Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (3): 1681-1688.DOI: 10.16085/j.issn.1000-6613.2020-0757
• Resources and environmental engineering • Previous Articles Next Articles
Comprehensive recovery of rare and precious metals from copper-rich solution of copper anode slime
ZHANG Fuyuan( ), ZHANG Jinchi, ZHANG Guang’an, ZHAO Zhuo
), ZHANG Jinchi, ZHANG Guang’an, ZHAO Zhuo
- School of Metallurgical Engineering, Anhui University of Technology, Ma’anshan 243032, Anhui, China
-
Received:2020-05-06Online:2021-03-17Published:2021-03-05 -
Contact:ZHANG Fuyuan
铜阳极泥分铜液综合回收稀贵金属
- 安徽工业大学冶金工程学院,安徽 马鞍山 243032
-
通讯作者:张福元 -
作者简介:张福元(1979—),男,博士,教授,研究方向为稀贵金属分离富集。E-mail:sanzhfy@163.com 。 -
基金资助:国家自然科学基金(U1703130)
CLC Number:
Cite this article
ZHANG Fuyuan, ZHANG Jinchi, ZHANG Guang’an, ZHAO Zhuo. Comprehensive recovery of rare and precious metals from copper-rich solution of copper anode slime[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1681-1688.
张福元, 张金池, 张广安, 赵卓. 铜阳极泥分铜液综合回收稀贵金属[J]. 化工进展, 2021, 40(3): 1681-1688.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-0757
Cu含量 /g·L-1 | As含量 /g·L-1 | As??含量 /g·L-1 | As??含量 /g·L-1 | Te含量 /g·L-1 | Fe含量 /g·L-1 | Se含量 /g·L-1 | Au含量 /mg·L-1 | Ag含量 /g·L-1 | Pt含量 /mg·L-1 | Pd含量 /mg·L-1 |
|---|---|---|---|---|---|---|---|---|---|---|
| 64.88 | 1.12 | 0.71 | 0.41 | 1.01 | 0.38 | 0.15 | 0.37 | 0.17 | 1.24 | 2.12 |
Cu含量 /g·L-1 | As含量 /g·L-1 | As??含量 /g·L-1 | As??含量 /g·L-1 | Te含量 /g·L-1 | Fe含量 /g·L-1 | Se含量 /g·L-1 | Au含量 /mg·L-1 | Ag含量 /g·L-1 | Pt含量 /mg·L-1 | Pd含量 /mg·L-1 |
|---|---|---|---|---|---|---|---|---|---|---|
