化工进展 ›› 2023, Vol. 42 ›› Issue (10): 5459-5469.DOI: 10.16085/j.issn.1000-6613.2022-2090
生物炭回收水中氮磷营养物质的研究进展与挑战
王书燕1,2( ), 张新波1,2(
), 张新波1,2( ), 彭安萍1,2, 刘阳1,2, NGO HUU HAO1, 郭文珊1, 温海涛1
), 彭安萍1,2, 刘阳1,2, NGO HUU HAO1, 郭文珊1, 温海涛1
- 1.天津城建大学环境与市政工程学院,基础设施防护和环境绿色生物技术国际联合研究中心,天津 300384
2.天津城建大学,天津市水质科学与技术重点实验室,天津 300384
-
收稿日期:2022-11-09修回日期:2023-04-29出版日期:2023-10-15发布日期:2023-11-11 -
通讯作者:张新波 -
作者简介:王书燕(1998—),女,硕士研究生,研究方向为环境功能材料。E-mail:7686430@qq.com。 -
基金资助:天津市科委创新平台项目(18PTZWHZ00140);天津科委重点项目(20JCZDJC00380)
Research progress and challenges in recovery of nitrogen and phosphorus nutrients from water by biochar
WANG Shuyan1,2( ), ZHANG Xinbo1,2(
), ZHANG Xinbo1,2( ), PENG Anping1,2, LIU Yang1,2, HAO NGO HUU1, GUO Wenshan1, WEN Haitao1
), PENG Anping1,2, LIU Yang1,2, HAO NGO HUU1, GUO Wenshan1, WEN Haitao1
- 1.Joint Research Centre for Protective Infrastructure Technology and Environmental Green Bioprocess, School of Environmental and Municipal Engineering, Tianjin Chengjian University, Tianjin 300384, China
