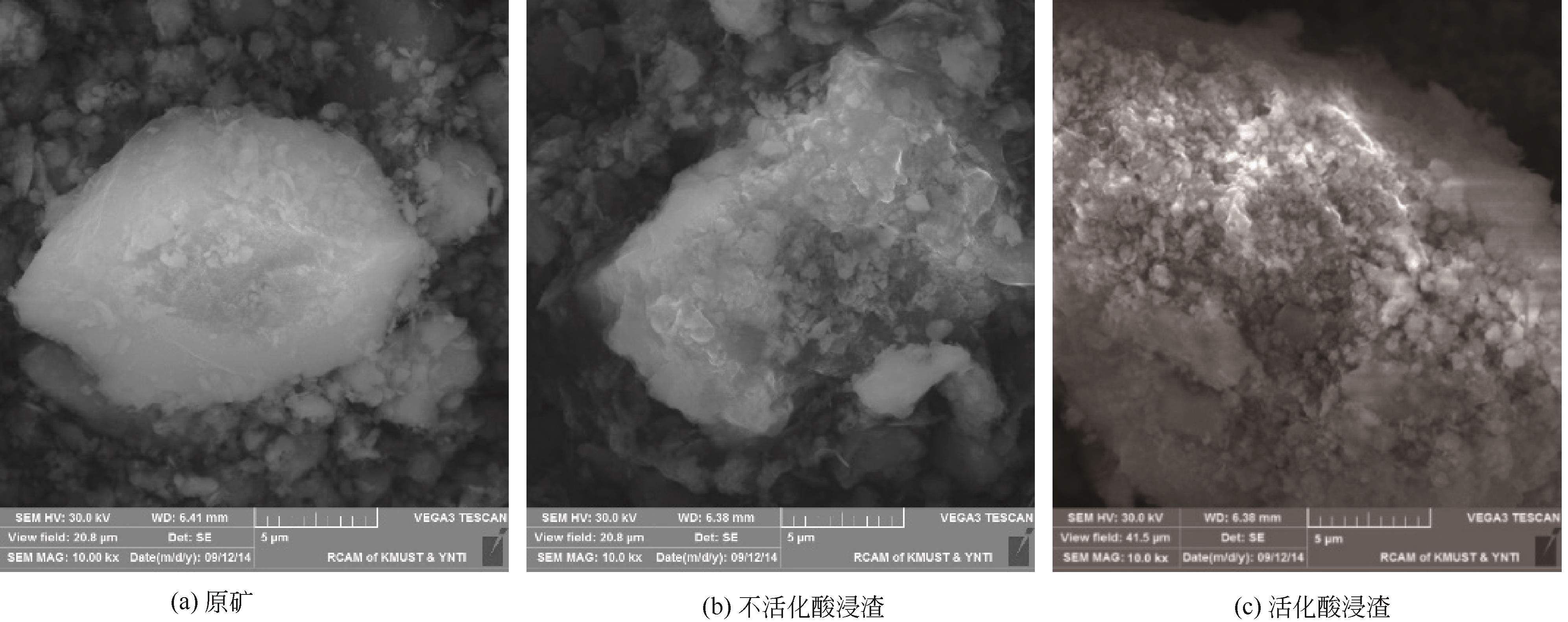
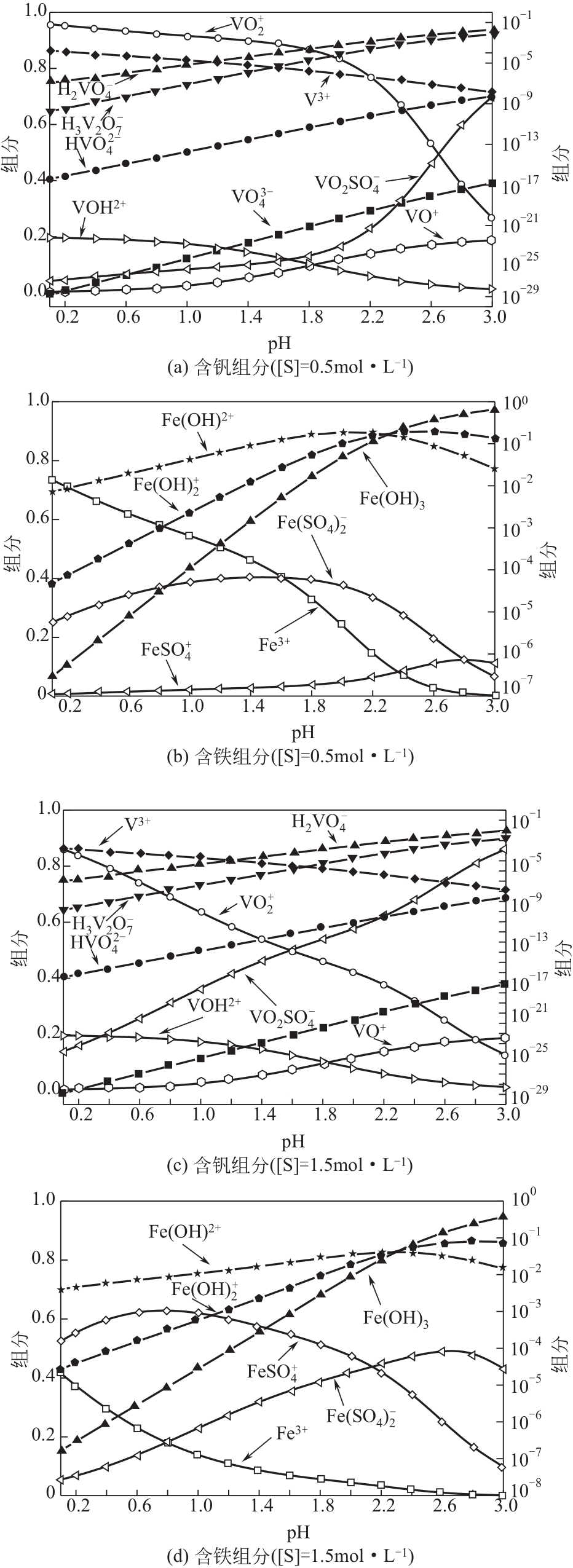
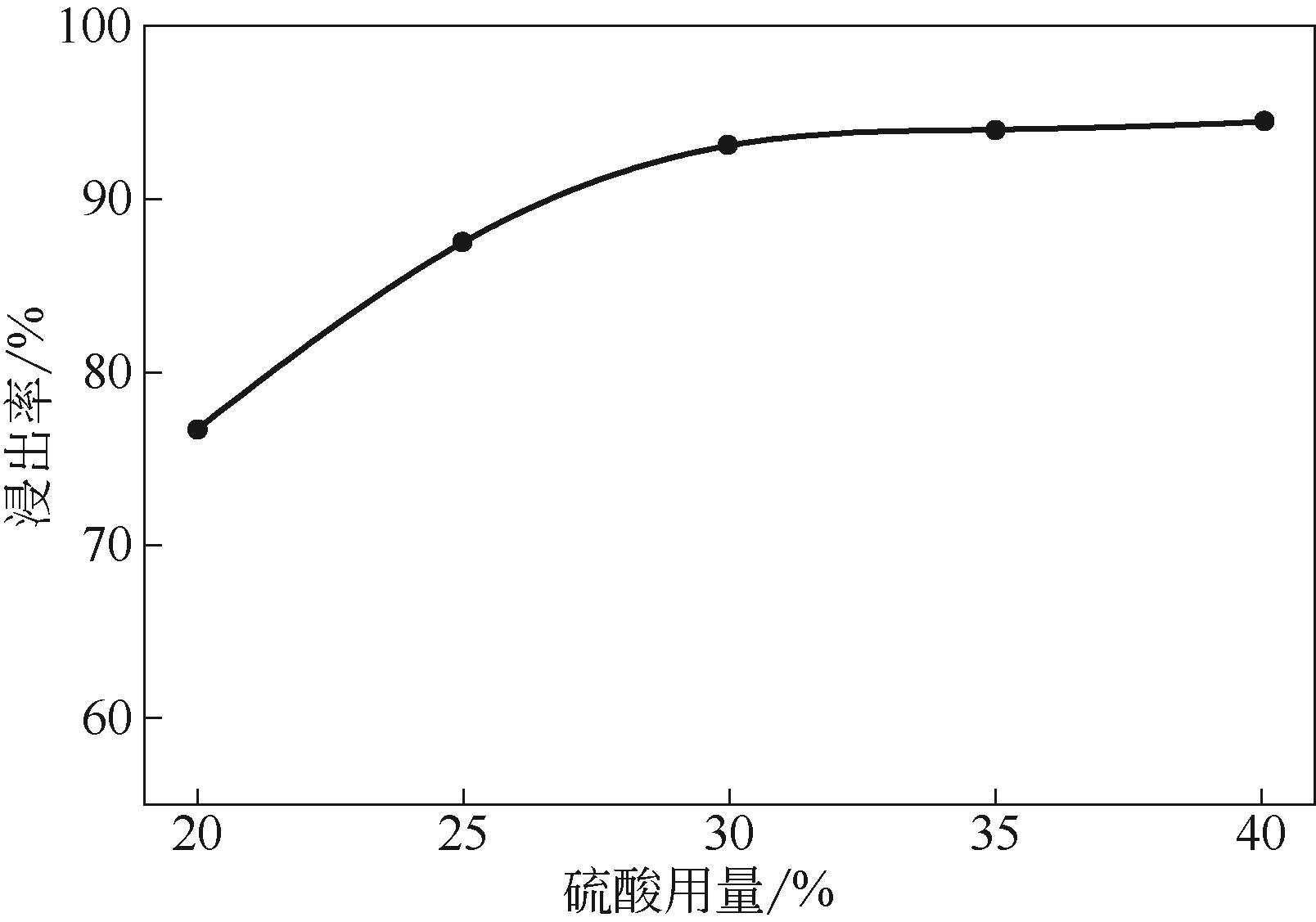
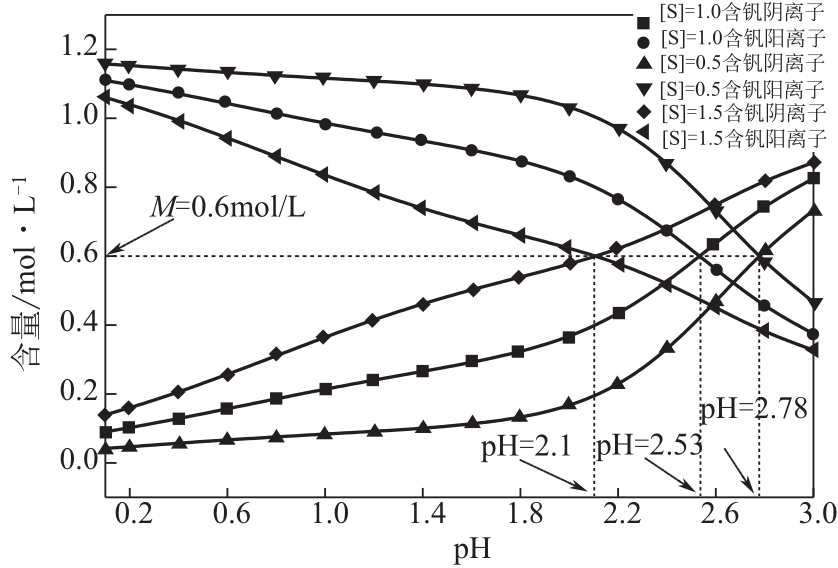
Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (10): 5360-5369.DOI: 10.16085/j.issn.1000-6613.2020-2103
• Special column:Resource recycling and value-added utilization • Previous Articles Next Articles
Analysis of thermodynamics of vanadium extraction from clay vanadium ore by direct atmospheric pressure activation
ZHANG Hao1( ), YE Guohua1,2(
), YE Guohua1,2( ), CHEN Ziyang2, XIE Yu1, ZUO Qi2
), CHEN Ziyang2, XIE Yu1, ZUO Qi2
- 1.Faculty of Land Resource Engineering, Kunming University of Science and Technology, Kunming 650093, Yunnan, China
2.State Key Laboratory of Complex Nonferrous Metal Resources Clean Utilization, Kunming 650093, Yunnan, China
-
Received:2020-10-20Revised:2020-12-14Online:2021-10-25Published:2021-10-10 -
Contact:YE Guohua
黏土钒矿直接常压活化酸浸提钒热力学分析
张豪1( ), 叶国华1,2(
), 叶国华1,2( ), 陈子杨2, 谢禹1, 左琪2
), 陈子杨2, 谢禹1, 左琪2
- 1.昆明理工大学国土资源工程学院,云南 昆明 650093
2.复杂有色金属资源清洁利用国家重点实验室,云南 昆明 650093
-
通讯作者:叶国华 -
作者简介:张豪(1994—),男,硕士研究生,研究方向为稀贵金属选冶。E-mail:m13629917490@163.com 。 -
基金资助:国家自然科学基金(51964028)
CLC Number:
Cite this article
ZHANG Hao, YE Guohua, CHEN Ziyang, XIE Yu, ZUO Qi. Analysis of thermodynamics of vanadium extraction from clay vanadium ore by direct atmospheric pressure activation[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5360-5369.
张豪, 叶国华, 陈子杨, 谢禹, 左琪. 黏土钒矿直接常压活化酸浸提钒热力学分析[J]. 化工进展, 2021, 40(10): 5360-5369.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-2103
| 成分 | 质量分数/% | 成分 | 质量分数/% |
|---|---|---|---|
| V | 1.20 | P | 0.26 |
