化工进展 ›› 2023, Vol. 42 ›› Issue (2): 907-916.DOI: 10.16085/j.issn.1000-6613.2022-0762
颗粒状NiO的制备及其电化学性能和CO2吸附性能
- 湖南城市学院材料与化学工程学院,湖南 益阳 413000
-
收稿日期:2022-04-26修回日期:2022-05-26出版日期:2023-02-25发布日期:2023-03-13 -
通讯作者:杨泛明 -
作者简介:杨泛明(1988—),男,博士,研究方向为新能源材料与新型吸附剂合成。E-mail:ychufei@163.com。 -
基金资助:湖南省自然科学基金(2019JJ50026)
Preparation of granular NiO for the electrochemical performance and CO2 adsorption performance
- College of Materials and Chemical Engineering, Hunan City University, Yiyang 413000, Hunan, China
-
Received:2022-04-26Revised:2022-05-26Online:2023-02-25Published:2023-03-13 -
Contact:YANG Fanming
摘要:
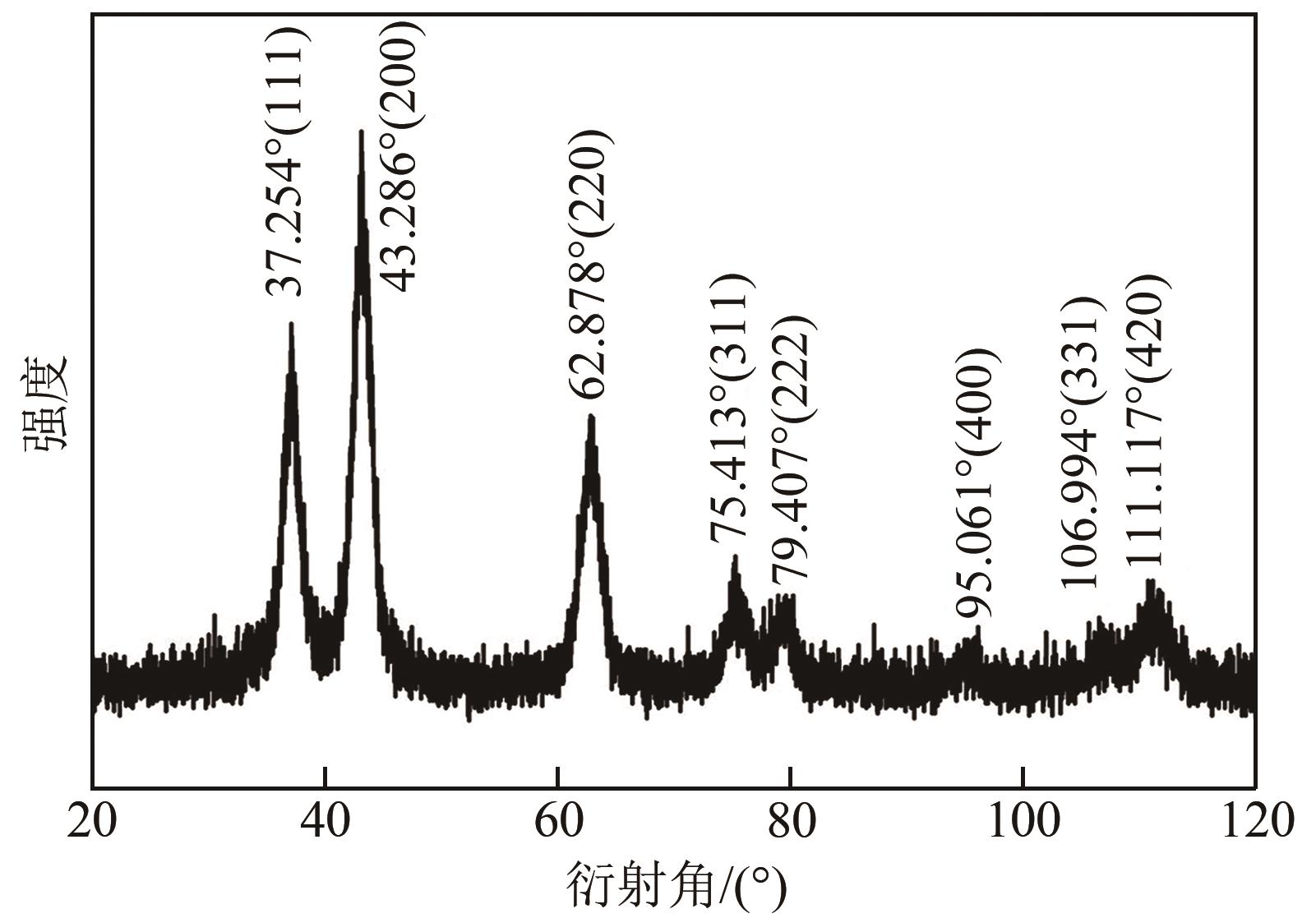
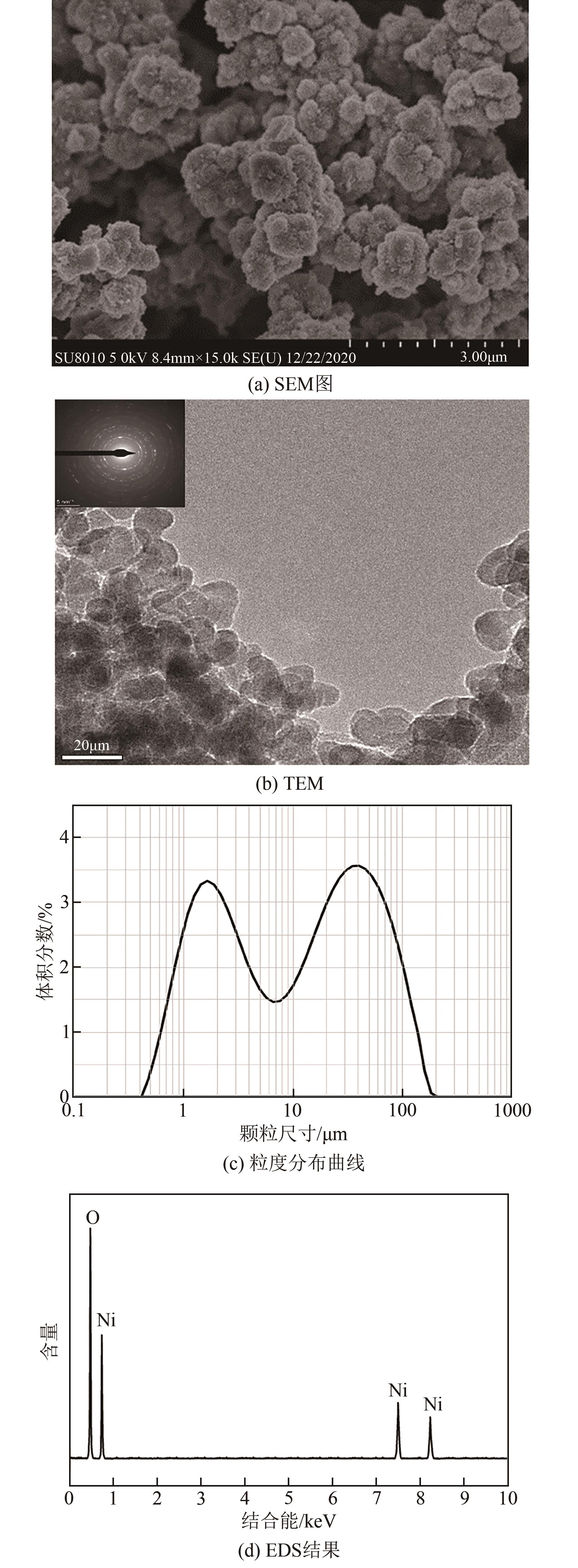
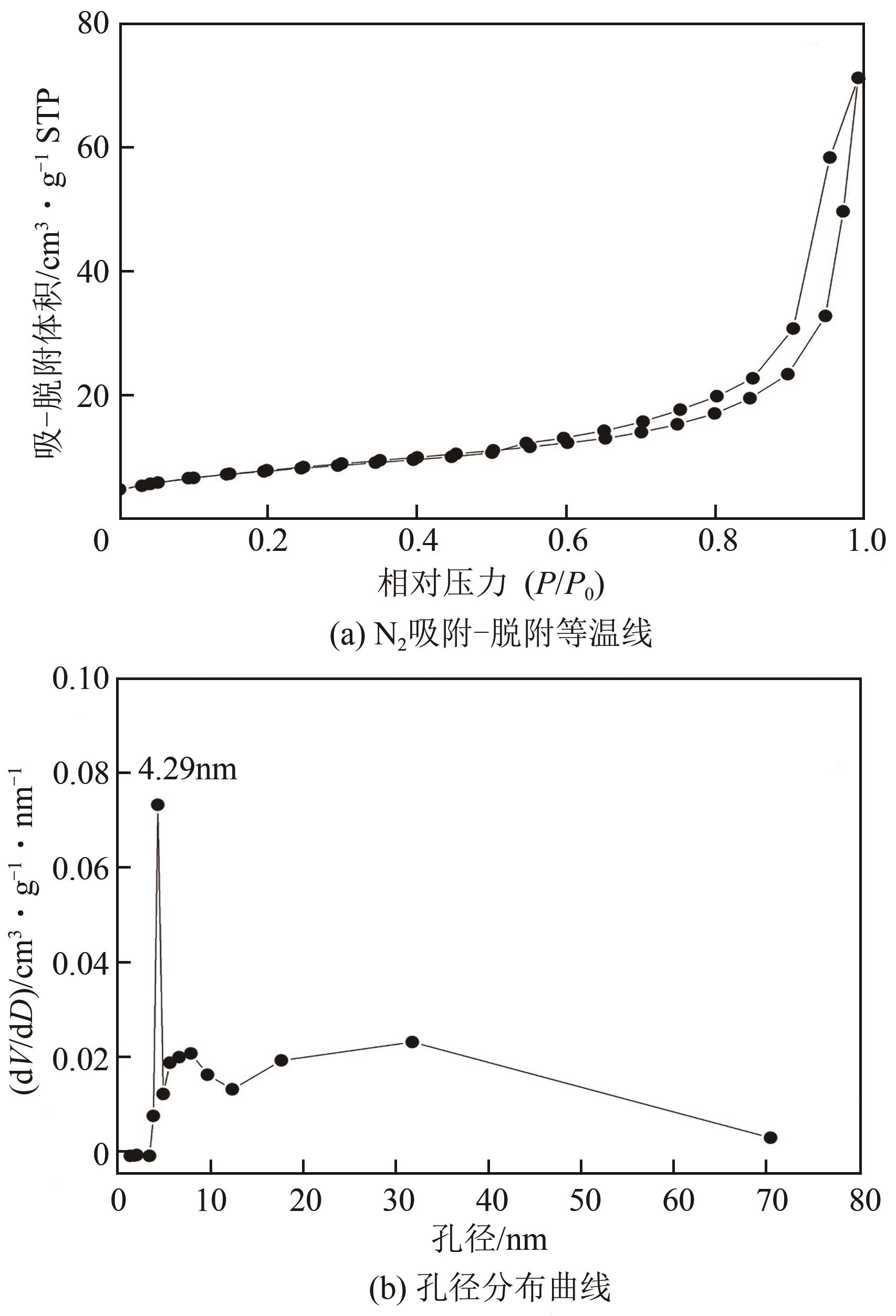
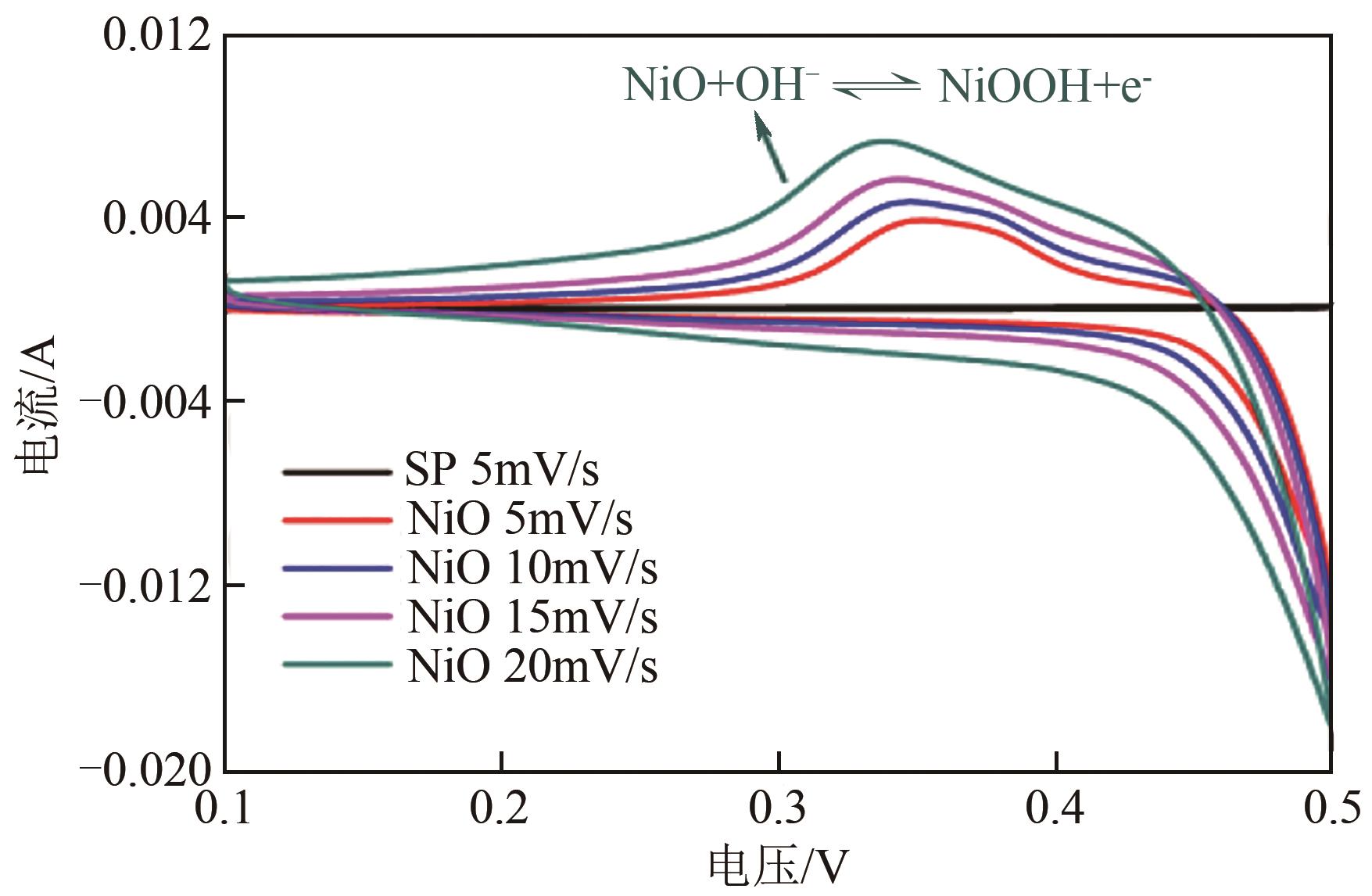
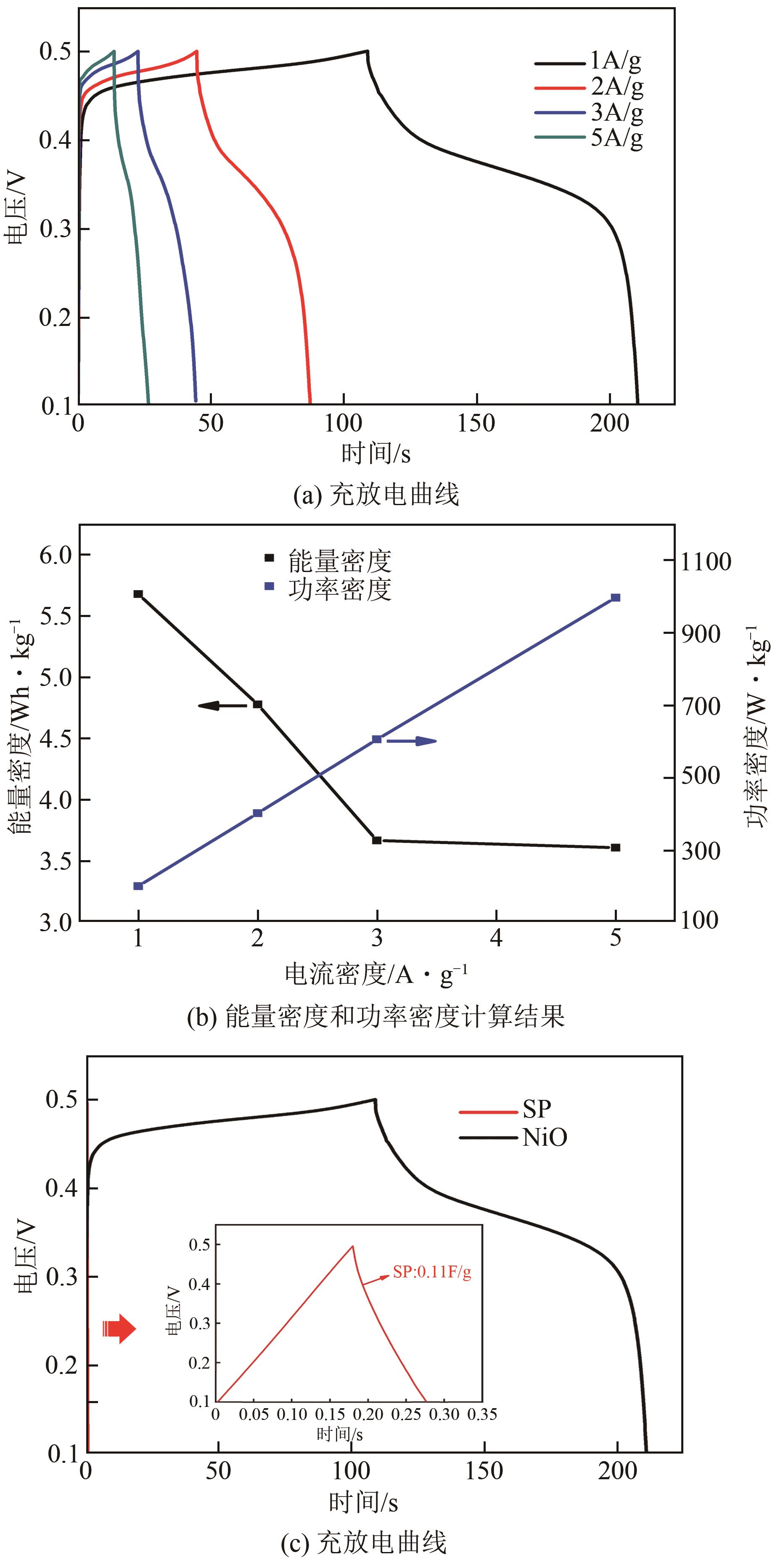
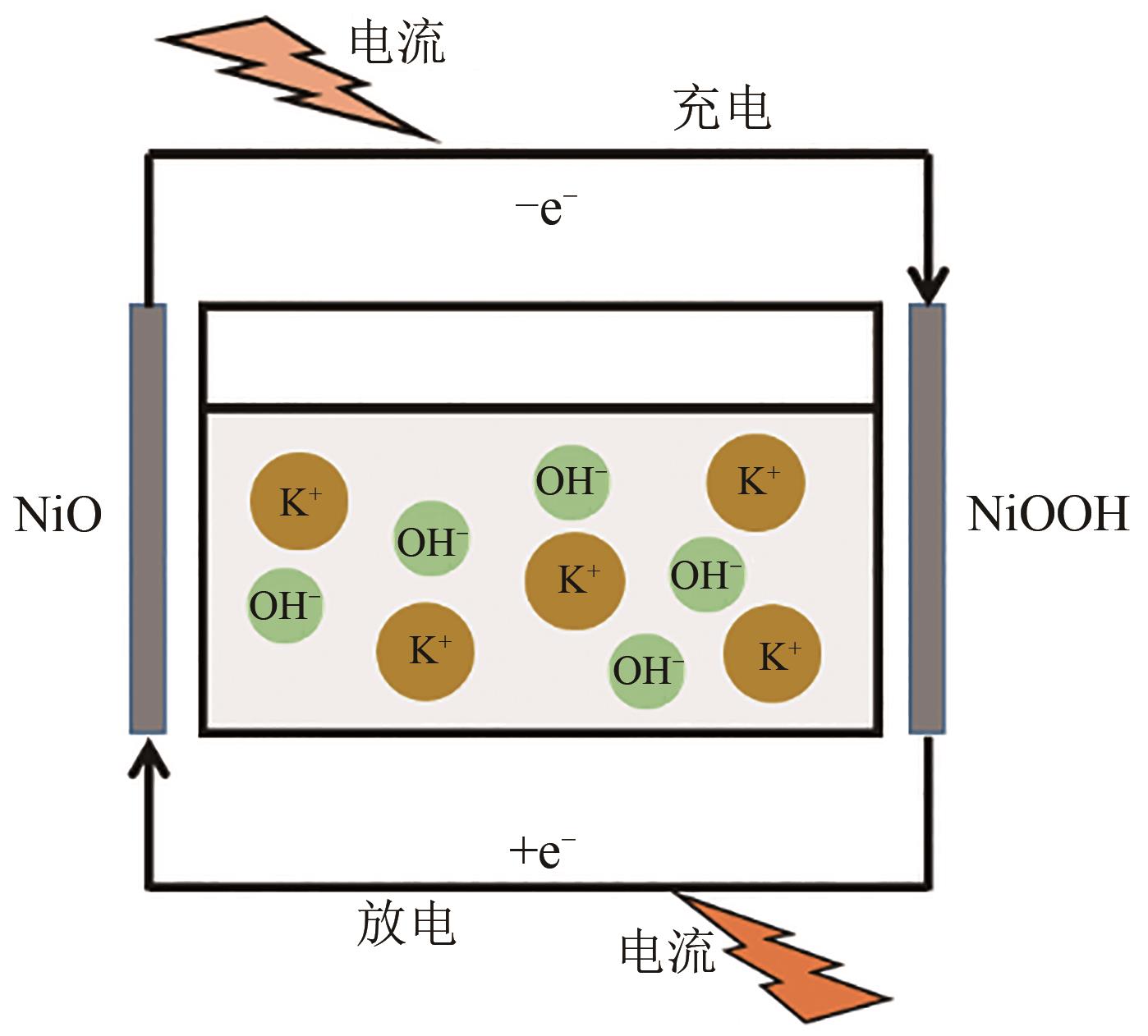
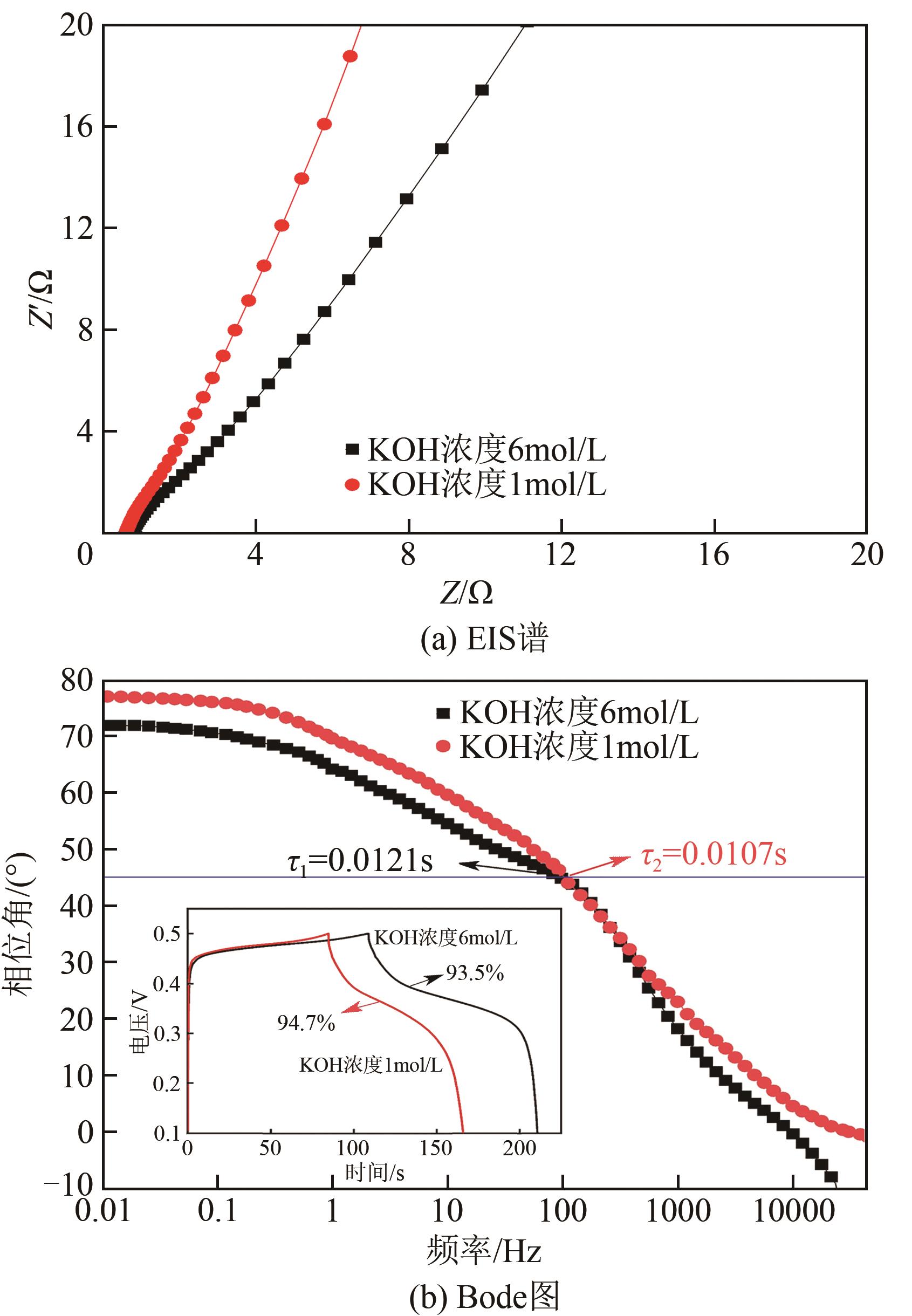
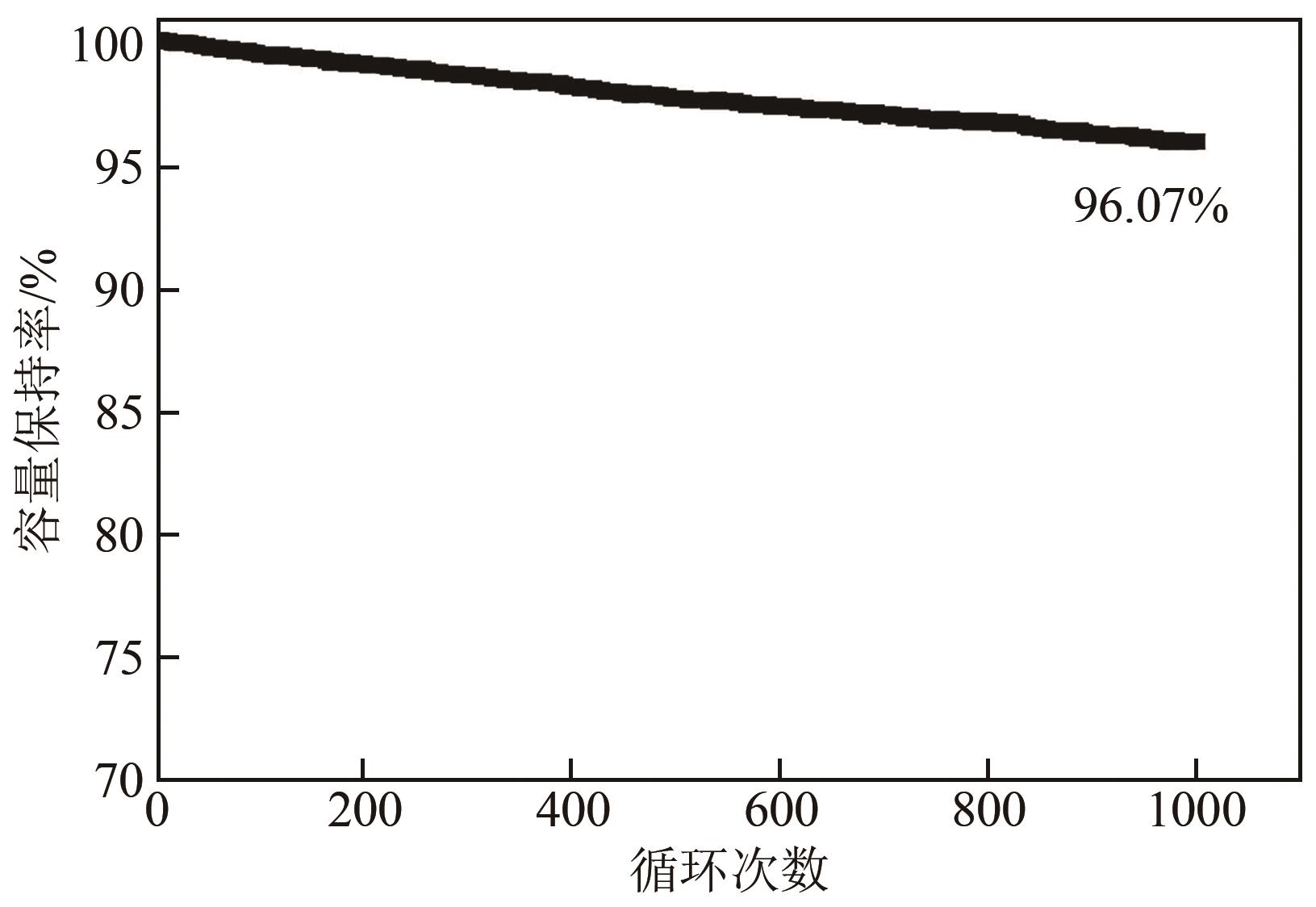
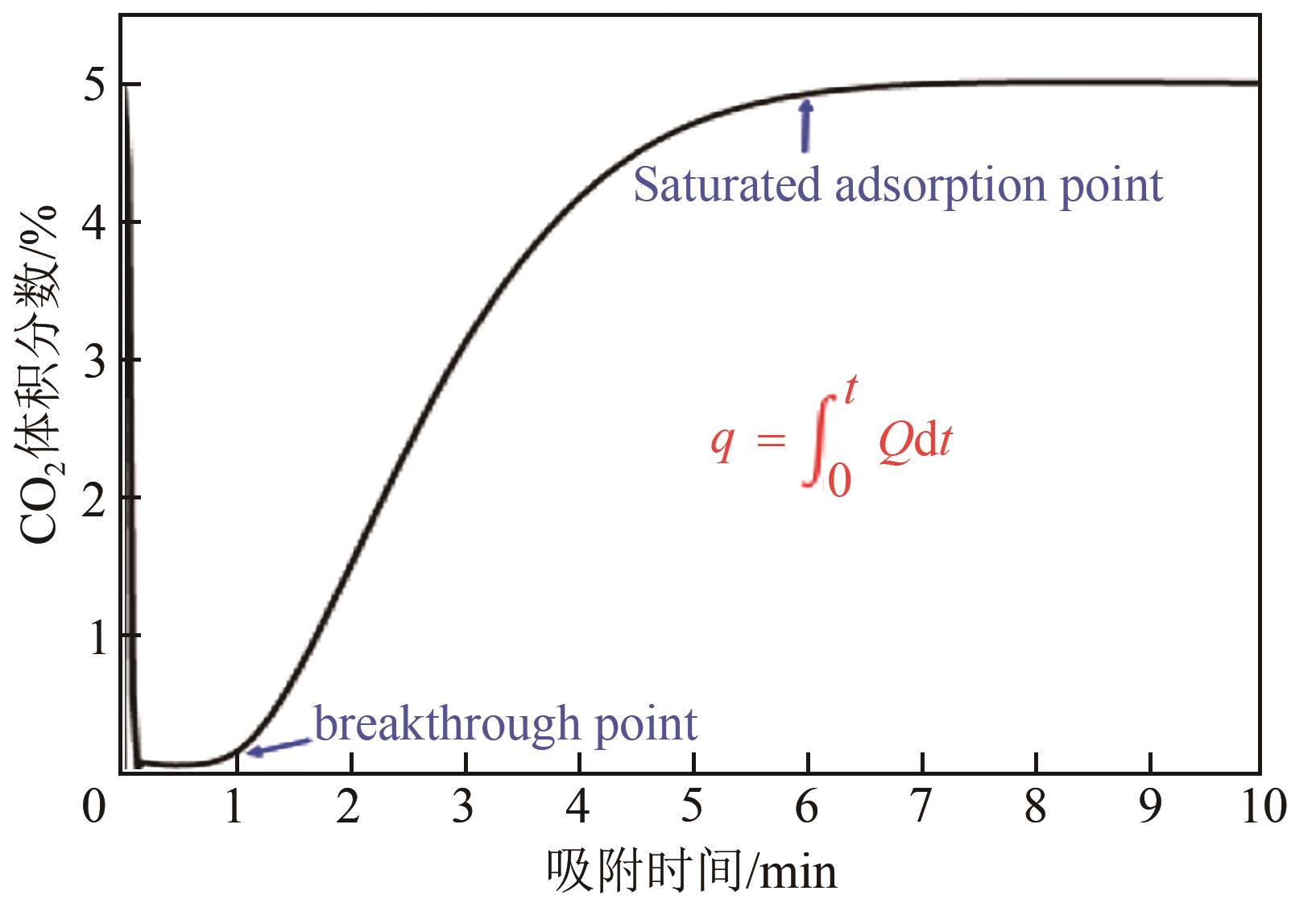
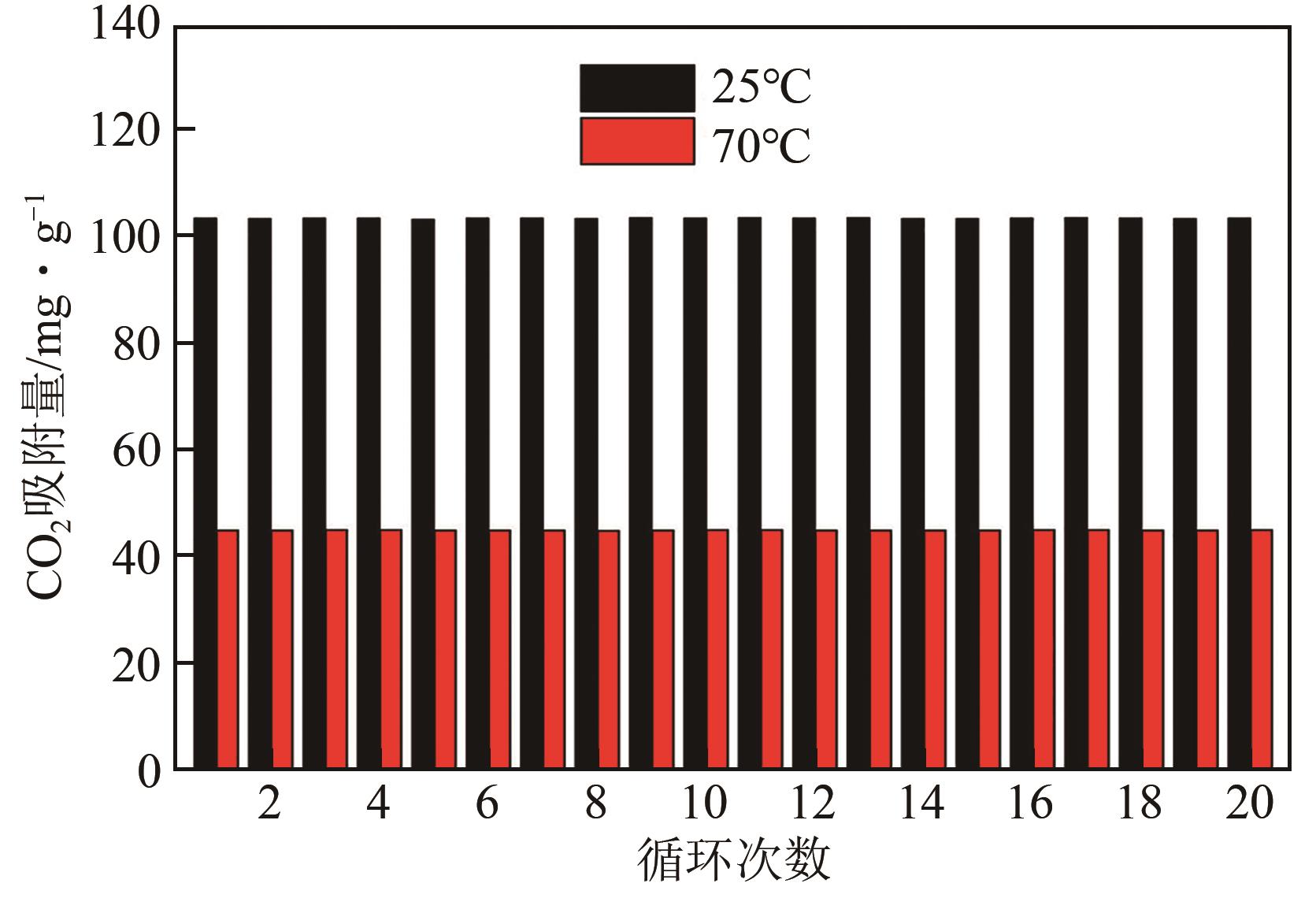
利用乙醇和去离子水的混合溶液为溶剂,通过静态水热处理合成NiO,并对其在KOH电解液中的电化学行为与CO2吸附性能进行探究。采用X射线衍射、X射线光电子能谱、扫描电子显微镜、能量色散谱和N2吸附-脱附技术对NiO的物相、形貌、组成和孔结构进行分析。结果表明,NiO为颗粒状介孔材料,属于立方系晶体。NiO中,Ni以Ni2+、Ni3+形式存在。以NiO为活性材料、6mol/L KOH为电解液组装三电极,可以产生赝电容。电流密度为1A/g,电压窗口为0.1~0.5V时,首次放电效率为93.5%,放电比容量为254.5F/g,能量密度为5.7Wh/kg,功率密度为200W/kg;循环1000次,放电比容量仅降低3.93%。NiO表面的CO2吸附过程符合Bangham模型;温度为25℃、气体流速为10mL/min时,CO2吸附量可达103.2mg/g;循环20次,吸附量保持稳定。
中图分类号:
引用本文
杨泛明, 贺国文. 颗粒状NiO的制备及其电化学性能和CO2吸附性能[J]. 化工进展, 2023, 42(2): 907-916.
YANG Fanming, HE Guowen. Preparation of granular NiO for the electrochemical performance and CO2 adsorption performance[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 907-916.
| 材料 | 电解液 | 电流密度i | 放电比容量 | 参考文献 |
