Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (2): 730-739.DOI: 10.16085/j.issn.1000-6613.2021-0489
• Industrial catalysis • Previous Articles Next Articles
Research progress of peroxymonosulfate activated by solid-phase cobalt-based catalyst in water treatment
XU Zetao( ), CAO Yiting, WANG Qiao(
), CAO Yiting, WANG Qiao( ), WANG Zhihong
), WANG Zhihong
- School of Civil and Traffic Engineering,Guangdong University of Technology, Guangzhou 510006, Guangdong, China
-
Received:2021-03-11Revised:2021-04-20Online:2022-02-23Published:2022-02-05 -
Contact:WANG Qiao
固相钴基催化剂活化过一硫酸盐在水处理中的研究进展
- 广东工业大学土木与交通工程学院,广东 广州 510006
-
通讯作者:王俏 -
作者简介:许泽涛(1996—),男,硕士研究生,研究方向为水污染处理技术。E-mail:2111909014@mail2.gdut.edu.cn 。 -
基金资助:国家自然科学基金青年基金(22006200);广东工业大学青年百人A启动项目(220413320)
CLC Number:
Cite this article
XU Zetao, CAO Yiting, WANG Qiao, WANG Zhihong. Research progress of peroxymonosulfate activated by solid-phase cobalt-based catalyst in water treatment[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 730-739.
许泽涛, 曹怡婷, 王俏, 王志红. 固相钴基催化剂活化过一硫酸盐在水处理中的研究进展[J]. 化工进展, 2022, 41(2): 730-739.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2021-0489
| 污染物 | 污染物浓度 | PMS浓度 | 催化剂 | 浓度/g·L-1 | pH | 温度/℃ | 时间/min | 去除率/% | 动力学常数/min-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 罗丹明B | 40mg/L | 0.1mol/L | Co-HAP-2 | 0.2 | 5.5 | 25 | 12 | 93.30 | 0.0332 | [ |
| 罗丹明B | 10mg/L | 0.05mg/L | Co3O4-Bi2O3 | 0.05 | 6 | 25 | 30 | 100 | — | [ |
| 亮红3BF | 0.1mmol/L | 0.4mmol/L | AC-CuCo2O4 | 0.2 | 10 | 25 | 10 | 98 | 0.476 | [ |
| 双酚A | 20mg/L | 0.3mmol/L | CoS | 0.05 | 7 | 25 | 10 | 90 | 0.37 | [ |
| 双酚A | 20mg/L | 0.3g/L | Co@NC-ZS-700 | 0.1 | 6.2 | 25 | 20 | 95 | — | [ |
| 双酚A | 80mg/L | 0.3g/L | Co-N-C-900 | 0.5 | — | 30 | 3 | 100 | 2.81 | [ |
| 2,4-二氯苯氧基乙酸 | 20mg/L | 1mmol/L | CuO-Co3O4@CeO2 | 0.07 | 6 | 25 | 45 | 97.50 | 0.1344 | [ |
| 2,4-二氯苯酚 | 50mg/L | 6mmol/L | CoOOH | 0.20 | 7 | 25 | 120 | 100 | 0.0462 | [ |
| 2,4-二氯苯酚 | 50mg/L | 1.26g/L | Co3Fe7-CoFe2O4 | 0.05 | 7.7 | 30 | 30 | 97.10 | 0.119 | [ |
| 阿特拉津 | 10μmol/L | 1mmol/L | CoBC500 | 0.1 | 5.3 | 25 | 10 | 99 | 0.76 | [ |
| 阿特拉津 | 23μmol/L | 0.3mmol/L | 3Co@Ⅰ | 0.2 | 7 | 25 | 30 | 96 | 0.1034 | [ |
| 二氢呋喃 | 10mg/L | 0.65mmol/L | Co-S@NC | 0.1 | 4.8 | 25 | 90 | 100.00 | 0.054 | [ |
| 喹克洛拉克 | 50mg/L | 20mmol/L | Co/NAC | 0.08 | — | 25 | 30 | 93 | 0.0022 | [ |
| 氯霉素 | 30mg/L | 10mmol/L | Co3O4-BC | 0.2 | 7 | 26 | 10 | 100 | 0.3361 | [ |