| 64.88 | 1.12 | 0.71 | 0.41 | 1.01 | 0.38 | 0.15 | 0.37 | 0.17 | 1.24 | 2.12 |
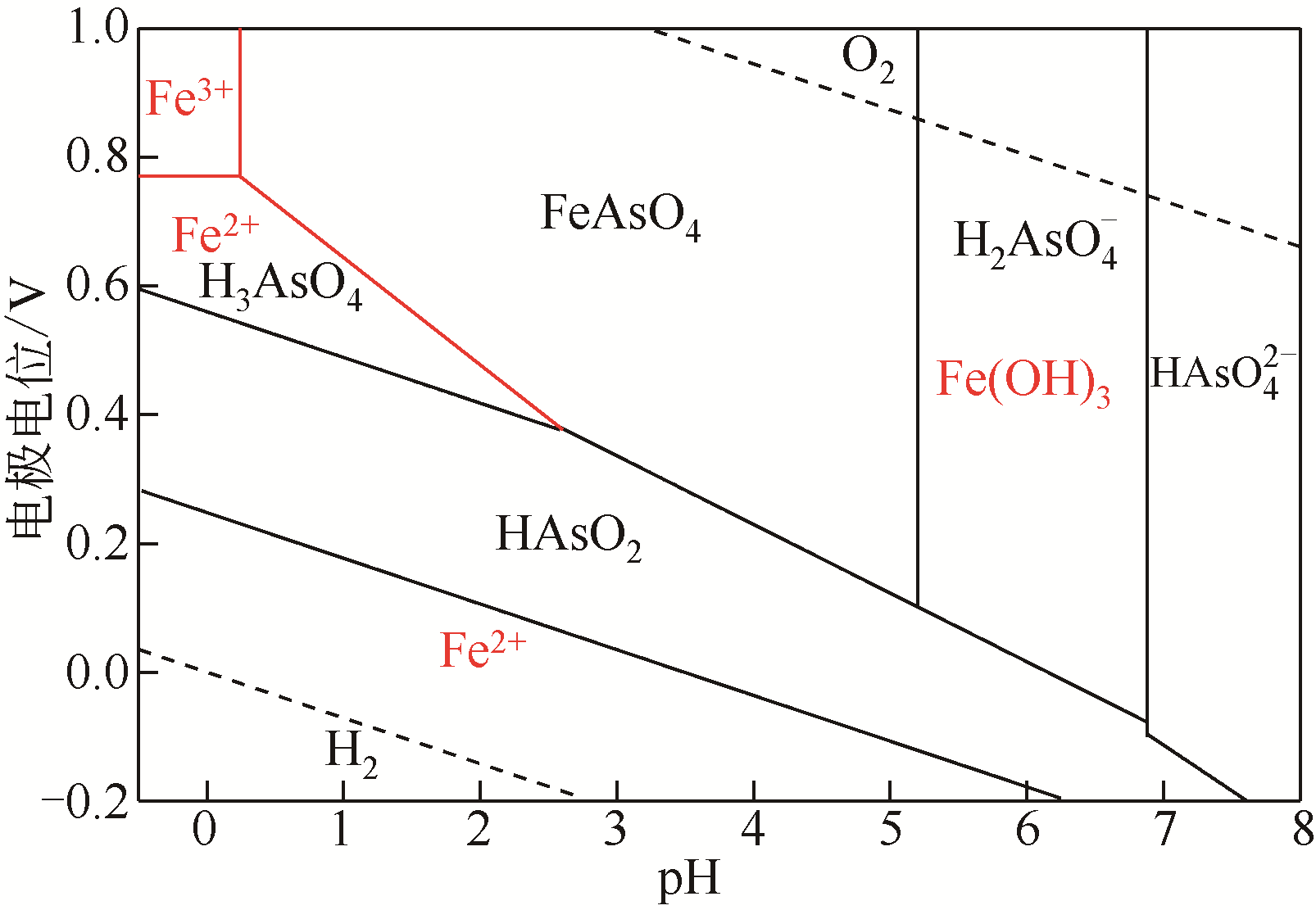
| [Fe3+]/[Fe2+] | 实际电位/V | [Fe3+]/[Fe2+] | 实际电位/V |
|---|---|---|---|
| 1/100000 | 0.416 | 1/10 | 0.700 |
| 1/10000 | 0.487 | 1 | 0.771 |
| 1/1000 | 0.558 | 10/1 | 0.842 |
| 1/100 | 0.629 | 100/1 | 0.913 |
| [Fe3+]/[Fe2+] | 实际电位/V | [Fe3+]/[Fe2+] | 实际电位/V |
|---|---|---|---|
| 1/100000 | 0.416 | 1/10 | 0.700 |
| 1/10000 | 0.487 | 1 | 0.771 |
| 1/1000 | 0.558 | 10/1 | 0.842 |
| 1/100 | 0.629 | 100/1 | 0.913 |
| 电极 | 电极反应 | 标准电极电位φ?/V |
|---|---|---|
| Fe3+/Fe2+ | Fe3++eFe2+ | 0.771 |
| Cu2+/Cu | Cu2++2eCu | 0.342 |
| HTeO | HTeO | 0.551 |
| [PdCl4]2-/Pd | [PdCl4]2-+2ePd+4Cl- | 0.591 |
| [PtCl6]2-/Pt | [PtCl6]2-+4ePt+6Cl- | 0.718 |
| [AuCl2]-/Au | [AuCl2]-+eAu+2Cl- | 1.154 |
| 电极 | 电极反应 | 标准电极电位φ?/V |
|---|---|---|
| Fe3+/Fe2+ | Fe3++eFe2+ | 0.771 |
| Cu2+/Cu | Cu2++2eCu | 0.342 |
| HTeO | HTeO | 0.551 |
| [PdCl4]2-/Pd | [PdCl4]2-+2ePd+4Cl- | 0.591 |
| [PtCl6]2-/Pt | [PtCl6]2-+4ePt+6Cl- | 0.718 |
| [AuCl2]-/Au | [AuCl2]-+eAu+2Cl- | 1.154 |
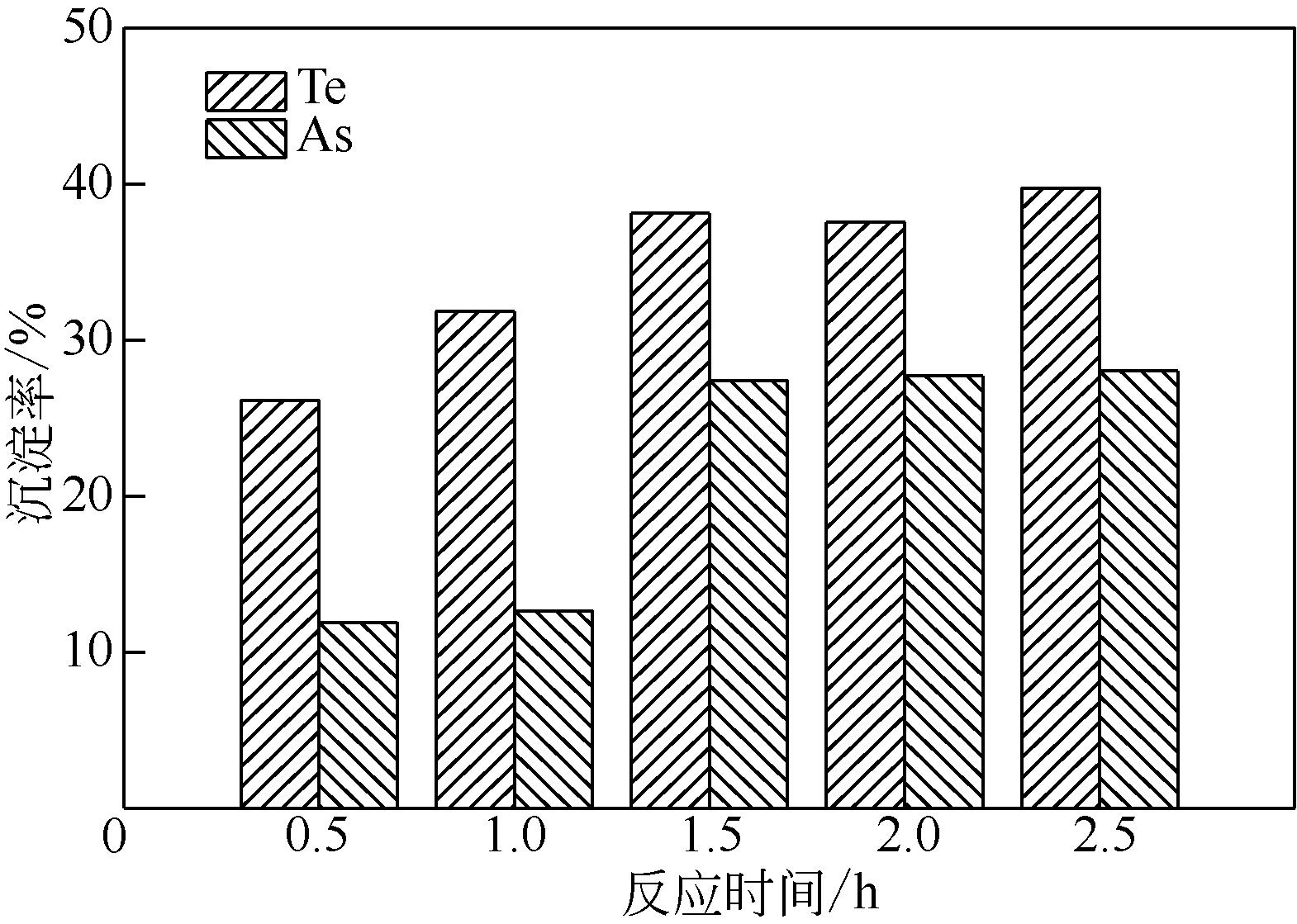
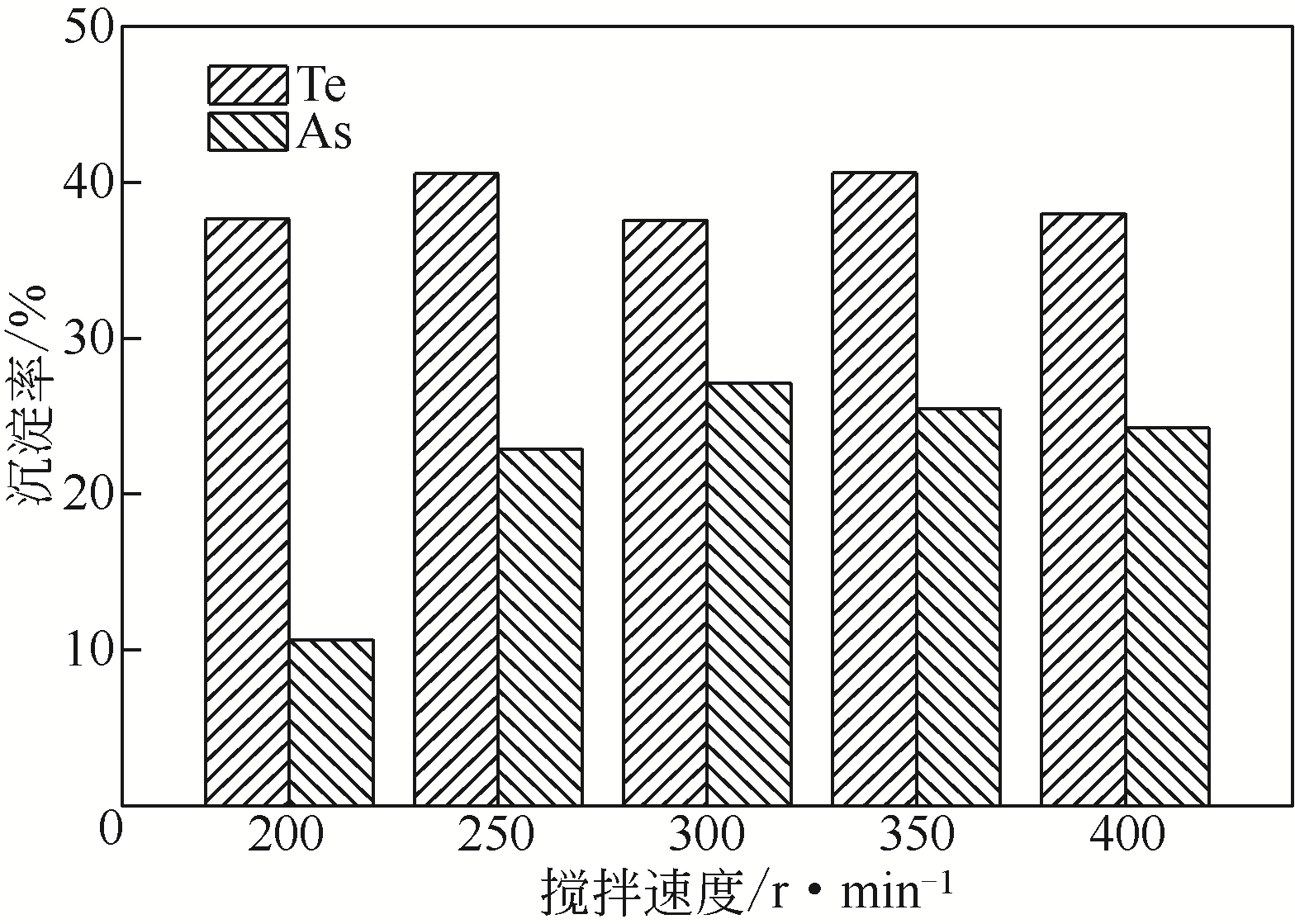
| 样品 | 浓度/g·L-1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Se | As | Te | Fe | Au① | Ag | Pt① | Pd① | |
| 沉淀率/% | — | 33.3 | 16.8 | 36.1 | — | 100 | 100 | 99.2 | 99.6 |
| 分铜液 | 84.13 | 0.15 | 3.28 | 1.55 | 0.22 | 0.35 | 0.21 | 1.33 | 2.42 |
| 沉淀后液1 | 83.17 | 0.11 | 2.70 | 0.96 | 5.97 | 0 | 0 | 0.02 | 0.01 |
| 沉淀后液2 | 79.66 | 0.10 | 2.78 | 0.99 | 5.88 | 0 | 0 | 0.01 | 0.02 |
| 沉淀后液 3 | 83.22 | 0.09 | 2.72 | 1.02 | 6.05 | 0 | 0 | 0 | 0 |
