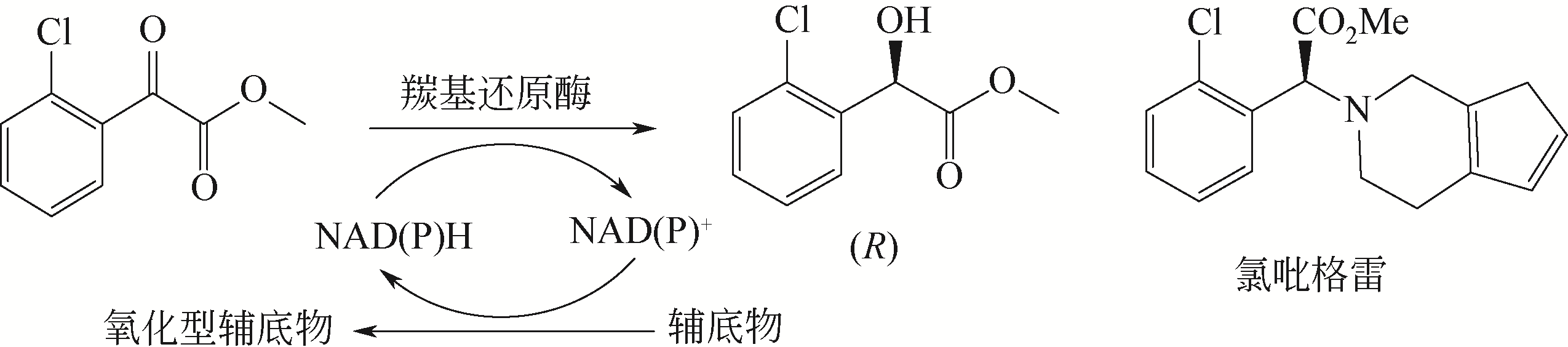
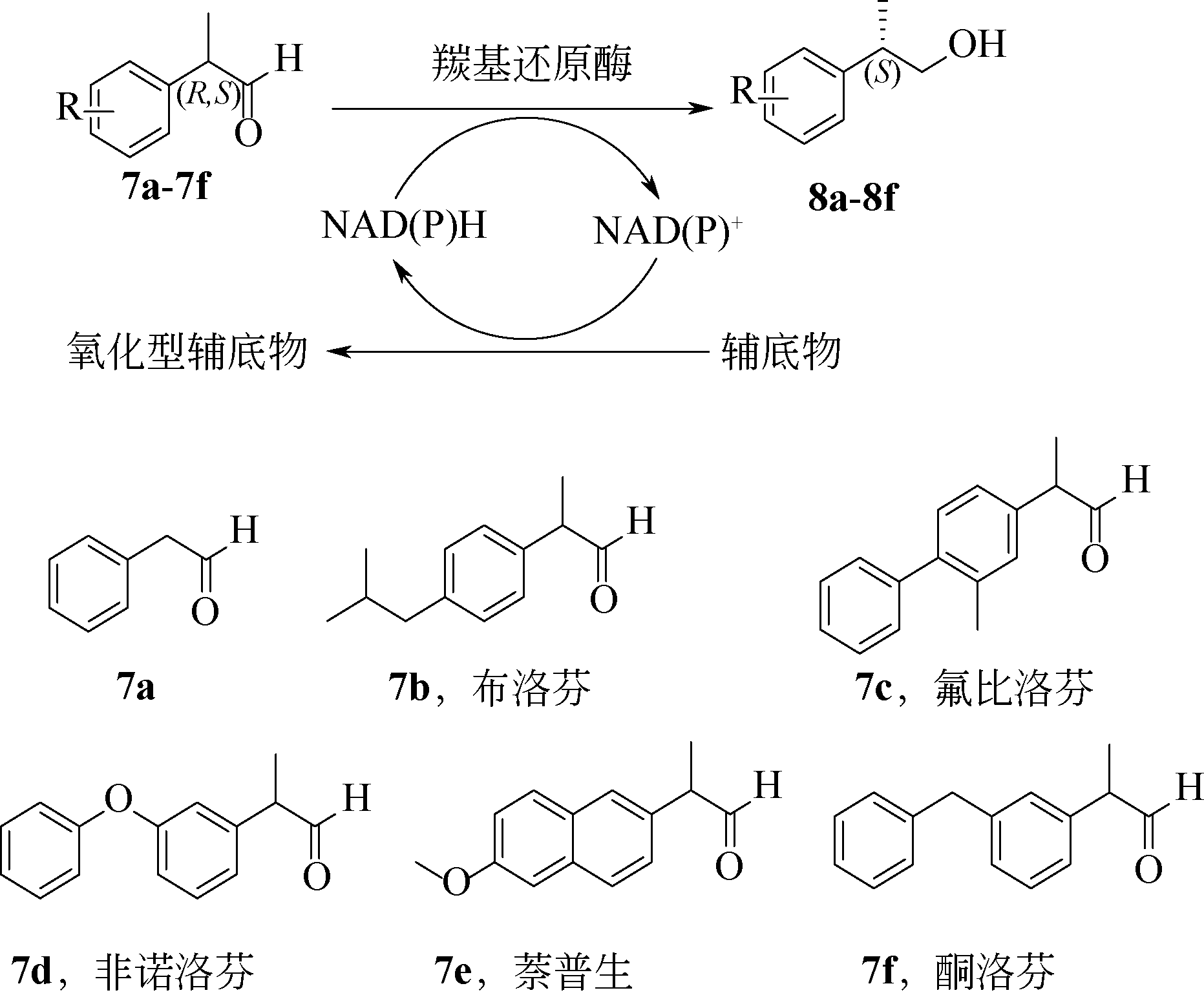
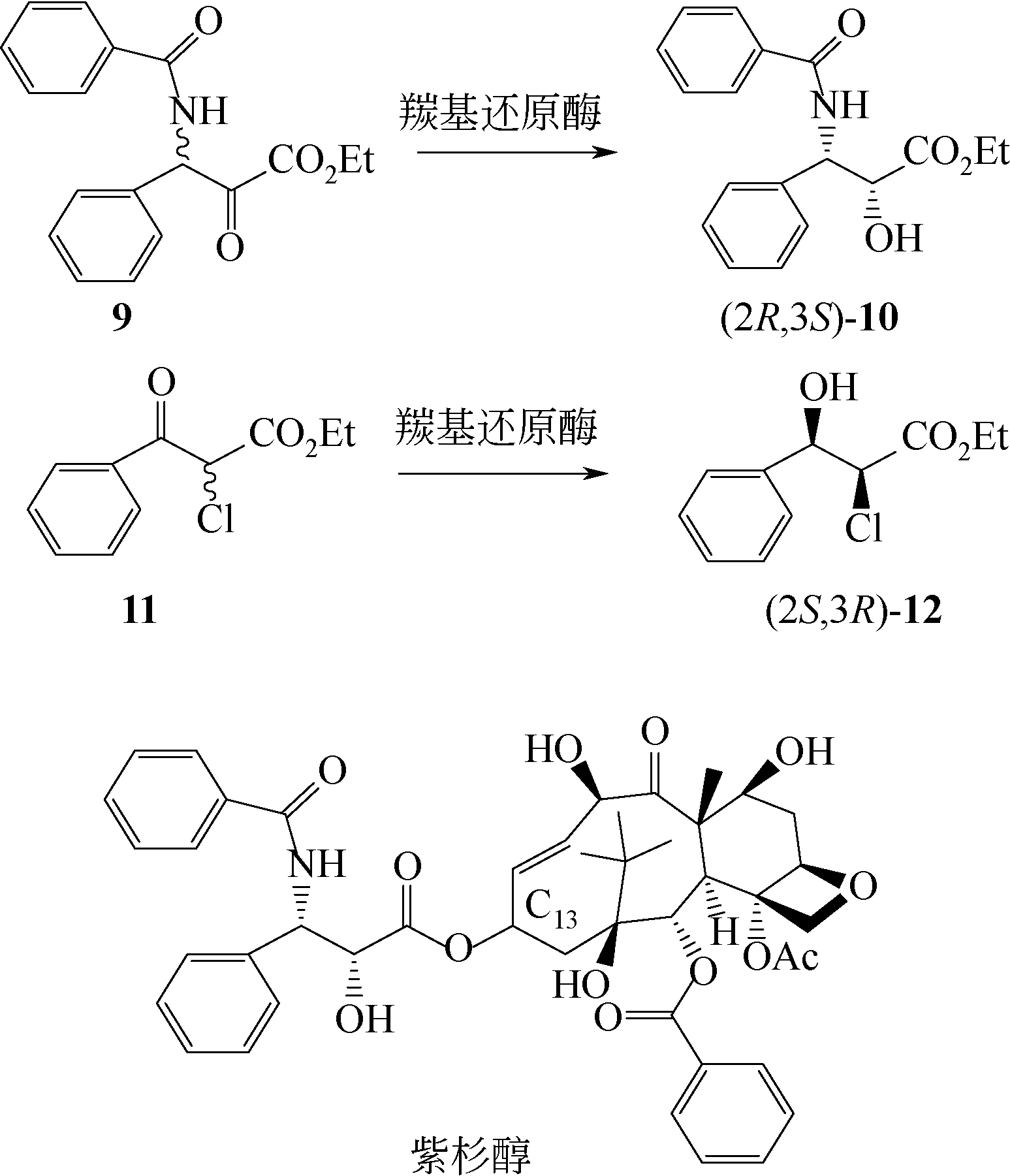
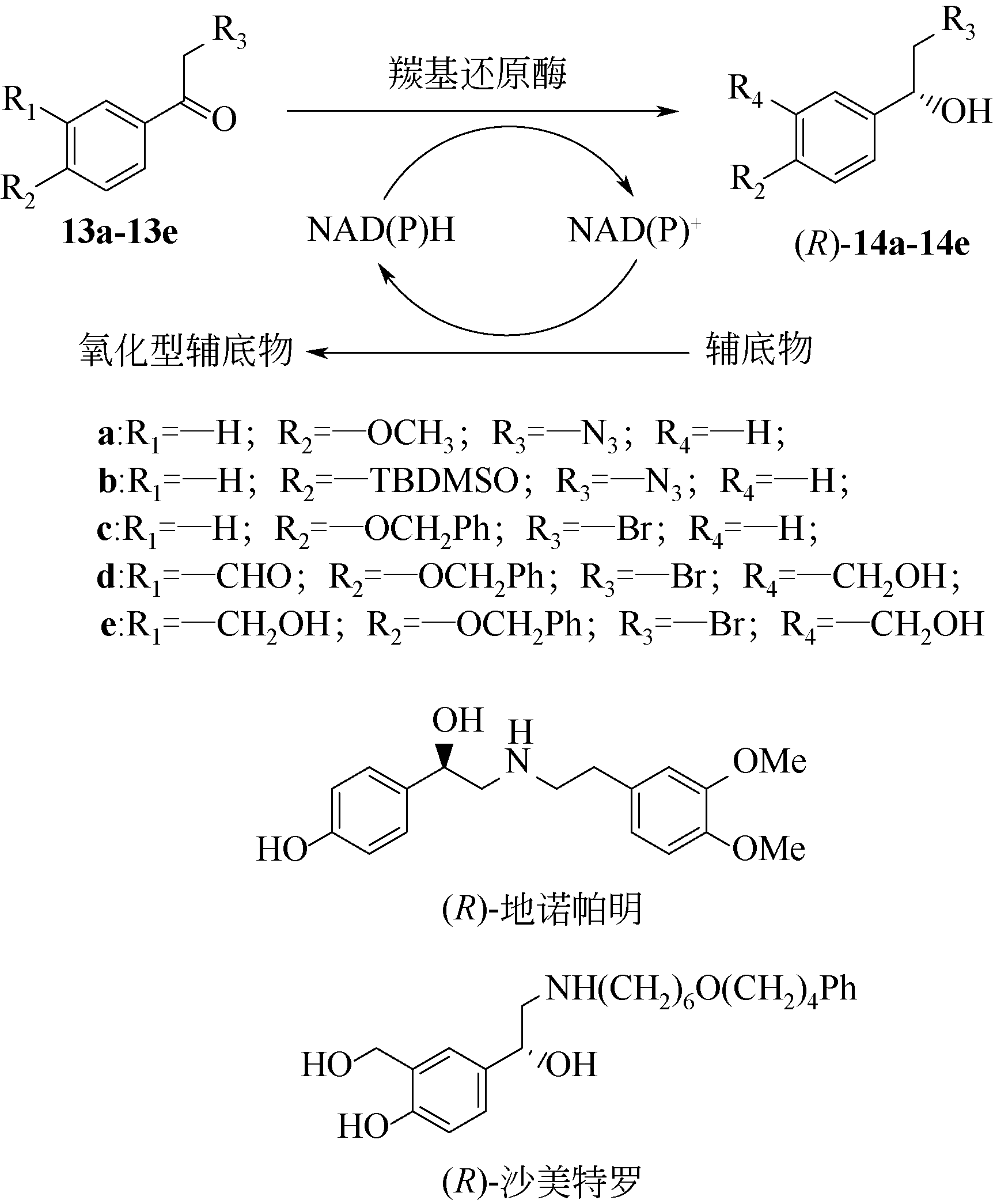
Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (3): 1142-1160.DOI: 10.16085/j.issn.1000-6613.2020-2124
• Special column:Green biomanufacturing • Previous Articles Next Articles
Stereoselective carbonyl reductases and their application in chiral alcohols synthesis
ZHANG Xiaojian1,2( ), LIU Qian1,2, LIU Zhiqiang1,2(
), LIU Qian1,2, LIU Zhiqiang1,2( ), ZHENG Yuguo1,2
), ZHENG Yuguo1,2
- 1.The National and Local Joint Engineering Research Center for Biomanufacturing of Chiral Chemicals, College of Biotechnology and Bioengineering, Zhejiang University of Technology, Hangzhou 310014, Zhejiang, China
2.Key Laboratory of Bioorganic Synthesis of Zhejiang Province, College of Biotechnology and Bioengineering, Zhejiang University of Technology, Hangzhou 310014, Zhejiang, China
-
Received:2020-10-22Online:2021-03-17Published:2021-03-05 -
Contact:LIU Zhiqiang
立体选择性羰基还原酶及其在手性醇合成中的应用
张晓健1,2( ), 刘倩1,2, 柳志强1,2(
), 刘倩1,2, 柳志强1,2( ), 郑裕国1,2
), 郑裕国1,2
- 1.浙江工业大学生物工程学院,手性生物制造国家地方联合工程研究中心,浙江 杭州 310014
2.浙江工业大学 生物工程学院,浙江省生物有机合成重点实验室,浙江 杭州 310014
-
通讯作者:柳志强 -
作者简介:张晓健(1980—),男,博士,讲师,研究方向为生物催化与转化。E-mail:xjzhang020@zjut.edu.cn 。 -
基金资助:国家自然科学基金(21672190)
CLC Number:
Cite this article
ZHANG Xiaojian, LIU Qian, LIU Zhiqiang, ZHENG Yuguo. Stereoselective carbonyl reductases and their application in chiral alcohols synthesis[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1142-1160.
张晓健, 刘倩, 柳志强, 郑裕国. 立体选择性羰基还原酶及其在手性醇合成中的应用[J]. 化工进展, 2021, 40(3): 1142-1160.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-2124
| 94 | ZHANG J, WITHOLT B, LI Z. Efficient NADPH recycling in enantioselective bioreduction of a ketone with permeabilized cells of a microorganism containing a ketoreductase and a glucose 6-phosphate dehydrogenase[J]. Advanced Synthesis & Catalysis, 2006, 348: 429-433. |
