| 1 |
ZHENG Lifang, WANG Xiaogang, YUE Lina, et al. Progress in the application of rare light metal beryllium[J]. Materials Science Forum, 2020, 977: 261-271.
|
| 2 |
朱学红, 冯慧, 张宏伟. 地缘政治视角下铍资源供应风险分析[J]. 中南大学学报(社会科学版), 2023, 29(5): 138-147.
|
|
ZHU Xuehong, FENG Hui, ZHANG Hongwei. Beryllium resource supply risk analysis from the geopolitical perspective[J]. Journal of Central South University (Social Sciences), 2023, 29(5): 138-147.
|
| 3 |
CHEN Zhaoxi, JIN Guangxu, CHEN Kaiyun, et al. Development and experimental study of beryllium window for ITER radial X-ray camera[J]. Fusion Engineering and Design, 2013, 88(12): 3280-3286.
|
| 4 |
邵伟华, 曾永杰, 常学勇, 等. 某含萤石铍矿综合回收铍和萤石的试验研究[J]. 矿产保护与利用, 2023, 43(1): 98-104.
|
|
SHAO Weihua, ZENG Yongjie, CHANG Xueyong, et al. Experimental study on comprehensive recovery of beryllium and fluorite from a fluorite-beryllium ore[J]. Conservation and Utilization of Mineral Resources, 2023, 43(1): 98-104.
|
| 5 |
ROUXEL Baptiste, MISCHLER Stefano, Roland LOGÉ, et al. Wear behaviour of novel copper alloy as an alternative to copper-beryllium[J]. Wear, 2023, 524: 204817.
|
| 6 |
中国有色金属工业协会专家委员会组织. 中国铍业[M]. 北京: 冶金工业出版社, 2015.
|
|
China Nonferrous Metals Industry Association Expert Committee Organization. China beryllium industry[M]. Beijing: Metallurgical Industry Press, 2015.
|
| 7 |
LU Ruilong, HAN Jingtao, LI Zhanhua, et al. Study of the mechanical properties and microstructure of spiral tubes and actuators for controlled extension fabricated with beryllium bronze strips[J]. Materials, 2023, 16(20): 6719.
|
| 8 |
PAVLOV Alexandr, SAGDOLDINA Zhuldyz, ZHILKASHINOVA Almira, et al. Synthesis and investigation of properties of beryllium ceramics modified with titanium dioxide nanoparticles[J]. Materials, 2023, 16(19): 6507.
|
| 9 |
宗国强, 肖吉昌. 氟化物熔盐的制备及其应用进展[J]. 化工进展, 2018, 37(7): 2455-2472.
|
|
ZONG Guoqiang, XIAO Jichang. Advances in the preparation and application of fluoride molten salts[J]. Chemical Industry and Engineering Progress, 2018, 37(7): 2455-2472.
|
| 10 |
贺云鹏. 高纯铍真空热压成型装置设计与研究[D]. 沈阳: 沈阳大学, 2021.
|
|
HE Yunpeng. Design and research of high-purity beryllium vacuum hot pressing device[D]. Shenyang: Shenyang University, 2021.
|
| 11 |
GERASIMOV M F, NIKIFOROV S V. Simulation of the thermoluminescence isothermal decay curves in Al2O3-BeO ceramics taking into account the energy distribution of trapping centers[J]. Bulletin of the Russian Academy of Sciences: Physics, 2023, 87(11): 1655-1660.
|
| 12 |
LAZUKINA O P, VOLKOVA E N, MALYSHEV K K, et al. Purity of alkaline-earth metals (according to materials in the exhibition-collection of extrapure substances)[J]. Inorganic Materials, 2021, 57(11): 1167-1172.
|
| 13 |
MATYASOVA V E, KOTSAR’ M L, ANAN’EV A V, et al. Ion-exchange processes in the reprocessing of sulfate solutions and pulps with production of high-purity beryllium compounds[J]. Atomic Energy, 2016, 119(6): 408-413.