2.Tianjin Key Laboratory of Aquatic Science and Technology, Tianjin Chengjian University, Tianjin 300384, China
-
Received:2022-11-09Revised:2023-04-29Online:2023-10-15Published:2023-11-11 -
Contact:ZHANG Xinbo
摘要:
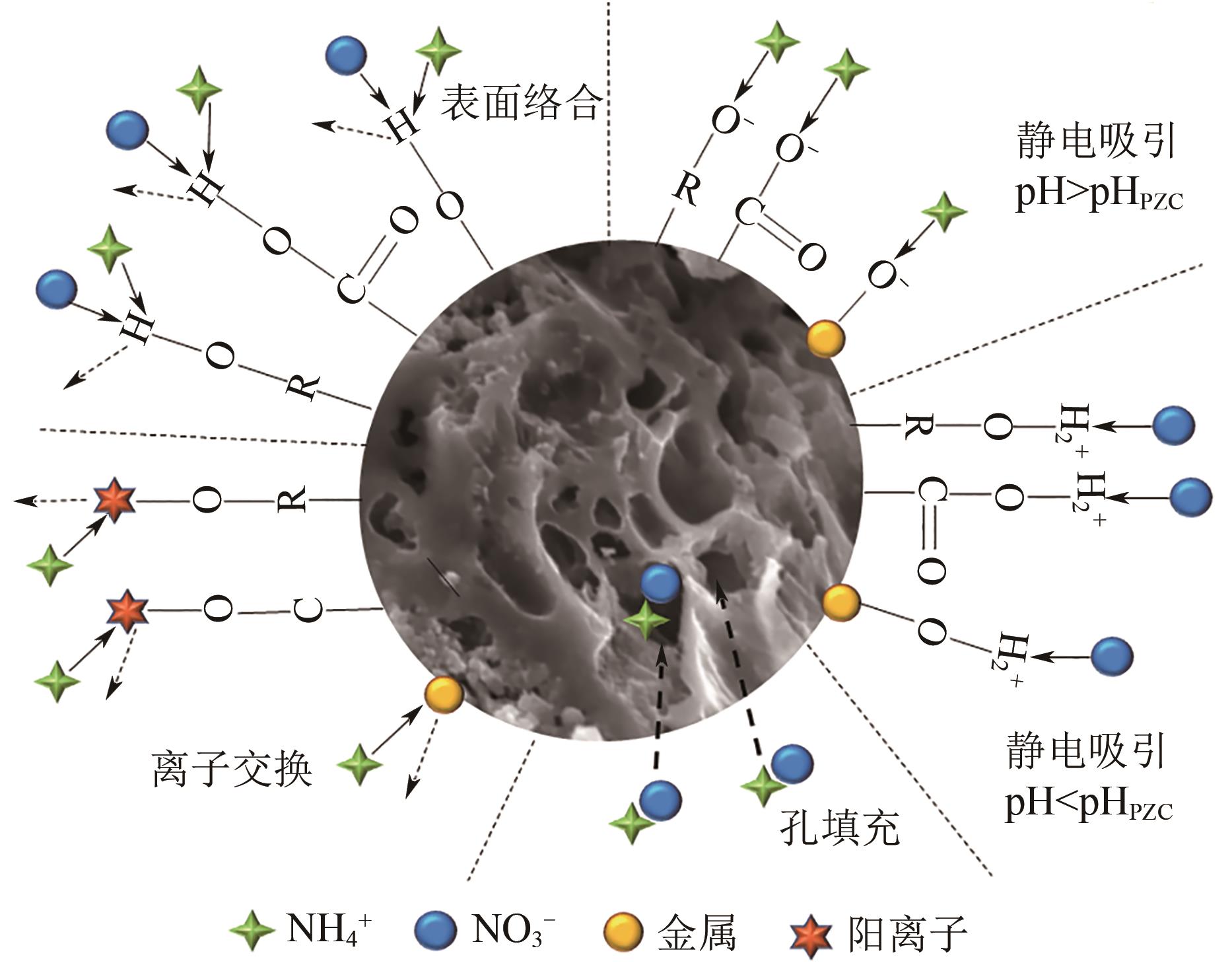
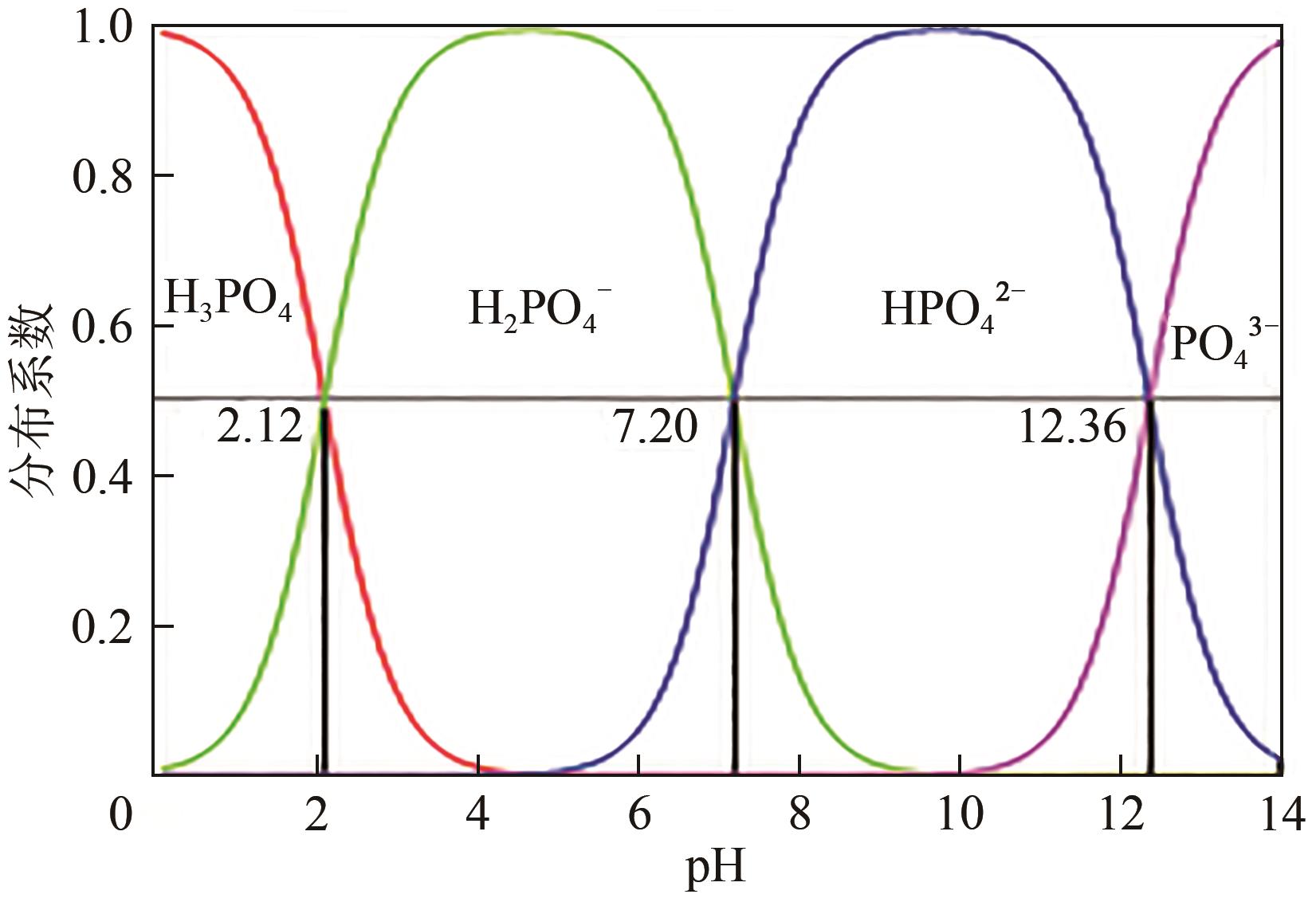
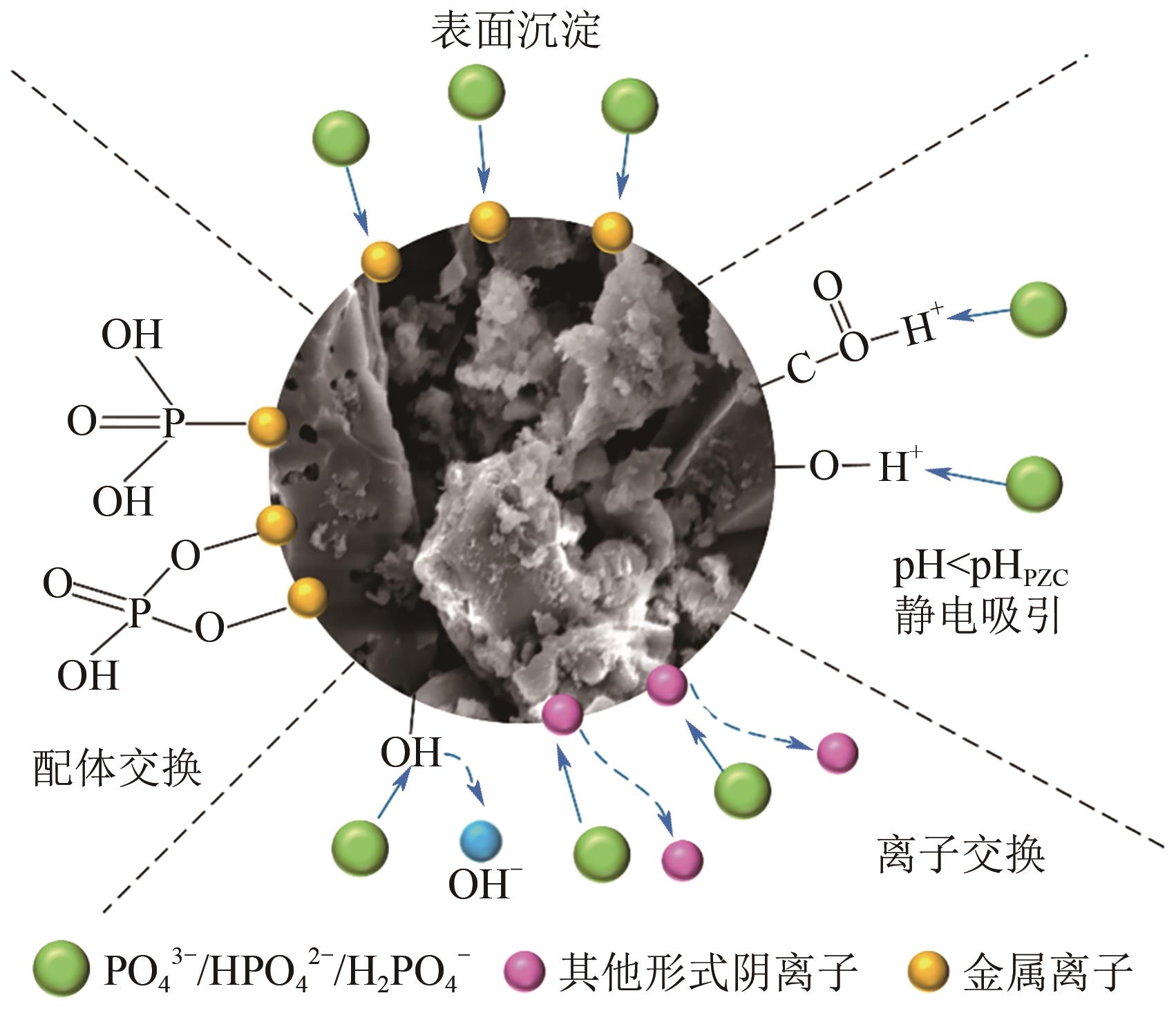
水中过量氮磷营养物质的存在会引发水体富营养化。生物炭具有比表面积大、孔隙率高、热稳定性强、表面官能团丰富等优点,在吸附去除水中污染物方面呈现出良好性能。近年来,生物炭作为一种经济高效的吸附剂回收水中的氮和磷而受到了广泛关注,但不同种类生物炭对水中氮或磷的吸附性能有较大的差异。本文综述了基于各种废弃生物质制备及改性的生物炭对水中氮和磷的吸附性能,探讨分析了影响生物炭吸附水中氮和磷性能的主要因素,包括制备条件、环境温度、溶液pH和共存离子等,阐述了生物炭吸附水中氮和磷的主要机理。同时指出了目前生物炭在实际应用中所面临的挑战,并进一步展望了未来生物炭的重点研究方向,从而为实际利用生物炭吸附回收水中的氮和磷提供理论依据。
中图分类号:
引用本文
王书燕, 张新波, 彭安萍, 刘阳, NGO HUU HAO, 郭文珊, 温海涛. 生物炭回收水中氮磷营养物质的研究进展与挑战[J]. 化工进展, 2023, 42(10): 5459-5469.
WANG Shuyan, ZHANG Xinbo, PENG Anping, LIU Yang, HAO NGO HUU, GUO Wenshan, WEN Haitao. Research progress and challenges in recovery of nitrogen and phosphorus nutrients from water by biochar[J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5459-5469.