| 平均值 | 82.02 | 0.10 | 2.73 | 0.99 | 5.97 | 0 | 0 | 0.01 | 0.01 |
| 样品 | 浓度/g·L-1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Se | As | Te | Fe | Au① | Ag | Pt① | Pd① | |
| 沉淀率/% | — | 33.3 | 16.8 | 36.1 | — | 100 | 100 | 99.2 | 99.6 |
| 分铜液 | 84.13 | 0.15 | 3.28 | 1.55 | 0.22 | 0.35 | 0.21 | 1.33 | 2.42 |
| 沉淀后液1 | 83.17 | 0.11 | 2.70 | 0.96 | 5.97 | 0 | 0 | 0.02 | 0.01 |
| 沉淀后液2 | 79.66 | 0.10 | 2.78 | 0.99 | 5.88 | 0 | 0 | 0.01 | 0.02 |
| 沉淀后液 3 | 83.22 | 0.09 | 2.72 | 1.02 | 6.05 | 0 | 0 | 0 | 0 |
| 平均值 | 82.02 | 0.10 | 2.73 | 0.99 | 5.97 | 0 | 0 | 0.01 | 0.01 |
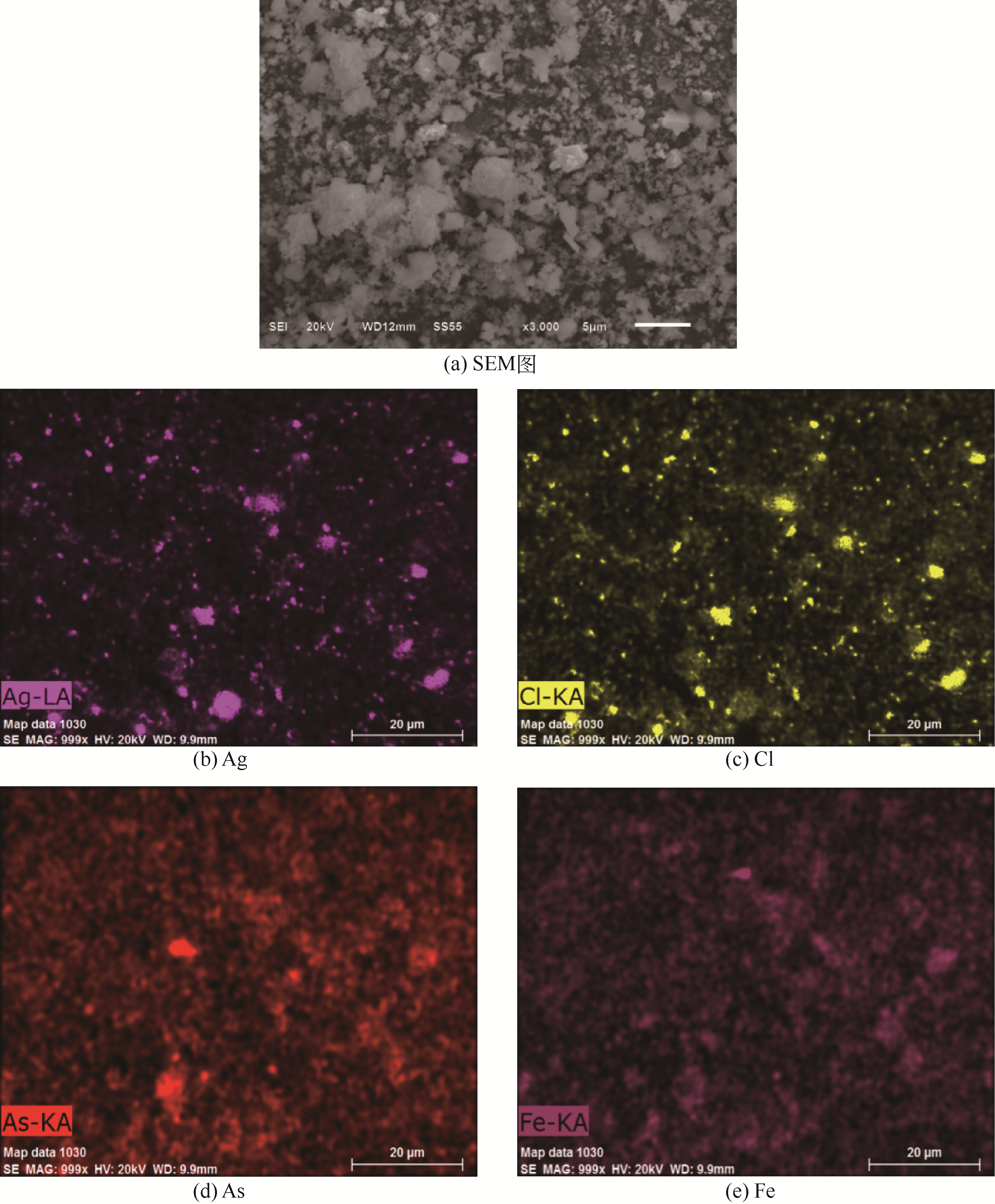
| 成分 | 质量分数/% | 成分 | 质量分数/% |
|---|---|---|---|
| Te | 18.24 | S | 0.538 |
| As | 15.53 | Se | 0.894 |
| Fe | 8.74 | Sb | 0.342 |
| Ag | 10.54 | Pt① | 1010 |
| Bi | 8.22 | Pd① | 320 |
| Ti | 4.27 | Au① | 124 |
| Cl | 3.38 |
| 成分 | 质量分数/% | 成分 | 质量分数/% |
|---|---|---|---|
| Te | 18.24 | S | 0.538 |
| As | 15.53 | Se | 0.894 |
| Fe | 8.74 | Sb | 0.342 |
| Ag | 10.54 | Pt① | 1010 |
| Bi | 8.22 | Pd① | 320 |
| Ti | 4.27 | Au① | 124 |
| Cl | 3.38 |
| 1 | LEE T D, EBONG A U. A review of thin film solar cell technologies and challenges[J]. Renewable & Sustainable Energy Reviews, 2017, 70: 1286-1297. |
| 2 | LIU Ming, WANG Cong, ZHOU Liqing. Development of small pixel HgCdTe infrared detectors[J]. Chinese Physics B, 2019, 28(3): 17-25. |
| 3 | LI Zelun, QI Shaojun, LIANG Yana, et al. Plasma surface functionalization of carbon nanofibres with silver, palladium and platinum nanoparticles for cost-effective and high-performance supercapacitors[J]. Micromachines, 2019, 10(1): 2. |
