiae, RECORD Eric, Antonio SUÁREZ, et al. Production of the Phanerochaete flavido-alba laccase in Aspergillus niger for synthetic dyes decolorization and biotransformation[J]. World Journal of Microbiology & Biotechnology, 2014, 30(1): 201-211. |
| 30 | GOMAA Ola M, MOMTAZ Osama A. Copper induction and differential expression of laccase in Aspergillus flavus [J]. Brazilian Journal of Microbiology, 2015, 46(1): 285-292. |
| 31 | LIU Enshi, SEGATO Fernando, WILKINS Mark R. Fed-batch production of Thermothelomyces thermophilus lignin peroxidase using a recombinant Aspergillus nidulans strain in stirred-tank bioreactor[J]. Bioresource Technology, 2021, 325: 124700. |
| 32 | BIKO Odwa D, Marinda VILJOEN-BLOOM, VAN ZYL Willem H. Medium optimization for enhanced production of recombinant lignin peroxidase in Pichia pastoris [J]. Biotechnology Letters, 2023, 45(1): 105-113. |
| 33 | LI Dongmei, YOUNGS Heather L, GOLD Michael H. Heterologous expression of a thermostable manganese peroxidase from Dichomitus squalens in Phanerochaete chrysosporium [J]. Archives of Biochemistry and Biophysics, 2001, 385(2): 348-356. |
| 34 | SATO Toshitsugu, IRIE Toshikazu, YOSHINO Fumihiko. Heterologous expression of the Pleurotus ostreatus MnP3 gene by the laccase gene promoter in Lentinula edodes [J]. Bioscience, Biotechnology, and Biochemistry, 2017, 81(8): 1553-1556. |
| 64 | SANA Barindra, CHIA Kuan Hui Burton, RAGHAVAN Sarada S, et al. Development of a genetically programed vanillin-sensing bacterium for high-throughput screening of lignin-degrading enzyme libraries[J]. Biotechnology for Biofuels, 2017, 10: 32. |
| 65 | SU Xiaolan, YANG Jianhua, YUAN Huiling, et al. Directed evolution of laccase for improved thermal stability facilitated by droplet-based microfluidic screening system[J]. Journal of Agricultural and Food Chemistry, 2022, 70(42): 13700-13708. |
| 66 | KHODAKARAMI Atefeh, GOODARZI Negar, Mahshideh HOSEINZADEHDEHK-ORDI, et al. Rational design toward developing a more efficient laccase: Catalytic efficiency and selectivity[J]. International Journal of Biological Macromolecules, 2018, 112: 775-779. |
| 67 | XU Kaizhong, WANG Haoran, WANG Yajing, et al. Enhancement in catalytic activity of CotA-laccase from Bacillus pumilus W3 via site-directed mutagenesis[J]. Journal of Bioscience and Bioengineering, 2020, 129(4): 405-411. |
| 68 | DING Zundan, GUAN Feifei, XU Guoshun, et al. MPEPE, a predictive approach to improve protein expression in E. coli based on deep learning[J]. Computational and Structural Biotechnology Journal, 2022, 20: 1142-1153. |
| 35 | EIBES G M, LÚ-CHAU T A, RUIZ-DUEÑAS F J, et al. Effect of culture temperature on the heterologous expression of Pleurotus eryngii versatile peroxidase in Aspergillus hosts[J]. Bioprocess and Biosystems Engineering, 2009, 32(1): 129-134. |
| 36 | LÚ-CHAU T A, RUIZ-DUEÑAS F J, CAMARERO S, et al. Effect of pH on the stability of Pleurotus eryngii versatile peroxidase during heterologous production in Emericella nidulans [J]. Bioprocess and Biosystems Engineering, 2004, 26(5): 287-293. |
| 37 | Ina SCHÜTTMANN, BOUWS Henning, SZWEDA Renata T, et al. Induction, characterization, and heterologous expression of a carotenoid degrading versatile peroxidase from Pleurotus sapidus [J]. Journal of Molecular Catalysis B: Enzymatic, 2014, 103: 79-84. |
| 38 | BEHRENS Christoph J, LINKE Diana, ALLISTER Aldrige B, et al. Variants of PpuLcc, a multi-dye decolorizing laccase from Pleurotus pulmonarius expressed in Pichia pastoris [J]. Protein Expression and Purification, 2017, 137: 34-42. |
| 39 | KIM Su-Jin, LEE Jeong-Ah, KIM Yong-Hwan, et al. Optimization of the functional expression of Coprinus cinereus peroxidase in Pichia pastoris by varying the host and promoter[J]. Journal of Microbiology and Biotechnology, 2009, 19(9): 966-971. |
| 40 | VARMAN Arul M, FOLLENFANT Rhiannon, LIU Fang, et al. Hybrid phenolic-inducible promoters towards construction of self-inducible systems for microbial lignin valorization[J]. Biotechnology for Biofuels, 2018, 11(1): 182. |
| [1] | 崔堂武, 袁波, 凌晨, 方彬任, 毛向阳, 费强. 木质素降解酶的酶活测试方法的评价与分析[J]. 化工进展, 2020, 39(12): 5189-5202. |
| [2] | 陈宏文1,刘 薇1,杜 钰1,陈 国1,方柏山2. 工业微生物还原型辅酶Ⅱ的代谢调控研究进展[J]. 化工进展, 2012, 31(11): 2535-2541. |
| [3] | 赖超凤,李 爽,彭丽丽,王菊芳. 漆酶及其在有机合成中应用的研究进展 [J]. 化工进展, 2010, 29(7): 1300-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||