|---|---|---|---|---|
| NiO | 6mol/L KOH | 1A/g | 254.5F/g | 本文 |
| NiO | 3mol/L KOH | 1A/g | 75.67F/g | [ |
| NiO | 1mol/L KOH | 1A/g | 1386F/g | [ |
| NiO | 1mol/L KOH | 1A/g | 63F/g | [ |
| NiO | 6mol/L KOH | 1×10-3mA/cm2 | 1.012mF/cm2 | [ |
| NiO | 1mol/L KOH | 1×10-3mA/cm2 | 2.08F/cm2 | [ |
| NiO | 6mol/L KOH | 1A/g | <44.2F/g | [ |
表1 NiO的电容性质对比
| 材料 | 电解液 | 电流密度i | 放电比容量 | 参考文献 |
|---|---|---|---|---|
| NiO | 6mol/L KOH | 1A/g | 254.5F/g | 本文 |
| NiO | 3mol/L KOH | 1A/g | 75.67F/g | [ |
| NiO | 1mol/L KOH | 1A/g | 1386F/g | [ |
| NiO | 1mol/L KOH | 1A/g | 63F/g | [ |
| NiO | 6mol/L KOH | 1×10-3mA/cm2 | 1.012mF/cm2 | [ |
| NiO | 1mol/L KOH | 1×10-3mA/cm2 | 2.08F/cm2 | [ |
| NiO | 6mol/L KOH | 1A/g | <44.2F/g | [ |
| qe,exp/mg·g-1 | 准一级动力学模型 | 准二级动力学模型 | Elovich模型 | Bangham模型 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
qe,cal /mg·g-1 | k1/min | R2 | qe,cal /mg·g-1 | k2 /mg·g-1·min-1 | R2 | αE/mg·g-1·min-1 | βE/mg·g-1 | R2 | qe,cal/mg·g-1 | k/min | z | R2 | |||||
| 103.2 | 104.3 | 0.6204 | 0.9979 | 45.2 | 0.0164 | 0.9801 | 199.9 | 0.0386 | 0.9526 | 103.3 | 0.5903 | 1.1221 | 0.9999 | ||||
表2 25℃时NiO的CO2吸附动力学参数
| qe,exp/mg·g-1 | 准一级动力学模型 | 准二级动力学模型 | Elovich模型 | Bangham模型 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
qe,cal /mg·g-1 | k1/min | R2 | qe,cal /mg·g-1 | k2 /mg·g-1·min-1 | R2 | αE/mg·g-1·min-1 | βE/mg·g-1 | R2 | qe,cal/mg·g-1 | k/min | z | R2 | |||||
| 103.2 | 104.3 | 0.6204 | 0.9979 | 45.2 | 0.0164 | 0.9801 | 199.9 | 0.0386 | 0.9526 | 103.3 | 0.5903 | 1.1221 | 0.9999 | ||||
| 材料 | 温度/℃ | 气体流速或压力 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|---|---|
| NiO | 25 | 20mL/min | 134.7 | 本文 |
| NiO | 55 | 10mL/min | 73.5 | 本文 |
