化工进展 ›› 2025, Vol. 44 ›› Issue (2): 941-956.DOI: 10.16085/j.issn.1000-6613.2024-0260
天然化合物用于光引发体系的研究进展
- 南京林业大学材料科学与工程学院,江苏 南京 210037
-
收稿日期:2024-02-04修回日期:2024-04-14出版日期:2025-02-25发布日期:2025-03-10 -
通讯作者:连海兰 -
作者简介:李珺(1998—),女,硕士研究生,研究方向为光固化涂料。E-mail:w1464670849@163.com。 -
基金资助:江苏省研究生科研与实践创新计划(KYCX23-1189)
Research progress on the use of natural compounds in photoinitiating systems
LI Jun( ), ZHANG Yu, WU Xinyu, LIAN Hailan(
), ZHANG Yu, WU Xinyu, LIAN Hailan( )
)
- College of Materials Science and Engineering, Nanjing Forestry University, Nanjing 210037, Jiangsu, China
-
Received:2024-02-04Revised:2024-04-14Online:2025-02-25Published:2025-03-10 -
Contact:LIAN Hailan
摘要:
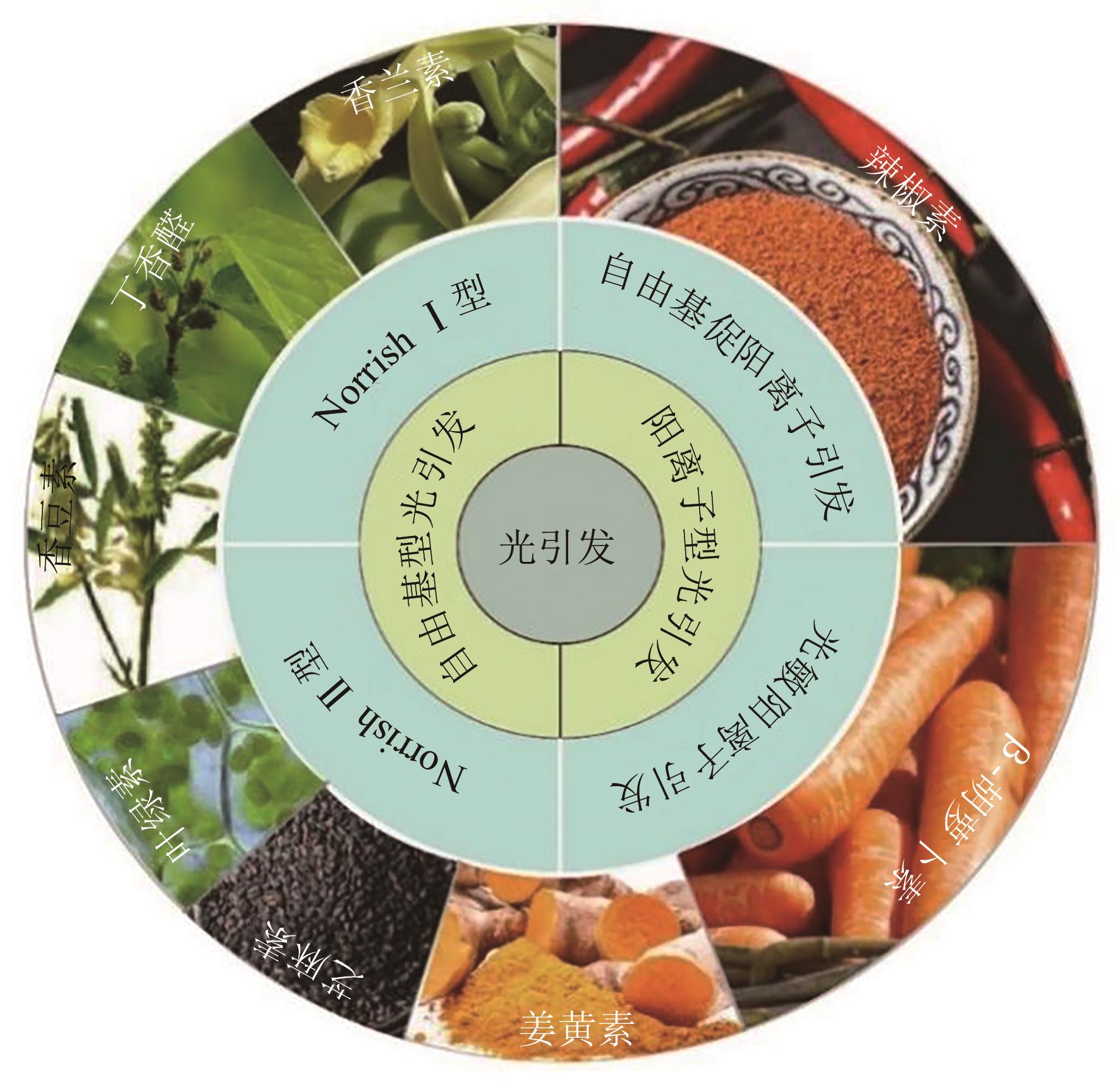
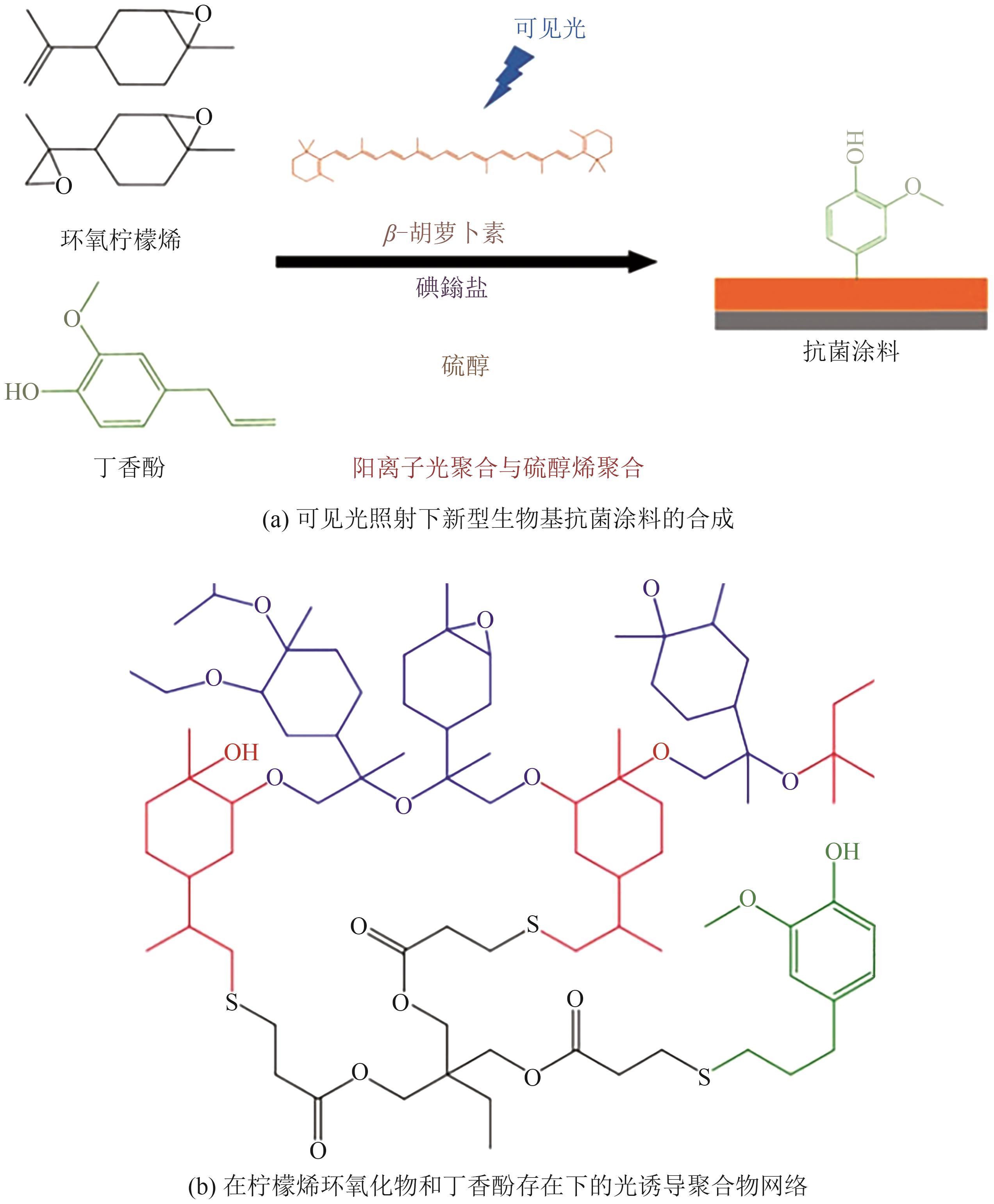
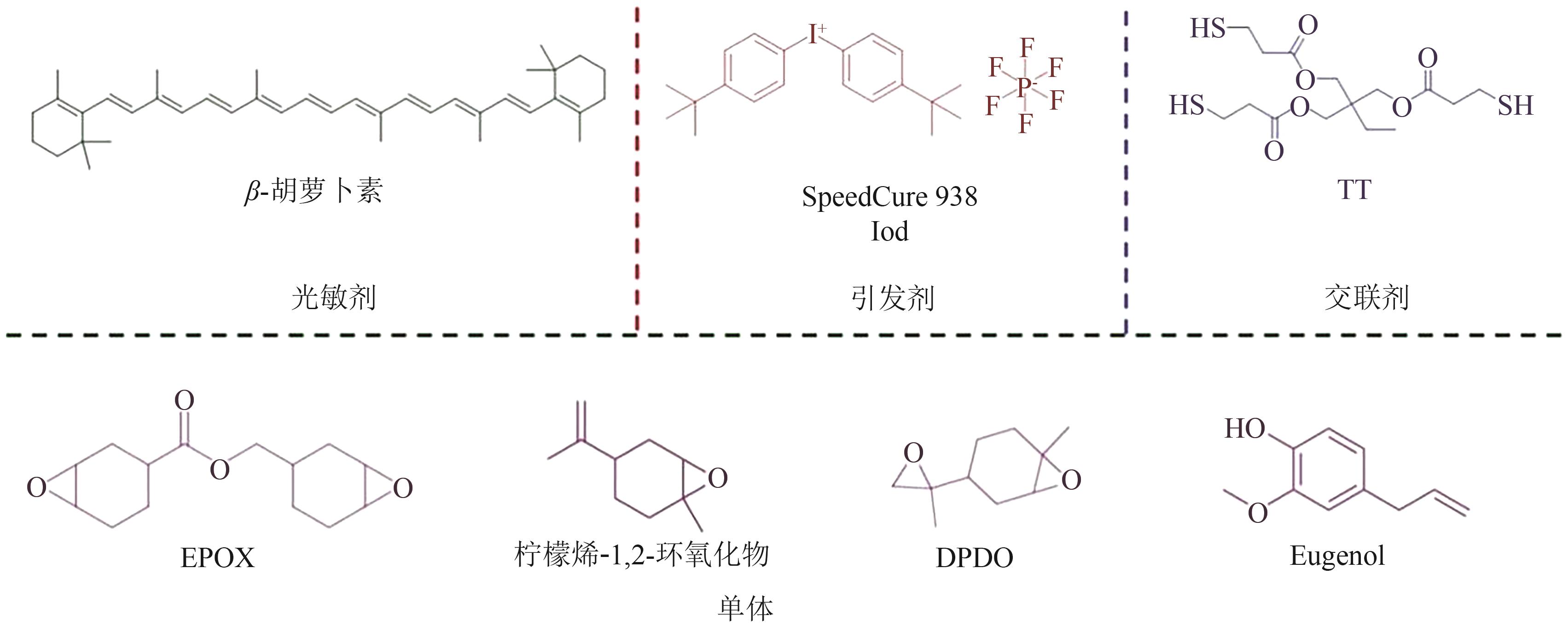
光聚合是一种较为理想的绿色合成途径,但目前大多数的商业光引发剂面临着毒性与环保问题,因此较为绿色的天然化合物成为光聚合研究领域的新热点。本文根据不同种类的天然化合物可通过其化学特性,直接或间接地参与光引发反应,提高体系的吸收波长范围,从而在发光二极管(LED)辐照下引发多种丙烯酸酯的自由基聚合反应和环氧化物的阳离子聚合反应等,概述了不同来源的天然化合物用于自由基聚合和阳离子聚合光引发体系的研究进展,展示了香兰素、丁香醛等多种天然化合物基光引发体系的独特优势和应用潜力,总结了目前所面临的提纯困难、产率低、氧阻聚等挑战,提出了后续研究应注重天然化合物的绿色提取与改性,以及天然化合物基阳离子光引发体系的开发等建议,为天然化合物用于光引发体系的设计及应用提供了参考。
中图分类号:
引用本文
李珺, 张毓, 吴新宇, 连海兰. 天然化合物用于光引发体系的研究进展[J]. 化工进展, 2025, 44(2): 941-956.
LI Jun, ZHANG Yu, WU Xinyu, LIAN Hailan. Research progress on the use of natural compounds in photoinitiating systems[J]. Chemical Industry and Engineering Progress, 2025, 44(2): 941-956.
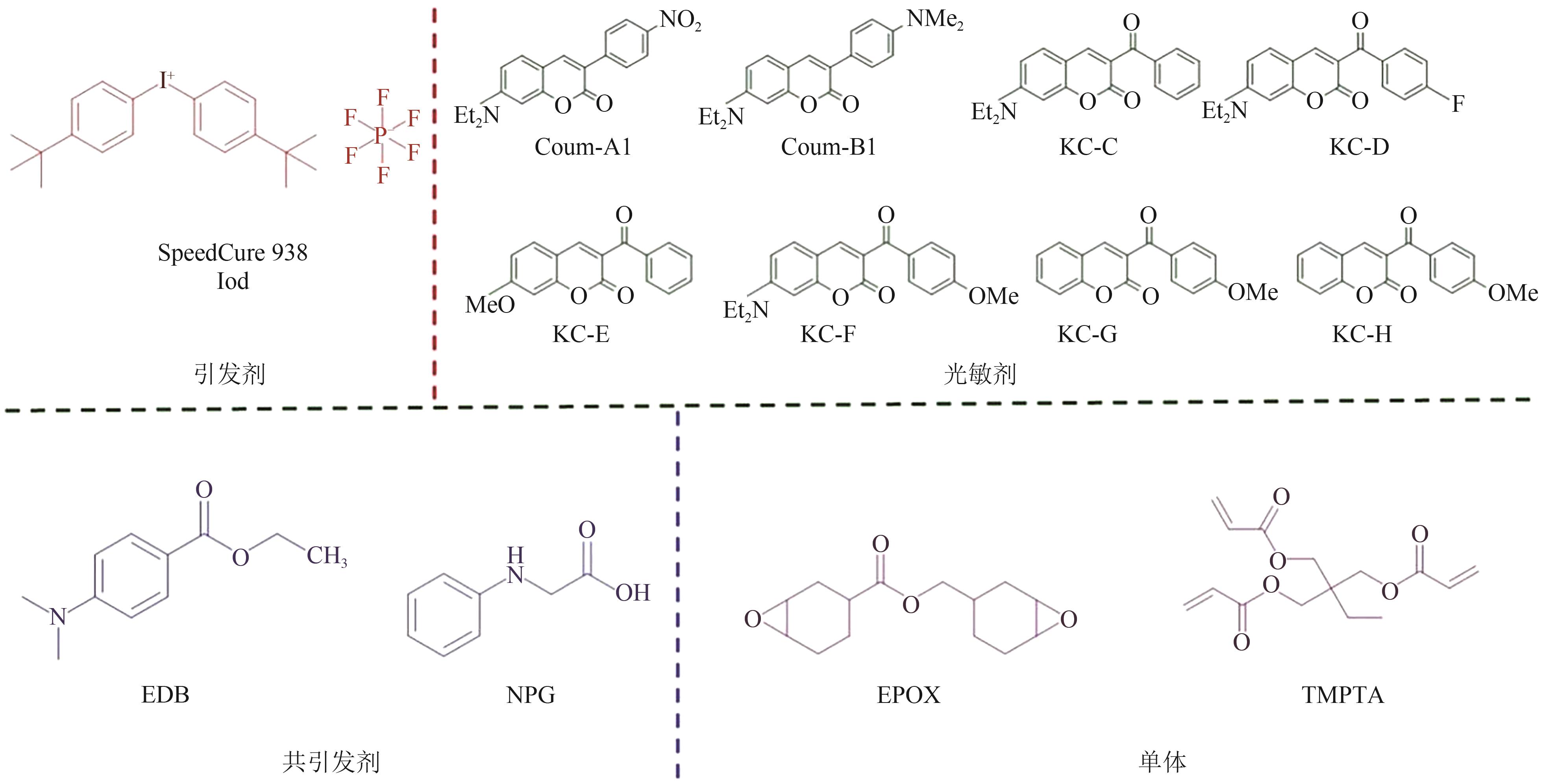
| 聚合类型 | 引发机理 | 使用的天然化合物 | 吸收波长范围/nm | 辐照设备 | 添加剂 | 引发单体类型 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 自由基 聚合 | Norrish Ⅰ型 (裂解型) | 香兰素 | 300~350 | Hg-Xe灯 | TMPTA/SOA | [ | |
| 丁香醛 | 300~385 | LED@385nm | TMPTA | [ | |||
| LED@400nm | |||||||
| 香豆素 | 400~480, 500~550 | LED@450nm | TMPTA/TAIC/TAC/APE | [ | |||
| 香豆素 | 280~480 | LED@375nm, LED@405nm | Iod,NPG/EDB | TMPTA | [ | ||
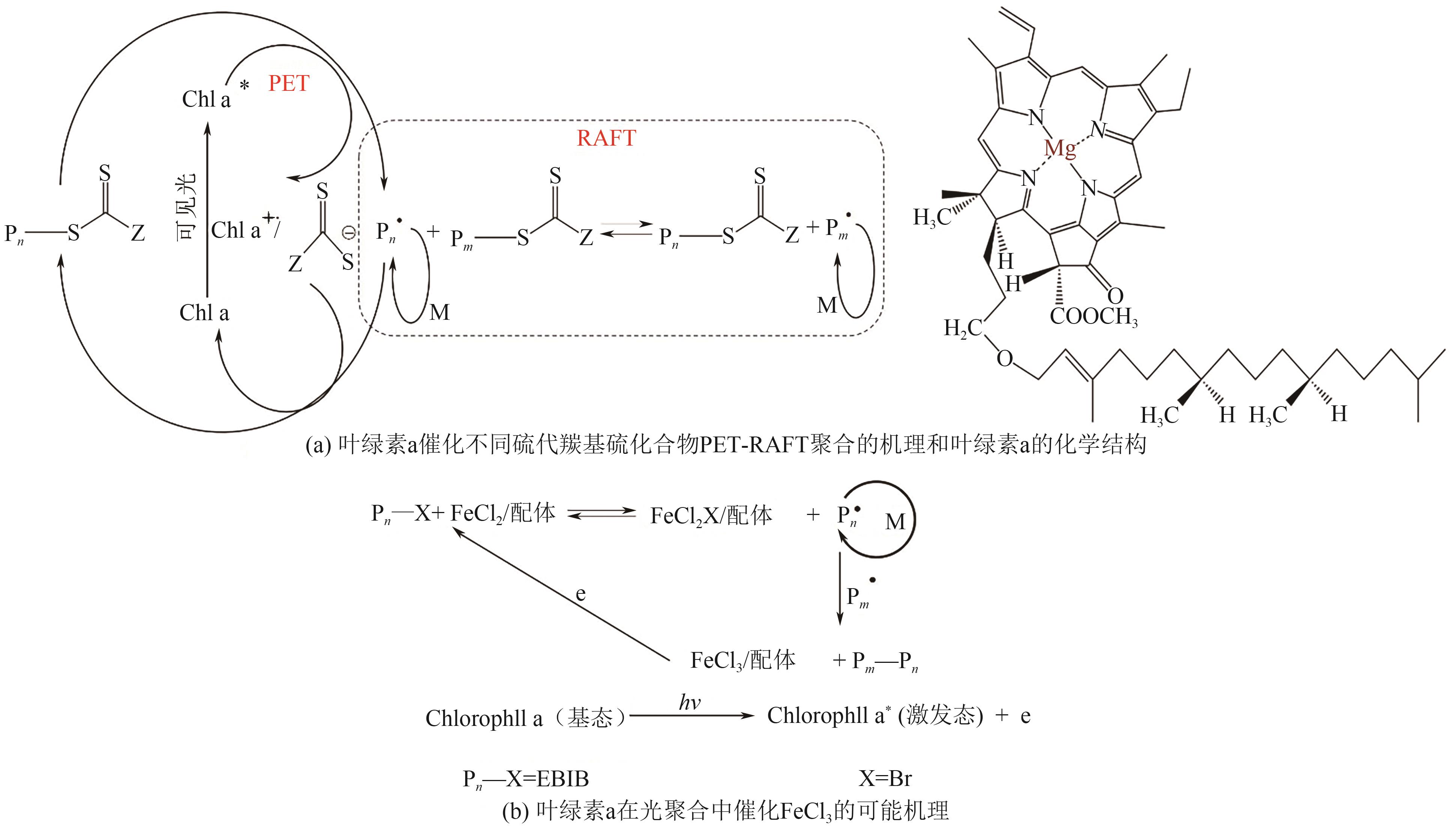
| Norrish Ⅱ型(夺氢型) | 叶绿酸a | 430~450, 640~660 | LED@430nm, LED@640nm | EBiB/ FeCl3·6H2O | MMA | [ | |
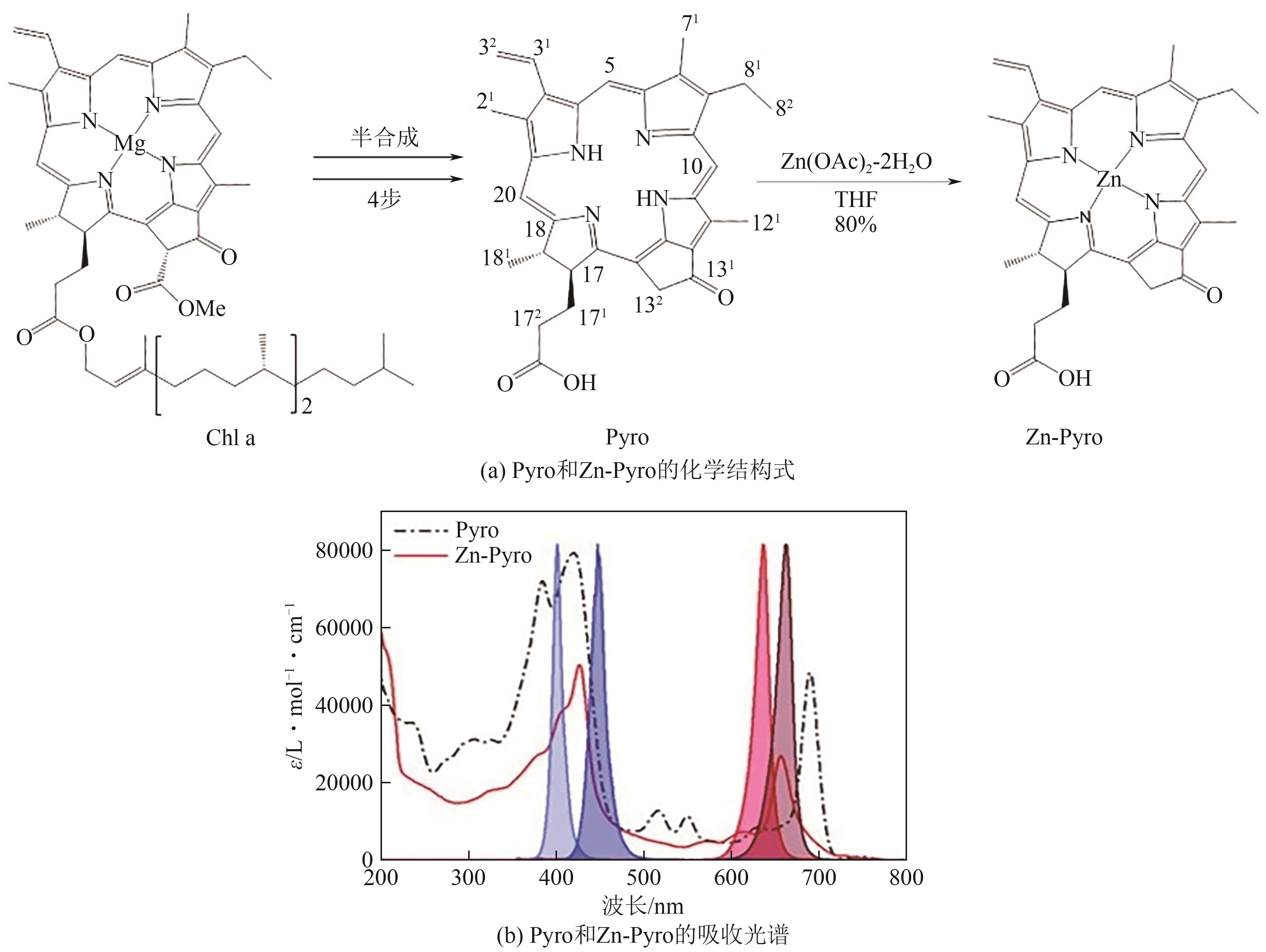
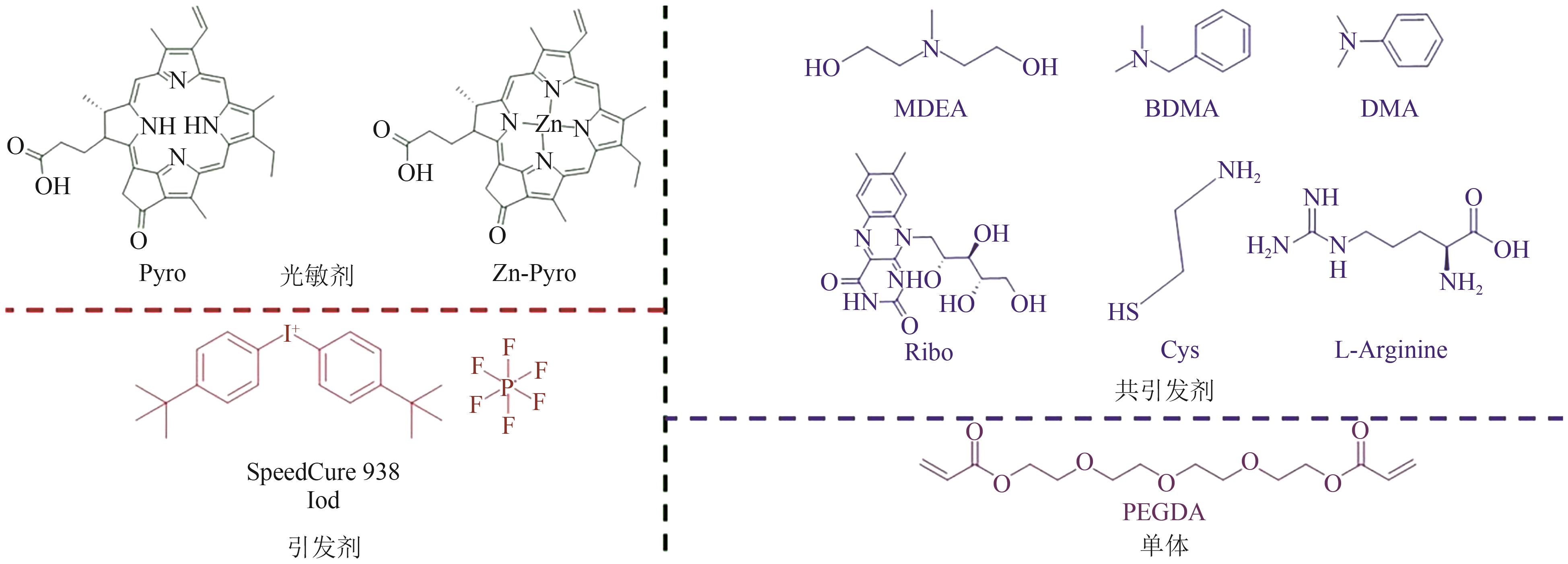
| 焦脱镁叶绿酸a | 420~427, 500~750 | LED@405nm, LED@455nm, LED@625nm, LED@660nm | Iod,MDEA/ BDMA/DMA/Ribo/ Cys/Argi | PEGDA | [ | ||
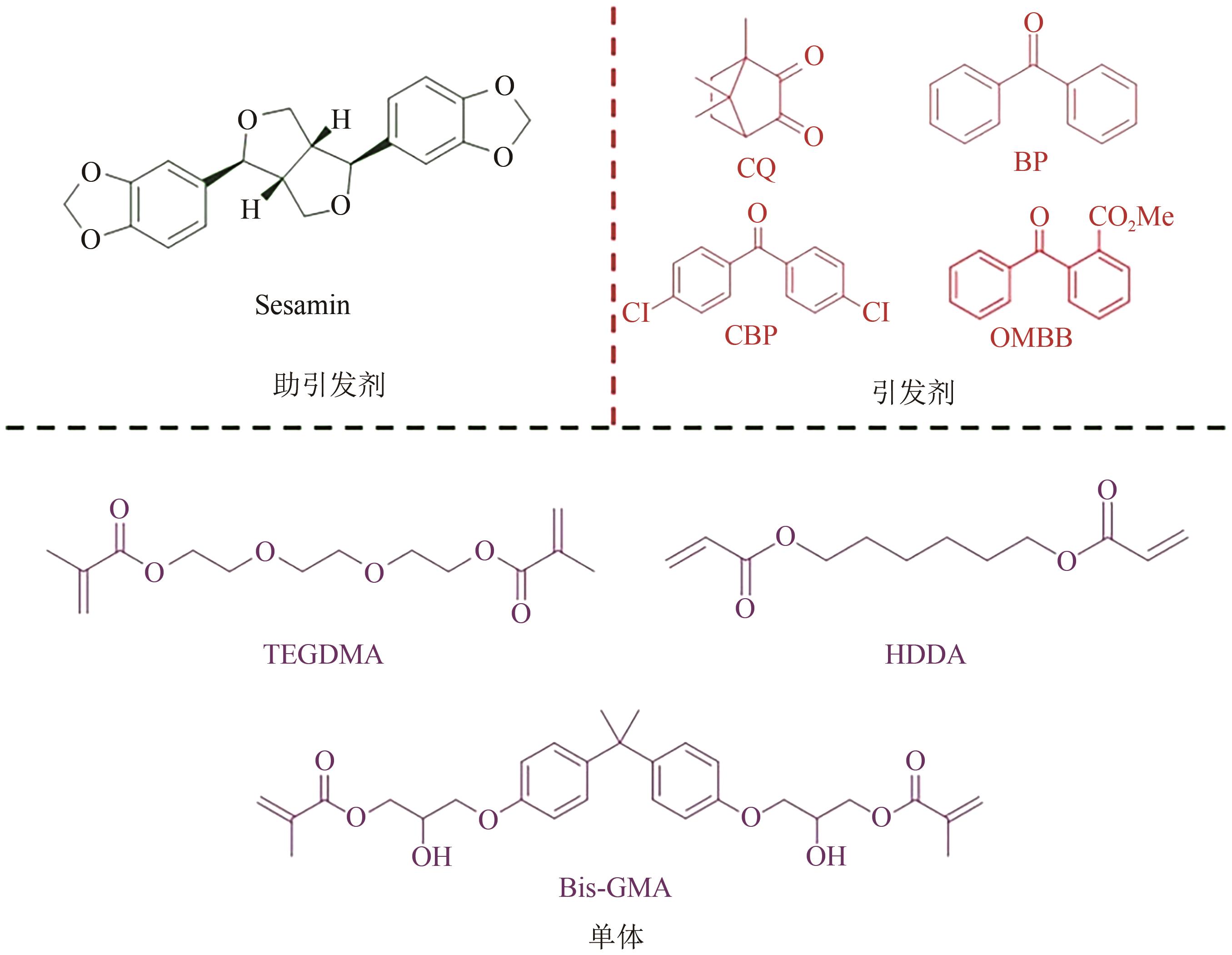