| 4 | LAZAREVIC T, RILAK A, BUGARCIC Z D. Platinum, palladium, gold and ruthenium complexes as anticancer agents: current clinical uses, cytotoxicity studies and future perspectives[J]. European Journal of Medicinal Chemistry, 2017, 142: 8-31. |
| 5 | LI Dong, GUO Xueyi, XU Zhipeng, et al. Leaching behavior of metals from copper anode slime using an alkali fusion-leaching process[J]. Hydrometallurgy, 2015, 157: 9-12. |
| 6 | KHANLARIAN M, RASHCHI F, SABA M. A modified sulfation-roasting-leaching process for recovering Se, Cu, and Ag from copper anode slimes at a lower temperature[J]. Journal of Environmental Management, 2019, 235: 303-309. |
| 7 | YANG Hongying, MA Zhiyuan, HUANG Songtao, et al. Intensification of pretreatment and pressure leaching of copper anode slime by microwave radiation[J]. Journal of Central South University, 2015, 22(12): 4536-4544. |
| 8 | 王小龙, 张昕红. 铜阳极泥处理工艺的探讨[J]. 矿冶, 2005, 14(4): 46-48. |
| WANG Xiaolong, ZHANG Xinhong. Discussion on process for treating copper anode slime[J]. Mining and Metallurgy, 2005, 14(4): 46-48. | |
| 9 | 郭学益, 肖彩梅, 钟菊芽, 等. 铜阳极泥处理过程中贵金属的行为[J]. 中国有色金属学报, 2010, 20(5): 990-998. |
| GUO Xueyi, XIAO Caimei, ZHONG Juya, et al. Behaviors of precious metals in process of copper anode slime treatment[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(5): 990-998. | |
| 10 | 阮胜寿, 汪劲松. 碱中和法从分铜液中提碲的试验研究[J]. 有色金属(冶炼部分), 2004(1): 31-32, 46. |
| RUAN Shengshou, WANG Jinsong. Study on tellurium extracting from copper leached solution by alkali neutralization process[J]. Nonferrous Metals(Extractive Metallurgy), 2004(1): 31-32, 46. | |
| 11 | 刘兴芝, 宋玉林, 武荣成, 等. 碲化铜法回收碲的物理化学原理[J]. 广东有色金属学报, 2002, 12(S1): 55-58. |