| 环丙沙星 | 10mg/L | 1.62mmol/L | Co-Fe/SiO2LC | 0.2 | 7 | 25 | 60 | 99.60 | 0.686 | [ |
| 环丙沙星 | 10mg/L | 1.3mmol/L | CoS2(HNSs) | 0.08 | 8 | 25 | 3 | 100.00 | 0.1209 | [ |
| 萘普生 | 0.043mmol/L | 2.5mmol/L | CoCNx/SBA-15 | 0.0375 | 6.4 | 25 | 55 | 100 | 0.0877 | [ |
| 5-磺基水杨酸 | 20mg/L | 150mg/L | CoTS | 0.1 | 7 | 30 | 60 | 100 | 0.0784 | [ |
| 四环素 | 30mg/L | 0.4g/L | ALCo-LDH | 0.2 | — | 25 | 30 | 96.10 | 0.980 | [ |
| 四环素 | 30mg/L | 0.3g/L | CoSx | 0.2 | 5 | 25 | 30 | 100 | 0.151 | [ |
| 三氯生 | 10mg/L | 0.05g/L | Co2Mn1O4 | 0.02 | 6.8 | 25 | 30 | 96.40 | 0.112 | [ |
| 污染物 | 污染物浓度 | PMS浓度 | 催化剂 | 浓度/g·L-1 | pH | 温度/℃ | 时间/min | 去除率/% | 动力学常数/min-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 罗丹明B | 40mg/L | 0.1mol/L | Co-HAP-2 | 0.2 | 5.5 | 25 | 12 | 93.30 | 0.0332 | [ |
| 罗丹明B | 10mg/L | 0.05mg/L | Co3O4-Bi2O3 | 0.05 | 6 | 25 | 30 | 100 | — | [ |
| 亮红3BF | 0.1mmol/L | 0.4mmol/L | AC-CuCo2O4 | 0.2 | 10 | 25 | 10 | 98 | 0.476 | [ |
| 双酚A | 20mg/L | 0.3mmol/L | CoS | 0.05 | 7 | 25 | 10 | 90 | 0.37 | [ |
| 双酚A | 20mg/L | 0.3g/L | Co@NC-ZS-700 | 0.1 | 6.2 | 25 | 20 | 95 | — | [ |
| 双酚A | 80mg/L | 0.3g/L | Co-N-C-900 | 0.5 | — | 30 | 3 | 100 | 2.81 | [ |
| 2,4-二氯苯氧基乙酸 | 20mg/L | 1mmol/L | CuO-Co3O4@CeO2 | 0.07 | 6 | 25 | 45 | 97.50 | 0.1344 | [ |
| 2,4-二氯苯酚 | 50mg/L | 6mmol/L | CoOOH | 0.20 | 7 | 25 | 120 | 100 | 0.0462 | [ |
| 2,4-二氯苯酚 | 50mg/L | 1.26g/L | Co3Fe7-CoFe2O4 | 0.05 | 7.7 | 30 | 30 | 97.10 | 0.119 | [ |
| 阿特拉津 | 10μmol/L | 1mmol/L | CoBC500 | 0.1 | 5.3 | 25 | 10 | 99 | 0.76 | [ |
| 阿特拉津 | 23μmol/L | 0.3mmol/L | 3Co@Ⅰ | 0.2 | 7 | 25 | 30 | 96 | 0.1034 | [ |
| 二氢呋喃 | 10mg/L | 0.65mmol/L | Co-S@NC | 0.1 | 4.8 | 25 | 90 | 100.00 | 0.054 | [ |
| 喹克洛拉克 | 50mg/L | 20mmol/L | Co/NAC | 0.08 | — | 25 | 30 | 93 | 0.0022 | [ |
| 氯霉素 | 30mg/L | 10mmol/L | Co3O4-BC | 0.2 | 7 | 26 | 10 | 100 | 0.3361 | [ |
| 环丙沙星 | 10mg/L | 1.62mmol/L | Co-Fe/SiO2LC | 0.2 | 7 | 25 | 60 | 99.60 | 0.686 | [ |
| 环丙沙星 | 10mg/L | 1.3mmol/L | CoS2(HNSs) | 0.08 | 8 | 25 | 3 | 100.00 | 0.1209 | [ |
| 萘普生 | 0.043mmol/L | 2.5mmol/L | CoCNx/SBA-15 | 0.0375 | 6.4 | 25 | 55 | 100 | 0.0877 | [ |
| 5-磺基水杨酸 | 20mg/L | 150mg/L | CoTS | 0.1 | 7 | 30 | 60 | 100 | 0.0784 | [ |
| 四环素 | 30mg/L | 0.4g/L | ALCo-LDH | 0.2 | — | 25 | 30 | 96.10 | 0.980 | [ |
| 四环素 | 30mg/L | 0.3g/L | CoSx | 0.2 | 5 | 25 | 30 | 100 | 0.151 | [ |