| Fe | 1.74 | S | 0.01 |
| CaO | 0.14 | Cr | 0.11 |
| SiO2 | 81.53 | K | 0.98 |
| MgO | 0.74 | Na | 0.03 |
| Mn | 0.01 | As | <0.10 |
| Al2O3 | 6.76 | TiO2 | 1.75 |
| 成分 | 质量分数/% | 成分 | 质量分数/% |
|---|---|---|---|
| V | 1.20 | P | 0.26 |
| Fe | 1.74 | S | 0.01 |
| CaO | 0.14 | Cr | 0.11 |
| SiO2 | 81.53 | K | 0.98 |
| MgO | 0.74 | Na | 0.03 |
| Mn | 0.01 | As | <0.10 |
| Al2O3 | 6.76 | TiO2 | 1.75 |
| 项目 | 价态质量分数/% | 合计质量分数/% | ||||
|---|---|---|---|---|---|---|
| V(Ⅲ) | V(Ⅳ) | V(Ⅴ) | 以V计 | 以V2O5计 | ||
| 含量 | 1.03 | 0.08 | 0.03 | 1.14 | 2.04 | |
| 分布率 | 90.18 | 6.84 | 2.98 | 100 | 100 | |
| 项目 | 价态质量分数/% | 合计质量分数/% | ||||
|---|---|---|---|---|---|---|
| V(Ⅲ) | V(Ⅳ) | V(Ⅴ) | 以V计 | 以V2O5计 | ||
| 含量 | 1.03 | 0.08 | 0.03 | 1.14 | 2.04 | |
| 分布率 | 90.18 | 6.84 | 2.98 | 100 | 100 | |
| 序号 | 方程式 | 298K标准电位/V |
|---|---|---|
| 1 | VOH2+ | 3.52 |
| 2 | V3++H2O | 2.92 |
| 3 | 2VO2++3H2O | 4.02 |
| 4 | 2VO | 1.03 |
| 5 | H3V2O | 7.38 |
| 6 | H2VO | 9.52 |
| 7 | HVO | 11.5 |
| 8 | V3++e- | -0.26 |
| 9 | VO+ | -0.04 |
| 10 | V2++H2O | -0.08 |
| 11 | V2++H2O | 0.13 |
| 12 | VOH2+ | 0.16 |
| 13 | 2VO++3H2O | 0.55 |
| 14 | 2VO2++5H2O | 1.10 |
| 15 | HV2O | 0.50 |
| 16 | HV2O | 0.72 |
| 17 | HV2O | 1.28 |
| 18 | HV2O | 1.96 |
| 序号 | 方程式 | 298K标准电位/V |
|---|---|---|
| 1 | VOH2+ | 3.52 |
| 2 | V3++H2O | 2.92 |
| 3 | 2VO2++3H2O | 4.02 |
| 4 | 2VO | 1.03 |
| 5 | H3V2O | 7.38 |
| 6 | H2VO | 9.52 |
| 7 | HVO | 11.5 |
| 8 | V3++e- | -0.26 |
| 9 | VO+ | -0.04 |
| 10 | V2++H2O | -0.08 |
| 11 | V2++H2O | 0.13 |
| 12 | VOH2+ | 0.16 |
| 13 | 2VO++3H2O | 0.55 |
| 14 | 2VO2++5H2O | 1.10 |
| 15 | HV2O | 0.50 |
| 16 | HV2O | 0.72 |
| 17 | HV2O | 1.28 |