| 芝麻素 | 250~300 | UV点光源 | BP,CBP,OMBB | HDDA/TPGDA/ TMPTA/TMPTMA | [ | ||
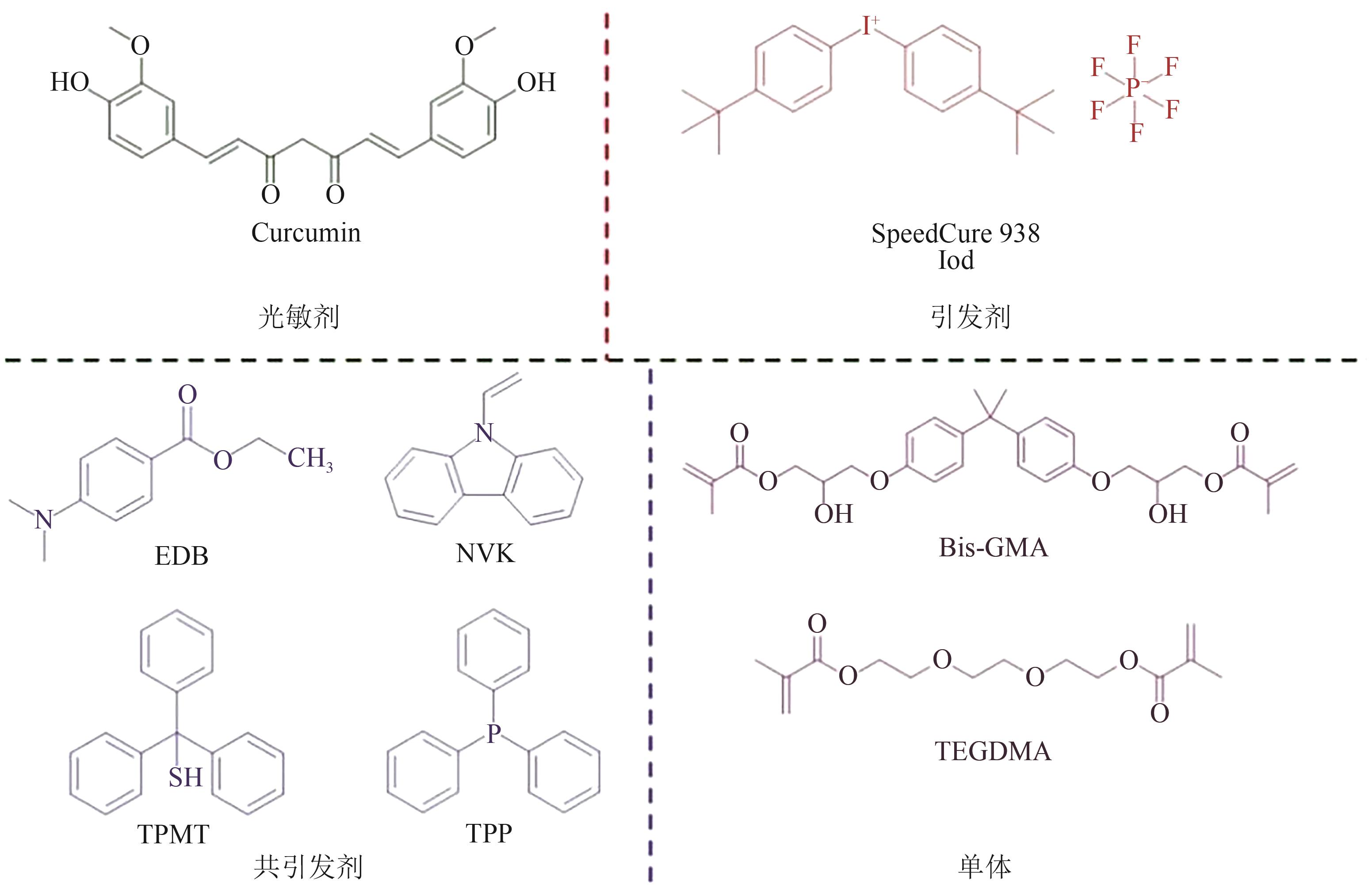
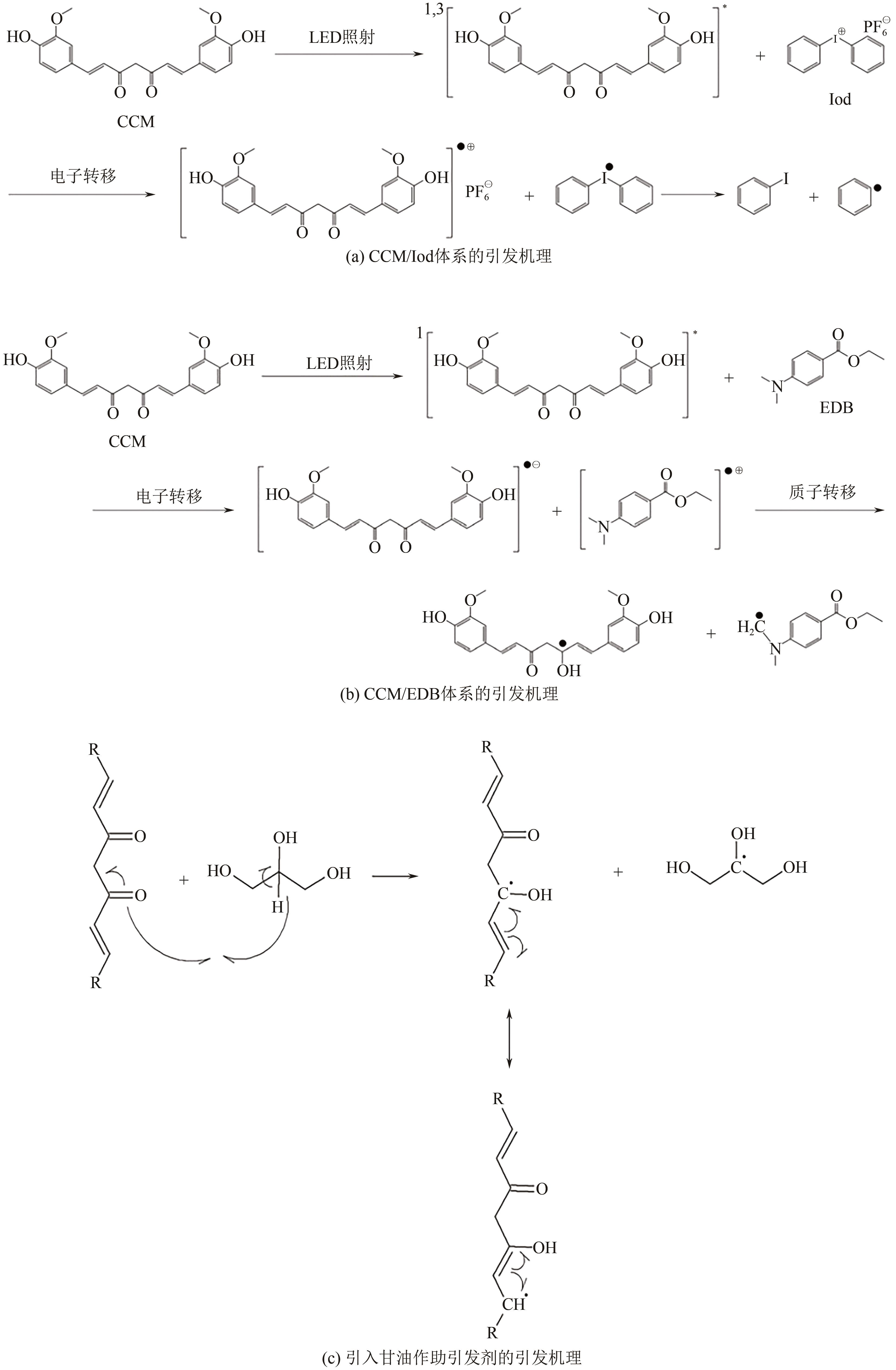
| 姜黄素 | 200~230, 400~450 | LED@(392,455,518,594,636)nm,白LED | Iod,EDB/NVK/ TPMT/TPP | bis-GMA/ TEGDMA | [ | ||
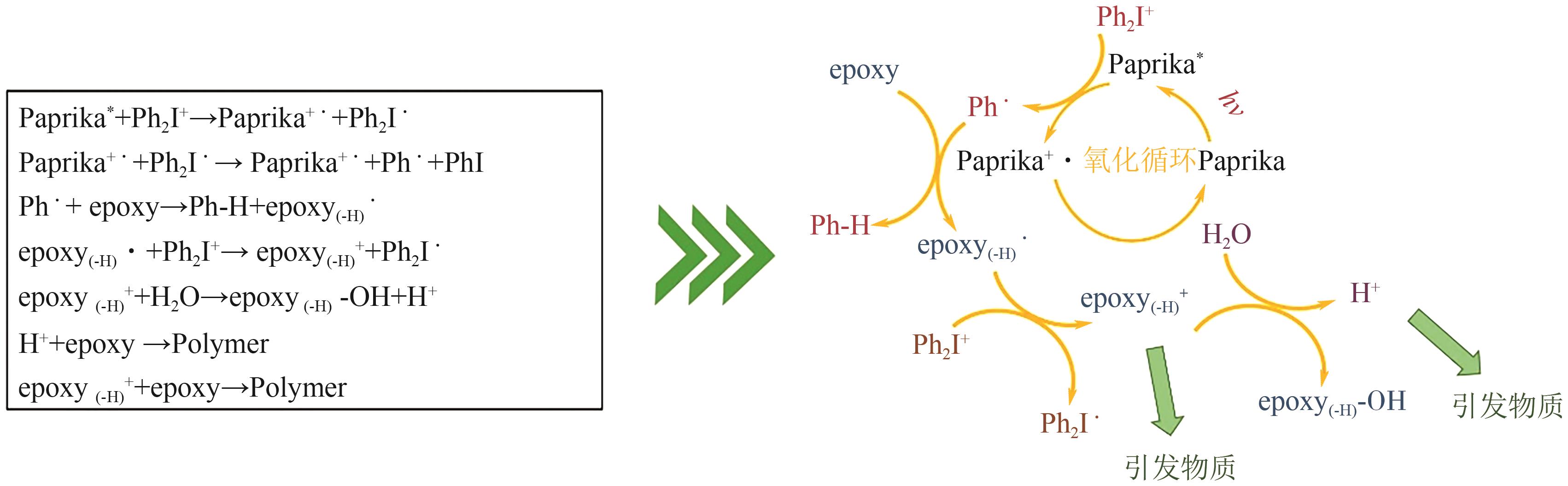
| 阳离子 聚合 | 自由基促 阳离子 | 辣椒粉 | 300~550 | 氙灯 | Iod | epoxidized gallic acid | [ |
| 光敏阳离子引发 | 姜黄素 | 250~550 | LED@(392,455,518,594,636)nm,白LED | ONI/NVK | bis-GMA/ TEGDMA | [ | |
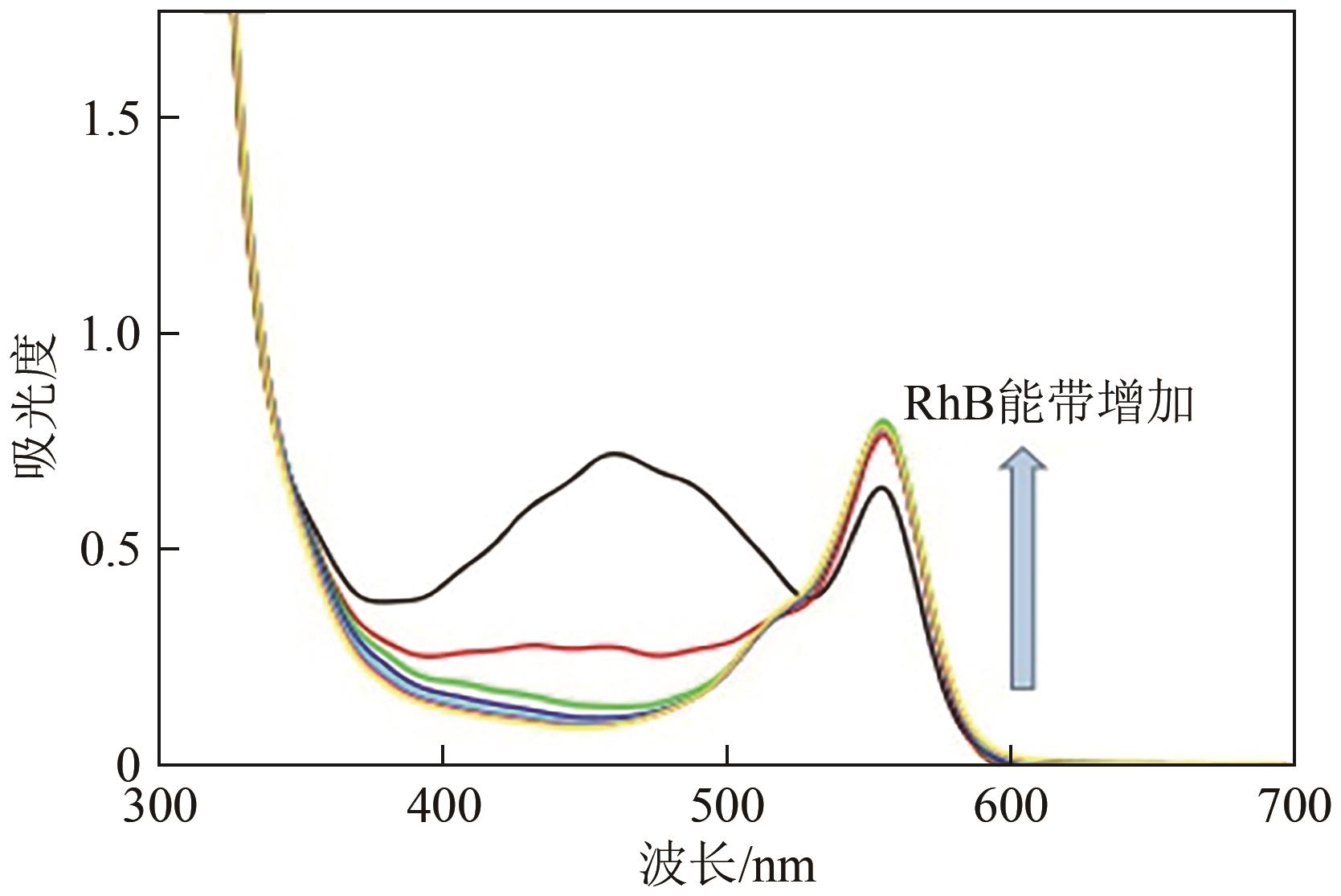
| β-胡萝卜素 | 400~500 | LED@405nm | Iod,TT | EPOX/Lim/DPDO | [ |
表1 基于天然化合物的光引发体系
| 聚合类型 | 引发机理 | 使用的天然化合物 | 吸收波长范围/nm | 辐照设备 | 添加剂 | 引发单体类型 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 自由基 聚合 | Norrish Ⅰ型 (裂解型) | 香兰素 | 300~350 | Hg-Xe灯 | TMPTA/SOA | [ | |
| 丁香醛 | 300~385 | LED@385nm | TMPTA | [ | |||
| LED@400nm | |||||||
| 香豆素 | 400~480, 500~550 | LED@450nm | TMPTA/TAIC/TAC/APE | [ | |||
| 香豆素 | 280~480 | LED@375nm, LED@405nm | Iod,NPG/EDB | TMPTA | [ | ||
| Norrish Ⅱ型(夺氢型) | 叶绿酸a | 430~450, 640~660 | LED@430nm, LED@640nm | EBiB/ FeCl3·6H2O | MMA | [ | |
| 焦脱镁叶绿酸a | 420~427, 500~750 | LED@405nm, LED@455nm, LED@625nm, LED@660nm | Iod,MDEA/ BDMA/DMA/Ribo/ Cys/Argi | PEGDA | [ | ||
| 芝麻素 | 250~300 | UV点光源 | BP,CBP,OMBB | HDDA/TPGDA/ TMPTA/TMPTMA | [ | ||
| 姜黄素 | 200~230, 400~450 | LED@(392,455,518,594,636)nm,白LED | Iod,EDB/NVK/ TPMT/TPP | bis-GMA/ TEGDMA | [ | ||
| 阳离子 聚合 | 自由基促 阳离子 | 辣椒粉 | 300~550 | 氙灯 | Iod | epoxidized gallic acid | [ |
| 光敏阳离子引发 | 姜黄素 | 250~550 | LED@(392,455,518,594,636)nm,白LED | ONI/NVK | bis-GMA/ TEGDMA | [ | |
| β-胡萝卜素 | 400~500 | LED@405nm | Iod,TT | EPOX/Lim/DPDO | [ |
| 1 | 曾国屏, 王刚, 张军, 等. UV LED固化水性UV涂料的研究进展[J]. 涂层与防护, 2019, 40(3): 24-30. |
| ZENG Guoping, WANG Gang, ZHANG Jun, et al. Research progress in UV LED curing waterborne UV coatings[J]. Coating and Protection, 2019, 40(3): 24-30. | |
| 2 | 徐杨, 刘毅, 苏晓磊. UV光固化阻焊油墨的制备及其性能[J]. 西安工程大学学报, 2020, 34(4): 92-98. |
| XU Yang, LIU Yi, SU Xiaolei. Preparation and properties of UV curing solder resist ink[J]. Journal of Xi’an Polytechnic University, 2020, 34(4): 92-98. | |
| 3 | 陈康, 李亚儒. 紫外光固化胶粘剂研究进展[J]. 山东化工, 2018, 47(13): 46-47, 49. |