| 三氯生 | 10mg/L | 0.05g/L | Co2Mn1O4 | 0.02 | 6.8 | 25 | 30 | 96.40 | 0.112 | [ |
| 1 | ZHAO Q, LU D, JIANG H, et al. Peroxymonosulfate-based cleaning technology for metal oxide-coated ceramic ultrafiltration membrane polluted by Alcian Blue 8GX dye: radical and non-radical oxidation cleaning mechanism[J]. Journal of Membrane Science, 2019, 573: 210-217. |
| 2 | XU H, WANG D, MA J, et al. A superior active and stable spinel sulfide for catalytic peroxymonosulfate oxidation of bisphenol S[J]. Applied Catalysis B: Environmental, 2018, 238: 557-567. |
| 3 | CHEN Z, BI S, ZHAO G, et al. Enhanced degradation of triclosan by cobalt manganese spinel-type oxide activated peroxymonosulfate oxidation process via sulfate radicals and singlet oxygen: mechanisms and intermediates identification[J]. Science of the Total Environment, 2020, 711: 134715. |
| 4 | ZHANG Q, HE D, LI X, et al. Mechanism and performance of singlet oxygen dominated peroxymonosulfate activation on CoOOH nanoparticles for 2,4-dichlorophenol degradation in water[J]. Journal of Hazardous Materials, 2020, 384: 121350. |
| 5 | CAO J, SUN S, LI X, et al. Efficient charge transfer in aluminum-cobalt layered double hydroxide derived from Co-ZIF for enhanced catalytic degradation of tetracycline through peroxymonosulfate activation[J]. Chemical Engineering Journal, 2020, 382: 122802. |
| 6 | WANG Y X, ZHOU L, DUAN X G, et al. Photochemical degradation of phenol solutions on Co3O4 nanorods with sulfate radicals[J]. Catalysis Today, 2015, 258: 576-584. |
| 7 | CAI H, LI J, YIN H, et al. Degradation of atrazine in aqueous solution through peroxymonosulfate activated by Co-modified nano-titanium dioxide[J]. Water Environment Research, 2020, 92 (9): 1363-1375. |
| 8 | DENG J, FENG S, ZHANG K, et al. Heterogeneous activation of peroxymonosulfate using ordered mesoporous Co3O4 for the degradation of chloramphenicol at neutral pH[J]. Chemical Engineering Journal, 2017, 308: 505-515. |
| 9 | ZHU C, ZHANG Y, FAN Z, et al. Carbonate-enhanced catalytic activity and stability of Co3O4 nanowires for 1O2-driven bisphenol A degradation via peroxymonosulfate activation: critical roles of electron and proton acceptors[J]. Journal of Hazardous Materials, 2020, 393: 122395. |
| 10 | XIE M, TANG J, FANG G, et al. Biomass Schiff base polymer-derived N-doped porous carbon embedded with CoO nanodots for adsorption and catalytic degradation of chlorophenol by peroxymonosulfate[J]. Journal of Hazardous Materials, 2020, 384: 121345. |