| MgO | 60 | 30mL/min | 87.56 | [ |
| TiO2 | 25 | 15 mL/min | 23.76 | [ |
| NiO/C | 25 | 5bar | 121.35 | [ |
| MgO/AC | 25 | 5bar | 105.17 | [ |
| Fe2O3 | 25 | 1bar | 109.12 | [ |
| MgO | 25 | 1.01atm | 70.88 | [ |
| CuO/ZnO/Al2O3 | 25 | 2MPa | 61.6 | [ |
表3 NiO与其他氧化物的CO2吸附性能对比
| 材料 | 温度/℃ | 气体流速或压力 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|---|---|
| NiO | 25 | 20mL/min | 134.7 | 本文 |
| NiO | 55 | 10mL/min | 73.5 | 本文 |
| MgO | 60 | 30mL/min | 87.56 | [ |
| TiO2 | 25 | 15 mL/min | 23.76 | [ |
| NiO/C | 25 | 5bar | 121.35 | [ |
| MgO/AC | 25 | 5bar | 105.17 | [ |
| Fe2O3 | 25 | 1bar | 109.12 | [ |
| MgO | 25 | 1.01atm | 70.88 | [ |
| CuO/ZnO/Al2O3 | 25 | 2MPa | 61.6 | [ |
| 1 | 陈雪丹, 陈硕翼, 乔志军, 等. 超级电容器的应用[J]. 储能科学与技术, 2016, 5(6): 800-806. |
| CHEN Xuedan, CHEN Shuoyi, QIAO Zhijun, et al. Applications of supercapacitors[J]. Energy Storage Science and Technology, 2016, 5(6): 800-806. | |
| 2 | 李璐, 高长青, 刘怡琳, 等. 氮杂化介孔碳制备方法及在超级电容器中的应用进展[J]. 无机盐工业, 2021, 53(3): 24-29, 77. |
| LI Lu, GAO Changqing, LIU Yilin, et al. Advances of preparation of nitrogen-doped mesoporous carbon and its application in super-capacitor[J]. Inorganic Chemicals Industry, 2021, 53(3): 24-29, 77. | |
| 3 | Justin RAJ C, RAJESH Murugesan, MANIKANDAN Ramu, et al. High electrochemical capacitor performance of oxygen and nitrogen enriched activated carbon derived from the pyrolysis and activation of squid gladius chitin[J]. Journal of Power Sources, 2018, 386: 66-76. |
| 4 | MA Xinlong, ZHAO Lei, YU Zhiqing, et al. Excellent compatibility of the gravimetric and areal capacitances of an electric-double-layer capacitor configured with S-doped activated carbon[J]. ChemSusChem, 2018, 11(21): 3766-3773. |