|
| 14 |
ZHAO Xu, XIA Hongyang, SU Yucheng, et al. Study on removal of beryllium from uranium beryllium ore wastewater by acid leaching activated carbon and its mechanism[J]. Journal of Radioanalytical and Nuclear Chemistry, 2023, 332(10): 4231-4242.
|
| 15 |
邓伟, 颜世强, 谭洪旗, 等. 我国铍矿资源概况及选矿技术研究现状[J]. 矿产综合利用, 2023(1): 148-154.
|
|
DENG Wei, YAN Shiqiang, TAN Hongqi, et al. General situation of beryllium ore resources and research status of mineral processing technology in China[J]. Multipurpose Utilization of Mineral Resources, 2023(1): 148-154.
|
| 16 |
张森, 鞠楠, 伍月, 等. 铍矿分布特点、主要类型与勘查开发现状[J]. 中国地质, 2023, 50(2): 410-424.
|
|
ZHANG Sen, JU Nan, WU Yue, et al. Distribution characteristics, main types and exploration and development status of beryllium deposit[J]. Geology in China, 2023, 50(2): 410-424.
|
| 17 |
贾福东, 张长青, 化志新, 等. 云南麻花坪钨铍矿床蓝柱石的鉴定特征及成分与成因分析[J]. 光谱学与光谱分析, 2020, 40(10): 3185-3192.
|
|
JIA Fudong, ZHANG Changqing, HUA Zhixin, et al. Identification characteristics, composition and genesis of euclase in mahuaping tungsten-beryllium polymetallic deposit in Yunnan Province, Southwest China[J]. Spectroscopy and Spectral Analysis, 2020, 40(10): 3185-3192.
|
| 18 |
李中. 铍矿石的浸出及回收工艺试验研究[D]. 衡阳: 南华大学, 2015.
|
|
LI Zhong. Study on the experimentalof beryllium ore leaching and recovery process[D]. Hengyang: University of South China, 2015.
|
| 19 |
雷湘. 高氟铍矿石的脱氟试验研究[J]. 湖南有色金属, 2004, 20(1): 21-22, 25.
|
|
LEI Xiang. Research on removing fluorine from beryllium ore containing high fluorine[J]. Hunan Nonferrous Metals, 2004, 20(1): 21-22, 25.
|
| 20 |
李卫. 高氟铍矿石的冶炼工艺研究[D]. 长沙: 中南大学, 2003.
|
|
LI Wei. Study on smelting process of high fluorine beryllium ore[D]. Changsha: Central South University, 2003.
|
| 21 |
肖超, 李婕, 吴海国. 硫酸法提取铍工艺中铝杂质分离的研究现状[J]. 稀有金属与硬质合金, 2013, 41(3): 8-10, 27.
|
|
XIAO Chao, LI Jie, WU Haiguo. The latest research on the aluminum separation during beryllium extraction with sulfuric acid[J]. Rare Metals and Cemented Carbides, 2013, 41(3): 8-10, 27.
|
| 22 |
郭培民, 赵沛, 王磊, 等. 一种从绿柱石中浸出铍的方法: CN112322893A[P]. 2021-02-05.
|
|
GUO Peimin, ZHAO Pei, WANG Lei, et al. A method for leaching beryllium from beryl: CN112322893A[P]. 2021-02-05.
|
| 23 |
郭培民, 王磊, 孔令兵, 等. 一种从含氟化铍混合物中提纯制备氢氧化铍的方法: CN113003591A[P]. 2021-06-22.
|
|
GUO Peimin, WANG Lei, KONG Lingbing, et al. A method for the purification and preparation of beryllium hydroxide from beryllium fluoride-containing mixtures: CN113003591A [P]. 2021-06-22.
|
| 24 |
郭培民, 王磊, 孔令兵, 等. 一种从含氟化铍混合物中提纯制备氟铍化铵的方法: CN112794343B[P]. 2022-12-16.