| 11 | WEI J, FENG Y, LIU Y, et al. MxCo3-xO4(M = Co, Mn, Fe) porous nanocages derived from metal–organic frameworks as efficient water oxidation catalysts[J]. Journal of Materials Chemistry A, 2015, 3 (44): 22300-22310. |
| 12 | YANG Q, CHOI H, AL-ABED S R, et al. Iron–cobalt mixed oxide nanocatalysts: heterogeneous peroxymonosulfate activation, cobalt leaching, and ferromagnetic properties for environmental applications[J]. Applied Catalysis B: Environmental, 2009, 88 (3/4): 462-469. |
| 13 | YAO Y, CAI Y, WU G, et al. Sulfate radicals induced from peroxymonosulfate by cobalt manganese oxides (CoxMn3-xO4) for Fenton-Like reaction in water[J]. Journal of Hazardous Materials, 2015, 296: 128-137. |
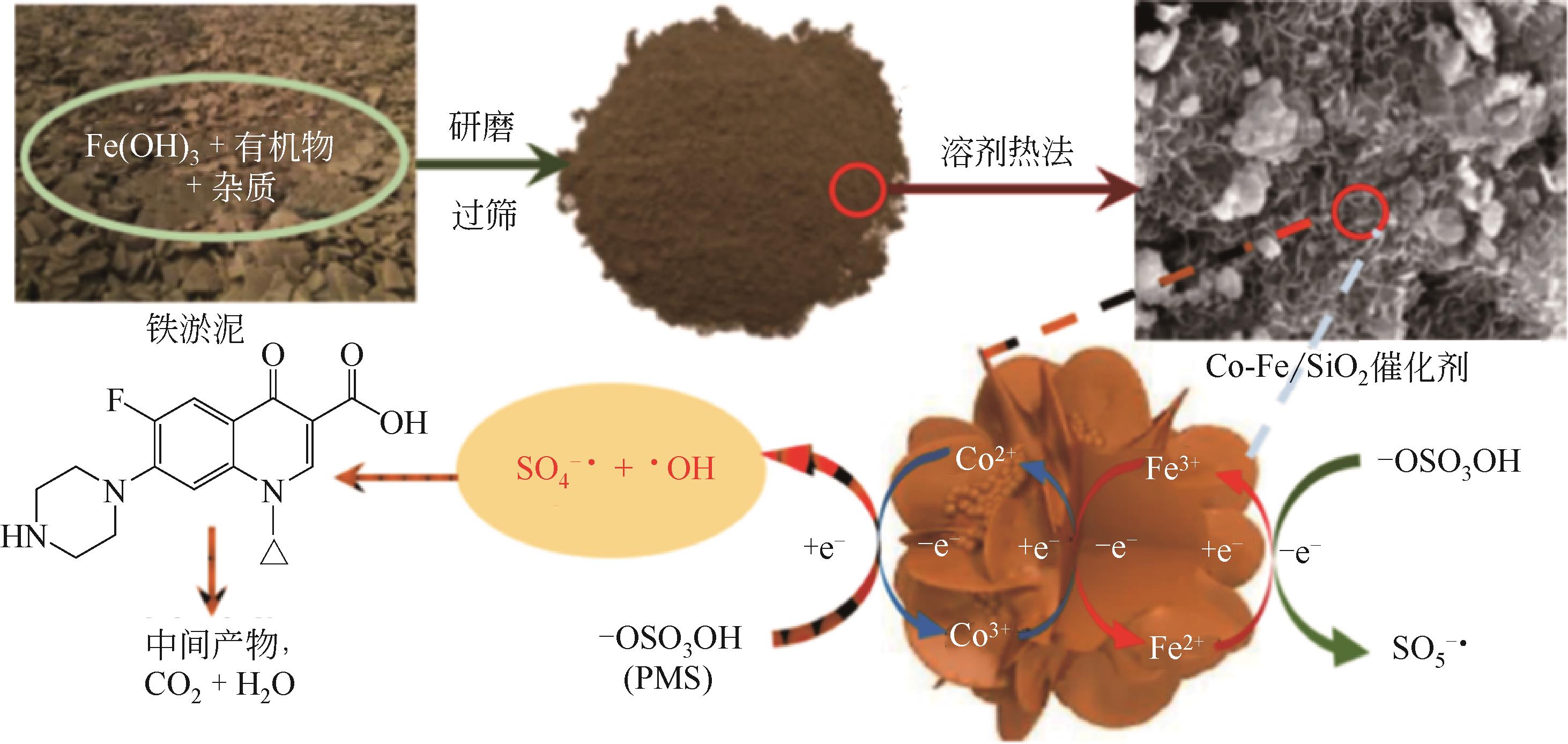
| 14 | ZHU S, XU Y, ZHU Z, et al. Activation of peroxymonosulfate by magnetic Co-Fe/SiO2 layered catalyst derived from iron sludge for ciprofloxacin degradation[J]. Chemical Engineering Journal, 2020, 384: 123298. |
| 15 | LI W, LI Y, ZHANG D, et al. CuO-Co3O4@CeO2 as a heterogeneous catalyst for efficient degradation of 2,4-dichlorophenoxyacetic acid by peroxymonosulfate[J]. Journal of Hazardous Materials, 2020, 381: 121209. |
| 16 | XU H, ZHANG Y, LI J, et al. Heterogeneous activation of peroxymonosulfate by a biochar-supported Co3O4 composite for efficient degradation of chloramphenicols[J]. Environmental Pollution, 2020, 257: 113610. |
| 17 | LIU B, GUO W, WANG H, et al. Activation of peroxymonosulfate by cobalt-impregnated biochar for atrazine degradation: the pivotal roles of persistent free radicals and ecotoxicity assessment[J]. Journal of Hazardous Materials, 2020, 398: 122768. |
| 18 | CHEN S, LIU X, GAO S, et al. CuCo2O4 supported on activated carbon as a novel heterogeneous catalyst with enhanced peroxymonosulfate activity for efficient removal of organic pollutants[J]. Environmental Research, 2020, 183: 109245. |
| 19 | 杨世迎, 张翱, 任腾飞, 等. 炭基材料催化过氧化物降解水中有机污染物:表面作用机制[J]. 化学进展, 2017, 29 (5): 539-552. |
| YANG Shiying, ZHANG Ao, REN Tengfei, et al. Surface mechanism of carbon-based materials for catalyzing peroxide degradation of organic pollutants in water[J]. Progress in Chemistry, 2017, 29 (5): 539-552. | |