| 5 | DUBEY Prashant, SHRIVASTAV Vishal, MAHESHWARI Priyanka H, et al. Recent advances in biomass derived activated carbon electrodes for hybrid electrochemical capacitor applications: Challenges and opportunities[J]. Carbon, 2020, 170: 1-29. |
| 6 | 张秋红, 左宋林, 卫歆雨, 等. 磷酸法活性炭作为离子液体超级电容器电极材料的研究[J]. 新型炭材料, 2018, 33(1): 61-70. |
| ZHANG Qiuhong, ZUO Songlin, WEI Xinyu, et al. H3PO4 activated carbons as the electrode materials of supercapacitors using an ionic liquid electrolyte[J]. New Carbon Materials, 2018, 33(1): 61-70. | |
| 7 | MA Jie, TAO Xueyu, ZHOU Shixiang, et al. Facile fabrication of Ag/PANI/g-C3N4 composite with enhanced electrochemical performance as supercapacitor electrode[J]. Journal of Electroanalytical Chemistry, 2019, 835: 346-353. |
| 8 | JEYARANJAN Aadithya, SAKTHIVEL Tamil Selvan, NEAL Craig J, et al. Scalable ternary hierarchical microspheres composed of PANI/rGO/CeO2 for high performance supercapacitor applications[J]. Carbon, 2019, 151: 192-202. |
| 9 | AHIRRAO Dinesh J, Ajay Kumar PAL, SINGH Vikalp, et al. Nanostructured porous polyaniline (PANI) coated carbon cloth (CC) as electrodes for flexible supercapacitor device[J]. Journal of Materials Science & Technology, 2021, 88: 168-182. |
| 10 | LI Z Y, AKHTAR M S, BUI P T M, et al. Predominance of two dimensional (2D) Mn2O3 nanowalls thin film for high performance electrochemical supercapacitors[J]. Chemical Engineering Journal, 2017, 330: 1240-1247. |
| 11 | JIANG Debin, ZHANG Bingyao, ZHENG Tianxu, et al. One-pot synthesis of η-Fe2O3 nanospheres/diatomite composites for electrochemical capacitor electrodes[J]. Materials Letters, 2018, 215: 23-26. |