| 95 | HE J, MAO X, SUN Z, et al. Microbial synthesis of ethyl (R)-4,4,4-trifluoro-3-hydroxybutanoate by asymmetric reduction of ethyl 4,4,4-trifluoroacetoacetate in an aqueous-organic solvent biphasic system[J]. Biotechnology Journal, 2007, 2: 260-265. |
| 96 | OLIVEIRA S S D, DIAS L R S, BARBOSA N C, et al. Enantioselective bioreduction of ethyl 4,4,4-trihalide-3-oxobutanoate by Kluyveromyces marxianus[J]. Tetrahedron Letters, 2013, 24: 3067-3070. |
| 97 | KARA S, SPICKERMANN D, WECKBECKER A. Bioreductions catalyzed by an alcohol dehydrogenase in non-aqueous media[J]. ChemCatChem, 2014, 6: 973-976. |
| 98 | PATEL R, CHU L, NANDURI V, et al. Enantioselective microbial reduction of 6-oxo-8-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-8-azaspiro[4.5]decane-7,9-dione[J]. Tetrahedron Asymmetr, 2005, 16(16): 2778-2783. |
| 99 | GOLDBERG S L, NANDURI V B, CHU L, et al. Enantioselective microbial reduction of 6-oxo-8-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-8-azaspiro [4.5] decane-7,9- dione: cloning and expression of reductases[J]. Enzyme and Microbial Technology, 2006, 39(7): 1441-1450. |
| 100 | ROBERGE C, KING A, PECORE V, et al. Asymmetric bioreduction of a keto ester to its corresponding (S)-hydroxy ester by Microbacterium sp. MB 5614[J]. Journal of Fermentation & Bioengineering, 1996, 81(6): 530-533. |
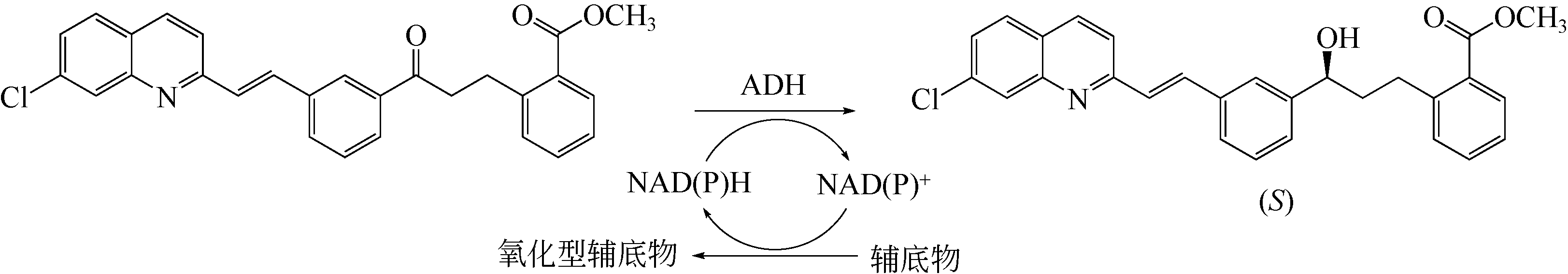
| 101 | LIANG J, BORUP B, MITCHELL V, et al. Ketoreductases for the production of (S,E)-methyl 2-(3-(3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-hroxypropyl)benzoate: US20130078692[P]. 2012-01-03. |
| 102 |
TSUTSUMI K, KATAYAMA T, UTSUMI N, et al. Practical asymmetric hydrogenation of 3-quinuclidinone catalyzed by the XylSkewphos/PICA-Ruthenium( ) complex[J]. Organic Process Research & Development, 2009, 13(3): 625-628. ) complex[J]. Organic Process Research & Development, 2009, 13(3): 625-628.