| CHEN Kang, LI Yaru. The research progress of ultraviolet curing adhesive[J]. Shandong Chemical Industry, 2018, 47(13): 46-47, 49. | |
| 4 | 马春雨, 陆伟星, 潘梦雅, 等. 紫外光固化涂料研究进展及其在钢板表面的应用[J]. 材料保护, 2020, 53(9): 100-106. |
| MA Chunyu, LU Weixing, PAN Mengya, et al. Research progress of UV curing coatings and its application on steel sheet surface[J]. Materials Protection, 2020, 53(9): 100-106. | |
| 5 | 陈健, 吴国民, 霍淑平, 等. 腰果酚光固化材料的研究进展[J]. 林产化学与工业, 2018, 38(6): 1-10. |
| CHEN Jian, WU Guomin, HUO Shuping, et al. Research progress of cardanol based UV-cured materials[J]. Chemistry and Industry of Forest Products, 2018, 38(6): 1-10. | |
| 6 | 何明俊, 胡孝勇, 柯勇. UV固化聚氨酯丙烯酸酯涂料的研究进展[J]. 中国胶粘剂, 2017, 26(10): 49-53. |
| HE Mingjun, HU Xiaoyong, KE Yong. Research progress of UV-curable polyurethane acrylate coating[J]. China Adhesives, 2017, 26(10): 49-53. | |
| 7 | 朱光达, 侯仪, 赵宁, 等. 光固化3D打印聚合物材料的研究进展[J]. 中国材料进展, 2022, 41(1): 68-80, 67. |
| ZHU Guangda, HOU Yi, ZHAO Ning, et al. Progress on photo-curing 3D printing polymer materials[J]. Materials China, 2022, 41(1): 68-80, 67. | |
| 8 | 胡传奇. 碳化硅陶瓷光固化增材制造工艺研究[D]. 北京: 中国建筑材料科学研究总院, 2021. |
| HU Chuanqi. Study on the additive manufacturing technology of silicon carbide ceramics based on UV curing[D]. Beijing: China Building Materials Research Institute, 2021. | |
| 9 | 李琪, 黄羿, 钱滨, 等. 橙黄光玻璃陶瓷的光固化成型与无压烧结[J]. 无机材料学报, 2022, 37(3): 289-296. |
| LI Qi, HUANG Yi, QIAN Bin, et al. Photo curing and pressureless sintering of orange-emitting glass-ceramics[J]. Journal of Inorganic Materials, 2022, 37(3): 289-296. | |
| 10 | 古孝雪, 于晶, 杨明英, 等. 丝素蛋白3D打印在生物医学领域中的应用[J]. 化学进展, 2022, 34(6): 1359-1368. |
| GU Xiaoxue, YU Jing, YANG Mingying, et al. Silk fibroin-based 3D printing strategies for biomedical applications[J]. Progress in Chemistry, 2022, 34(6): 1359-1368. | |
| 11 | 余刘洋, 李丹杰, 夏培斌, 等. 陶瓷光固化3D打印技术研究进展及应用[J]. 橡塑技术与装备, 2022, 48(1): 5-9. |
| YU Liuyang, LI Danjie, XIA Peibin, et al. Research progress and application of ceramic light-curing 3D printing technology[J]. China Rubber/Plastics Technology and Equipment, 2022, 48(1): 5-9. | |
| 12 | RHODES M C, BUCHER J R, PECKHAM J C, et al. Carcinogenesis studies of benzophenone in rats and mice[J]. Food and Chemical Toxicology, 2007, 45(5): 843-851. |