| 12 | MAHMOOD Majid, ZULFIQAR Sonia, WARSI Muhammad Farooq, et al. Nanostructured V2O5 and its nanohybrid with MXene as an efficient electrode material for electrochemical capacitor applications[J]. Ceramics International, 2022, 48(2): 2345-2354. |
| 13 | XIAO Huanhao, YAO Shunyu, LIU Hongda, et al. NiO nanosheet assembles for supercapacitor electrode materials[J]. Progress in Natural Science: Materials International, 2016, 26(3): 271-275. |
| 14 | CAI Guofa, WANG Xu, CUI Mengqi, et al. Electrochromo-supercapacitor based on direct growth of NiO nanoparticles[J]. Nano Energy, 2015, 12: 258-267. |
| 15 | DURAISAMY Navaneethan, NUMAN Arshid, FATIN Saiha Omar, et al. Facile sonochemical synthesis of nanostructured NiO with different particle sizes and its electrochemical properties for supercapacitor application[J]. Journal of Colloid and Interface Science, 2016, 471: 136-144. |
| 16 | SUN Wanting, XIAO Li, WU Xiang. Facile synthesis of NiO nanocubes for photocatalysts and supercapacitor electrodes[J]. Journal of Alloys and Compounds, 2019, 772: 465-471. |
| 17 | ZHOU Shengyu, WANG Shen, ZHOU Shijin, et al. An electrochromic supercapacitor based on an MOF derived hierarchical-porous NiO film[J]. Nanoscale, 2020, 12(16): 8934-8941. |
| 18 | DAI Kai, LIANG Changhao, DAI Jianming, et al. High-yield synthesis of carbon nanotube-porous nickel oxide nanosheet hybrid and its electrochemical capacitance performance[J]. Materials Chemistry and Physics, 2014, 143(3): 1344-1351. |
| 19 | QIAN Yue, LIU Rong, WANG Qiufan, et al. Efficient synthesis of hierarchical NiO nanosheets for high-performance flexible all-solid-state supercapacitors[J]. Journal of Materials Chemistry A, 2014, 2(28): 10917-10922. |