| 方法 | 温度/℃ | 停留时间 | 升温速率/℃·min-1 | 参考文献 |
|---|---|---|---|---|
| 慢速热解 | 100~1000 | >1h | <10 | [ |
| 快速热解 | 500~1000 | <2s | >120 | [ |
| 水热炭化 | 180~250 | 1~16h | — | [ |
| 气化 | >800 | 10~20s | — | [ |
| 烘烤 | 200~300 | 20~120min | <50 | [ |
| 微波热解 | 400~500 | 1~10min | — | [ |
表1 生物炭制备方法及条件
| 方法 | 温度/℃ | 停留时间 | 升温速率/℃·min-1 | 参考文献 |
|---|---|---|---|---|
| 慢速热解 | 100~1000 | >1h | <10 | [ |
| 快速热解 | 500~1000 | <2s | >120 | [ |
| 水热炭化 | 180~250 | 1~16h | — | [ |
| 气化 | >800 | 10~20s | — | [ |
| 烘烤 | 200~300 | 20~120min | <50 | [ |
| 微波热解 | 400~500 | 1~10min | — | [ |
| 原材料 | 制备 温度/℃ | 改性剂 | 吸附 目标物 | 初始浓度 /mg·L-1 | 最大吸附容量 /mg·g-1 | 吸附等温曲线 | 动力学模型 | 主要机理 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 竹子 | 370 | — | NH4+ | 1~10 | 6.38 | Freundlich | 准二级 | 静电吸引 | [ |
| 橘子、菠萝、 火龙果皮 | 300~600 | — | NH4+ | 10~100 | 4.71、5.60、2.65 | Langmuir | 准二级 | 阳离子交换、 静电吸引、 表面络合 | [ |
| 湿地植物 | 500 | — | NH4+ | 0~100 | 13.35 | Langmuir | 准二级 | 阳离子交换、 表面络合 | [ |
| 巨型芦苇 | 500 | — | NH4+ | 30~80 | 1.49 | Freundlich、 Langmuir | 准一级、 准二级 | 离子交换 | [ |
| 粪便污泥 | 600 | — | NH4+ | 100 | 12 | Langmuir | 准二级 | 静电吸引 | [ |
| 稻草 | 300~600 | — | NH3-N | 10~500 | 15.79±1.84 | — | 准二级 | 静电吸引 | [ |
| 木屑、稻壳 | 600 | — | NH4+ | 250~1400 | 44.64±0.462、 39.8±0.54 | Langmuir | 准二级 | 静电引力 | [ |
| 稻草、芦苇、 木屑和蛋壳 | 300~700 | — | NH4+ | 0~320 | 4.2、3.2、 3.3、2.2 | Langmuir | 准二级 | 静电吸引 | [ |
| 消化污泥 | 350~550 | — | NH4+ | 2~80 | 1.4 | Langmuir | 准二级 | 静电吸引 | [ |
| 污泥、核桃壳 | 600 | — | NH4+ | 50 | 22.85 | Freundlich | 准二级 | 粒子内表面扩散 | [ |
| 芝麻秸秆 | 300~700 | — | NH4+ | 5~500 | 93.61 | Langmuir-Freundlich | 准二级 | 粒子内表面扩散 | [ |
| 松木锯末、小麦秸秆 | 300, 550 | — | NH4+ | 10~100 | 5.38、2.08 | Redlich-Peterson | 准二级 | 静电吸引、 表面络合 | [ |
| 紫茎泽兰 | 300 | — | NH4+ | 5~100 | 1.909 | Langmuir-Freundlich | 准二级 | 静电吸引 | [ |
| 玉米芯 | 300~600 | — | NO3- | 0~2000 | 14.46 | Langmuir | 准二级 | 静电吸引 | [ |
| 猪骨、楠竹 | 550 | — | NH4+ | 10~400 | 13.97 | Langmuir | 准二级 | — | [ |
| 玉米秸秆 | 500 | KMnO4 | NH4+ | — | 23.80 | Langmuir | 准一级、 准二级 | — | [ |
| 玉米芯 | 400 | HNO3、NaOH | NH4+ | 10~100 | 22.6 | Langmuir | 准二级 | 离子交换、 静电吸引 | [ |
| 水稻秸秆 | 400、600 | KOH、FeCl3·H2O | NH4+、NO3- | 10~200 | 19.26、46.50 | Langmuir-Freundlich | 准二级 | 静电吸引、 表面络合 | [ |
| 污泥 | 400 | HCl、FeCl3 | NH4+ | 2~100 | 9.67 | Langmuir | 准二级 | 静电吸引、 离子交换 | [ |
| 大豆秸秆 | 500 | MgCl2、AlCl3 | NH4+、NO3- | 50 | 0.70、40.63 | — | 准二级 | 静电吸引 | [ |
| 棕榈叶 | 700 | Mg、Al | NO3- | 10~50 | 28.06 | Langmuir | 准一级 | 离子交换、 表面络合 | [ |
| 杨木屑 | 550 | AlCl3·6H2O | NO3- | 0~2000 | 89.58 | Langmuir-Freundlich | 准二级 | 静电吸引 | [ |
| 玉米秸秆 | 550 | FeCl3 | NO3- | 2.5~5 | 14 | Langmuir | — | 静电吸引 | [ |
| 稻壳 | 450 | MgCl2、 膨润土 | NH4+ | 60 | 50.27 | — | 准二级 | 离子交换、 静电吸引 | [ |
| 毛竹 | 300~500 | 蒙脱石 | NH4+ | 20~6000 | 12.52 | Redlich-Peterson | Elovich | 范德华力 | [ |
| 金竹 | 460 | 蒙脱石 | NO3- | 5~400 | 9 | Langmuir | — | 静电吸引 | [ |
| 油菜秸秆 | 750 | 赤泥 | NH4+ | 27.15~407.14 | 2.97 | Langmuir | 准二级 | 孔填充、静电 吸引、表面沉淀 | [ |
表2 生物炭吸附水中氮的研究
| 原材料 | 制备 温度/℃ | 改性剂 | 吸附 目标物 | 初始浓度 /mg·L-1 | 最大吸附容量 /mg·g-1 | 吸附等温曲线 | 动力学模型 | 主要机理 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 竹子 | 370 | — | NH4+ | 1~10 | 6.38 | Freundlich | 准二级 | 静电吸引 | [ |
| 橘子、菠萝、 火龙果皮 | 300~600 | — | NH4+ | 10~100 | 4.71、5.60、2.65 | Langmuir | 准二级 | 阳离子交换、 静电吸引、 表面络合 | [ |
| 湿地植物 | 500 | — | NH4+ | 0~100 | 13.35 | Langmuir | 准二级 | 阳离子交换、 表面络合 | [ |
| 巨型芦苇 | 500 | — | NH4+ | 30~80 | 1.49 | Freundlich、 Langmuir | 准一级、 准二级 | 离子交换 | [ |
| 粪便污泥 | 600 | — | NH4+ | 100 | 12 | Langmuir | 准二级 | 静电吸引 | [ |
| 稻草 | 300~600 | — | NH3-N | 10~500 | 15.79±1.84 | — | 准二级 | 静电吸引 | [ |
| 木屑、稻壳 | 600 | — | NH4+ | 250~1400 | 44.64±0.462、 39.8±0.54 | Langmuir | 准二级 | 静电引力 | [ |
| 稻草、芦苇、 木屑和蛋壳 | 300~700 | — | NH4+ | 0~320 | 4.2、3.2、 3.3、2.2 | Langmuir | 准二级 | 静电吸引 | [ |
| 消化污泥 | 350~550 | — | NH4+ | 2~80 | 1.4 | Langmuir | 准二级 | 静电吸引 | [ |
| 污泥、核桃壳 | 600 | — | NH4+ | 50 | 22.85 | Freundlich | 准二级 | 粒子内表面扩散 | [ |
| 芝麻秸秆 | 300~700 | — | NH4+ | 5~500 | 93.61 | Langmuir-Freundlich | 准二级 | 粒子内表面扩散 | [ |
| 松木锯末、小麦秸秆 | 300, 550 | — | NH4+ | 10~100 | 5.38、2.08 | Redlich-Peterson | 准二级 | 静电吸引、 表面络合 | [ |
| 紫茎泽兰 | 300 | — | NH4+ | 5~100 | 1.909 | Langmuir-Freundlich | 准二级 | 静电吸引 | [ |
| 玉米芯 | 300~600 | — | NO3- | 0~2000 | 14.46 | Langmuir | 准二级 | 静电吸引 | [ |
| 猪骨、楠竹 | 550 | — | NH4+ | 10~400 | 13.97 | Langmuir | 准二级 | — | [ |
| 玉米秸秆 | 500 | KMnO4 | NH4+ | — | 23.80 | Langmuir | 准一级、 准二级 | — | [ |
| 玉米芯 | 400 | HNO3、NaOH | NH4+ | 10~100 | 22.6 | Langmuir | 准二级 | 离子交换、 静电吸引 | [ |
| 水稻秸秆 | 400、600 | KOH、FeCl3·H2O | NH4+、NO3- | 10~200 | 19.26、46.50 | Langmuir-Freundlich | 准二级 | 静电吸引、 表面络合 | [ |
| 污泥 | 400 | HCl、FeCl3 | NH4+ | 2~100 | 9.67 | Langmuir | 准二级 | 静电吸引、 离子交换 | [ |
| 大豆秸秆 | 500 | MgCl2、AlCl3 | NH4+、NO3- | 50 | 0.70、40.63 | — | 准二级 | 静电吸引 | [ |
| 棕榈叶 | 700 | Mg、Al | NO3- | 10~50 | 28.06 | Langmuir | 准一级 | 离子交换、 表面络合 | [ |
| 杨木屑 | 550 | AlCl3·6H2O | NO3- | 0~2000 | 89.58 | Langmuir-Freundlich | 准二级 | 静电吸引 | [ |
| 玉米秸秆 | 550 | FeCl3 | NO3- | 2.5~5 | 14 | Langmuir | — | 静电吸引 | [ |
| 稻壳 | 450 | MgCl2、 膨润土 | NH4+ | 60 | 50.27 | — | 准二级 | 离子交换、 静电吸引 | [ |
| 毛竹 | 300~500 | 蒙脱石 | NH4+ | 20~6000 | 12.52 | Redlich-Peterson | Elovich | 范德华力 | [ |
| 金竹 | 460 | 蒙脱石 | NO3- | 5~400 | 9 | Langmuir | — | 静电吸引 | [ |
| 油菜秸秆 | 750 | 赤泥 | NH4+ | 27.15~407.14 | 2.97 | Langmuir | 准二级 | 孔填充、静电 吸引、表面沉淀 | [ |
| 原料 | 热解温度/℃ | 改性剂 | 磷初始浓度 /mg·L-1 | 最大吸附量 /mg·g-1 | 吸附等温线 | 动力学 模型 | 主要机理 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 稻草 | 800 | — | 0~200 | 5.58 | Langmuir | 准一级 | — | [ |
| 紫茎泽兰 | 300 | — | 5~100 | 2.32 | Langmuir-Freundlich | 准二级 | 静电吸引、配体交换 | [ |