|
|
GUO Peimin, WANG Lei, KONG Lingbing, et al. A method for the purification and preparation of ammonium beryllium fluoride from beryllium fluoride-containing mixtures: CN112794343B[P]. 2022-12-16.
|
| 25 |
郭培民, 曾志彦, 王磊, 等. 一种金属铍珠及金属铍锭的制备方法: CN113059154B[P]. 2022-09-16.
|
|
GUO Peimin, ZENG Zhiyan, WANG Lei, et al. A preparation method of beryllium metal beads and beryllium metal ingots: CN113059154B[P]. 2022-09-16.
|
| 26 |
刘光启. 化学化工物性数据手册-无机卷[M]. 北京: 化学工业出版社, 2002.
|
|
LIU Guangqi. Handbook of physical properties of chemistry and chemical engineering-inorganic volume[M]. Beijing: Chemical Industry Press, 2002.
|
| 27 |
稀有金属手册编辑委员会. 稀有金属手册.下[M]. 北京: 冶金工业出版社, 1995.
|
|
Editorial Board of the Handbook of Rare Metals. Handbook of rare metals. Next[M]. Beijing: Metallurgical Industry Press, 1995.
|
| 28 |
郭培民, 赵沛. 冶金资源高效利用[M]. 北京: 冶金工业出版社, 2012.
|
|
GUO Peimin, ZHAO Pei. Efficient utilization of metallurgical resources[M]. Beijing: Metallurgical Industry Press, 2012.
|
| 29 |
EVEREST David Anthony. The chemistry of beryllium[M]. Amsterdam: Elsevier Publishing Company, 1964.
|
| 30 |
马进伟, 方浩, 陈茜茜, 等. 基于焓-熵-(火用)平衡的无盖板PV/T系统热力学分析与优化[J]. 化工进展, 2022, 41(4): 1840-1847.
|
|
MA Jinwei, FANG Hao, CHEN Qianqian, et al. Thermodynamic analysis and optimization of unglazed PV/T system based on enthalpy-entropy-exergy equilibrium[J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1840-1847.
|
| 31 |
宋昌斌, 李润超. 碳酸锂在水中的溶解度和超溶解度的测定及热力学分析[J]. 化工进展, 2016, 35(8): 2350-2354.
|
|
SONG Changbin, LI Runchao. Measurement and thermodynamic analysis of the solubility and supersolubility of lithium carbonate in water[J]. Chemical Industry and Engineering Progress, 2016, 35(8): 2350-2354.
|
| 32 |
张琳. F-、Al3+、CaF2络合平衡在废液制冰晶石中的应用[D]. 青岛: 山东科技大学, 2010.
|
|
ZHANG Lin. Application of F-, Al3+, CaF2 complex balance in making cryolite from waste water[D]. Qingdao: Shandong University of Science and Technology, 2010.