| 20 | ZHOU N, ZU J, YANG L, et al. Cobalt (0/Ⅱ) incorporated N-doped porous carbon as effective heterogeneous peroxymonosulfate catalyst for quinclorac degradation[J]. Journal of Colloid and Interface Science, 2020, 563: 197-206. |
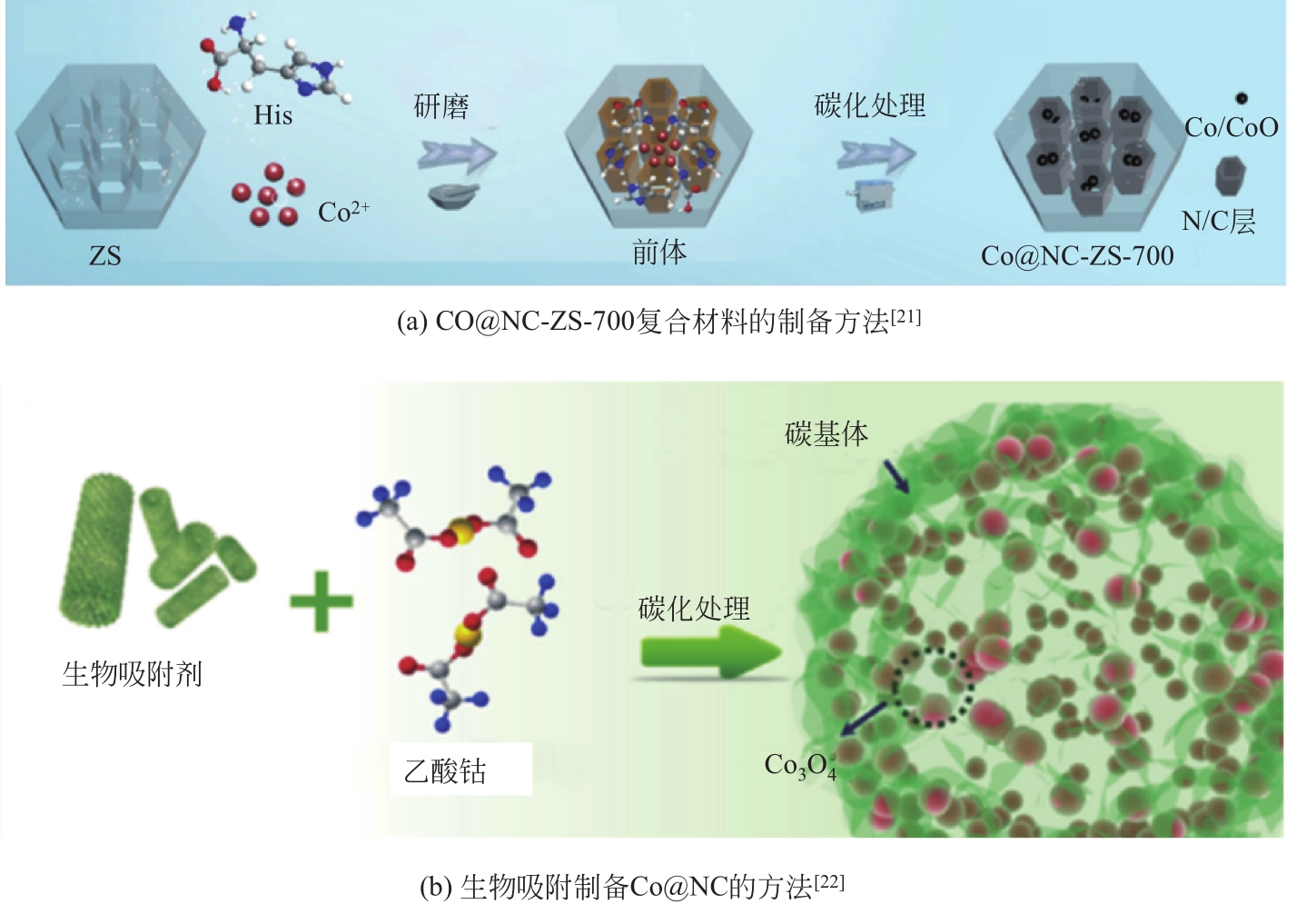
| 21 | LUO J, BO S, AN Q, et al. Designing ordered composites with confined Co-N/C layers for efficient pollutant degradation: structure-dependent performance and PMS activation mechanism[J]. Microporous and Mesoporous Materials, 2020, 293: 109810. |
| 22 | DU W, ZHANG Q, SHANG Y, et al. Sulfate saturated biosorbent-derived Co-S@NC nanoarchitecture as an efficient catalyst for peroxymonosulfate activation[J]. Applied Catalysis B: Environmental, 2020, 262: 118302. |
| 23 | 赵朝成, 吴光锐. MOFs复合材料催化降解水中有机污染物的应用研究进展[J]. 化工进展, 2019, 38 (4): 1775-1784. |
| ZHAO Chaocheng, WU Guangrui. Research progress on the mechanism and applications of MOFs composite materials for catalytic degradation of organic pollutants in the solution[J]. Chemical Industry and Engineering Progress, 2019, 38(4): 1775-1784. | |
| 24 | ZENG T, ZHANG X, WANG S, et al. Spatial confinement of a Co3O4 catalyst in hollow metal-organic frameworks as a nanoreactor for improved degradation of organic pollutants[J]. Environmental Science & Technology, 2015, 49 (4): 2350-2357. |
| 25 | LI Z, TANG X, HUANG G, et al. Bismuth MOFs based hierarchical Co3O4-Bi2O3 composite: an efficient heterogeneous peroxymonosulfate activator for azo dyes degradation[J]. Separation and Purification Technology, 2020, 242: 116825. |
| 26 | WU X, ZHAO W, HUANG Y, et al. A mechanistic study of amorphous CoSx cages as advanced oxidation catalysts for excellent peroxymonosulfate activation towards antibiotics degradation[J]. Chemical Engineering Journal, 2020, 381: 122768. |
| 27 | PANG Y, KONG L, CHEN D, et al. Facilely synthesized cobalt doped hydroxyapatite as hydroxyl promoted peroxymonosulfate activator for degradation of Rhodamine B[J]. Journal of Hazardous Materials, 2020, 384: 121447. |
| 28 | DING Y, HU Y, PENG X, et al. Micro-nano structured CoS: an efficient catalyst for peroxymonosulfate activation for removal of bisphenol A[J]. Separation and Purification Technology, 2020, 233: 116022. |
| 29 | CHEN M, WANG N, ZHU L. Single-atom dispersed Co-N-C: a novel adsorption-catalysis bifunctional material for rapid removing bisphenol A[J]. Catalysis Today, 2020, 348: 187-193. |