| 18 | HV2O | 1.96 |
| 序号 | 方程式 | ΔS | 比热容/J·K-1 | 电势/V | ||||
|---|---|---|---|---|---|---|---|---|
| 333K | 373K | 298K | 333K | 373K | ||||
| 19 | MnO+2H+ | -105.55 | 36.00 | 98.33 | 7.65 | 6.56 | 5.62 | |
| 20 | Mn2++2e- | 17.13 | -19.97 | -20.51 | -1.18 | -1.18 | -1.17 | |
| 21 | MnO+2H++2e- | -88.42 | 13.85 | 15.10 | -0.73 | -0.74 | -0.76 | |
| 22 | Mn3O4+2H++2e- | -37.19 | 17.72 | 19.63 | 0.46 | 0.46 | 0.45 | |
| 23 | Mn3O4+8H++2e- | -353.31 | 119.18 | 126.25 | 1.82 | 1.76 | 1.68 | |
| 24 | Mn2O3+6H++2e- | -262.91 | 89.56 | 93.59 | 1.44 | 1.40 | 1.34 | |
| 25 | 3Mn2O3+2H++2e- | -81.02 | 30.32 | 28.28 | 0.69 | 0.67 | 0.66 | |
| 26 | MnO2+4H++2e- | -159.61 | 54.81 | 56.95 | 1.23 | 1.20 | 1.17 | |
| 27 | 2MnO2+2H++2e- | -56.32 | 20.07 | 20.30 | 1.02 | 1.00 | 0.99 | |
| 28 | 2MnO2+2H++2e- | -432.00 | 230.80 | 131.90 | 1.69 | 1.64 | 1.58 | |
| 29 | MnO4-+4H+3e- | 76.68 | -53.11 | -57.13 | -0.44 | -0.43 | -0.41 | |
| 30 | Fe2++2e- | 269.23 | -14.23 | -11.61 | 0.77 | 0.82 | 0.88 | |
| 31 | Fe3++e- | -128.41 | 75.29 | 80.46 | 6.57 | 5.54 | 4.62 | |
| 32 | Fe(OH)2+2H+ | -277.31 | 121.81 | 124.94 | 1.53 | 0.87 | 0.32 | |
| 33 | Fe(OH)3+3H+ | 435.68 | -116.71 | -158.32 | 1.06 | 1.01 | 0.95 | |
| 34 | Fe(OH)3+3H+ | -51.72 | 22.18 | 23.33 | -0.05 | -0.06 | -0.07 | |
| 35 | Fe(OH)2+2H++2e- | -28.56 | 78.82 | 77.35 | 0.27 | 0.27 | 0.26 | |
| 36 | Fe(OH)3+H++e- | -200.91 | 9.42 | -2.33 | 0.34 | 0.32 | 0.30 | |
| 37 | V3++H2O | 367.08 | 1.58 | 8.21 | 1.04 | 1.01 | 0.99 | |
| 38 | VO2++H2O | -281.24 | 55.14 | 15.04 | 0.96 | 0.93 | 0.90 | |
| 39 | V2O5+6H++2e- | -65.27 | -182.17 | -35.63 | 2.15 | 1.62 | 1.14 | |