| 13 | DI GIANNI A, BONGIOVANNI R, PRIOLA A, et al. UV-cured fluorinated coatings for plastics: Effect of the photoinitiator and of the substrate filler on adhesion[J]. International Journal of Adhesion and Adhesives, 2004, 24(6): 513-518. |
| 14 | PEIJNENBURG Ad, Jenneke RIETHOF-POORTMAN, BAYKUS Hakan, et al. AhR-agonistic, anti-androgenic, and anti-estrogenic potencies of 2-isopropylthioxanthone (ITX) as determined by in vitro bioassays and gene expression profiling[J]. Toxicology in Vitro, 2010, 24(6): 1619-1628. |
| 15 | MOMO Federico, FABRIS Sabrina, STEVANATO Roberto. Interaction of isopropylthioxanthone with phospholipid liposomes[J]. Biophysical Chemistry, 2007, 127(1/2): 36-40. |
| 16 | ABRAHAMSE Heidi, HAMBLIN Michael R. New photosensitizers for photodynamic therapy[J]. Biochemical Journal, 2016, 473(4): 347-364. |
| 17 | LI Li, CHEN Yisha, CHEN Weijie, et al. Photodynamic therapy based on organic small molecular fluorescent dyes[J]. Chinese Chemical Letters, 2019, 30(10): 1689-1703. |
| 18 | MAOKA Takashi. Carotenoids as natural functional pigments[J]. Journal of Natural Medicines, 2020, 74(1): 1-16. |
| 19 | PARK Jung Hwa, GATEWOOD Barbara M, RAMASWAMY Gita N. Naturally occurring quinones and flavonoid dyes for wool: Insect feeding deterrents[J]. Journal of Applied Polymer Science, 2005, 98(1): 322-328. |
| 20 | ZVEZDINA S V, BEREZIN M B, BEREZIN B D. Natural dyes based on chlorophyll and protoporphyrin derivatives[J]. Russian Journal of Coordination Chemistry, 2010, 36(9): 711-714. |
| 21 | VIERA Isabel, Antonio PÉREZ-GÁLVEZ, ROCA María. Green natural colorants[J]. Molecules, 2019, 24(1): 154-171. |
| 22 | 唐招贤. 可用于LED光聚合的α-二羰基类光引发剂的合成与性能研究[D]. 北京: 北京化工大学, 2022. |
| TANG Zhaoxian. Synthesis and properties research of α-dicarbonyl-based photoinitiators for LED photolymerization[D].Beijing: Beijing University of Chemical Technology, 2022. | |
| 23 | 周颖, 郝康安, 王少凡, 等. 可见光响应型染料基多功能光引发体系的研究进展[J]. 化工进展, 2023, 42(12): 6438-6451. |
| ZHOU Ying, HAO Kang’an, WANG Shaofan, et al. Research progress of visible-light-responsive dye-based multi-functional photoinitiating systems[J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6438-6451. | |