| LIU Xingzhi, SONG Yulin, WU Rongcheng, et al. Physicochemical principle for recovering tellurium by copper telluride method[J]. Journal of Guangdong Non-ferrous Metals, 2002, 12(S1): 55-58. | |
| 12 | 余洪, 胡显智, 字富庭, 等. 置换法回收硫代硫酸盐浸金液中金的研究进展[J]. 稀有金属, 2015, 39(5): 473-480. |
| YU Hong, HU Xianzhi, ZI Futing, et al. Progress in gold cementation from ammonium thiosulfate solution[J]. Chinese Journal of Rare Metals, 2015, 39(5): 473-480. | |
| 13 | 郑雅杰, 陈昆昆, 孙召明. SO2还原沉金后液回收硒碲及捕集铂钯[J]. 中国有色金属学报, 2011, 21(9): 2258-2264. |
| ZHENG Yajie, CHEN Kunkun, SUN Zhaoming. Recycling Se and Te and capturing Pt and Pd from solution after precipitating gold by SO2 reduction[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(9): 2258-2264. | |
| 14 | 张福元, 郑雅杰, 孙召明, 等. 采用亚硫酸钠还原法从沉金后液中回收稀贵金属[J]. 中国有色金属学报, 2015, 25(8): 2293-2299. |
| ZHANG Fuyuan, ZHENG Yajie, SUN Zhaoming, et al. Recovery of rare and precious metals from precipitated gold solution by Na2SO3 reduction[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(8): 2293-2299. | |
| 15 | 王欣欣, 郑雅杰, 靳利娥, 等. 焦亚硫酸钠复合还原硒、碲、金、铂、钯的热力学及动力学特征分析[J]. 中国有色金属学报, 2017, 27(9): 1943-1950. |
| WANG Xinxin, ZHENG Yajie, JIN Li’e, et al. Thermodynamic and dynamic characteristics analysis of reducing Se, Te, Au, Pd and Pt of precipitated gold solution by Na2S2O5 compound[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(9): 1943-1950. | |
| 16 | YI Qingping, FAN Ruiyi, XIE Feng, et al. Selective recovery of Au(III) and Pd(II) from waste PCBs using ethylenediamine modified persimmon tannin adsorbent[J]. Procedia Environmental Sciences, 2016, 31: 185-194. |