| 紫茎泽兰 | 600 | — | 20~300 | 13.6 | Langmuir | 准二级 | — | [ |
| 美人蕉 | 800 | — | 5~500 | 9.47 | Langmuir | 准二级 | — | [ |
| 竹子 | 600 | Mg/Al或Mg/Fe LDH | 5~600 | 172 | Langmuir | 准二级 | 离子交换 | [ |
| 白菜/油菜 | 500 | Mg/Al LDO | 5~500 | 127.2~132.8 | Langmuir | 准二级 | 静电吸引、配体交换、 离子交换 | [ |
| 椰枣叶 | 700 | Mg/Al LDH | 10~50 | 177.97 | Langmuir | 准一级 | 配体交换 | [ |
| 甘蔗叶 | 550 | Mg/Al LDH | 5~500 | 81.83 | Langmuir | 准二级 | 静电吸引、配体交换 | [ |
| 香蒲 | 400~600 | La(OH)3 | 5~500 | 36.06 | Langmuir | 准二级 | 配体交换、静电吸引 | [ |
| 法国梧桐 | 600 | La(OH)3 | 30~100 | 148.11 | Langmuir | 准二级 | 静电吸引、配体交换 | [ |
| 小麦秸秆 | 550 | 壳聚糖和La(OH)3 | 25~300 | 108.86 | Langmuir | 准二级 | 静电吸引、配体交换 | [ |
| 菠萝皮 | 300 | Fe2O3和La(OH)3 | 100~400 | 101.16 | Langmuir | 准二级 | 表面沉淀、配体交换、 静电吸引 | [ |
| 橡木 | 500 | La(OH)3 | 1~400 | 46.37 | Langmuir | 准二级 | 静电吸引、表面沉淀、 配体交换 | [ |
| 脱水污泥 | 400~800 | La(OH)3 | 50~300 | 93.91 | Langmuir | 准二级 | 静电吸引、配体交换 | [ |
| 美人蕉 | 800 | La(NO3)3 | 5~500 | 37.37 | Langmuir | 准二级 | 静电吸引、离子交换、 配体交换 | [ |
| 核桃壳 | 400 | LaCl3 | 0~50 | 12.18 | Langmuir | 准二级 | — | [ |
| 玉米秸秆 | 800 | NaLa(CO3)2、FeCl3 | 50~400 | 217.84 | Langmuir | 准二级 | 静电吸引、配体交换 | [ |
| 紫薇落叶 | 600 | Mg(OH)2 | 10~300 | 121.95 | Sips | 准二级 | 配体交换 | [ |
| 柏木木屑 | 400~600 | MgCl2 | 50~250 | 43.5~66.7 | Langmuir | 准二级 | 表面沉淀 | [ |
| 山核桃木屑/竹子 | 600 | MgCl2/AlCl3/FeCl3 | 15~1000 | 119.6 | Langmuir-Freundlich | — | 静电吸引、表面沉淀 | [ |
| 甘蔗叶 | 550 | MgCl2 | 1~500 | 398.71 | Langmuir | 准二级 | 静电吸引、表面沉淀 | [ |
| 毛竹 | 400~600 | MgCl2 | 19.7~498 | 344~370 | Langmuir-Freundlich | 准二级 | 配体交换、静电吸引 | [ |
| 香蕉秸秆/木薯秸秆/ 玉米秸秆/杉木秸秆/ 芋头秸秆/油茶壳 | 430 | MgCl2 | 20~350 | 6.77~31.15 | Langmuir | 准二级 | 静电吸引、表面沉淀 | [ |
| 玉米秸秆 | 550 | MgCl2 | 4~200 | 60.95 | Langmuir-Freundlich | 准二级 | 静电吸引、表面沉淀 | [ |
| 胡萝卜 | 400 | MgCl2 | 25~350 | 138 | Langmuir | 准一级 | — | [ |
| 玉米芯 | 800 | MgCl2 | 3~25 | 8.44 | Langmuir | 准二级 | — | [ |
| 杨树 | 550 | AlCl3 | 50~1600 | 57.49 | Langmuir-Freundlich | 准二级 | — | [ |
| 麦秸 | 600 | CaCl2 | 200~1000 | 213.22±13.57 | Langmuir-Freundlich | 准二级 | 表面沉淀 | [ |
| 花生壳/甘蔗渣 | 460~850 | MgCl2、CaCl2 | 3~5800 | 111.80~129.79 | Sips | 准二级 | 表面沉淀 | [ |
| 稻壳/木材 | 500 | Fe2(SO4)3、FeSO4 | 25~150 | 24.9~27.6 | Freundlich | 准二级 | 静电吸引、配体交换 | [ |
| 水葫芦 | 450 | FeCl3、FeCl2 | 0.186~150 | 5.068 | Langmuir-Freundlich | 准二级 | 静电吸引、配体交换 | [ |
| 活性污泥 | 550 | FeCl3 | 5~1000 | 111 | Freundlich | 准二级 | 静电吸引、离子交换、 配体交换 | [ |
| 玉米秸秆 | 550 | FeCl3 | 10~50 | 98 | Langmuir | — | 离子交换、表面沉淀、 配体交换 | [ |
| 核桃壳 | 600 | FeCl2、MgCl2 | 5~400 | 6.945 | Langmuir-Freundlich | 准二级 | 静电吸引 | [ |
| 酒糟 | 600 | 磷石膏 | 5~500 | 102.4 | Freundlich | 准二级 | 静电吸引、表面沉淀、 配体交换 | [ |
| 油菜 | 700 | 煤矸石 | 5~200 | 7.9 | Langmuir | 准二级 | 静电吸引、配体交换、 表面沉淀 | [ |
| 羊粪 | 800 | 牡蛎壳、LaCl3 | 5~200 | 88.34 | Langmuir | 准二级 | 配体交换 | [ |
| 脱水污泥 | 800 | 石灰石 | 50~500 | 231.28 | Langmuir | 准二级 | 表面沉淀、配体交换 | [ |
表3 生物炭吸附水中磷酸盐的研究
| 原料 | 热解温度/℃ | 改性剂 | 磷初始浓度 /mg·L-1 | 最大吸附量 /mg·g-1 | 吸附等温线 | 动力学 模型 | 主要机理 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| 稻草 | 800 | — | 0~200 | 5.58 | Langmuir | 准一级 | — | [ |
| 紫茎泽兰 | 300 | — | 5~100 | 2.32 | Langmuir-Freundlich | 准二级 | 静电吸引、配体交换 | [ |
| 紫茎泽兰 | 600 | — | 20~300 | 13.6 | Langmuir | 准二级 | — | [ |
| 美人蕉 | 800 | — | 5~500 | 9.47 | Langmuir | 准二级 | — | [ |
| 竹子 | 600 | Mg/Al或Mg/Fe LDH | 5~600 | 172 | Langmuir | 准二级 | 离子交换 | [ |
| 白菜/油菜 | 500 | Mg/Al LDO | 5~500 | 127.2~132.8 | Langmuir | 准二级 | 静电吸引、配体交换、 离子交换 | [ |
| 椰枣叶 | 700 | Mg/Al LDH | 10~50 | 177.97 | Langmuir | 准一级 | 配体交换 | [ |
| 甘蔗叶 | 550 | Mg/Al LDH | 5~500 | 81.83 | Langmuir | 准二级 | 静电吸引、配体交换 | [ |
| 香蒲 | 400~600 | La(OH)3 | 5~500 | 36.06 | Langmuir | 准二级 | 配体交换、静电吸引 | [ |
| 法国梧桐 | 600 | La(OH)3 | 30~100 | 148.11 | Langmuir | 准二级 | 静电吸引、配体交换 | [ |
| 小麦秸秆 | 550 | 壳聚糖和La(OH)3 | 25~300 | 108.86 | Langmuir | 准二级 | 静电吸引、配体交换 | [ |
| 菠萝皮 | 300 | Fe2O3和La(OH)3 | 100~400 | 101.16 | Langmuir | 准二级 | 表面沉淀、配体交换、 静电吸引 | [ |
| 橡木 | 500 | La(OH)3 | 1~400 | 46.37 | Langmuir | 准二级 | 静电吸引、表面沉淀、 配体交换 | [ |
| 脱水污泥 | 400~800 | La(OH)3 | 50~300 | 93.91 | Langmuir | 准二级 | 静电吸引、配体交换 | [ |
| 美人蕉 | 800 | La(NO3)3 | 5~500 | 37.37 | Langmuir | 准二级 | 静电吸引、离子交换、 配体交换 | [ |
| 核桃壳 | 400 | LaCl3 | 0~50 | 12.18 | Langmuir | 准二级 | — | [ |
| 玉米秸秆 | 800 | NaLa(CO3)2、FeCl3 | 50~400 | 217.84 | Langmuir | 准二级 | 静电吸引、配体交换 | [ |
| 紫薇落叶 | 600 | Mg(OH)2 | 10~300 | 121.95 | Sips | 准二级 | 配体交换 | [ |
| 柏木木屑 | 400~600 | MgCl2 | 50~250 | 43.5~66.7 | Langmuir | 准二级 | 表面沉淀 | [ |
| 山核桃木屑/竹子 | 600 | MgCl2/AlCl3/FeCl3 | 15~1000 | 119.6 | Langmuir-Freundlich | — | 静电吸引、表面沉淀 | [ |
| 甘蔗叶 | 550 | MgCl2 | 1~500 | 398.71 | Langmuir | 准二级 | 静电吸引、表面沉淀 | [ |
| 毛竹 | 400~600 | MgCl2 | 19.7~498 | 344~370 | Langmuir-Freundlich | 准二级 | 配体交换、静电吸引 | [ |
| 香蕉秸秆/木薯秸秆/ 玉米秸秆/杉木秸秆/ 芋头秸秆/油茶壳 | 430 | MgCl2 | 20~350 | 6.77~31.15 | Langmuir | 准二级 | 静电吸引、表面沉淀 | [ |
| 玉米秸秆 | 550 | MgCl2 | 4~200 | 60.95 | Langmuir-Freundlich | 准二级 | 静电吸引、表面沉淀 | [ |
| 胡萝卜 | 400 | MgCl2 | 25~350 | 138 | Langmuir | 准一级 | — | [ |
| 玉米芯 | 800 | MgCl2 | 3~25 | 8.44 | Langmuir | 准二级 | — | [ |
| 杨树 | 550 | AlCl3 | 50~1600 | 57.49 | Langmuir-Freundlich | 准二级 | — | [ |
| 麦秸 | 600 | CaCl2 | 200~1000 | 213.22±13.57 | Langmuir-Freundlich | 准二级 | 表面沉淀 | [ |
| 花生壳/甘蔗渣 | 460~850 | MgCl2、CaCl2 | 3~5800 | 111.80~129.79 | Sips | 准二级 | 表面沉淀 | [ |
| 稻壳/木材 | 500 | Fe2(SO4)3、FeSO4 | 25~150 | 24.9~27.6 | Freundlich | 准二级 | 静电吸引、配体交换 | [ |
| 水葫芦 | 450 | FeCl3、FeCl2 | 0.186~150 | 5.068 | Langmuir-Freundlich | 准二级 | 静电吸引、配体交换 | [ |
| 活性污泥 | 550 | FeCl3 | 5~1000 | 111 | Freundlich | 准二级 | 静电吸引、离子交换、 配体交换 | [ |
| 玉米秸秆 | 550 | FeCl3 | 10~50 | 98 | Langmuir | — | 离子交换、表面沉淀、 配体交换 | [ |
| 核桃壳 | 600 | FeCl2、MgCl2 | 5~400 | 6.945 | Langmuir-Freundlich | 准二级 | 静电吸引 | [ |