|
| 103 | UZURA A, NOMOTO F, SAKODA A, et al. Stereoselective synthesis of (R)-3-quinuclidinol through asymmetric reduction of 3-quinuclidinone with 3-quinuclidinone reductase of Rhodotorula rubra[J]. Applied Microbiology and Biotechnology, 2009, 83(4): 617-626. |
| 104 | CHEN Y H, JIANG Q H, SUN L L, et al. Magnetic combined cross-cinked enzyme cggregates of ketoreductase and alcohol dehydrogenase: an efficient and stable biocatalyst for asymmetric synthesis of (R)-3-quinuclidinol with regeneration of coenzymes in situ[J]. Catalysts, 2018, 8(8): 334. |
| 105 | CHEN Q, XIE B, ZHOU L, et al. A tailor-made self-sufficient whole-cell biocatalyst enables scalable enantioselective synthesis of (R)-3-quinuclidinol in a high space-time yield[J]. Organic Process Research & Development, 2019, 23(9): 1813-1821. |
| 1 | LOCKHART R, MARTINELLI R. Proposed correlation of data for isothermal two-phase, two-component flow in pipes[J]. Chemical Engineering Progress, 1949, 45(1): 39-48. |
| 2 | NI Y, XU J H. Biocatalytic ketone reduction: a green and efficient access to enantiopure alcohols[J]. Biotechnology Advances, 2012, 30: 1279-1288. |
| 106 | CHEN Y Z, LIN H, XU X Y, et al. Preparation the key intermediate of angiotensin‐converting enzyme (ACE) inhibitors: high enantioselective production of ethyl (R)‐2‐hydroxy‐4‐phenylbutyrate with Candida boidinii CIOC21[J]. Advanced Synthesis & Catalysis, 2010, 350(3): 426-430. |
| 107 | 汪云, 王利群, 何玉财, 等. 两相体系中固定化黏红酵母CCZU-G5催化合成(R)-2-羟基-4-苯基丁酸乙酯[J]. 化工进展, 2013, 32(3): 661-665. |
| WANG Yun, WANG Liqun, HE Yucai, et al. Biosynthesis of ethyl(R)-2-hydroxy-4-phenylbutyrate catalyzed by immobilized Rhodotorula mucilaginosa CCZU-G5 in biphasic system[J]. Chemical Industry and Engineering Progress, 2013, 32(3): 661-665. | |
| 108 | XIA S W, CHEN Y Z, ZHOU J R, et al. Enzyme-catalyzed asymmetric synthesis of optically active (R)-and (S)-ethyl-4-phenyl-4-hydroxybutyrate with microbial cells[J]. Biocatalysis and Biotransformation, 2013, 31(1): 66-70. |
| 109 | ZHANG W, NI Y, SUN Z, et al. Biocatalytic synthesis of ethyl(R)-2-hydroxy-4-phenylbutyrate with Candida krusei SW2026: a practical process for high enantiopurity and product titer[J]. Process Biochemistry, 2009, 44(11): 1270-1275. |
| 110 | LI N, NI Y, SUN Z. Purification and characterization of carbonyl reductase from Candida krusei SW 2026 involved in enantioselective reduction of ethyl 2-oxo-4-phenylbutyrate[J]. Journal of Molecular Catalysis B: Enzymatic, 2010, 66(1/2): 190-197. |
| 111 | QIAN X L, PAN J, SHEN N D, et al. Efficient production of ethyl (R)-2-hydroxy-4-phenylbutyrate using a cost-effective reductase expressed in Pichia pastoris[J]. Biochemical Engineering Journal, 2014, 91: 72-77. |
| 112 | WANG Z, ZHOU S, ZHANG S, et al. Semi-rational engineering of a thermostable aldo-keto reductase from Thermotoga maritima for synthesis of enantiopure ethyl-2-hydroxy-4-phenylbutyrate (EHPB)[J]. Scientific Reports, 2017, 7(1): 4007. |
| 113 | QIAN W Z, OU L, LI C X, et al. Evolution of glucose dehydrogenase for cofactor regeneration in bioredox processes with denaturing agents[J]. ChemBioChem, 2020, 21(18): 2680-2688. |
| 114 | JEONG M, LEE Y M, HONG S H,et al. Optimization of enantioselective synthesis of methyl (R)-2-chloromandelate by whole cells of Saccharomyces cerevisiae[J]. Biotechnology Letters, 2010, 32(10): 1529-1531. |
| 115 | NI Y, LI C X, ZHANG J, et al. Efficient reduction of ethyl2-oxo-4-phenylbutyrate at 620g·L-1 by a bacterial reductase with broad substrate spectrum[J]. Advance Synthesis & Catalysis, 2011, 353(8): 1213-1217. |
| 116 | MA H, YANG L, NI Y, et al. Stereospecific reduction of rethyl o‐chlorobenzoylformate at 300g·L-1 without additional cofactor using a carbonyl reductase mined from Candida glabrata[J]. Advanced Synthesis & Catalysis, 2012, 354(9): 1765-1772. |
| 117 | NI Y, PAN J, MA H M, et al. Bioreduction of methyl o-chlorobenzoylformate at 500g·L-1 without external cofactors for efficient production of enantiopure clopidogrel intermediate[J]. Tetrahedron Letters, 2012, 53(35): 4715-4717. |
| 118 | ZHENG G W, LIU Y Y, CHEN Q, et al. Preparation of structurally diverse chiral alcohols by engineering ketoreductase CgKR1[J]. ACS Catalysis, 2017, 7(10): 7174-7181. |
| 119 | NELSON T D, LEBLOND C R, FRANTZ D E, et al. Stereoselective synthesis of a potent thrombin inhibitor by a novel P2-P3 lactone ring opening[J]. Journal of Organic Chemistry, 2004, 69(11): 3620-3627. |
| 120 | BALÁZS E, ANTAL S, GÁBOR S, et al. Stereoselective production of (S)-1-aralkyl- and 1-arylethanols by freshly harvested and lyophilized yeast cells[J]. Tetrahedron Asymmetry, 2006, 17(2): 268-274. |
| 121 | SIMON R C, BUSTO E, RICHTER N, et al. Chemoenzymatic synthesis of enantiomerically pure syn‐configured 1‐aryl‐3‐methylisochroman derivatives[J]. European Journal of Organic Chemistry, 2014(1): 111-121. |
| 122 | HAQ S F, SHANBHAG A P, KARTHIKEYAN S, et al. A strategy to identify a ketoreductase that preferentially synthesizes pharmaceutically relevant (S)-alcohols using whole-cell biotransformation[J]. Microbial Cell Factories, 2018, 17(1): 192. |
| 123 | GIACOMINI D, GALLETTI P, QUINTAVALLA A, et al. Highly efficient asymmetric reduction of arylpropionic aldehydes by horse liver alcohol dehydrogenase through dynamic kinetic resolution[J]. Cheminform, 2007, 39: 4038-4040. |
| 124 | GALLETTI P, EMER E, GUCCIARDO G, et al. Chemoenzymatic synthesis of (2S)-2-arylpropanols through a dynamic kinetic resolution of 2-arylpropanals with alcohol dehydrogenases[J]. Organic & Biomolecular Chemistry, 2010, 8(18): 4117-4123. |
| 125 | FRIEST J A, MAEZATO Y, BROUSSY S, et al. Use of a robust dehydrogenase from an archael hyperthermophile in asymmetric catalysis-dynamic reductive kinetic resolution entry into (S)-Profens[J]. Journal of the American Chemical Society, 2010, 132(17): 5930-5931. |
| 126 | QUAGLIA D, PORI M, GALLETTI P, et al. His-tagged horse liver alcohol dehydrogenase: immobilization and application in the bio-based enantioselective synthesis of (S)-arylpropanols[J]. Process Biochemistry, 2013, 48(5/6): 810-818. |