| 40 | V2O4+4H+ | -247.78 | -300.58 | -46.77 | 2.20 | 1.64 | 1.13 | |
| 序号 | 方程式 | ΔS | 比热容/J·K-1 | 电势/V | ||||
|---|---|---|---|---|---|---|---|---|
| 333K | 373K | 298K | 333K | 373K | ||||
| 19 | MnO+2H+ | -105.55 | 36.00 | 98.33 | 7.65 | 6.56 | 5.62 | |
| 20 | Mn2++2e- | 17.13 | -19.97 | -20.51 | -1.18 | -1.18 | -1.17 | |
| 21 | MnO+2H++2e- | -88.42 | 13.85 | 15.10 | -0.73 | -0.74 | -0.76 | |
| 22 | Mn3O4+2H++2e- | -37.19 | 17.72 | 19.63 | 0.46 | 0.46 | 0.45 | |
| 23 | Mn3O4+8H++2e- | -353.31 | 119.18 | 126.25 | 1.82 | 1.76 | 1.68 | |
| 24 | Mn2O3+6H++2e- | -262.91 | 89.56 | 93.59 | 1.44 | 1.40 | 1.34 | |
| 25 | 3Mn2O3+2H++2e- | -81.02 | 30.32 | 28.28 | 0.69 | 0.67 | 0.66 | |
| 26 | MnO2+4H++2e- | -159.61 | 54.81 | 56.95 | 1.23 | 1.20 | 1.17 | |
| 27 | 2MnO2+2H++2e- | -56.32 | 20.07 | 20.30 | 1.02 | 1.00 | 0.99 | |
| 28 | 2MnO2+2H++2e- | -432.00 | 230.80 | 131.90 | 1.69 | 1.64 | 1.58 | |
| 29 | MnO4-+4H+3e- | 76.68 | -53.11 | -57.13 | -0.44 | -0.43 | -0.41 | |
| 30 | Fe2++2e- | 269.23 | -14.23 | -11.61 | 0.77 | 0.82 | 0.88 | |
| 31 | Fe3++e- | -128.41 | 75.29 | 80.46 | 6.57 | 5.54 | 4.62 | |
| 32 | Fe(OH)2+2H+ | -277.31 | 121.81 | 124.94 | 1.53 | 0.87 | 0.32 | |
| 33 | Fe(OH)3+3H+ | 435.68 | -116.71 | -158.32 | 1.06 | 1.01 | 0.95 | |
| 34 | Fe(OH)3+3H+ | -51.72 | 22.18 | 23.33 | -0.05 | -0.06 | -0.07 | |
| 35 | Fe(OH)2+2H++2e- | -28.56 | 78.82 | 77.35 | 0.27 | 0.27 | 0.26 | |
| 36 | Fe(OH)3+H++e- | -200.91 | 9.42 | -2.33 | 0.34 | 0.32 | 0.30 | |
| 37 | V3++H2O | 367.08 | 1.58 | 8.21 | 1.04 | 1.01 | 0.99 | |
| 38 | VO2++H2O | -281.24 | 55.14 | 15.04 | 0.96 | 0.93 | 0.90 | |
| 39 | V2O5+6H++2e- | -65.27 | -182.17 | -35.63 | 2.15 | 1.62 | 1.14 | |