| 17 | GAMEZ S, GARCES K, TORRE E D L, et al. Precious metals recovery from waste printed circuit boards using thiosulfate leaching and ion exchange resin[J]. Hydrometallurgy, 2019, 186: 1-11. |
| 18 | SCHOEMAN E, BRADSHAW S M, AKDOGAN G, et al. The extraction of platinum and palladium from a synthetic cyanide heap leach solution with strong base anion exchange resins[J]. International Journal of Mineral Processing, 2017, 162: 27-35. |
| 19 | 张福元, 徐娟, 赵卓, 等. 沉金后液中铂、钯、碲的组态特征及其碲捕集稀贵金属机理[J]. 稀有金属, 2021, 45(2): 217-225. |
| ZHANG Fuyuan, XU Juan, ZHAO Zhuo, et al. Configuration characteristics of rare and precious metals and mechanism of gold, platinum and palladium captured by tellurium of degoldized solution[J]. Chinese Journal of Rare Metals, 2021, 45(2): 217-225. | |
| 20 | OGURI K, SHIMODA G, TATSUMI Y. Quantitative determination of gold and the platinum-group elements in geological samples using improved NiS fire-assay and tellurium coprecipitation with inductively coupled plasma-mass spectrometry (ICP-MS)[J]. Chemical Geology, 1999, 157(3/4): 189-197. |
| 21 | 何一芳, 张学彬. 共沉淀分离富集-ICP-AES法测定铜灰渣中金、铂、钯[J]. 贵金属, 2014, 35(2): 59-63. |
| HE Yifang, ZHANG Xuebin. Determination of gold, platinum and palladium in copper slag by ICP-AES with coprecipitation separation and enrichment[J]. Precious Metals, 2014, 35(2): 59-63. | |
| 22 | 谭文进, 郑允, 贺小塘, 等. 碱熔-碲共沉淀富集-电感耦合等离子体原子发射光谱法测定石油化工废催化剂不溶渣中铂钯[J]. 冶金分析, 2016, 36(2): 43-48. |