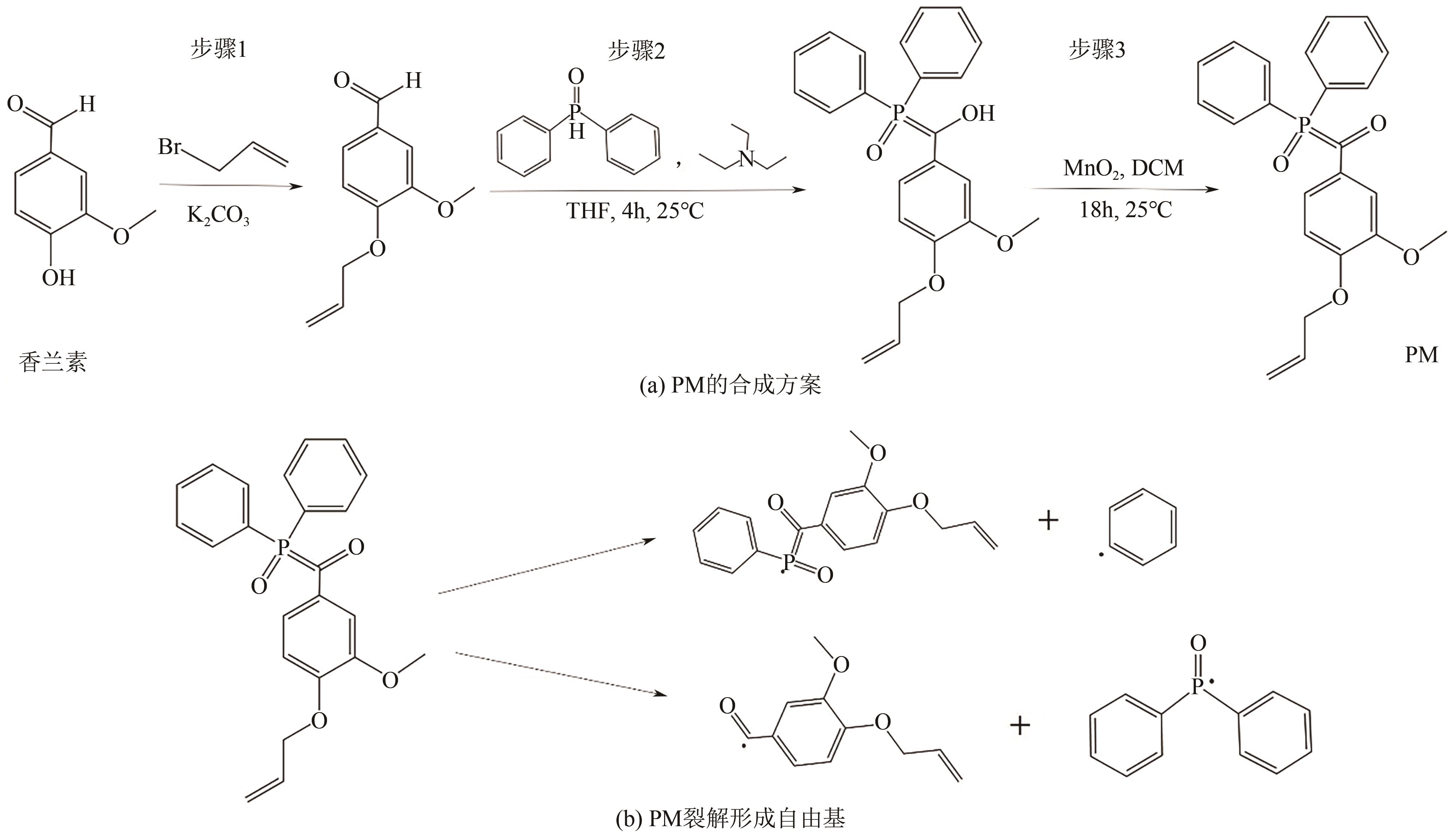
| 24 | BRELOY L, NEGRELL C, A-S MORA, et al. Vanillin derivative as performing type I photoinitiator[J]. European Polymer Journal, 2020, 132(1): 109727. |
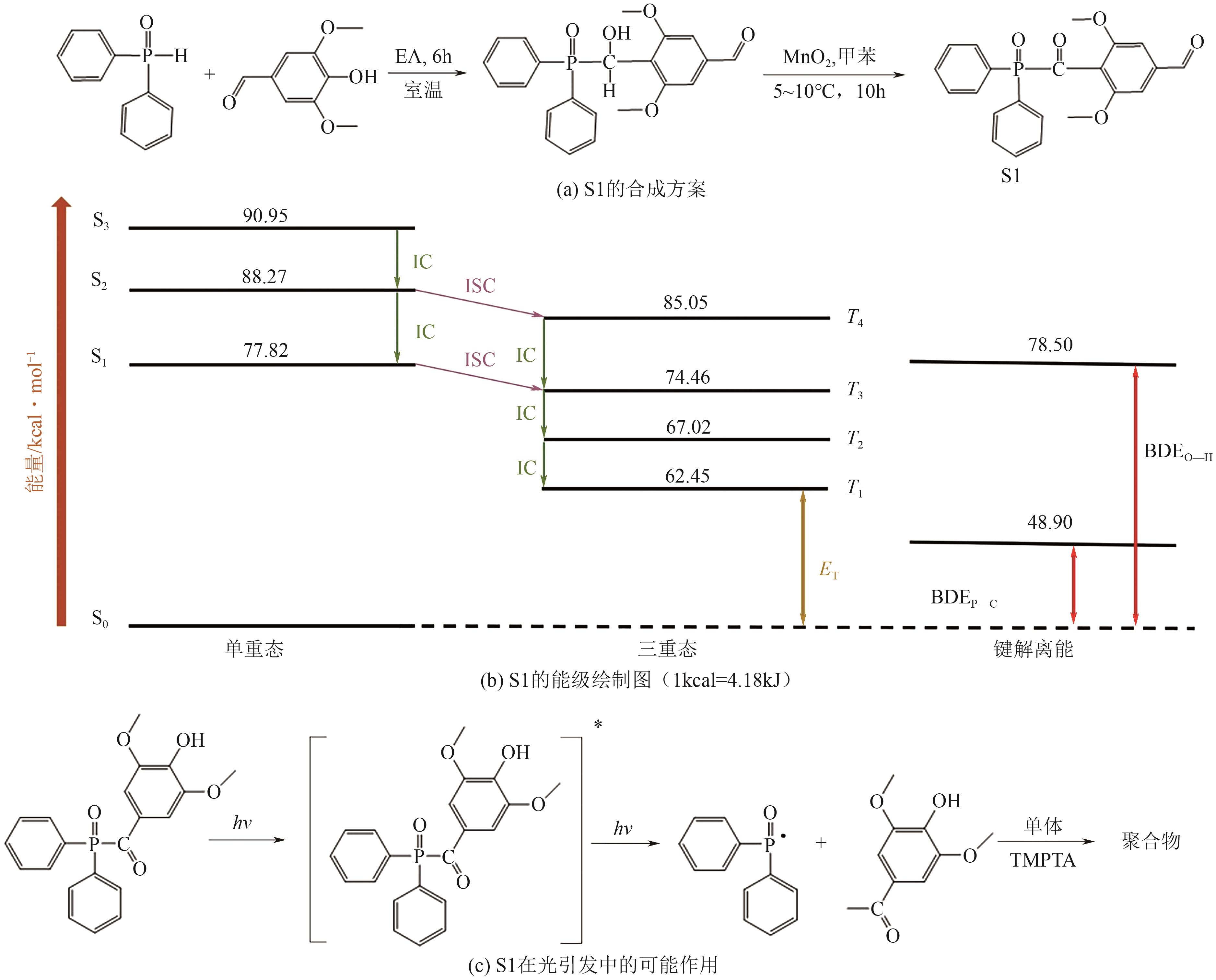
| 25 | LI Qianmin, LI Huimin, WANG Zhen, et al. Bifunctional free radical photoinitiator based on syringaldehyde[J]. Polymers for Advanced Technologies, 2022, 33(5): 1617-1627. |
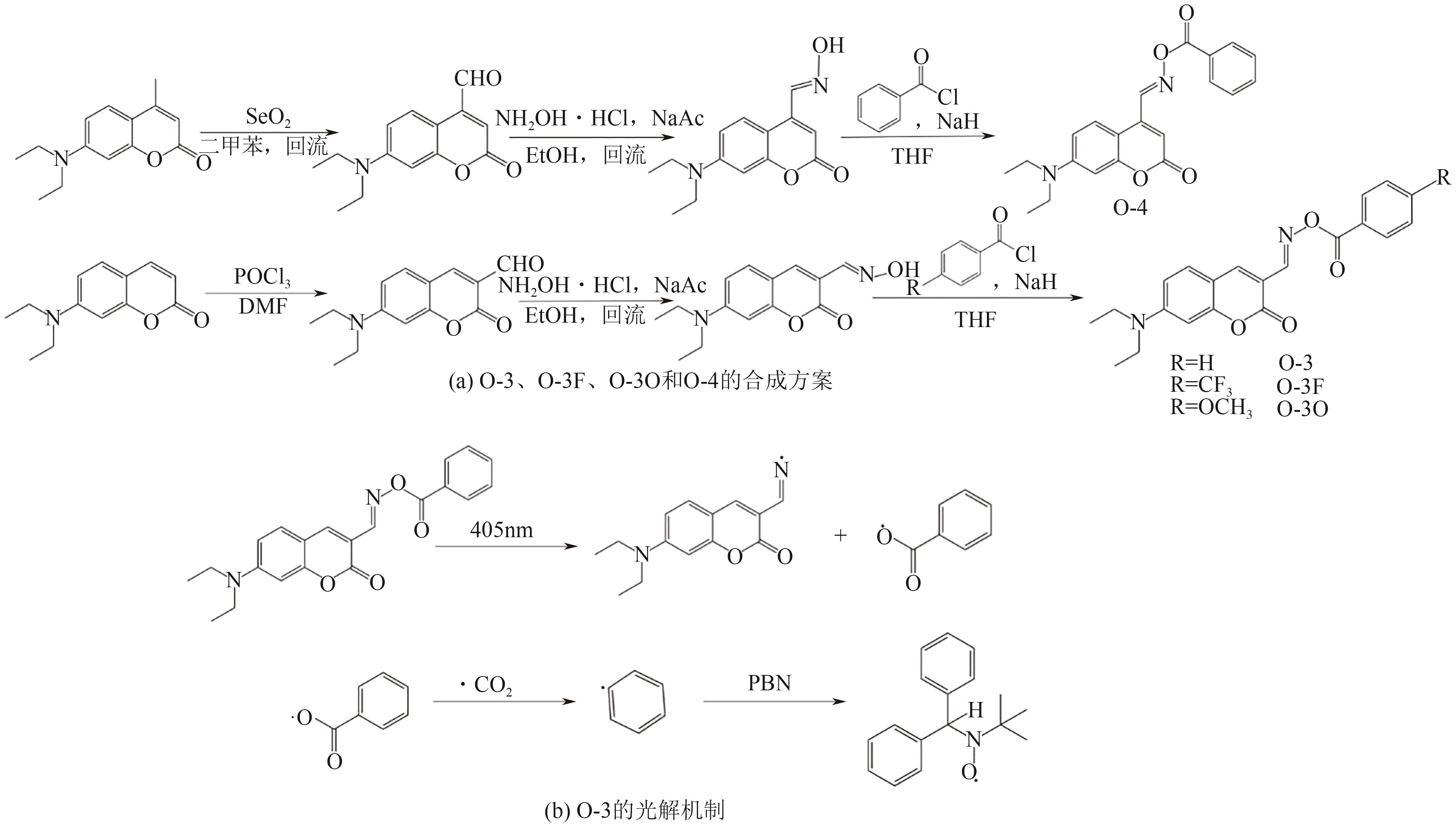
| 26 | LI Zhiquan, ZOU Xiucheng, ZHU Guigang, et al. Coumarin-based oxime esters: Photobleachable and versatile unimolecular initiators for acrylate and thiol-based click photopolymerization under visible light-emitting diode light irradiation[J]. ACS Applied Materials & Interfaces, 2018, 10(18): 16113-16123. |
| 27 | ABDALLAH Mira, DUMUR Frédéric, HIJAZI Akram, et al. Keto-coumarin scaffold for photoinitiators for 3D printing and photocomposites[J]. Journal of Polymer Science, 2020, 58(8): 1115-1129. |
| 28 | WANG Guoxiang, HE Jieyu, LIU Lichao, et al. Photo-induced atom transfer radical polymerization of MMA with chlorophll A as photoinitiator[J]. Journal of Polymer Research, 2016, 23(5): 101. |
| 29 | BRELOY Louise, Vlasta BREZOVÁ, RICHETER Sébastien, et al. Bio-based porphyrins pyropheophorbide a and its Zn-complex as visible-light photosensitizers for free-radical photopolymerization[J]. Polymer Chemistry, 2022, 13(12): 1658-1671. |
| 30 | WANG Kemin, JIANG Shan, YU Qiang. Sesamin as a co-initiator for UV photopolymerization[J]. Polymer Science Series B, 2011, 53(3): 176-180. |
| 31 | ZHAO Jiacheng, Jacques LALEVÉE, LU Hongxu, et al. A new role of curcumin: As a multicolor photoinitiator for polymer fabrication under household UV to red LED bulbs[J]. Polymer Chemistry, 2015, 6(28): 5053-5061. |
| 32 | Pauline SAUTROT-BA, MALVAL Jean-Pierre, Mathilde WEISS-MAURIN, et al. Paprika, Gallic acid, and visible light: The green combination for the synthesis of biocide coatings[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(1): 104-109. |
| 33 | ABDALLAH Mira, HIJAZI Akram, LIN Jui-Teng, et al. Coumarin derivatives as photoinitiators in photo-oxidation and photo-reduction processes and a kinetic model for simulations of the associated polymerization profiles[J]. ACS Applied Polymer Materials, 2020, 2(7): 2769-2780. |