| 酒糟 | 600 | 磷石膏 | 5~500 | 102.4 | Freundlich | 准二级 | 静电吸引、表面沉淀、 配体交换 | [ |
| 油菜 | 700 | 煤矸石 | 5~200 | 7.9 | Langmuir | 准二级 | 静电吸引、配体交换、 表面沉淀 | [ |
| 羊粪 | 800 | 牡蛎壳、LaCl3 | 5~200 | 88.34 | Langmuir | 准二级 | 配体交换 | [ |
| 脱水污泥 | 800 | 石灰石 | 50~500 | 231.28 | Langmuir | 准二级 | 表面沉淀、配体交换 | [ |
| 1 | JIANG Dan, CHU Bei, AMANO Yoshimasa, et al. Removal and recovery of phosphate from water by Mg-laden biochar: Batch and column studies[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 558: 429-437. |
| 2 | SHAKOOR Muhammad Bilal, YE Zhilong, CHEN Shaohua. Engineered biochars for recovering phosphate and ammonium from wastewater: A review[J]. Science of the Total Environment, 2021, 779: 146240. |
| 3 | JIANG Yanhong, LI Anyu, DENG Hua, et al. Characteristics of nitrogen and phosphorus adsorption by Mg-loaded biochar from different feedstocks[J]. Bioresource Technology, 2019, 276: 183-189. |
| 4 | YIN Qianqian, WANG Ruikun, ZHAO Zhenghui. Application of Mg-Al-modified biochar for simultaneous removal of ammonium, nitrate, and phosphate from eutrophic water[J]. Journal of Cleaner Production, 2018, 176: 230-240. |
| 5 | FENG Qianwei, CHEN Miao, WU Pan, et al. Simultaneous reclaiming phosphate and ammonium from aqueous solutions by calcium alginate-biochar composite: Sorption performance and governing mechanisms[J]. Chemical Engineering Journal, 2022, 429: 132166. |
| 6 | HUANG Yimin, LEE Xinqing, GRATTIERI Matteo, et al. Modified biochar for phosphate adsorption in environmentally relevant conditions[J]. Chemical Engineering Journal, 2020, 380: 122375. |
| 7 | RAHIMI Shadi, MODIN Oskar, MIJAKOVIC Ivan. Technologies for biological removal and recovery of nitrogen from wastewater[J]. Biotechnology Advances, 2020, 43: 107570. |
| 8 | YIN Qianqian, ZHANG Bingdong, WANG Ruikun, et al. Biochar as an adsorbent for inorganic nitrogen and phosphorus removal from water: A review[J]. Environmental Science and Pollution Research, 2017, 24(34): 26297-26309. |
| 9 | KAZEMI SHARIAT PANAHI Hamed, DEHHAGHI Mona, Yong Sik OK, et al. A comprehensive review of engineered biochar: Production, characteristics, and environmental applications[J]. Journal of Cleaner Production, 2020, 270: 122462. |
| 10 | 钟金魁, 丁莉, 李柳, 等. 玉米秸秆生物炭对铜的吸附及其影响因素[J]. 安全与环境学报, 2020, 20(5): 1862-1870. |
| ZHONG Jinkui, DING Li, LI Liu, et al. Adsorption of copper content rate on maize straw biochar and influential factors concerned[J]. Journal of Safety and Environment, 2020, 20(5): 1862-1870. | |
| 11 | VIKRANT Kumar, KIM Ki-Hyun, Yong Sik OK, et al. Engineered/designer biochar for the removal of phosphate in water and wastewater[J]. Science of the Total Environment, 2018, 616/617: 1242-1260. |
| 12 | 陈梅, 王芳, 张德俐, 等. 生物炭结构性质对氨氮的吸附特性影响[J]. 环境科学, 2019, 40(12): 5421-5429. |
| CHEN Mei, WANG Fang, ZHANG Deli, et al. Effect of biochar structure on adsorption characteristics of ammonia nitrogen[J]. Environmental Science, 2019, 40(12): 5421-5429. | |
| 13 | MASEBINU S O, AKINLABI E T, MUZENDA E, et al. A review of biochar properties and their roles in mitigating challenges with anaerobic digestion[J]. Renewable and Sustainable Energy Reviews, 2019, 103: 291-307. |
| 14 | KUMAR Adarsh, SAINI Komal, BHASKAR Thallada. Hydochar and biochar: Production, physicochemical properties and techno-economic analysis[J]. Bioresource Technology, 2020, 310: 123442. |
| 15 | QAMBRANI Naveed Ahmed, RAHMAN Md Mukhlesur, WON Seunggun, et al. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review[J]. Renewable and Sustainable Energy Reviews, 2017, 79: 255-273. |
| 16 | ZHU Danchen, CHEN Yingquan, YANG Haiping, et al. Synthesis and characterization of magnesium oxide nanoparticle-containing biochar composites for efficient phosphorus removal from aqueous solution[J]. Chemosphere, 2020, 247: 125847. |
| 17 | MOHAN Dinesh, SARSWAT Ankur, Yong Sik OK, et al. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review[J]. Bioresource Technology, 2014, 160: 191-202. |
| 18 | XIANG W, ZHANG X, CHEN J, et al. Biochar technology in wastewater treatment: A critical review[J]. Chemosphere, 2020, 252: 126539. |
| 19 | BRIDGWATER A V. Review of fast pyrolysis of biomass and product upgrading[J]. Biomass and Bioenergy, 2012, 38: 68-94. |
| 20 | SAHA Nepu, SABA Akbar, Toufiq REZA M.. Effect of hydrothermal carbonization temperature on pH, dissociation constants, and acidic functional groups on hydrochar from cellulose and wood[J]. Journal of Analytical and Applied Pyrolysis, 2019, 137: 138-145. |
| 21 | BARSKOV Stan, ZAPPI Mark, BUCHIREDDY Prashanth, et al. Torrefaction of biomass: A review of production methods for biocoal from cultured and waste lignocellulosic feedstocks[J]. Renewable Energy, 2019, 142: 624-642. |
| 22 | FOONG Shin Ying, LIEW Rock Keey, YANG Yafeng, et al. Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: Progress, challenges, and future directions[J]. Chemical Engineering Journal, 2020, 389: 124401. |
| 23 | MUTSENGERERE S, CHIHOBO C H, MUSADEMBA D, et al. A review of operating parameters affecting bio-oil yield in microwave pyrolysis of lignocellulosic biomass[J]. Renewable and Sustainable Energy Reviews, 2019, 104: 328-336. |
| 24 | YIN Qianqian, ZHANG Bingdong, WANG Ruikun, et al. Phosphate and ammonium adsorption of sesame straw biochars produced at different pyrolysis temperatures[J]. Environmental Science and Pollution Research, 2018, 25(5): 4320-4329. |
| 25 | HOU Jie, HUANG Lei, YANG Zhimin, et al. Adsorption of ammonium on biochar prepared from giant reed[J]. Environmental Science and Pollution Research, 2016, 23(19): 19107-19115. |