| 20 | 徐舟, 侯程, 王诗琴, 等. 氧化镍/碳纳米管构筑准固态不对称超级电容器及电化学性能[J]. 化工进展, 2020, 39(10): 4088-4094. |
| XU Zhou, HOU Cheng, WANG Shiqin, et al. Quasi-solid-state asymmetric supercapacitor constructed with NiO/CNT composites and its electrochemical performance[J]. Chemical Industry and Engineering Progress, 2020, 39(10): 4088-4094. | |
| 21 | LIU Ao, ZHANG Haitao, WANG Gao, et al. Sandwich-like NiO/rGO nanoarchitectures for 4V solid-state asymmetric-supercapacitors with high energy density[J]. Electrochimica Acta, 2018, 283: 1401-1410. |
| 22 | LI Shuting, DUAN Yanan, TENG Ying, et al. MOF-derived tremelliform Co3O4/NiO/Mn2O3 with excellent capacitive performance[J]. Applied Surface Science, 2019, 478: 247-254. |
| 23 | SHI Jicheng, DENG Han, LU Lu, et al. Performance of nickel–zinc battery with ZnO/activated carbon/3D network carbon felt as zinc negative electrode[J]. Journal of Applied Electrochemistry, 2021, 51(12): 1675-1687. |
| 24 | ZHU Chunmei, HE Ying, LIU Yijun, et al. ZnO@MOF@PANI core-shell nanoarrays on carbon cloth for high-performance supercapacitor electrodes[J]. Journal of Energy Chemistry, 2019, 35: 124-131. |
| 25 | CAO Junming, LI Junzhi, LI La, et al. Mn-doped Ni/Co LDH nanosheets grown on the natural N-dispersed PANI-derived porous carbon template for a flexible asymmetric supercapacitor[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(12): 10699-10707. |
| 26 | LIANG Xiao, ZHAO Lei, WANG Qiufan, et al. A dynamic stretchable and self-healable supercapacitor with a CNT/graphene/PANI composite film[J]. Nanoscale, 2018, 10(47): 22329-22334. |
| 27 | KOLLE J M, FAYAZ M, SAYARI A. Understanding the effect of water on CO2 adsorption[J]. Chemical Reviews, 2021, 121(13): 7280-7345. |