| 127 | KEARNS J, KAYSER M M. Application of yeast-catalyzed reductions to synthesis of (2R,3S)-phenylisoserine[J]. Tetrahedron Letters, 1994, 35(18): 2845-2848. |
| 128 | PATEL R N, BANERJEE A, MCNAMEE C G, et al. Enzymatic reduction method for the preparation of compounds useful for preparing taxanes: US5686298 A[P]. 1995. |
| 129 | APPLEGATE G A, CHELOHA R W, NELSON D L, et al. A new dehydrogenase from Clostridium acetobutylicum for asymmetric synthesis: dynamic reductive kinetic resolution entry into the taxotere side chain[J]. Chemical Communications, 2011, 47(8): 2420-2422. |
| 130 | LIANG J, JENNE S J, MUNDORFF E, et al. Methods of using engineered ketoreductase polypeptides for the stereoselective reduction of acetophenones: US8512973[P]. 2013-08-20. |
| 131 | LI H, WANG R, WANG A, et al. Rapidly and precisely crosslinked enzymes using bio-orthogonal chemistry from cell lysate for the synthesis of (S)-1-(2,6-dichloro-3-fluorophenyl) ethanol[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(16): 6466-6478. |
| 132 | CHING C, GRUBER J M, HUISMAN G W, et al. Ketoreductases and uses thereof: US8415127[P]. 2008-02-08. |
| 133 | PATEL R N, CHU L, MUELLER R. Diastereoselective microbial reduction of (S)-[3-chloro-2-oxo-1-(phenylmethyl)propyl]carbamic acid,1,1-dimethylethyl ester[J]. Tetrahedron Asymmetry, 2003, 14(20): 3105-3109. |
| 134 | WU K, ZHENG K, XIONG L, et al. Efficient synthesis of an antiviral drug intermediate using an enhanced short-chain dehydrogenase in an aqueous-organic solvent system[J]. Applied Microbiology and Biotechnology, 2019, 103(11): 4417-4427. |
| 135 | YADAV J S, REDDY P T, NANDA S, et al. Stereoselective synthesis of (R)-(-)-denopamine, (R)-(-)-tembamide and (R)-(-)-aegeline via asymmetric reduction of azidoketones by Daucus carota in aqueous medium[J]. Tetrahedron Asymmetry, 2002, 12(24): 3381-3385. |
| 136 | GOSWAMI J, BEZBARUAH R L, GOSWAMI A, et al. A convenient stereoselective synthesis of (R)-(-)-denopamine and (R)-(-)-salmeterol[J]. Cheminform, 2002, 12(24): 3343-3348. |
| 137 | ZHANG R, REN J, WANG Y, et al. Isolation and characterization of a novel Rhodococcus strain with switchable carbonyl reductase and para-acetylphenol hydroxylase activities[J]. Journal of Industrial Microbiology & Biotechnology, 2013, 40(1): 11-20. |
| 138 | LIN H, CHEN Y Z, XU X Y, et al. Preparation of key intermediates of adrenergic receptor agonists: highly enantioselective production of (R)-α-halohydrins with Saccharomyces cerevisiae CGMCC 2.396[J]. Journal of Molecular Catalysis B: Enzymatic, 2009, 57(1/2/3/4): 1-5. |
| 139 | ROCHA L C, FERREIRA H V, PIMENTA E F, et al. Biotransformation of α-bromoacetophenones by the marine fungus Aspergillus sydowii[J]. Marine Biotechnology, 2010, 12(5): 552-557. |
| 140 | TOKOSHIMA D, HANAYA K, SHOJI M, et al. Whole-cell yeast-mediated preparation of (R)-2-chloro-1-(3-nitrophenyl)ethanol as a synthetic precursor for (R)-phenylephrine[J]. Journal of Molecular Catalysis B: Enzymatic, 2013, 97: 95-99. |
| 3 | MATSUDA T, YAMANAKA R, NAKAMURA K. Biocatalytic asymmetric reduction of C̿ O and activated C̿ C bonds in stereoselective synthesis: stereoselective synthesis of drugs and natural products[M]. John Wiley & Sons, Inc, 2013. |
| 4 | CHEN X, ZHENG Y G, LIU Z Q, et al. Stereoselective determination of 2-benzamidomethyl-3-oxobutanoate and methyl-2-benzoylamide-3-hydroxybutanoate by chiral high-performance liquid chromatography in biotransformation[J]. Journal of Chromatography B, 2015, 974: 57-64. |
| 141 | XU G C, YU H L, ZHANG X Y, et al. Access to optically active aryl halohydrins using a substrate-tolerant carbonyl reductase discovered from Kluyveromyces thermotolerans[J]. ACS Catalysis, 2012, 2(12): 2566-2571. |
| 142 | ALVIZO O, COLLIER S J, HENNEMANN J, et al. Ketoreductase polypeptides for the preparation of phenylephrine: US9834758[P]. 2017-12-05. |
| 143 | PENG G J, CHO Y C, FU T K, et al. Enantioselective synthesis of (S)-phenylephrine by recombinant Escherichia coli cells expressing the short-chain dehydrogenase/reductase gene from Serratia quinivorans BCRC 14811[J]. Process Biochemistry, 2013, 48(10): 1509-1515. |
| 144 | KUAN Y C, XU Y B, WANG W C, et al. Enantioselective synthesis of (R)-phenylephrine by Serratia marcescens BCRC10948 cells that homologously express SM_SDR[J]. Enzyme & Microbial Technology, 2018, 110: 14-19. |
| 145 | Alba DÍAZ-RODRÍGUEZ, Wioleta BORZĘCKA, LAVANDERA Iván, et al. Stereodivergent preparation of valuable γ- or δ-hydroxy esters and lactones through one-pot cascade or tandem chemoenzymatic protocols[J]. ACS Catalysis, 2013, 4(2): 386-393. |
| 146 | JI X J, HUANG H, ZHU J G, et al. Engineering Klebsiella oxytoca for efficient 2,3-butanediol production through insertional inactivation of acetaldehyde dehydrogenase gene[J]. Applied Microbiology & Biotechnology, 2010, 85(6): 1751-1758. |
| 147 | WANG Z, SONG Q Q, YU M, et al. Characterization of a stereospecific acetoin(diacetyl) reductase from Rhodococcus erythropolis WZ010 and its application for the synthesis of (2S,3S)-2,3-butanediol[J]. Applied Microbiology & Biotechnology, 2014, 98(2): 641-650. |
| 148 | HE Y Z, CHEN F X, SUN M J, et al. Efficient (3S)-acetoin and (2S,3S)-2,3-butanediol production from meso-2,3-butanediol using whole-cell biocatalysis[J]. Molecules, 2018, 23(3): 691. |
| 149 | SINGH A, CHISTI Y, BANERJEE U C. Stereoselective biocatalytic hydride transfer to substituted acetophenones by the yeast Metschnikowia koreensis[J]. Process Biochemistry, 2012, 47(12): 2398-2404. |
| 150 | VITALE P, D'INTRONO C, PERNA F M, et al. Kluyveromyces marxianus CBS 6556 growing cells as a new biocatalyst in the asymmetric reduction of substituted acetophenones[J]. Tetrahedron Asymmetry, 2013, 44(35): 389-394. |
| 151 | ROCHA-MARTÍN J, VEGA D, BOLIVAR J M, et al. Characterization and further stabilization of a new anti-Prelog specific alcohol dehydrogenase from Thermus thermophilus HB27 for asymmetric reduction of carbonyl compounds[J]. Bioresource Technology, 2012, 103(1): 343-350. |