| 26 | TANG Yao, ALAM Md Samrat, KONHAUSER Kurt O, et al. Influence of pyrolysis temperature on production of digested sludge biochar and its application for ammonium removal from municipal wastewater[J]. Journal of Cleaner Production, 2019, 209: 927-936. |
| 27 | XU Defu, CAO Junmin, LI Yingxue, et al. Effect of pyrolysis temperature on characteristics of biochars derived from different feedstocks: A case study on ammonium adsorption capacity[J]. Waste Management, 2019, 87: 652-660. |
| 28 | KIZITO Simon, WU Shubiao, KIPKEMOI KIRUI W, et al. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry[J]. Science of the Total Environment, 2015, 505: 102-112. |
| 29 | FAN Ruemei, CHEN Ching-lung, LIN Jui-yen, et al. Adsorption characteristics of ammonium ion onto hydrous biochars in dilute aqueous solutions[J]. Bioresource Technology, 2019, 272: 465-472. |
| 30 | HU Xiaojian, ZHANG Xinbo, Huu Hao NGO, et al. Comparison study on the ammonium adsorption of the biochars derived from different kinds of fruit peel[J]. Science of the Total Environment, 2020, 707: 135544. |
| 31 | CUI Xiaoqiang, HAO Hulin, ZHANG Changkuan, et al. Capacity and mechanisms of ammonium and cadmium sorption on different wetland-plant derived biochars[J]. Science of the Total Environment, 2016, 539: 566-575. |
| 32 | BAI Xiaofeng, LI Zifu, ZHANG Yaozhong, et al. Recovery of ammonium in urine by biochar derived from faecal sludge and its application as soil conditioner[J]. Waste and Biomass Valorization, 2017, 9(9): 1619-1628. |
| 33 | PARK Man Ho, JEONG Sangjae, KIM Jae Young. Adsorption of NH3-N onto rice straw-derived biochar[J]. Journal of Environmental Chemical Engineering, 2019, 7(2): 103039. |
| 34 | YIN Qianqian, LIU Mengtian, REN Huaipu. Biochar produced from the co-pyrolysis of sewage sludge and walnut shell for ammonium and phosphate adsorption from water[J]. Journal of Environmental Management, 2019, 249: 109410. |
| 35 | YANG Hye In, LOU Kangyi, RAJAPAKSHA Anushka Upamali, et al. Adsorption of ammonium in aqueous solutions by pine sawdust and wheat straw biochars[J]. Environmental Science and Pollution Research, 2017, 25(26): 25638-25647. |
| 36 | CHENG Ning, WANG Bing, FENG Qianwei, et al. co-adsorption performance and mechanism of nitrogen and phosphorus onto eupatorium adenophorum biochar in water[J]. Bioresource Technology, 2021, 340: 125696. |
| 37 | ZHAO Haihua, XUE Yingwen, LONG Li, et al. Adsorption of nitrate onto biochar derived from agricultural residuals[J]. Water Science and Technology, 2017, 77(2): 548-554. |
| 38 | 刘艳艳, 田质涛, 毛卫康. 猪骨/竹共热解生物炭对氨氮、Cd2+和Cu2+的吸附特性研究[J]. 环境科学与技术, 2022, 45(4): 29-37. |
| LIU Yanyan, TIAN Zhitao, MAO Weikang. Characteristics of adsorption of ammonia nitrogen,Cd2+and Cu2+ on paorcine-bone/bamboo co-pyrolysis biochar[J]. Environmental Science & Technology, 2022, 45(4): 29-37. | |
| 39 | 何强, 张逸卓, 申海旭, 等. 改性玉米秸秆生物炭对化粪池出水中氨氮的吸附[J]. 水处理技术, 2022, 48(12): 19-23. |
| HE Qiang, ZHANG Yizhuo, SHEN Haixu, et al. Adsorption of ammonia nitrogen in septic tank effluent by modified corn straw biochar[J]. Technology of Water Treatment, 2022, 48(12): 19-23. | |
| 40 | Thi Mai VU, TRINH Van Tuyen, DOAN Dinh Phuong, et al. Removing ammonium from water using modified corncob-biochar[J]. Science of the Total Environment, 2017, 579: 612-619. |
| 41 | CHANDRA Subhash, MEDHA Isha, BHATTACHARYA Jayanta. Potassium-iron rice straw biochar composite for sorption of nitrate, phosphate, and ammonium ions in soil for timely and controlled release[J]. Science of the Total Environment, 2020, 712: 136337. |
| 42 | WU Ruofan, ZHAI Xu, DAI Kuai, et al. Synthesis of acidified magnetic sludge-biochar and its role in ammonium nitrogen removal: Perception on effect and mechanism[J]. Science of the Total Environment, 2022, 832: 154780. |
| 43 | ALAGHA Omar, MANZAR Mohammad Saood, ZUBAIR Mukarram, et al. Comparative adsorptive removal of phosphate and nitrate from wastewater using biochar-MgAl LDH nanocomposites: Coexisting anions effect and mechanistic studies[J]. Nanomaterials, 2020, 10(2): 336. |
| 44 | YIN Qianqian, REN Huaipu, WANG Ruikun, et al. Evaluation of nitrate and phosphate adsorption on Al-modified biochar: Influence of Al content[J]. Science of the Total Environment, 2018, 631/632: 895-903. |
| 45 | MIN Li, ZHONGSHENG Zhang, Li ZHE, et al. Removal of nitrogen and phosphorus pollutants from water by FeCl3- impregnated biochar[J]. Ecological Engineering, 2020, 149: 105792. |
| 46 | JING Huanping, LI Yuan, WANG Xuejiang, et al. Simultaneous recovery of phosphate, ammonium and humic acid from wastewater using a biochar supported Mg(OH)2/bentonite composite[J]. Environmental Science: Water Research & Technology, 2019, 5(5): 931-943. |
| 47 | CHEN Liang, CHEN Xiao Long, ZHOU Chun Hui, et al. Environmental-friendly montmorillonite-biochar composites: Facile production and tunable adsorption-release of ammonium and phosphate[J]. Journal of Cleaner Production, 2017, 156(10): 648-659. |
| 48 | Eva VIGLAŠOVá, Michal GALAMBOŠ, Zuzana DANKOVá, et al. Production, characterization and adsorption studies of bamboo-based biochar/montmorillonite composite for nitrate removal[J]. Waste Management, 2018, 79: 385-394. |
| 49 | ZHAO Zhipeng, WANG Bing, FENG Qianwei, et al. Recovery of nitrogen and phosphorus in wastewater by red mud-modified biochar and its potential application[J]. Science of the Total Environment, 2023, 860: 160289. |
| 50 | 王芳君, 桑倩倩, 邓颖, 等. 磁性铁基改性生物炭去除水中氨氮[J]. 环境科学, 2021, 42(4): 1913-1922. |