| 28 | HAN Jun, ZHANG Li, ZHAO Bo, et al. The N-doped activated carbon derived from sugarcane bagasse for CO2 adsorption[J]. Industrial Crops and Products, 2019, 128: 290-297. |
| 29 | CHO Seho, YU Hye-Ryeon, CHOI Tae Hoon, et al. Surface functionalization and CO2 uptake on carbon molecular sieves: Experimental observation and theoretical study[J]. Applied Surface Science, 2018, 447: 8-14. |
| 30 | YANG Fanming, LIU Ying, CHEN Lang, et al. Synthesis of amine-modified solid Fe-Zr adsorbents for CO2 adsorption[J]. Journal of Chemical Technology & Biotechnology, 2016, 91(8): 2340-2348. |
| 31 | BIEN Caitlin E, LIU Qiao, WADE Casey R. Assessing the role of metal identity on CO2 adsorption in MOFs containing M-OH functional groups[J]. Chemistry of Materials, 2020, 32(1): 489-497. |
| 32 | GAO Wanlin, ZHOU Tuantuan, WANG Qiang. Controlled synthesis of MgO with diverse basic sites and its CO2 capture mechanism under different adsorption conditions[J]. Chemical Engineering Journal, 2018, 336: 710-720. |
| 33 | TUMULURI Uma, HOWE Joshua D, MOUNFIELD William P, et al. Effect of surface structure of TiO2 nanoparticles on CO2 adsorption and SO2 resistance[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(10): 9295-9306. |
| 34 | LI Shuangshuang, LIU Guilong, ZHANG Siran, et al. Cerium-modified Ni-La2O3/ZrO2 for CO2 methanation[J]. Journal of Energy Chemistry, 2020, 43: 155-164. |
| 35 | NUNES Willian G, MIRANDA Andre N, FREITAS Bruno, et al. Charge-storage mechanism of highly defective NiO nanostructures on carbon nanofibers in electrochemical supercapacitors[J]. Nanoscale, 2021, 13(21): 9590-9605. |