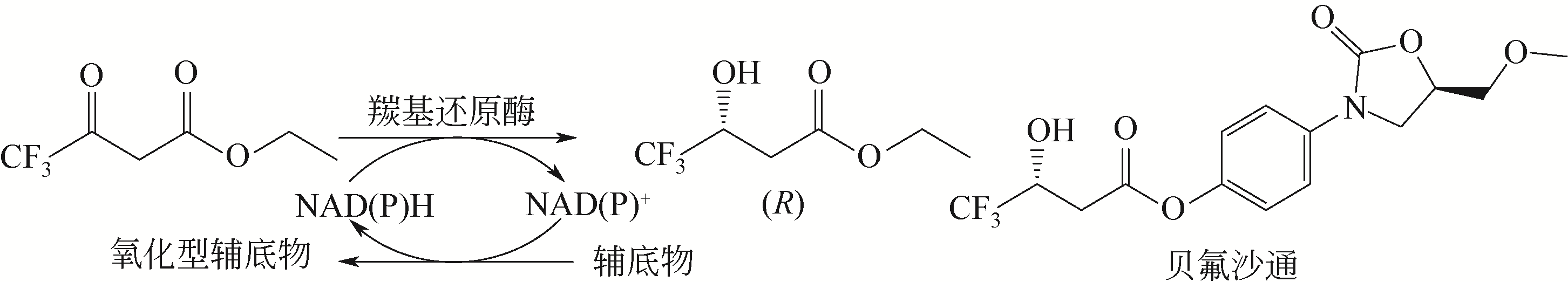
| 152 | WU X, ZHANG C, ORITA I, et al. Thermostable alcohol dehydrogenase from Thermococcus kodakarensis KOD1 for enantioselective bioconversion of aromatic secondary alcohols[J]. Applied and Environmental Microbiology, 2013, 79(7): 2209-2217. |
| 153 | ZHANG R Z, GENG Y W, XU Y, et al. Carbonyl reductase SCRII from Candida parapsilosis catalyzes anti-Prelog reaction to (S)-1-phenyl-1,2-ethanediol with absolute stereochemical selectivity[J]. Bioresource Technology, 2011, 102(2): 483-489. |
| 154 | ZHANG R Z, ZHANG B T, XU Yet al. Efficicent (R)-phenylethanol production with enantioselectivity-alerted (S)-carbonyl reductase II and NADPH regeneration[J]. PLoS One, 2013, 8(12): e83586. |
| 155 | RAO J X, ZHANG R Z, LIANG H B, et al. Efficient chiral synthesis by Saccharomyces cerevisiae spore encapsulation of Candida parapsilosis Glu228Ser/(S)-carbonyl reductase Ⅱ and Bacillus sp. YX-1 glucose dehydrogenase in organic solvents[J]. Microbial Cell Factories, 2019, 18(1): 87. |
| 156 | QIN F, QIN B, MORI T, et al. Engineering of Candida glabrata ketoreductase 1 for asymmetric reduction of α-halo ketones[J]. ACS Catalysis, 2016, 6: 6135-6140. |
| 5 | LUO X, WANG Y J, ZHENG Y G, et al. Cloning and characterization of a NADH-dependent aldo-keto reductase from a newly isolated Kluyveromyces lactis XP1461[J]. Enzyme and Microbial Technology, 2015, 77: 68-77. |
| 6 | KALUZNA W A, MATSUDA T, SEWELL A K, et al. Systematic investigation of Saccharomyces cerevisiae enzymes catalyzing carbonyl reductions[J]. Journal of the American Chemical Society, 2004, 126: 12827-12832. |
| 7 | XU Q, TAO W Y, HUANG H, et al. Highly efficient synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate by a novel carbonyl reductase from Yarrowia lipolytica and using mannitol or sorbitol as cosubstrate[J]. Biochemical Engineering Journal, 2016, 106: 61-67. |
| 8 | CHEN X, LIU Z Q, HUANG J F, et al. Asymmetric synthesis of optically active methyl-2-benzamido-methyl-3-hydroxy-butyrate by robust short-chain alcohol dehydrogenases from Burkholderia gladioli[J]. Chemical Communication, 2015, 51(61): 12328-12331. |
| 9 | WANG L J, LI C X, NI Y, et al. Highly efficient synthesis of chiral alcohols with a novel NADH-dependent reductase from Streptomyces coelicolor[J]. Biotechnology Technology, 2011, 102: 7023-7028. |
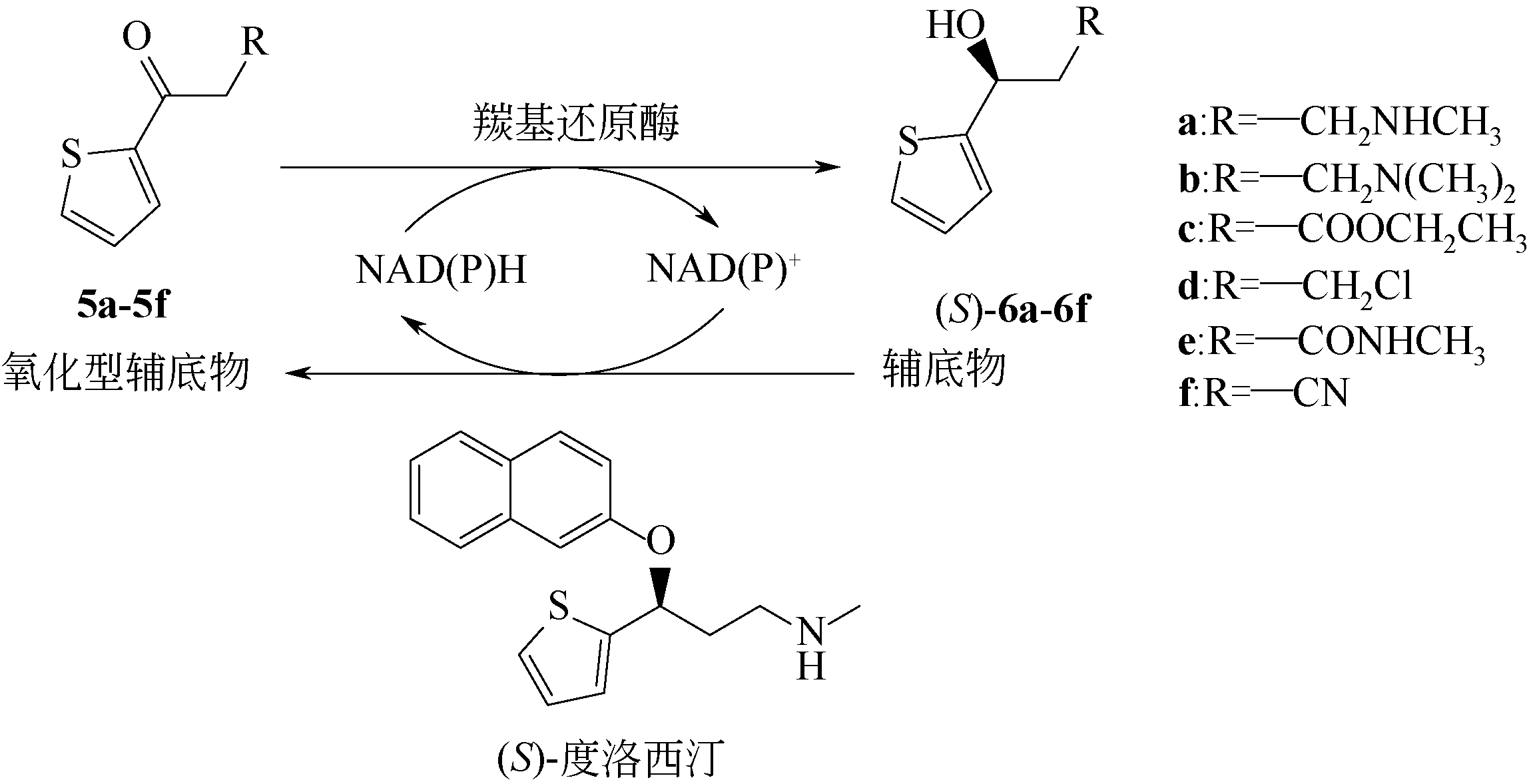
| 10 | CHEN X, LIU Z Q, LIN C P, et al. Chemoenzymatic synthesis of (S)-duloxetine using carbonyl reductase from Rhodosporidium toruloides[J]. Bioorganic Chemistry, 2016, 65: 82-89. |
| 11 | HOLLMANN F, ARENDS I W C E, HOLTMANN D. Enzymatic reductions for the chemist[J]. Green Chemistry, 2011, 13(9): 2285-2314. |
| 12 | ZHENG Y G, YIN H H, YU D F, et al. Recent advances in biotechnological applications of alcohol dehydrogenases[J]. Applied Microbiology & Biotechnology, 2017, 101(3): 1-15. |
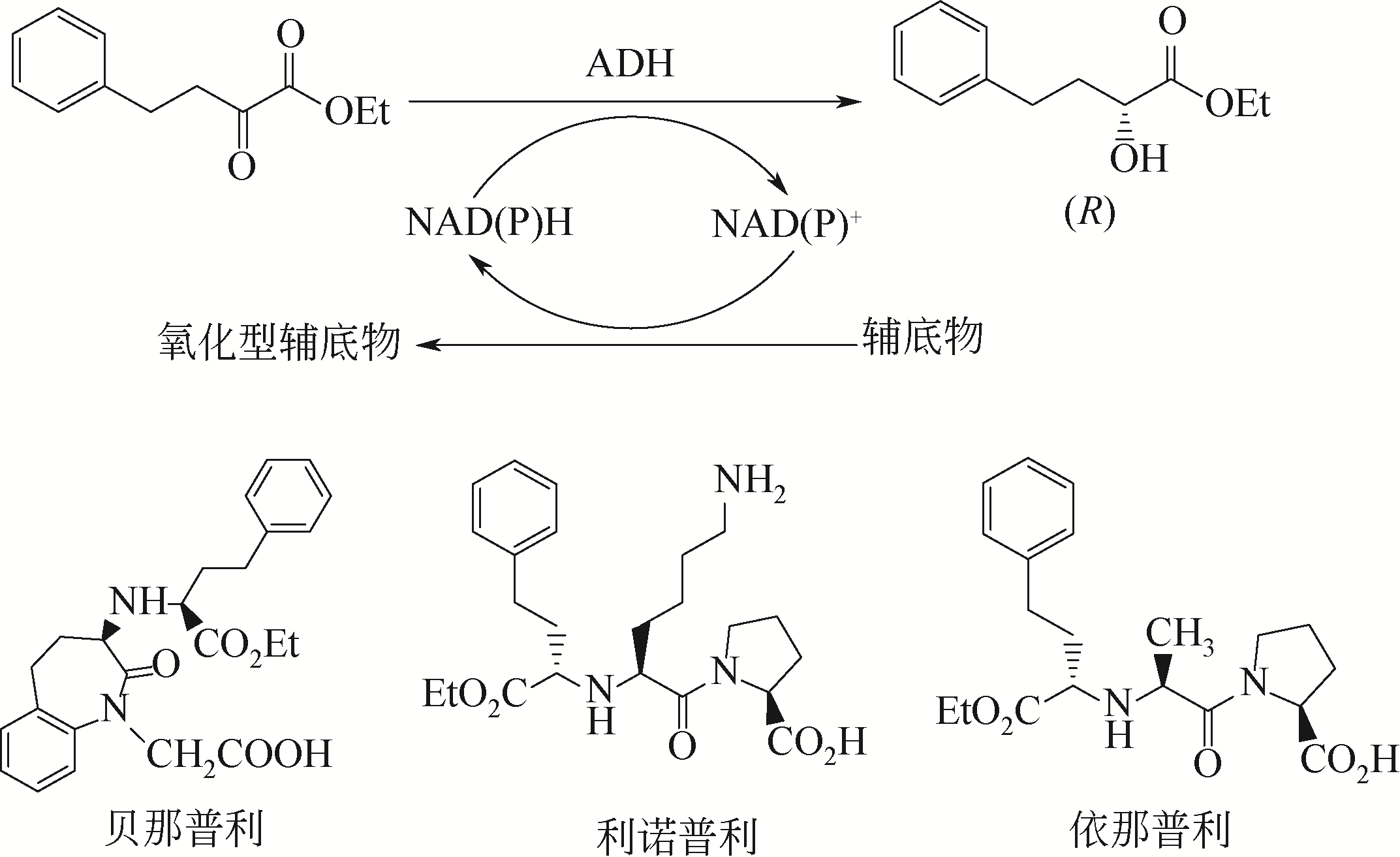
| 13 | FREY J, RUSCHE H, SCHINK B, et al. Cloning, functional expression and characterization of a bifunctional 3-hydroxybutanal dehydrogenase/reductase involved in acetone metabolism by Desulfococcus biacutus[J]. BMC Microbiology, 2016, 16(1): 280. |
| 14 | PENNING T M. The aldo-keto reductases (AKRs): overview[J]. Chemico-Biological Interactions, 2015, 234: 236-246. |
| 15 | JORNVALL H, PERSSON M, JEFFERY J. Alcohol and polyol dehydrogenases are both divided into two protein types, and structural properties cross-relate the different enzyme activities within each type[J]. Proceedings of the National Academy of Sciences of the United States of America, 1981, 78(7): 4226-4230. |
| 16 | PERSSON B, KROOK M, JÖRNVALL H. Characteristics of short-chain alcohol dehydrogenases and related enzymes[J]. European Journal of Biochemistry, 2010, 200(2): 537-543. |
| 17 | KALLBERG Y, OPPERMANN U, PERSSON B. Classification of the short-chain dehydrogenase/reductase superfamily using hidden Markov models[J]. FEBS Journal, 2010, 277(10): 2375-2386. |
| 18 | KAVANAGH K, JORNVALL H, PERSSON B, et al. The SDR superfamily: functional and structural diversity within a family of metabolic and regulatory enzymes[J]. Cellular and Molecular Life Sciences, 2008, 65(24): 3895-3906. |
| 19 | PERSSON B, KALLBERG Y. Classification and nomenclature of the superfamily of short-chain dehydrogenases/reductases (SDRs)[J]. Chemico-Biological Interactions, 2013, 202(1/2/3): 111-115. |
| 20 | MAETINS B M, MACEDO-RIBERIRO S, BRESSER J, et al. Structural basis for stereo-specific catalysis in NAD+-dependent (R)-2-hydroxyl glutarate dehydrogenase from Acidaminococcus fermentans[J]. The FEBS Journal, 2005, 272: 269-281. |
| 21 | LESK A M. NAD-binding domains of dehydrogenases[J]. Current Opinion in Structural Biology, 1995, 5(6): 775-83. |
| 22 | FILLING C, BERNDT K D, BENACH J, et al. Critical residues for structure and catalysis in short-chain dehydrogenases/reductases[J]. Journal of Biological Chemistry, 2002, 277(28): 25677. |
| 23 | KOUMANOV A, BENACH J, ATRIAN S, et al. The catalytic mechanism of Drosophila alcohol dehydrogenase: evidence for a proton relay modulated by the coupled ionization of the active site Lysine/Tyrosine pair and a NAD+ ribose OH switch[J]. Proteins-Structure Function & Bioinformatics, 2010, 51(2): 289-298. |
| 24 | OPPERMANU U C T, FILLING C, BERNDT K D, et al. Active site directed mutagenesis of 3β/17β-hydroxysteroid dehydrogenase establishes differential effects on short-chain dehydrogenase/reductase reactions[J]. Biochemistry, 1997, 36(1): 34-40. |
| 25 | JÖRNVALL H, HEDLUND J, BERGMAN T, et al. Origin and evolution of medium chain alcohol dehydrogenases[J]. Chemico-Biological Interactions, 2013, 202(1/2/3): 91-96. |
| 26 | TANEJA B, MANDE S C. Conserved structural features and sequence patterns in the GroES fold family[J]. Protein Engineering, 1999, 12: 815-818. |