| WANG Fangjun, SANG Qianqian, DENG Ying, et al. Synthesis of magnetic iron modifying biochar for ammonia nitrogen removal from water[J]. Environmental Science, 2021, 42(4): 1913-1922. | |
| 51 | ZHANG Ming, SONG Ge, GELARDI Danielle L, et al. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water[J]. Water Research, 2020, 186: 116303. |
| 52 | NARDIS Bárbara Olinda, FRANCA José Romão, CARNEIRO Jefferson Santana da Silva, et al. Production of engineered-biochar under different pyrolysis conditions for phosphorus removal from aqueous solution[J]. Science of the Total Environment, 2022, 816: 151559. |
| 53 | LUO Yuan, XIE Kun, FENG Yiyang, et al. Synthesis of a La(OH)3 nanorod/walnut shell biochar composite for reclaiming phosphate from aqueous solutions[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 610: 125736. |
| 54 | 易蔓, 李婷婷, 李海红, 等. Ca/Mg负载改性沼渣生物炭对水中磷的吸附特性[J]. 环境科学, 2019, 40(3): 1318-1327. |
| YI Man, LI Tingting, LI Haihong, et al. Characteristics of phosphorus adsorption in aqueous solution by Ca/Mg loaded biogas residue biochar[J]. Environmental Science, 2019, 40(3): 1318-1327. | |
| 55 | FANG Le, LI Jiang-shan, DONATELLO Shane, et al. Use of Mg/Ca modified biochars to take up phosphorus from acid-extract of incinerated sewage sludge ash (ISSA) for fertilizer application[J]. Journal of Cleaner Production, 2020, 244: 118853. |
| 56 | LUO Haoyu, WANG Yijie, WEN Xiaoqing, et al. Key roles of the crystal structures of MgO-biochar nanocomposites for enhancing phosphate adsorption[J]. Science of the Total Environment, 2021, 766: 142618. |
| 57 | LIU Xiaoning, SHEN Feng, QI Xinhua. Adsorption recovery of phosphate from aqueous solution by CaO-biochar composites prepared from eggshell and rice straw[J]. Science of the Total Environment, 2019, 666: 694-702. |
| 58 | LIU LingYan, ZHANG ChunHong, CHEN ShuangRong, et al. Phosphate adsorption characteristics of La(OH)3-modified, canna-derived biochar[J]. Chemosphere, 2022, 286: 131773. |
| 59 | WAN Stefan, WANG Shengsen, LI Yuncong, et al. Functionalizing biochar with Mg-Al and Mg-Fe layered double hydroxides for removal of phosphate from aqueous solutions[J]. Journal of Industrial and Engineering Chemistry, 2017, 47: 246-253. |
| 60 | ZHANG Zhaoran, YAN Liangguo, YU Haiqin, et al. Adsorption of phosphate from aqueous solution by vegetable biochar/layered double oxides: Fast removal and mechanistic studies[J]. Bioresource Technology, 2019, 284: 65-71. |
| 61 | LI Ronghua, WANG Jim J, ZHOU Baoyue, et al. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios[J]. Science of the Total Environment, 2016, 559: 121-129. |
| 62 | XU Qinyuan, CHEN Zhongbing, WU Zhengsong, et al. Novel lanthanum doped biochars derived from lignocellulosic wastes for efficient phosphate removal and regeneration[J]. Bioresource Technology, 2019, 289: 121600. |
| 63 | JIA Ziyue, ZENG Wei, XU Huanhuan, et al. Adsorption removal and reuse of phosphate from wastewater using a novel adsorbent of lanthanum-modified platanus biochar[J]. Process Safety and Environmental Protection, 2020, 140: 221-232. |
| 64 | LIAO Taiwan, LI Ting, SU Xiangde, et al. La(OH)3-modified magnetic pineapple biochar as novel adsorbents for efficient phosphate removal[J]. Bioresource Technology, 2018, 263: 207-213. |
| 65 | WANG Zhanghong, SHEN Dekui, SHEN Fei, et al. Phosphate adsorption on lanthanum loaded biochar[J]. Chemosphere, 2016, 150: 1-7. |
| 66 | LI Jing, LI Bing, HUANG Haiming, et al. Investigation into lanthanum-coated biochar obtained from urban dewatered sewage sludge for enhanced phosphate adsorption[J]. Science of the Total Environment, 2020, 714: 136839. |
| 67 | 罗元, 谢坤, 冯弋洋, 等. 镧改性核桃壳生物炭制备及吸附水体磷酸盐性能[J]. 化工进展, 2021, 40(2): 1121-1129. |
| LUO Yuan, XIE Kun, FENG Yiyang, et al. Preparation of lanthanum modified walnut shell biochar and adsorption of phosphate from aqueous solutions[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 1121-1129. | |
| 68 | QU Jianhua, AKINDOLIE Modupe Sarah, FENG Yan, et al. One-pot hydrothermal synthesis of NaLa(CO3)2 decorated magnetic biochar for efficient phosphate removal from water: Kinetics, isotherms, thermodynamics, mechanisms and reusability exploration[J]. Chemical Engineering Journal, 2020, 394: 124915. |
| 69 | HADDAD Khouloud, JELLALI Salah, JEGUIRIM Mejdi, et al. Investigations on phosphorus recovery from aqueous solutions by biochars derived from magnesium-pretreated cypress sawdust[J]. Journal of Environmental Management, 2018, 216: 305-314. |
| 70 | ALHUJAILY Ahmad, MAO Yingzheng, ZHANG Jialong, et al. Facile fabrication of Mg-Fe-biochar adsorbent derived from spent mushroom waste for phosphate removal[J]. Journal of the Taiwan Institute of Chemical Engineers, 2020, 117: 75-85. |
| 71 | LI Ronghua, WANG Jim J, ZHOU Baoyue, et al. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment[J]. Journal of Cleaner Production, 2017, 147: 96-107. |
| 72 | DE CARVALHO EUFRáSIO PINTO Marina, DAVID DA SILVA Demetrius, AMORIM GOMES Ana Luiza, et al. Biochar from carrot residues chemically modified with magnesium for removing phosphorus from aqueous solution[J]. Journal of Cleaner Production, 2019, 222: 36-46. |
| 73 | JENA Jyotsnarani, Trupti DAS, SARKAR Ujjaini. Explicating proficiency of waste biomass-derived biochar for reclaiming phosphate from source-separated urine and its application as a phosphate biofertilizer[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104648. |
| 74 | LI Xiaoyun, XIE Yanhua, JIANG Fei, et al. Enhanced phosphate removal from aqueous solution using resourceable nano-CaO2/BC composite: Behaviors and mechanisms[J]. Science of the Total Environment, 2020, 709: 136123. |