| 36 | GHAEMI A, MASHHADIMOSLEM H, ZOHOURIAN IZADPANAH P. NiO and MgO/activated carbon as an efficient CO2 adsorbent: Characterization, modeling, and optimization[J]. International Journal of Environmental Science and Technology, 2022, 19(2): 727-746. |
| 37 | SHI Jinsong, CUI Hongmin, XU Jianguo, et al. Design and fabrication of hierarchically porous carbon frameworks with Fe2O3 cubes as hard template for CO2 adsorption[J]. Chemical Engineering Journal, 2020, 389: 124459. |
| 38 | ELVIRA Gutiérrez-Bonilla, FRANCISCO Granados-Correa, Sánchez-Mendieta VÍCTOR, et al. MgO-based adsorbents for CO2 adsorption: Influence of structural and textural properties on the CO2 adsorption performance[J]. Journal of Environmental Sciences, 2017, 57: 418-428. |
| 39 | SMYRNIOTI Maria, TAMPAXIS Christos, STERIOTIS Theodore, et al. Study of CO2 adsorption on a commercial CuO/ZnO/Al2O3 catalyst[J]. Catalysis Today, 2020, 357: 495-502. |
| 40 | KULAL Nagendra, VASISTA Vaishnavi, SHANBHAG Ganapati V. Identification and tuning of active sites in selected mixed metal oxide catalysts for cyclic carbonate synthesis from epoxides and CO2 [J]. Journal of CO2 Utilization, 2019, 33: 434-444. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [7] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [8] | 史天茜, 石永辉, 武新颖, 张益豪, 秦哲, 赵春霞, 路达. Fe2+对厌氧氨氧化EGSB反应器运行性能的影响[J]. 化工进展, 2023, 42(9): 5003-5010. |
| [9] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [10] | 张丽宏, 金要茹, 程芳琴. 煤气化渣资源化利用[J]. 化工进展, 2023, 42(8): 4447-4457. |
| [11] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [12] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [13] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [14] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| [15] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||