| 27 | MAN H, GARGIULO S, FRANK A, et al. Structure of the NADH-dependent thermostable alcohol dehydrogenase TADH from Thermus sp. ATN1 provides a platform for engineering specificity and improved compatibility with inorganic cofactor-regeneration catalysts[J]. Journal of Molecular Catalysis B: Enzymatic, 2014, 105: 1-6. |
| 28 | MAN H, LODERER C, MARION B, et al. Structure of NADH‐dependent carbonyl reductase (CPCR2) from Candida parapsilosis provides insight into mutations that improve catalytic properties[J]. ChemCatChem, 2014, 6(4): 1103-1111. |
| 29 | HEDLUND J, JÖRNVALL H, PERSSON B. Subdivision of the MDR superfamily of medium chain dehydrogenases/reductases through iterative hidden Markov model refinement[J]. BMC Bioinformatics, 2010, 11: 534. |
| 30 | JÖRNVALL H, HEDLUND J, BERGMAN T, et al. Superfamilies SDR and MDR: from early ancestry to present forms. emergence of three lines, a Zn-metalloenzyme, and distinct variabilities[J]. Biochemical & Biophysical Research Communications, 2010, 396(1): 125-130. |
| 31 | HYNDMAN D, BAUMAN D R, HEREDIA V V, et al. The aldo-keto reductase superfamily homepage[J]. Chemico-Biological Interactions, 2003, 143/144: 621-631. |
| 32 | LAPTHORN A J, ZHU X F, ELLIS E M. The diversity of microbial aldo/keto reductases from Escherichia coli K12[J]. Chemico-Biological Interactions, 2013, 202: 168-177. |
| 33 | JEZ J M, BENNETT M J, SCHLEGEL B P, et al. Comparative anatomy of the aldo-keto reductase superfamily[J]. Biochemical Journal, 1997, 326(3): 625-636. |
| 34 | MINDINICH R D, PENNING T M. Aldo-keto reductase (AKR) superfamily: genomics and annotation[J]. Human Genomics, 2009, 3(1): 362-370. |
| 35 | PENNING T M, DRURY J E. Human aldo-keto reductases: function, gene regulation, and single nucleotide polymorphisms[J]. Archives of Biochemistry & Biophysics, 2007, 464(2): 241-250. |
| 36 | NI Y, LI C X, MA H M, et al. Biocatalytic properties of a recombinant aldo-keto reductase with broad substrate spectrum and excellent stereoselectivity[J]. Applied Microbiology and Biotechnology, 2011, 89(4): 1111-1118. |
| 37 | NING C X, SU E Z, WEI D Z. Characterization and identification of three novel aldo-keto reductases from Lodderomyces elongisporus for reducing ethyl 4-chloroacetoacetate[J]. Archives of Biochemistry & Biophysics, 2014, 564: 219-228. |
| 38 | GUO R Y, NIE Y, MU X Q, et al. Genomic mining-based identification of novel stereospecific aldo-keto reductases toolbox from Candida parapsilosis for highly enantioselective reduction of carbonyl compounds[J]. Journal of Molecular Catalysis B: Enzymatic, 2014, 105: 66-73. |
| 39 | XU Y P, GUAN Y H, YU H L, et al. Improved o-chlorobenzoylformate bioreduction by stabilizing aldo-keto reductase YtbE with additives[J]. Journal of Molecular Catalysis B: Enzymatic, 2014, 104: 108-114. |
| 40 | ELLIS E M. Microbial aldo-keto reductases[J]. FEMS Microbiology Letters, 2002, 216: 123-131. |
| 41 | RUIZ F X, COUSIDO-SIAH A, MITSCHLER A, et al. X-ray structure of the V301L aldo-keto reductase 1B10 complexed with NADP+ and the potent aldose reductase inhibitor fidarestat: implications for inhibitor binding and selectivity[J]. Chemico-biological Interactions, 2013, 202(1-3): 178-185. |
| 42 | YAMAMOTO K, HIGASHIURA A, SUZUKI M, et al. Identification, characterization, and crystal structure of an aldo-keto reductase (AKR2E4) from the silkworm Bombyx mori [J]. Biochemical & Biophysical Research Communications, 2013, 538(1): 156-163. |
| 43 | COSTANZO L D, PENNING T M, CHRISTIANSON D W. Aldo-keto reductases in which the conserved catalytic histidine is substituted[J]. Chemico-Biological Interactions, 2009, 178(1/2/3): 127-133. |
| 44 | LIU X, WANG C, ZHANG L J, et al. Structural and mutational studies on an aldo-keto reductase AKR5C3 from Gluconobacter oxydans[J]. Protein Society, 2014, 23: 1540-1549. |
| 45 | HONG S H, NAM H K, KIM K R, et al. Molecular characterization of an aldo-keto reductase from Marivirga tractuosa that converts retinal to retinol[J]. Journal of Biotechnology, 2014, 169: 23-33. |
| 46 | QUINTARD A, ALEXAKIS A. Stereoselective synthesis of drugs and natural products[M]. New York: Wiley, 2013. |
| 47 | WEI P, GUO Z, WU X, et al. Significantly enhancing the biocatalytic synthesis of chiral alcohols by semi-rationally engineering an anti-Prelog carbonyl reductase from Acetobacter sp. CCTCC M209061[J]. Molecular Catalysis, 2019, 479: 110613. |
| 48 | GONG X M, QIN Z, LI F L, et al. Development of an engineered ketoreductase with simultaneously improved thermostability and activity for making a bulky atorvastatin precursor[J]. ACS Catalysis, 2019, 9(1): 147-153. |
| 49 | HONDA K, INOUE M, ONO T, et al. Improvement of operational stability of Ogataea minuta carbonyl reductase for chiral alcohol production[J]. Journal of Bioscience and Bioengineering, 2017, 123(6): 673-678. |
| 50 | QIN F, QIN B, ZHANG W, et al. Discovery of a switch between Prelog and anti-Prelog reduction toward halogen-substituted acetophenones in short-chain dehydrogenase/reductases[J]. ACS Catalysis, 2018, 8(7): 6012-6020. |
| 51 | ZHANG W, ZHU T, LI H, et al. Key sites insight on the stereoselectivity of four mined aldo-keto reductases toward α-keto esters and halogen-substituted acetophenones[J]. Applied Microbiology and Biotechnology, 2019, 103(15): 6119-6128. |
| 52 | WU H, TIAN C Y, SONG X K, et al. Methods for the regeneration of nicotinamide coenzymes[J]. Green Chemistry, 2013, 15: 1773-1789. |
| 53 | KARA S, SCHRITTWIESER J H, HOLLMANNF, et al. Recent trends and novel concepts in cofactor-dependent biotransformations[J]. Applied Microbiology and Biotechnology, 2014, 98: 1517-1529. |
| 54 | RODRIGUEZ C, LAVANDERA I, GOTOR V. Recent advances in cofactor regeneration systems applied to biocatalyzed oxidative processes[J]. Current Organic Chemistry, 2012, 16: 2525-2541. |
| 55 | WECKBECKER A, GROGER H, HUMMEL W. Regeneration of nicotinamide coenzymes: principles and applications for the synthesis of chiral compounds[J]. Advances in Biochemical Engineering/Biotechnology, 2010, 120: 195-242. |