| 75 | AJMAL Zeeshan, MUHMOOD Atif, DONG Renjie, et al. Probing the efficiency of magnetically modified biomass-derived biochar for effective phosphate removal[J]. Journal of Environmental Management, 2020, 253: 109730. |
| 76 | CAI Ru, WANG Xin, JI Xionghui, et al. Phosphate reclaim from simulated and real eutrophic water by magnetic biochar derived from water hyacinth[J]. Journal of Environmental Management, 2017, 187: 212-219. |
| 77 | YANG Qi, WANG Xiaolin, LUO Wei, et al. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge[J]. Bioresource Technology, 2018, 247: 537-544. |
| 78 | TAO Xuefeng, HUANG Tao, Bo LYU. Synthesis of Fe/Mg-biochar nanocomposites for phosphate removal[J]. Materials, 2020, 13(4): 816. |
| 79 | WANG Bing, LIAN Guoqi, LEE Xinqing, et al. Phosphogypsum as a novel modifier for distillers grains biochar removal of phosphate from water[J]. Chemosphere, 2020, 238: 124684. |
| 80 | WANG Bing, MA Yuena, LEE Xinqing, et al. Environmental-friendly coal gangue-biochar composites reclaiming phosphate from water as a slow-release fertilizer[J]. Science of the Total Environment, 2021, 758: 143664. |
| 81 | FENG Yiyang, LUO Yuan, HE Qiuping, et al. Performance and mechanism of a biochar-based Ca-La composite for the adsorption of phosphate from water[J]. Journal of Environmental Chemical Engineering, 2021, 9(3): 105267. |
| 82 | XUE Junbing, WANG Haixia, LI Peng, et al. Efficient reclaiming phosphate from aqueous solution using waste limestone modified sludge biochar: Mechanism and application as soil amendments[J]. Science of the Total Environment, 2021, 799: 149454. |
| 83 | YANG Jie, ZHANG Mingliang, WANG Haixia, et al. Efficient recovery of phosphate from aqueous solution using biochar derived from co-pyrolysis of sewage sludge with eggshell[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 105354. |
| 84 | 罗元, 谢坤, 张克强, 等. 生物炭及其金属改性材料脱除水体磷酸盐研究进展[J]. 环境化学, 2020, 39(8): 2175-2186. |
| LUO Yuan, XIE Kun, ZHANG Keqiang, et al. Research progress on removal of phosphate from aqueous solution by biochar and its metal modified materials[J]. Environmental Chemistry, 2020. 39(8): 2175-2186. | |
| 85 | DENG Yu, LI Min, ZHANG Zhan, et al. Comparative study on characteristics and mechanism of phosphate adsorption on Mg/Al modified biochar[J]. Journal of Environmental Chemical Engineering, 2021, 9(2): 105079. |
| 86 | YAO Ying, GAO Bin, CHEN Jianjun, et al. Engineered biochar reclaiming phosphate from aqueous solutions: mechanisms and potential application as a slow-release fertilizer[J]. Environmental Science & Technology, 2013, 47(15): 8700-8708. |
| 87 | NAKARMI Amita, BOURDO Shawn E, RUHL Laura, et al. Benign zinc oxide betaine-modified biochar nanocomposites for phosphate removal from aqueous solutions[J]. Journal of Environmental Management, 2020, 272: 111048. |
| 88 | LIU Hongbo, SHAN Jinhua, CHEN Zhongbing, et al. Efficient recovery of phosphate from simulated urine by Mg/Fe bimetallic oxide modified biochar as a potential resource[J]. Science of the Total Environment, 2021, 784: 147546. |
| 89 | ZHANG Xu, GANG Daniel Dianchen, SUN Peizhe, et al. Goethite dispersed corn straw-derived biochar for phosphate recovery from synthetic urine and its potential as a slow-release fertilizer[J]. Chemosphere, 2021, 262: 127861. |
| 90 | KUMAR Aman, SINGH Ekta, MISHRA Rahul, et al. Biochar as environmental armour and its diverse role towards protecting soil, water and air[J]. Science of the Total Environment, 2022, 806: 150444. |
| 91 | ZHOU Hongxu, MARGENOT Andrew J, LI Yunkai, et al. Phosphorus pollution control using waste-based adsorbents: Material synthesis, modification, and sustainability[J]. Critical Reviews in Environmental Science and Technology, 2021, 52(12): 2023-2059. |
| 92 | YANG Hailan, Shujing Y E, ZENG Zhuotong, et al. Utilization of biochar for resource recovery from water: A review[J]. Chemical Engineering Journal, 2020, 397: 125502. |
| 93 | TONINI Davide, SAVEYN Hans G M, HUYGENS Dries. Environmental and health co-benefits for advanced phosphorus recovery[J]. Nature Sustainability, 2019, 2(11): 1051-1061. |
| [1] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [2] | 赖诗妮, 江丽霞, 李军, 黄宏宇, 小林敬幸. 含碳掺氨燃料的研究进展[J]. 化工进展, 2023, 42(9): 4603-4615. |
| [3] | 王浩然, 殷全玉, 方明, 侯建林, 李军, 何斌, 张明月. 近临界水处理废弃烟梗工艺优化[J]. 化工进展, 2023, 42(9): 5019-5027. |
| [4] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [5] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [6] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| [7] | 李佳, 樊星, 陈莉, 李坚. 硝酸生产尾气中NO x 和N2O联合脱除技术研究进展[J]. 化工进展, 2023, 42(7): 3770-3779. |
| [8] | 陈娜, 张肖静, 张楠, 马冰冰, 张涵, 杨浩洁, 张宏忠. 淬灭酶对亚硝化-混合自养脱氮系统的影响[J]. 化工进展, 2023, 42(7): 3816-3823. |
| [9] | 丁文金, 刘卓齐, 卢海臣, 孙红娟, 彭同江. CH3COONa-NH4OH-H2O体系下磷石膏矿化CO2-联产高纯CaCO3[J]. 化工进展, 2023, 42(7): 3824-3833. |
| [10] | 张杉, 仲兆平, 杨宇轩, 杜浩然, 李骞. 磷酸盐改性高岭土对生活垃圾热解过程中重金属的富集[J]. 化工进展, 2023, 42(7): 3893-3903. |
| [11] | 王蕴青, 杨国锐, 延卫. 过渡金属磷化物的改性方法及其在电化学析氢中的应用[J]. 化工进展, 2023, 42(7): 3532-3549. |
| [12] | 蒋博龙, 崔艳艳, 史顺杰, 常嘉城, 姜楠, 谭伟强. 过渡金属Co3O4/ZnO-ZIF氧还原催化剂Co/Zn-ZIF模板法制备及其产电性能[J]. 化工进展, 2023, 42(6): 3066-3076. |
| [13] | 任建鹏, 吴彩文, 刘慧君, 吴文娟. 木质素-聚苯胺复合材料的制备及对刚果红的吸附[J]. 化工进展, 2023, 42(6): 3087-3096. |
| [14] | 李白雪, 信欣, 朱羽蒙, 刘琴, 刘鑫. SASD-A体系构建及进水不同S/N对脱氮工艺的影响机制[J]. 化工进展, 2023, 42(6): 3261-3271. |
| [15] | 吴锋振, 刘志炜, 谢文杰, 游雅婷, 赖柔琼, 陈燕丹, 林冠烽, 卢贝丽. 生物质基铁/氮共掺杂多孔炭的制备及其活化过一硫酸盐催化降解罗丹明B[J]. 化工进展, 2023, 42(6): 3292-3301. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||