| 34 | BRELOY Louise, OUARABI Célia Ait, BROSSEAU Arnaud, et al. β-carotene/limonene derivatives/eugenol: Green synthesis of antibacterial coatings under visible-light exposure[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(24): 19591-19604. |
| 35 | IBRAHIM Ahmad, STEFANO Luciano, TARZI Olga, et al. High-performance photoinitiating systems for free radical photopolymerization. Application to holographic recording[J]. Photochemistry and Photobiology, 2013, 89(6): 1283-1290. |
| 36 | BANERJEE Goutam, CHATTOPADHYAY Pritam. Vanillin biotechnology: The perspectives and future[J]. Journal of the Science of Food and Agriculture, 2019, 99(2): 499-506. |
| 37 | FACHE Maxence, BOUTEVIN Bernard, CAILLOL Sylvain. Vanillin production from lignin and its use as a renewable chemical[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(1): 35-46. |
| 38 | NAVARUCKIENE Aukse, BRIDZIUVIENE Danguole, RAUDONIENE Vita, et al. Influence of vanillin acrylate-based resin composition on resin photocuring kinetics and antimicrobial properties of the resulting polymers[J]. Materials, 2021, 14(3): 653-673. |
| 39 | LEBEDEVAITE Migle, OSTRAUSKAITE Jolita, SKLIUTAS Edvinas, et al. Photoinitiator free resins composed of plant-derived monomers for the optical µ-3D printing of thermosets[J]. Polymers, 2019, 11(1): 116. |
| 40 | ZHU Jiangyu, TAN Xiaomiao, HAFID Halimatun Saadiah, et al. Enhancement of biomass yield and lipid accumulation of freshwater microalga Euglena gracilis by phenolic compounds from basic structures of lignin[J]. Bioresource Technology, 2021, 321: 124441. |
| 41 | SHAHZAD Sumayya, MATEEN Somaiya, KAUSAR Tasneem, et al. Effect of syringic acid and syringaldehyde on oxidative stress and inflammatory status in peripheral blood mononuclear cells from patients of myocardial infarction[J]. Naunyn-Schmiedeberg’s Archives of Pharmacology, 2020, 393(4): 691-704. |
| 42 | DUMUR Frédéric. Recent advances on coumarin-based photoinitiators of polymerization[J]. European Polymer Journal, 2022, 163: 110962. |
| 43 | MA Xiaoyu, GU Renquan, YU Liujian, et al. Conjugated phenothiazine oxime esters as free radical photoinitiators[J]. Polymer Chemistry, 2017, 8(39): 6134-6142. |