| 40 | V2O4+4H+ | -247.78 | -300.58 | -46.77 | 2.20 | 1.64 | 1.13 | |
| 序号 | 方程式 | lgK? |
|---|---|---|
| 41 | VO | 24.70 |
| 42 | VO | 11.54 |
| 43 | VO | 21.10 |
| 44 | 2VO | 46.45 |
| 45 | VO | 29.37 |
| 46 | VO++H+ | -1.83 |
| 47 | V(OH)2++H+ | 2.96 |
| 48 | Fe3++OH- | 11.89 |
| 49 | Fe3++2OH- | 23.62 |
| 50 | Fe3++3OH- | 35.30 |
| 51 | Fe3++SO | 2.03 |
| 52 | Fe3++2SO | 2.98 |
| 53 | H+HSO | -11.60 |
| 54 | H+SO | 1.96 |
| 55 | H++OH- | 14.00 |
| 序号 | 方程式 | lgK? |
|---|---|---|
| 41 | VO | 24.70 |
| 42 | VO | 11.54 |
| 43 | VO | 21.10 |
| 44 | 2VO | 46.45 |
| 45 | VO | 29.37 |
| 46 | VO++H+ | -1.83 |
| 47 | V(OH)2++H+ | 2.96 |
| 48 | Fe3++OH- | 11.89 |
| 49 | Fe3++2OH- | 23.62 |
| 50 | Fe3++3OH- | 35.30 |
| 51 | Fe3++SO | 2.03 |
| 52 | Fe3++2SO | 2.98 |
| 53 | H+HSO | -11.60 |
| 54 | H+SO | 1.96 |
| 55 | H++OH- | 14.00 |
| 浸出工艺 | 产品名称 | 质量/g | 钒品位/% | 浸出率/% |
|---|---|---|---|---|
| 活化酸浸 | 浸渣 | 85.50 | 0.18 | 92.58 |
| 浸出液 | 228 | 8.24 | ||
| 浸出原料 | 100 | 2.04 | ||
| 不活化酸浸 | 浸渣 | 86.80 | 0.54 | 76.86 |
| 浸出液 | 230 | 6.76 | ||
| 浸出原料 | 100 | 2.04 |
| 浸出工艺 | 产品名称 | 质量/g | 钒品位/% | 浸出率/% |
|---|---|---|---|---|
| 活化酸浸 | 浸渣 | 85.50 | 0.18 | 92.58 |
| 浸出液 | 228 | 8.24 | ||
| 浸出原料 | 100 | 2.04 | ||
| 不活化酸浸 | 浸渣 | 86.80 | 0.54 | 76.86 |
| 浸出液 | 230 | 6.76 | ||
| 浸出原料 | 100 | 2.04 |
| 产品 | 化学成分/% | |||||||
|---|---|---|---|---|---|---|---|---|
| V2O5 | Fe | SiO2 | MgO | CaO | Na | K | P | |
| 活化酸浸液 | 8.24 | 6.56 | <0.01 | 2.03 | 0.01 | 0.04 | 3.31 | 0.97 |
| 不活化酸浸液 | 6.76 | 6.48 | <0.01 | 1.14 | 0.08 | 0.04 | 1.92 | 0.95 |
| 产品 | 化学成分/% | |||||||
|---|---|---|---|---|---|---|---|---|