| 30 | ZHOU Y, ZHANG Y, HU X. Synergistic coupling Co3Fe7 alloy and CoFe2O4 spinel for highly efficient removal of 2,4-dichlorophenol by activating peroxymonosulfate[J]. Chemosphere, 2020, 242: 125244. |
| 31 | LI W, LI S, TANG Y, et al. Highly efficient activation of peroxymonosulfate by cobalt sulfide hollow nanospheres for fast ciprofloxacin degradation[J]. Journal of Hazardous Materials, 2020, 389: 121856. |
| 32 | DONG X, DUAN X, SUN Z, et al. Natural illite-based ultrafine cobalt oxide with abundant oxygen-vacancies for highly efficient Fenton-like catalysis[J]. Applied Catalysis B: Environmental, 2020, 261: 118214. |
| 33 | HOU J, LIN J, FU H, et al. Vitamin B12 derived CoCNx composite confined in SBA-15 as highly effective catalyst to activate peroxymonosulfate for naproxen degradation[J]. Chemical Engineering Journal, 2020, 389: 124344. |
| 34 | LI Meng-Chia, GHANBARI Farshid, CHANG Fang-Chih, et al. Enhanced degradation of 5-sulfosalicylic acid using peroxymonosulfate activated by ordered porous silica-confined Co3O4 prepared via a solvent-free confined space strategy[J]. Separation and Purification Technology, 2020, 249: 116972. |
| 35 | GHANBARI F, AHMADI M, GOHARI F. Heterogeneous activation of peroxymonosulfate via nanocomposite CeO2-Fe3O4 for organic pollutants removal: the effect of UV and US irradiation and application for real wastewater[J]. Separation and Purification Technology, 2019, 228: 115732. |
| 36 | FARSHID Ghanbaria, Martínez-Huitleb CARLOS A. Electrochemical advanced oxidation processes coupled with peroxymonosulfate for the treatment of real washing machine effluent: A comparative study[J]. Journal of Electroanalytical Chemistry, 2019, 847: 113182. |
| 37 | YUAN R, JIANG M, GAO S, et al. 3D mesoporous α-Co(OH)2 nanosheets electrodeposited on nickel foam: a new generation of macroscopic cobalt-based hybrid for peroxymonosulfate activation[J]. Chemical Engineering Journal, 2020, 380: 122447. |
| 38 | JAAFARZADEH N, GHANBARI F, AHMADI M. Efficient degradation of 2,4-dichlorophenoxyacetic acid by peroxymonosulfate/magnetic copper ferrite nanoparticles/ozone: a novel combination of advanced oxidation processes[J]. Chemical Engineering Journal, 2017, 320: 436-447. |
| 39 | BACHA A U R, NABI I, CHENG H, et al. Photoelectrocatalytic degradation of endocrine-disruptor bisphenol-A with significantly activated peroxymonosulfate by Co-BiVO4 photoanode[J]. Chemical Engineering Journal, 2020, 389: 124482. |