| 56 | LIU Z Q, YE J J, YANG Z, et al. Upscale production of ethyl (S)-4-chloro-3-hydroxybutanoate by using carbonyl reductase coupled with glucose dehydrogenase in aqueous-organic solvent system[J]. Applied Microbiology & Biotechnology, 2015, 99(5): 239-2129. |
| 57 | ZHANG X J, WANG W Z, ZHOU R, et al. Asymmetric synthesis of tert-butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate using a self-sufficient biocatalyst based on carbonyl reductase and cofactor co-immobilization[J]. Bioprocess and Biosystems Engineering, 2020, 43(1): 21-31. |
| 58 | KALIAPERUMAL T, GUMMADI S N, CHADHA A. Synthesis of both enantiomers of ethyl-4-chloro-3-hydroxbutanoate from a prochiral ketone using Candida parapsilosis ATCC 7330[J]. Tetrahedron Asymmetry, 2011, 22(14/15): 1548-1552. |
| 59 | PATEL R N, MCNAMEE C G, BANERJEE A, et al. Stereoselective reduction of β-keto esters by Geotrichum candidum[J]. Enzyme & Microbial Technology, 1992, 14(9): 731-738. |
| 60 | YE Q, OUYANG P, YING H. A review-biosynthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate ester: recent advances and future perspectives[J]. Applied Microbiology & Biotechnology, 2011, 89(3): 513-522. |
| 61 | YOU Z Y, LIU Z Q, ZHENG Y G. Characterization of a newly synthesized carbonyl reductase and construction of a biocatalytic process for the synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate with high space-time yield[J]. Applied Microbiology & Biotechnology, 2014, 98(4): 1671-1680. |
| 62 | CAI P, AN M, XU L, et al. Development of a substrate-coupled biocatalytic process driven by an NADPH-dependent sorbose reductase from Candida albicans for the asymmetric reduction of ethyl4-chloro-3-oxobutanoate[J]. Biotechnology Letters, 2012, 34(12): 2223-2227. |
| 63 | LUO W, DU H J, BONKU E M, et al. An alkali-tolerant carbonyl reductase from Bacillus subtilis by gene mining: identification and application[J]. Catalysis Letters, 2019, 149(11): 2973-2983. |
| 64 | YANG Z Y, YE W T, XIE Y, et al. Efficient asymmetric synthesis of ethyl (S)-4-Chloro-3-hydroxybutyrate using alcohol dehydrogenase SmADH31 with high tolerance of substrate and product in a monophasic aqueous system[J]. Organic Process Research & Development, 2020, 24(6): 1068-1076. |
| 65 | LUO X, WANG Y J, SHEN W, et al. Activity improvement of a Kluyveromyces lactis aldo-keto reductase KlAKR via rational design[J]. Journal of Biotechnology, 2016, 224: 20-26. |
| 66 | WANG Y J, YING B B, SHEN W, et al. Rational design of Kluyveromyces marxianus ZJB14056 aldo-keto reductase KmAKR to enhance diastereoselectivity and activity[J]. Enzyme & Microbial Technology, 2017, 107: 32-40. |
| 67 | YU H, QIU S, CHENG F, et al. Improving the catalytic efficiency of aldo-keto reductase KmAKR towards t-butyl 6-cyano-(3R,5R)-dihydroxyhexanoate via semi-rational design[J]. Bioorganic Chemistry, 2019, 90: 103018. |
| 68 | QIU S, CHENG F, JIN L J, et al. Co-evolution of activity and thermostability of an aldo-keto reductase KmAKR for asymmetric synthesis of statin precursor dichiral diols[J]. Bioorganic Chemistry, 2020, 103: 104228. |
| 69 | WU X, WANG L, WANG S, et al. Stereoselective introduction of two chiral centers by a single diketoreductase: an efficient biocatalytic route for the synthesis of statin side chains[J]. Amino Acids, 2010, 39(1): 305-308. |
| 70 | WU X, CHEN C, LIU N, et al. Preparation of ethyl 3R,5S-6-(benzyloxy)-3,5-dihydroxy-hexanoate by recombinant diketoreductase in a biphasic system[J]. Bioresource Technology, 2011, 102(3): 3649-3652. |
| 71 | WU X, JIANG J, CHEN Y. Correlation between intracellular cofactor concentrations and biocatalytic efficiency: coexpression of diketoreductase and glucose dehydrogenase for the preparation of chiral diol for statin drugs[J]. ACS Catalysis, 2014, 1(12): 1661-1664. |
| 72 | HE X, CHEN S, WU J, et al. Highly efficient enzymatic synthesis of tert-butyl (S)-6-chloro-5-hydroxy-3-oxohexanoate with a mutant alcohol dehydrogenase of Lactobacillus kefir[J]. Applied Microbiology and Biotechnology, 2015, 99(21): 8963-8975. |
| 73 | LIU Z Q, WU L, ZHANG X J, et al. Directed evolution of carbonyl reductase from Rhodosporidium toruloides and its application in stereoselective synthesis of tert-butyl (3R,5S)-6-Chloro-3,5-dihydroxyhexanoate[J]. Journal of Agricultural & Food Chemistry, 2017, 65(18): 3721. |
| 74 | LIU Z Q, WU L, ZHENG L, et al. Biosynthesis of tert-butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate by carbonyl reductase from Rhodosporidium toruloides in mono and biphasic media[J]. Bioresource Technology, 2017, 249: 161. |
| 75 | ZHANG X J, ZHENG L, WU D, et al. Production of tert-butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate using carbonyl reductase coupled with glucose dehydrogenase with high space-time yield[J]. Biotechnology Progress, 2020, 36 (1): 21672190. |
| 76 | 吴结丰, 徐利敏. β-内酰胺的研究进展[J]. 甘肃石油和化工, 2007, 21(3): 23-27. |
| WU Jiefeng, XU Limin. Research progress of β-lactam[J]. Gansu Petroleum and Chemical Industry, 2007, 21(3): 23-27. | |
| 77 | LIU Z Q, DONG S C, YIN H H, et al. Enzymatic synthesis of an ezetimibe intermediate using carbonyl reductase coupled with glucose dehydrogenase in an aqueous-organic solvent system[J]. Bioresource Technology, 2017, 229: 26-32. |
| 78 | ZHANG X J, ZHOU R, WU D, et al. Efficient production of an ezetimibe intermediate using carbonyl reductase coupled with glucose dehydrogenase[J]. Biotechnology Progress, 2020, DOI:org/10.1002/btpr.3068. |
| 79 | MATSUYAMA A, YAMAMOTO H, KAWADA N, et al. Industrial production of (R)-1,3-butanediol by new biocatalysts[J]. Journal of Molecular Catalysis B: Enzymatic, 2001, 11(4/5/6): 513-521. |
| 80 | YAMAMOTO H, MATSUYAMA A, KOBAYASHI Y. Synthesis of 1,3-butanediol by enantioselective oxidation using whole recombinant cells expressing-specific secondary alcohol dehydrogenase[J]. Bioence Biotechnology and Biochemistry, 2014, 66(4): 925-927. |
| 81 | ZHENG R C, GE Z, QIU Z K, et al. Asymmetric synthesis of (R)-1,3-butanediol from 4-hydroxy-2-butanone by a newly isolated strain Candida krusei ZJB-09162[J]. Applied Microbiology & Biotechnology, 2012, 94(4): 969-976. |