| V2O5 | Fe | SiO2 | MgO | CaO | Na | K | P | |
| 活化酸浸液 | 8.24 | 6.56 | <0.01 | 2.03 | 0.01 | 0.04 | 3.31 | 0.97 |
| 不活化酸浸液 | 6.76 | 6.48 | <0.01 | 1.14 | 0.08 | 0.04 | 1.92 | 0.95 |
| 1 | ZHAO Yunliang, WANG Wei, ZHANG Yimin, et al. In-situ investigation on mineral phase transition during roasting of vanadium-bearing stone coal[J]. Advanced Powder Technology, 2017, 28(3): 1103-1107. |
| 2 | LI Hongyi, FANG Haixing, WANG Kang, et al. Asynchronous extraction of vanadium and chromium from vanadium slag by stepwise sodium roasting-water leaching[J]. Hydrometallurgy, 2015, 156: 124-135. |
| 3 | CAI Zhenlei, ZHANG Yimin. Phase transformations of vanadium recovery from refractory stone coal by novel NaOH molten roasting and water leaching technology[J]. RSC Advances, 2017, 7(2): 36917-36922. |
| 4 | JI Yilong, SHEN Shaobo, LIU Jianhua, et al. Cleaner and effective process for extracting vanadium from vanadium slag by using an innovative three-phase roasting reaction[J]. Journal of Cleaner Production, 2017, 149: 1068-1078. |
| 5 | ZHANG Ying, ZHANG Ting’an, DREISINGER D, et al. Recovery of vanadium from calcification roasted-acid leaching tailing by enhanced acid leaching[J]. Journal of Hazardous Materials, 2019, 369: 632-641. |
| 6 | PENG Hao, GUO Jing, ZHENG Xiaogang, et al. Leaching kinetics of vanadium from calcification roasting converter vanadium slag in acidic medium[J]. Journal of Environmental Chemical Engineering, 2016, 256: 98-106. |
| 7 | LI Meng, ZHENG Shili, LIU Biao, et al. A clean and efficient method for recovery of vanadium from vanadium slag: nonsalt roasting and ammonium carbonate leaching processes[J]. Mineral Processing and Extractive Metallurgy Review, 2017, 38: 228-237. |