| 44 | GROENENBOOM Cornelis J, HAGEMAN Hendrik J, OOSTERHOFF Pieter, et al. Photoinitiators and photoinitiation Part 11. The photodecomposition of some O-acyl 2-oximinoketones[J]. Journal of Photochemistry and Photobiology A: Chemistry, 1997, 107(1/2/3): 261-269. |
| 45 | MIYAKE Yusuke, TAKAHASHI Hirona, AKAI Nobuyuki, et al. Structure and reactivity of radicals produced by photocleavage of oxime ester compounds studied by time-resolved electron paramagnetic resonance spectroscopy[J]. Chemistry Letters, 2014, 43(8): 1275-1277. |
| 46 | 李雪纯, 孙芳. 自由基型光引发剂简介及研究进展[J]. 大学化学, 2021, 36(6): 5-14. |
| LI Xuechun, SUN Fang. Introduction and progress of free radical photoinitiators[J]. University Chemistry, 2021, 36(6): 5-14. | |
| 47 | SPECHT Dnolad P, MARTIC Peter A, FARID Samir. Ketocoumarins[J]. Tetrahedron, 1982, 38(9): 1203-1211. |
| 48 | WILLIAMS J L R, SPECHT D P, FARID S. Ketocoumarins as photosensitizers and photoinitiators[J]. Polymer Engineering & Science, 1983, 23(18): 1022-1024. |
| 49 | SHANMUGAM Sivaprakash, XU Jiangtao, BOYER Cyrille. Utilizing the electron transfer mechanism of chlorophyll a under light for controlled radical polymerization[J]. Chemical Science, 2015, 6(2): 1341-1349. |
| 50 | HEWLINGS Susan J, KALMAN Douglas S. Curcumin: A review of its effects on human health[J]. Foods, 2017, 6(10): 92. |
| 51 | DE OLIVEIRA Deborah Stolte Bezerra Lisbôa, DE OLIVEIRA Luiza Stolte Bezerra Lisbôa, ALARCON Rafael Turra, et al. Use of curcumin and glycerol as an effective photoinitiating system in the polymerization of urethane dimethacrylate[J]. Journal of Thermal Analysis and Calorimetry, 2017, 128(3): 1671-1682. |
| 52 | ALBRECHT W N, STEPHENSON R L. Health hazards of tertiary amine catalysts[J]. Scandinavian Journal of Work, Environment & Health, 1988, 14(4): 209-219. |
| 53 | ELAD Dov, YOUSSEFYEH Raymond D. The photochemical conversion of acetals to carboxylic esters[J]. Tetrahedron Letters, 1963, 4(30): 2189-2191. |
| 54 | WANG Kemin, YANG Dongzhi, XIAO Ming, et al. Sesamin as a co-initiator for unfilled dental restorations[J]. Acta Biomaterialia, 2009, 5(7): 2508-2517. |