| 40 | HU P, LONG M. Cobalt-catalyzed sulfate radical-based advanced oxidation: a review on heterogeneous catalysts and applications[J]. Applied Catalysis B: Environmental, 2016, 181: 103-117. |
| 41 | WU Y, FANG Z, SHI Y, et al. Activation of peroxymonosulfate by BiOCl@Fe3O4 catalyst for the degradation of atenolol: kinetics, parameters, products and mechanism[J]. Chemosphere, 2019, 216: 248-257. |
| 42 | WANG Q, SHAO Y, GAO N, et al. Activation of peroxymonosulfate by Al2O3-based CoFe2O4 for the degradation of sulfachloropyridazine sodium: Kinetics and mechanism[J]. Separation and Purification Technology, 2017, 189: 176-185. |
| 43 | DUAN X, SU C, MIAO J, et al. Insights into perovskite-catalyzed peroxymonosulfate activation: maneuverable cobalt sites for promoted evolution of sulfate radicals[J]. Applied Catalysis B: Environmental, 2018, 220: 626-634. |
| 44 | LI Y, MA S, XU S, et al. Novel magnetic biochar as an activator for peroxymonosulfate to degrade bisphenol A: emphasizing the synergistic effect between graphitized structure and CoFe2O4[J]. Chemical Engineering Journal, 2020, 387: 124094. |
| 45 | LI H, WAN J, MA Y, et al. Degradation of refractory dibutyl phthalate by peroxymonosulfate activated with novel catalysts cobalt metal-organic frameworks: mechanism, performance, and stability[J]. Journal of Hazardous Materials, 2016, 318: 154-163. |
| [1] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [2] | WU Fengzhen, LIU Zhiwei, XIE Wenjie, YOU Yating, LAI Rouqiong, CHEN Yandan, LIN Guanfeng, LU Beili. Preparation of biomass derived Fe/N co-doped porous carbon and its application for catalytic degradation of Rhodamine B via peroxymonosulfate activation [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3292-3301. |
| [3] | PAN Jie, WANG Mingxin, GAO Shengwang, XIA Xunfeng, HAN Xue. Nitrogen-sulfur doped biochar/permonosulfate for degradation of sulfisoxazole in water [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4204-4212. |
| [4] | ZHEN Jianzheng, NIE Shisong, PAN Shiyuan, LYU Weiyang, YAO Yuyuan. Research progress on advanced activation of peroxymonosulfate by multidimensional carbon-supported metal catalyst for degradation of organic pollutants in water [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1858-1872. |