| 8 | LI Meng, LIU Biao, ZHENG Shili, et al. A cleaner vanadium extraction method featuring non-salt roasting and ammonium bicarbonate leaching[J]. Journal of Cleaner Production, 2017, 149: 206-217. |
| 9 | ZHANG Xuefei, LIU Fengguo, XUE Xiangxin, et al. Effects of microwave and conventional blank roasting on oxidation behavior, microstructure and surface morphology of vanadium slag with high chromium content[J]. Journal of Alloys and Compounds, 2016, 686: 356-365. |
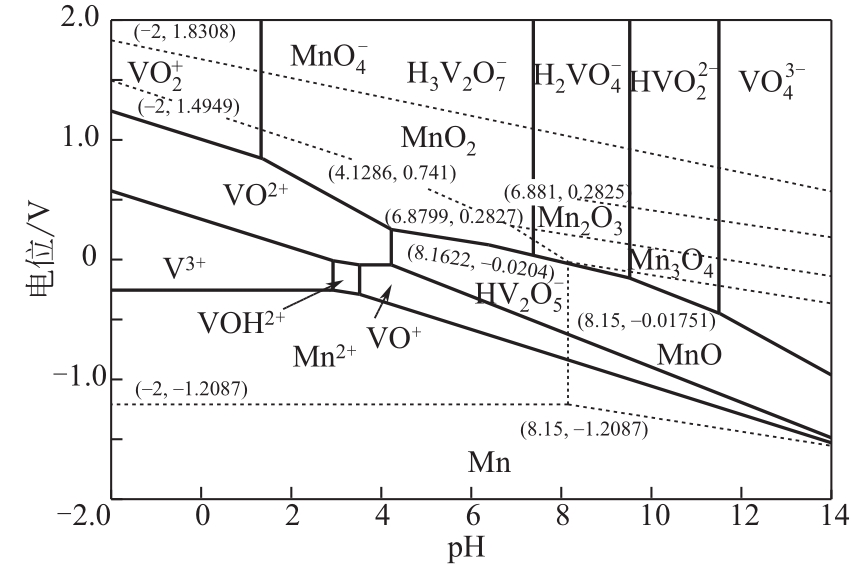
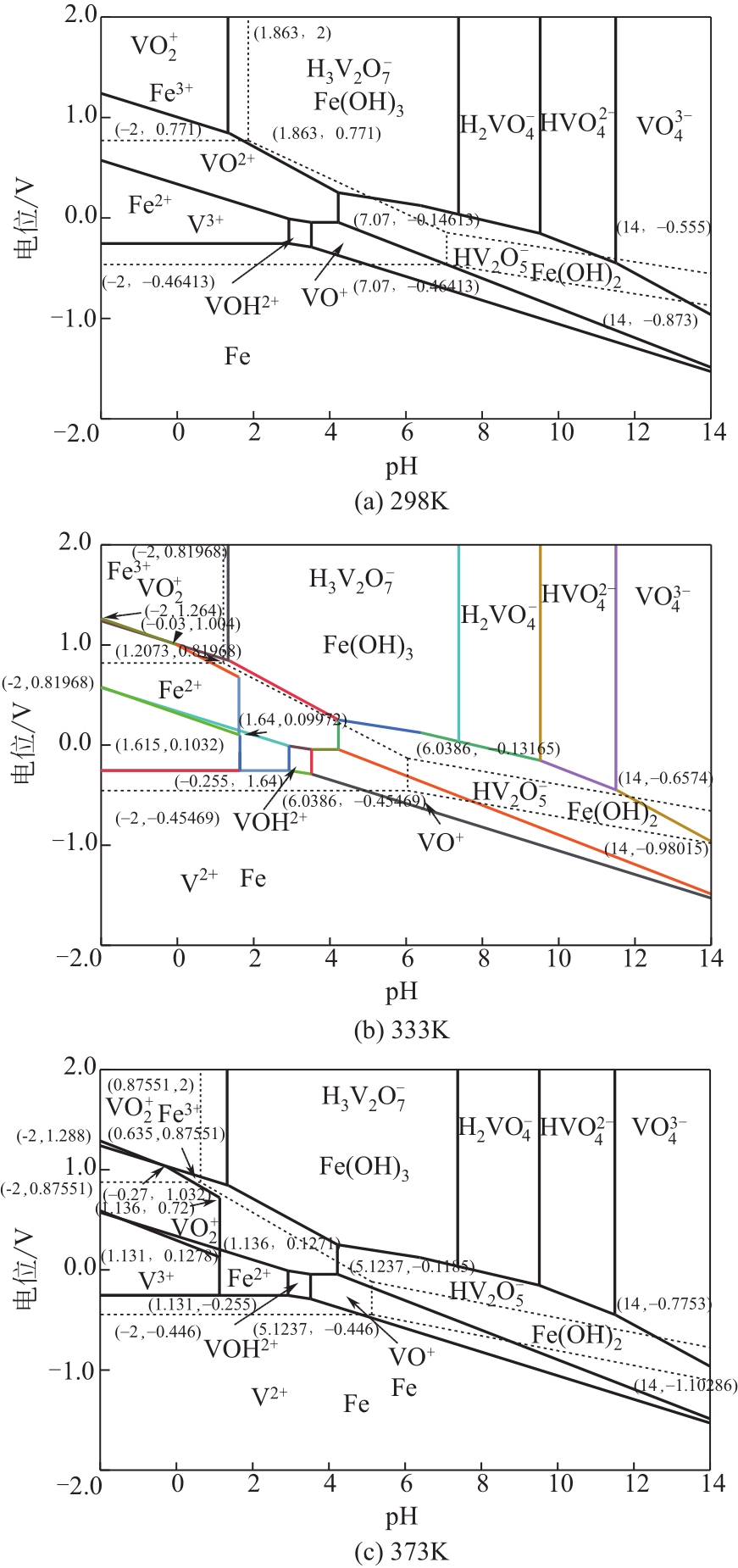
| 10 | 张延安, 牟望重, 豆志河, 等. 转炉钒渣氧压酸浸过程V-Fe-H2O系的电位-pH图[J]. 中国有色金属学报, 2011, 21(11): 2936-2945. |
| ZHANG Yan’an, MOU Wangzhong, DOU Zhihe, et al. Potential-pH diagrams for V-Fe-H2O system during oxygen pressure acid leaching of vanadium-bearing converter slags[J]. Chinese Journal of Nonferrous Metals, 2011, 21(11): 2936-2945. | |
| 11 | 杨显万. 高温水溶液热力学数据计算手册[M]. 北京: 冶金工业出版社, 1983: 523-674. |
| YANG Xianwan. Handbook of Thermodynamic Data in Aqueous Solutions at High Temperature[M]. Beijing: Metallurgical Industry Press, 1983: 523-674. | |
| 12 | 伊赫桑·巴伦. 纯物质热化学数据手册[M]. 程乃良, 牛四通, 徐桂英, 译. 北京: 科学出版社, 2003: 716-726. |
| BARIN I. Thermochemical data of pure substances[M]. CHENG Nailiang, NIU Sitong, XU Guiying, trans. Beijing: Science Press, 2003: 716-726. | |
| 13 |
CHEN B F, HUANG S, LIU B, et al. Thermodynamic analysis for separation of vanadium and chromium in V( )-Cr(Ⅲ)-H2O system[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(3): 57-65. )-Cr(Ⅲ)-H2O system[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(3): 57-65.