| 55 | Aina RIBAS-MASSONIS, CICUJANO Magalí, DURAN Josep, et al. Free-radical photopolymerization for curing products for refinish coatings market[J]. Polymers, 2022, 14(14): 2856. |
| 56 | 李晖, 蒋洪石, 韩秋萍. 二芳基碘鎓盐的应用研究进展[J]. 运城学院学报, 2008, 26(5): 55-57. |
| LI Hui, JIANG Hongshi, HAN Qiuping. Research progress in application of diaryl iodonium salt[J]. Journal of Yuncheng University, 2008, 26(5): 55-57. | |
| 57 | 钟彦, 王新伟, 王顺花, 等. 鎓盐类阳离子聚合光引发剂的研究进展[J]. 信息记录材料, 2010, 11(2): 28-34. |
| ZHONG Yan, WANG Xinwei, WANG Shunhua, et al. Research progress of onium salt photoinitiators for cationic polymerization[J]. Information Recording Materials, 2010, 11(2): 28-34. | |
| 58 | CRIVELLO James V, RAJARAMAN Surésh, MOWERS William A, et al. Free radical accelerated cationic polymerizations[J]. Macromolecular Symposia, 2000, 157(1): 109-120. |
| 59 | PODEMSKA Karolina, Radosław PODSIADŁY, SZYMCZAK Agnieszka Marzena, et al. Diazobenzo[a]fluorene derivatives as visible photosensitizers for cationic polymerization[J]. Dyes and Pigments, 2012, 95(1): 74-78. |
| 60 | RIBEIRO Bernardo Dias, BARRETO Daniel Weingart, COELHO Maria Alice Zarur. Technological aspects of β-carotene production[J]. Food and Bioprocess Technology, 2011, 4(5): 693-701. |
| 61 | CRIVELLO James V, BULUT Umut. Curcumin: A naturally occurring long-wavelength photosensitizer for diaryliodonium salts[J]. Journal of Polymer Science A: Polymer Chemistry, 2005, 43(21): 5217-5231. |
| 62 | HAN Weixiang, FU Hongyuan, XUE Tanlong, et al. Facilely prepared blue-green light sensitive curcuminoids with excellent bleaching properties as high performance photosensitizers in cationic and free radical photopolymerization[J]. Polymer Chemistry, 2018, 9(14): 1787-1798. |
| [1] | 周颖, 郝康安, 王少凡, 黄安荣, 吴翀, 左晓玲. 可见光响应型染料基多功能光引发体系的研究进展[J]. 化工进展, 2023, 42(12): 6438-6451. |
| [2] | 陈彦彤, 李旭东, 陶冶, 黄红缨, 叶招莲, 盖鑫磊. 有机激发三重态参与的光化学反应研究进展[J]. 化工进展, 2020, 39(8): 3344-3353. |
| [3] | 李大刚, 陈崇城, 陈晓玲, 李云龙, 黄茂坤. 紫外激发丙酮-乙二醇引发制备离子型聚丙烯酰胺及其絮凝性能[J]. 化工进展, 2017, 36(02): 689-694. |
| [4] | 张香平, 白银鸽, 闫瑞一, 高红帅. 离子液体萃取分离有机物研究进展[J]. 化工进展, 2016, 35(06): 1587-1605. |
| [5] | 李大刚1,袁淑芳1,李云龙1,林松柏1, 2. 紫外激发丙酮-乙二醇引发丙烯酰胺聚合[J]. 化工进展, 2012, 31(11): 2553-2557. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||