| [5] | LYU Peng, HE Changfan, HE Lin, LI Xingang, SUI Hong. Degradation characteristics and enhancement mechanism of heavy oily sludge by heterogeneous oxidation [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 6149-6157. |
| [6] | XUE Yuwei, YE Xiaozhen, ZENG Jing, WANG Yongquan, HONG Junming. Pretreatment of tobacco sugar flavoring wastewater by nano layered iron-manganese bimetallic catalysts activating peroxymonosulfate [J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5661-5668. |
| [7] | WANG Wenxia, LIU Xiaofeng, CHEN Xi, XU Yanhong, MENG Zhenbang, ZHENG Junxia, AN Taicheng. Research advances of synthesis and applications of porous g-C3N4-based photocatalyst [J]. Chemical Industry and Engineering Progress, 2022, 41(1): 300-309. |
| [8] | Yuqing ZHANG, Xiulan SONG, Pei BI. Effects of pH on the production of short-chain fatty acids from waste activated sludge enhanced by potassium peroxymonosulfate [J]. Chemical Industry and Engineering Progress, 2020, 39(9): 3786-3793. |
| [9] | Jie XU, Shiqian GAO, Jing XIA, Ke ZHANG, Zichun SHAO, Lanjing WANG, Yongjing TIAN. Activatoin of peroxymonosulfate by Sr-doped LaCo0.5Cu0.5O3 perovskite [J]. Chemical Industry and Engineering Progress, 2020, 39(9): 3525-3534. |
| [10] | Peng SUN, Kaikai ZHANG, Yu ZHANG, Yanrong ZHANG. Simultaneous removal of Cu2+ and p-nitroaniline from aqueous solution by biochar/peroxymonosulfate system [J]. Chemical Industry and Engineering Progress, 2020, 39(10): 4268-4274. |
| [11] | Jiangming YE,Shaohua LIANG,Siwen ZHANG,Rongyue SUN,Xiaolong BI. Self-activation mechanism of the spent calcium-sorbent under environmental conditions [J]. Chemical Industry and Engineering Progress, 2019, 38(9): 4302-4307. |
| [12] | Yiping CHEN, Guanshang XIA, Chaohong ZHENG, Si WU. Degradation of ciprofloxacin by advanced oxidation process with carbon nanotubes/peroxymonosulfate [J]. Chemical Industry and Engineering Progress, 2019, 38(04): 2037-2045. |
| [13] | GU Zhenchuan, GAO Naiyun, AN Na, CHEN Juxiang. Chloride ion activate peroxymonosulfate for degradation of trimethoprim in aqueous solution [J]. Chemical Industry and Engineering Progress, 2018, 37(05): 1992-1998. |
| [14] | LIU Liyan, SUN Zhirou, YE Wenbo, TAN Wei. Degradation of Acid Red B with Fe3O4 activated peroxymonosulfate with ultrasound irradiation [J]. Chemical Industry and Engineering Progress, 2016, 35(11): 3663-3668. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||