|
| 14 | 刘景文, 阳征斐, 周鹏, 等. V(Ⅴ)-Fe(Ⅲ)-S(Ⅵ)-H2O系热力学研究与钒铁分离方法理论[J]. 中国有色金属学报, 2020, 30(4): 912-919. |
LIU Jingwen, YANG Zhengfei, ZHOU Peng, et al. V(Ⅴ)-Fe(Ⅲ)-S( )-H2O thermodynamic study and separation theory of ferrovanadium[J]. Chinese Journal of Nonferrous Metals, 2020, 30(4): 912-919. )-H2O thermodynamic study and separation theory of ferrovanadium[J]. Chinese Journal of Nonferrous Metals, 2020, 30(4): 912-919.
|
|
| 15 | 何伟. 黏土钒矿不磨不焙烧常压活化酸浸提钒的研究[D]. 昆明: 昆明理工大学, 2014. |
| HE Wei. Extraction of vanadium from clay vanadium ore by activated acid under normal pressure without grinding and roasting[D]. Kunming: Kunming University of Science and Technology, 2014. |
| [1] | WANG Peng, ZHANG Yang, FAN Bingqiang, HE Dengbo, SHEN Changshuai, ZHANG Hedong, ZHENG Shili, ZOU Xing. Process and kinetics of hydrochloric acid leaching of high-carbon ferrochromium [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 510-517. |
| [2] | MA Yi, CAO Shiwei, WANG Jiajun, LIN Liqun, XING Yan, CAO Tengliang, LU Feng, ZHAO Zhenlun, ZHANG Zhijun. Research progress in recovery of spent cathode materials for lithium-ion batteries using deep eutectic solvents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 219-232. |
| [3] | LI Weihua, YU Qianwen, YIN Junquan, WU Yinkai, SUN Yingjie, WANG Yan, WANG Huawei, YANG Yufei, LONG Yuyang, HUANG Qifei, GE Yanchen, HE Yiyang, ZHAO Lingyan. Leaching behavior of heavy metals from broken ton bags filled with fly ash in acid rain environment [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4917-4928. |
| [4] | LU Yang, ZHOU Jinsong, ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao. Leaching mechanism of Hg-absorption products on CeO2/TiO2 sorbentsin syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3875-3883. |
| [5] | CHANG Zhankun, ZHANG Chi, SU Bingqin, ZHANG Congzheng, WANG Jian, QUAN Xiaohui. Effect of H2S gaseous substrate on sludge bioleaching treatment efficiency [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2733-2743. |
| [6] | LI Weihua, WU Yinkai, SUN Yingjie, YIN Junquan, XIN Mingxue, ZHAO Youjie. Progress on evaluation methods for toxic leaching of heavy metals from MSW incineration fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2666-2677. |
| [7] | CHEN Shaoqin, HU Ling, LEI Tianya, WANG Rong, SHU Jiancheng, CHEN Mengjun. Mechanical activation for zinc enhanced leaching from zinc calcine [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1649-1658. |
| [8] | LI Jingjing, ZHAO Yao, XU Fengchi, LI Kangjian. Heavy metal leaching characteristics of porous asphalt mixture containing MSWI-BAA under different stormwater runoff flow rates [J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5520-5530. |
| [9] | ZHANG Xinyuan, ZHANG Bolin, ZHANG Shengen. Research progress on recovery of spent vanadium-titanium based deNO x catalyst with alkaline process [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 580-594. |
| [10] | CHEN Yu, LIU Chong, QIU Yuhui, BI Zixin, MU Tiancheng. Ionic liquids and deep eutectic solvents for green recycle of spent lithium-ion batteries [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 485-496. |
| [11] | YU Zhengwei, ZHANG Xiaoxia, LEI Jie, LI Ao, WANG Guangying, DING Xiang, LONG Hongming. Comprehensive recovery of cerium and manganese from waste CeO x -MnO x -based SCR denitrification catalysts by reductive acid leaching [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 5122-5131. |
| [12] | JI Dongli, YE Jiliang, HE Shaolin, YUAN Hongying, XU Longtan, LI Ruolin, WANG Shuai, SONG Yang, QI Zhibin, GE Yanbing. Analysis of influence of oil shale in-situ mining on some groundwater index [J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4057-4064. |
| [13] | CHENG Mingqiang, RU Juanjian, HUA Yixin, WANG Ding, GENG Xiao, ZHANG Wenwen, HUANG Haoming, WANG Daoxiang. Progress of deep eutectic solvents in recovery of cathode materials from spent lithium ion batteries [J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3293-3305. |
| [14] | HE Changfan, ZHAO Xiaohang, ZHANG Xueying, HE Lin, SUI Hong, LI Xingang. Peroxymonosulfate-ferrate-FeS system soil column leaching to remediate o-dichlorobenzene contaminated soil [J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2743-2752. |
| [15] | CHEN Lei, YAN Xingqing, HU Yanwei, YU Shuai, YANG Kai, CHEN Shaoyun, GUAN Hui, YU Jianliang, MAHGEREFTEH Haroun, MARTYNOV Sergey. Research progress on fracture control of accidental leakage and decompression in CO2 pipeline transportation [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1241-1255. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||