|
| 33 |
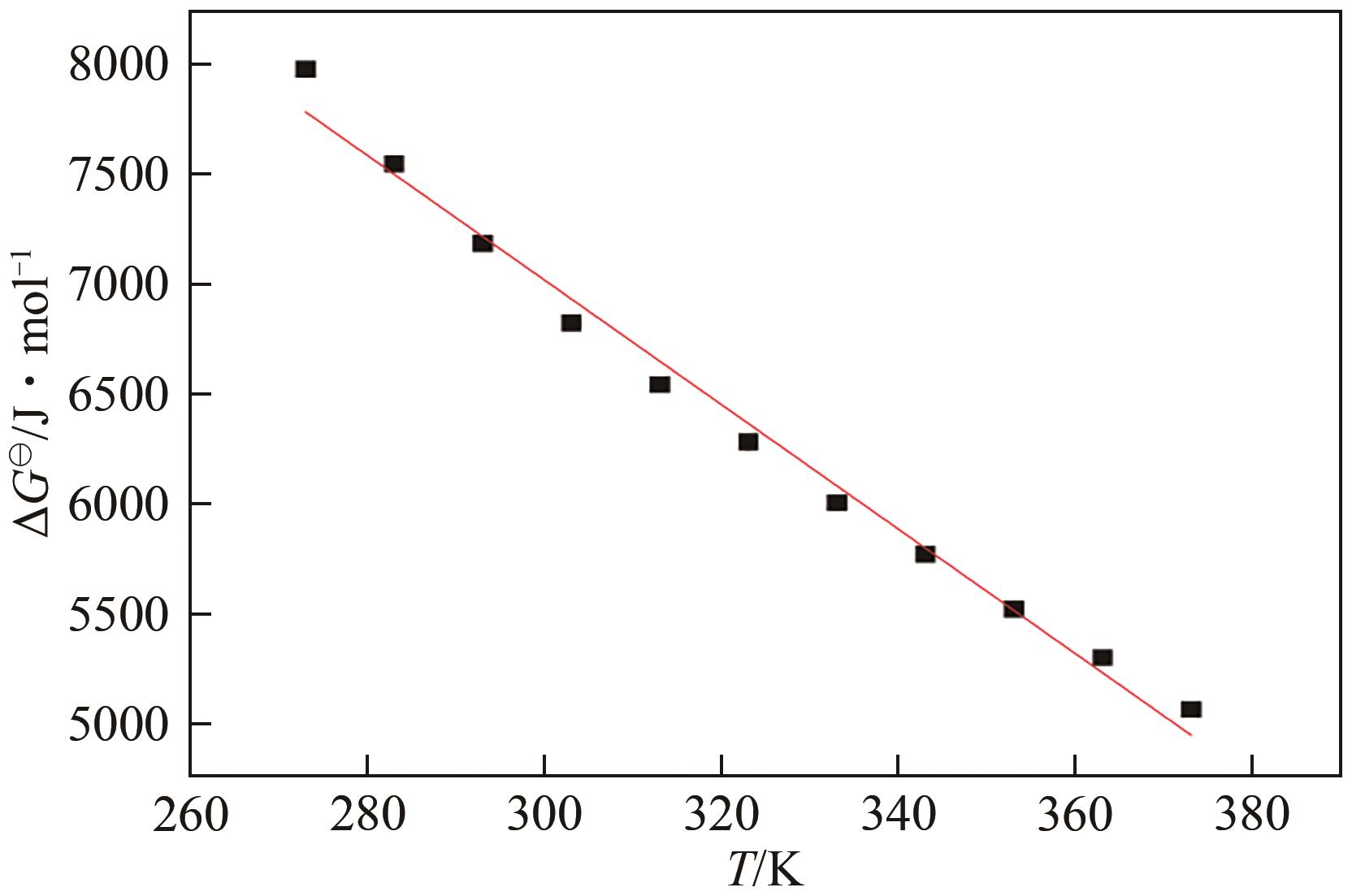
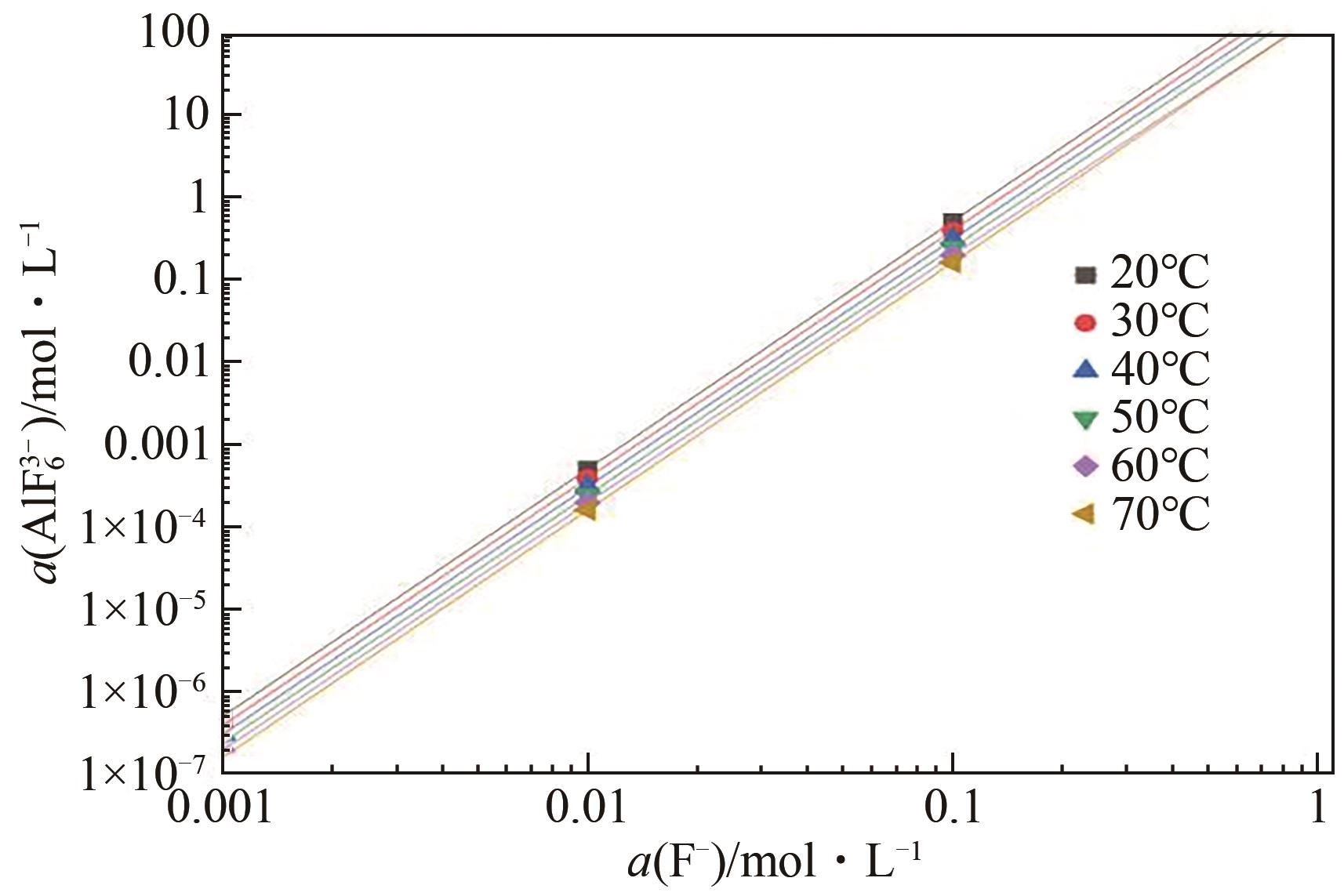
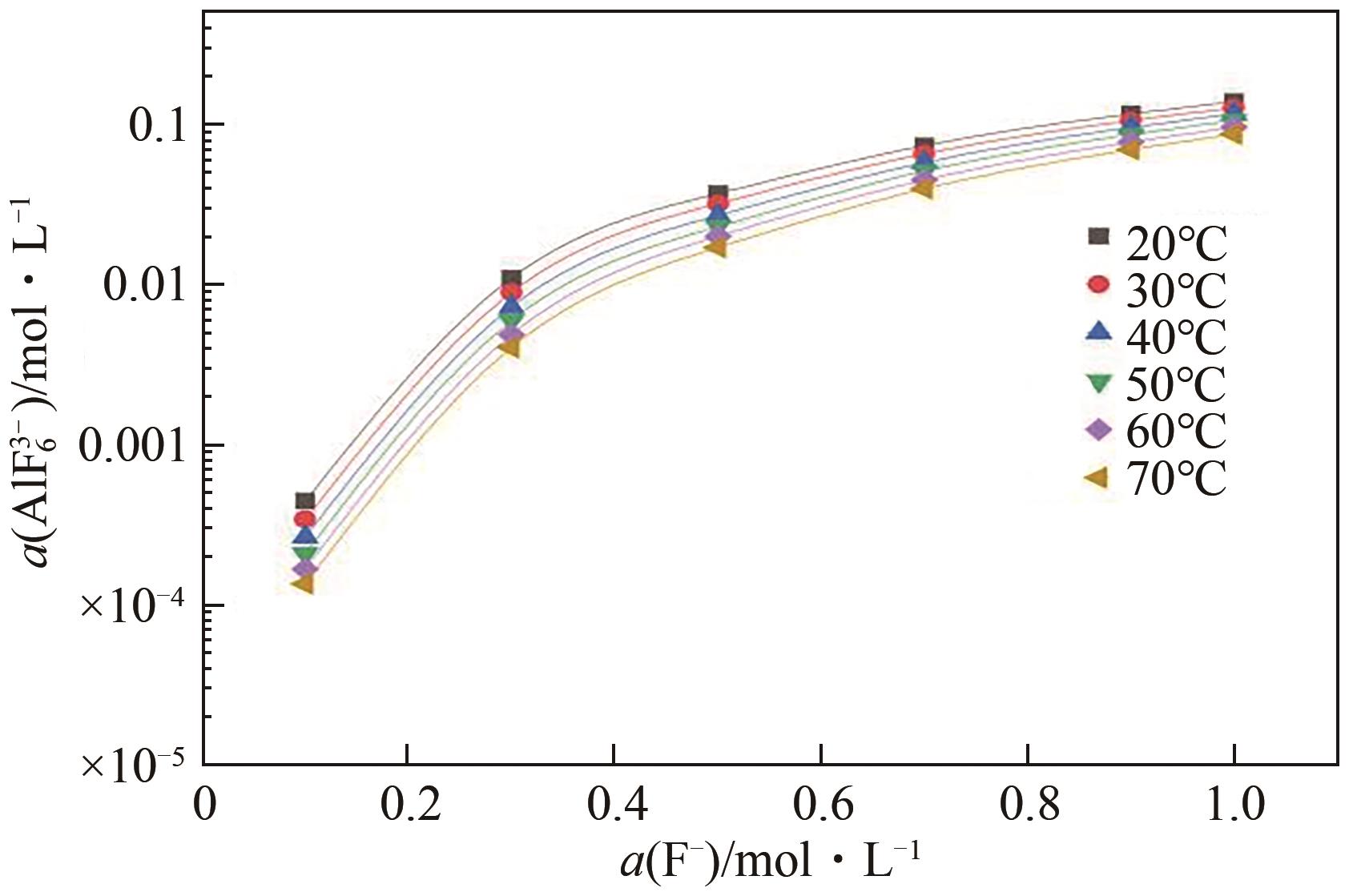
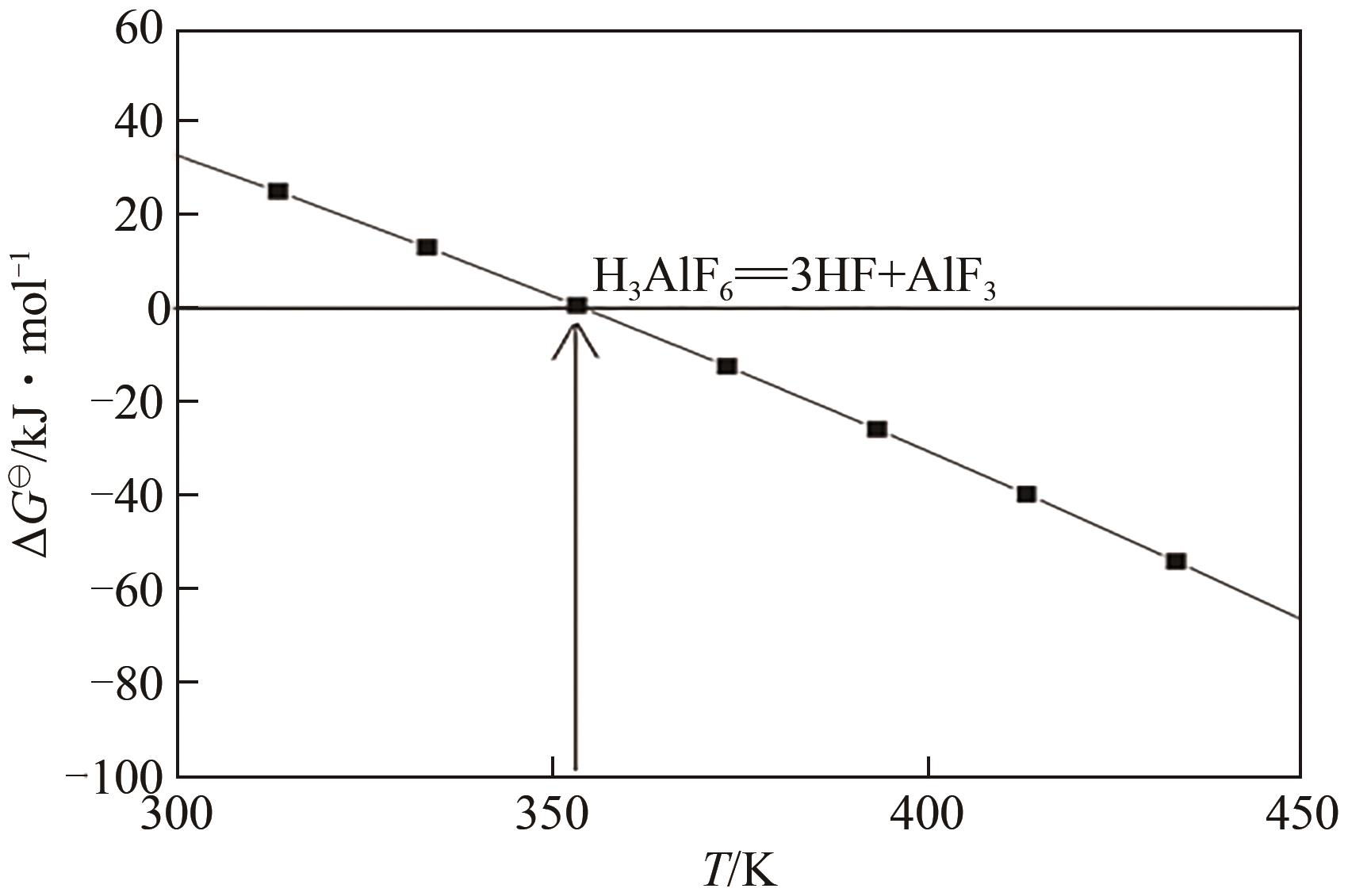
申耀宗, 郭培民, 王磊, 等. 氢氟酸解离铍矿新路线中氧化物杂质的浸出热力学[J]. 有色金属(冶炼部分), 2023(10): 42-48.
|
|
SHEN Yaozong, GUO Peimin, WANG Lei, et al. Leaching thermodynamics of oxide impurities in a new route of beryllium ore dissociation by hydrofluoric acid[J]. Nonferrous Metals (Extractive Metallurgy), 2023(10): 42-48.
|
 ), 郭培民1,2(
), 郭培民1,2( ), 王磊1,2, 孔令兵1,2, 郭庆3, 谢奕斌3
), 王磊1,2, 孔令兵1,2, 郭庆3, 谢奕斌3
 ), GUO Peimin1,2(
), GUO Peimin1,2( ), WANG Lei1,2, KONG Lingbing1,2, GUO Qing3, XIE Yibin3
), WANG Lei1,2, KONG Lingbing1,2, GUO Qing3, XIE Yibin3