| 82 | NOYORI R, IKEDA T, OHKUMA T, et al. Stereoselective hydrogenation via dynamic kinetic resolution[J]. Journal of the American Chemical Society, 1989, 111(25): 8291-8327. |
| 83 | SHIMODA K, KUBOTA N, HAMADA H, et al. Production of (2R,3S)-2-benzamidomethyl-3-hydroxybutanoates by immobilized plant cells of Parthenocissus tricuspidata[R]. Biochemistry Insights, 2009. Doi. 10.4137/BCI.S961. |
| 84 | GANDOLFI R, CESAROTTI E, MOLINARI F, et al. Asymmetric reductions of ethyl 2-(benzamidomethyl)-3-oxobutanoate by yeasts[J]. Tetrahedron Asymmetry, 2009, 20(4): 43-414. |
| 85 | RIMOLDI I, CESAROTTI E, ZERLA D, et al. 3-(Hydroxy(phenyl) methyl) azetidin-2-ones obtained via catalytic asymmetric hydrogenation or by biotransformation[J]. Tetrahedron Asymmetry, 2011, 22(5): 597-602. |
| 86 | YASOHARA Y, YANO M, KAWANO S, et al. Method for producing optically active2-(substituted aminomethyl)-3-hydroxybutyric acid ester: US2009104671[P]. 2008-04-09. |
| 87 | VOLKMANN R A, KELBAUGH P R, NASON D M, et al. 2-Thioalkyl penems: an efficient synthesis of sulopenem, a (5R,6S)-6-(1(R)-hydroxyethyl)-2-[(cis-1-oxo-3-thiolanyl) thio]-2-penem antibacterial[J]. Journal of Organic Chemistry, 1992, 57(16): 4352-4361. |
| 88 | LIANG J, MUNDORFF E, VOLADRI R, et al. Highly enantioselective reduction of a small heterocyclic ketone: biocatalytic reduction of tetrahydrothiophene-3-one to the corresponding (R)-alcohol[J]. Organic Process Research & Development, 2010, 14(1): 188-192. |
| 89 | WHEELER W J, KUO F. An asymmetric synthesis of duloxetine hydrochloride, a mixed uptake inhibitor of serotonin and norepinephrine, and ITS C-14 labeled isotopomers[J]. Journal of Labelled Compounds and Radiopharmaceuticals, 1995, 36(3): 213-223. |
| 90 | SONI P, KANSAL H, BANERJEE U C. Optimization of process parameters for the production of carbonyl reductase by Candida viswanathii in a laboratory-scale fermentor[J]. Journal of Industrial Microbiology & Biotechnology, 2008, 35(3): 167-173. |
| 91 | SAVILE C, GRUBER J M, MUNDORFF E, et al. Ketoreductase polypeptides for the production of a3-aryl-3-hydroxypropanamine from a 3-aryl-3-ketopropanamine: US8673607[P]. 2014-03-18. |
| 92 | WANG Y J, LIU X Q, LUO X, et al. Cloning, expression and enzymatic characterization of an aldo-keto reductase from Candida albicans XP1463[J]. Journal of Molecular Catalysis B: Enzymatic, 2015, 122: 44-50. |
| 93 | CURET O, DAMOISEAU G, AUBIN N, et al. Befloxatone, a new reversible and selective monoamine oxidase-A inhibitor[J]. Journal of Pharmacology and Experimental Therapeutics, 1996, 277: 253-264. |
| [1] | LI Huahua, LI Yihang, JIN Beichen, LI Longxin, CHENG Shao’an. Research progress of Anammox bio-electrochemical coupling wastewater treatment system [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2678-2690. |
| [2] | YUE Xin, LI Chunying, SUN Dao’an, LI Jiangwei, DU Yongmei, MA Hui, LYU Jian. Progress on heterogeneous catalysts for cyclopropanation of diazo compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2390-2401. |
| [3] | MENG Lingding, MAO Menglei, LIAO Qiyong, MENG Zihui, LIU Wenfang. Recent advance in stability of carbonic anhydrase and formate dehydrogenase [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 436-447. |
| [4] | GAO Bo, FENG Xudong, LI Chun. Visual and high-throughput method for detecting the activity of aspartate transcarbamylase [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2054-2059. |
| [5] | ZHANG Yan, WANG Wei, XIE Rui, JU Xiaojie, LIU Zhuang, CHU Liangyin. Controllable fabrication of polymeric microparticles loaded with enzyme@ZIF-8 [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2022-2028. |
| [6] | LI Qingyuan, WANG Chao, XU Shipei, ZHANG Xueqin, QIU Mingjian, LIU Mengyao, CONG Mengxiao. Research progress on reaction process and catalysts for PBS precursor of 1,4-butanediol synthesis [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5771-5782. |
| [7] | LU Zeping, PEI Xinhua, XUE Yu, ZHANG Xiaoguang, HU Yi. Chemical modification of porcine pancreatic lipase with betaine ionic liquid to improve its enzymatic properties [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 6045-6052. |
| [8] | LI Qing, LIU Wujun, GUO Xiaojia, WANG Qian, ZHAO Zongbao. Chiral NAD analogs as cofactors for biocatalysis [J]. Chemical Industry and Engineering Progress, 2021, 40(9): 5214-5221. |
| [9] | JU Shuyun, WU Jianping, YANG Lirong. Advances in the molecular modification and application of D-amino acid oxidase [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1215-1225. |
| [10] | Zhufan LIN, Shao’an CHENG, Zhengzhong MAO, Ruonan GU, Jiawei YANG. Recent advances in the construction and influencing factors of bio-electrochemical nitrogen removal systems [J]. Chemical Industry and Engineering Progress, 2020, 39(9): 3766-3776. |
| [11] | Cheng ZHU,Guochao XU,Wei DAI,Jieyu ZHOU,Ye NI. Effect of position 127 on the activity and enantioselectivity of alcohol dehydrogenase KpADH [J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5504-5511. |
| [12] | Tian JIANG, Xudong FENG, Yan LI, Chu LI. The biocatalysis and enzyme modification of substrate specificity [J]. Chemical Industry and Engineering Progress, 2019, 38(01): 606-614. |
| [13] | YU Bo, LIU Chao, LIU Jindong, DING Wanyu, CHAI Weiping. Preparation of mesoporous zirconium phosphate and its catalytic performace in the preparation of cellulose from glucose [J]. Chemical Industry and Engineering Progress, 2018, 37(06): 2236-2241. |
| [14] | YAN Xingchen, ZHAO Qianru, WANG Kaifeng, GUO Yuxin, JIANG Ling, HUANG He. Auto-induced expression of trehalose synthetase and novel process for catalytic production of trehalose [J]. Chemical Industry and Engineering Progress, 2018, 37(05): 1949-1955. |
| [15] | WANG Rui, XU Yaohui, WANG Kewei, WU Minchen. Expression of PvEH3,a Phaseolus vulgaris epoxide hydrolase,and synthesis of chiral vicinal diols [J]. Chemical Industry and Engineering Progress, 2018, 37(05): 1933-1939. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||