| TAN Wenjin, ZHENG Yun, HE Xiaotang, et al. Determination of platinum and palladium in insoluble slag of waste catalyst for petrochemical industry by inductively coupled plasma atomic emission spectrometry after alkali fusion-tellurium coprecipitation enrichment[J]. Metallurgical Analysis, 2016, 36(2): 43-48. | |
| 23 | 杨显万. 高温水溶液热力学数据计算手册[M]. 北京: 冶金工业出版社, 1983. |
| YANG Xianwan. Handbook of thermodynamic data calculation for high temperature aqueous solution[M]. Beijing: Metallurgical Industry Press, 1983. | |
| 24 | 吴维昌, 冯洪清, 吴开冶. 标准电极电位数据手册[M]. 北京: 科学出版社, 1991. |
| WU Weichang, FENG Hongqing, WU Kaiye. Standard electrode potential datasheet[M]. Beijing: Science Press, 1991. | |
| 25 | 张金池, 张福元, 赵卓, 等. 铜阳极泥分铜液高效分离稀散金属碲[J]. 稀有金属, DOI: 10.13373/j.cnki.cjrm.XY19060043. |
| ZHANG Jinchi, ZHANG Fuyuan, ZHAO Zhuo, et al. Efficiently separating rare metal of tellurium from copper-rich solution of copper anode slime[J]. Chinese Journal of Rare Metals, DOI: 10.13373/j.cnki.cjrm.XY19060043.. | |
| 26 | 任曼, 邓海琳, 龚国洪, 等.分离富集金、铂、钯的碲共沉淀物研究[J]. 冶金分析, 2005, 25(3): 13-15. |
| REN Man, DENG Hailin, GONG Guohong, et al. Study on Te-coprecipitates for separation and preconcentration of gold, platinum, palladium[J]. Metallurgical Analysis, 2005, 25(3): 13-15. |
| [1] | MA Yi, CAO Shiwei, WANG Jiajun, LIN Liqun, XING Yan, CAO Tengliang, LU Feng, ZHAO Zhenlun, ZHANG Zhijun. Research progress in recovery of spent cathode materials for lithium-ion batteries using deep eutectic solvents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 219-232. |
| [2] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [3] | ZHAO Wei, ZHAO Deyin, LI Shihan, LIU Hongda, SUN Jin, GUO Yanqiu. Synthesis and application of triazine drag reducing agent for nature gas pipeline [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 391-399. |
| [4] | ZHANG Jie, WANG Fangfang, XIA Zhonglin, ZHAO Guangjin, MA Shuangchen. Current SF6 emission, emission reduction and future prospects under “carbon peaking and carbon neutrality” [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 447-460. |
| [5] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [6] | ZHAO Jingchao, TAN Ming. Effect of surfactants on the reduction of industrial saline wastewater by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 529-535. |
| [7] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [8] | SHU Bin, CHEN Jianhong, XIONG Jian, WU Qirong, YU Jiangtao, YANG Ping. Necessity analysis of promoting the development of green methanol under the goal of carbon neutrality [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4471-4478. |
| [9] | DONG Jiayu, WANG Simin. Experimental on ultrasound enhancement of para-xylene crystallization characteristics and regulation mechanism [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4504-4513. |
| [10] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [11] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [12] | ZHU Chuanqiang, RU Jinbo, SUN Tingting, XIE Xingwang, LI Changming, GAO Shiqiu. Characteristics of selective non-catalytic reduction of NO x with solid polymer denitration agent [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4939-4946. |
| [13] | WANG Baoying, WANG Huangying, YAN Junying, WANG Yaoming, XU Tongwen. Research progress of polymer inclusion membrane in metal separation and recovery [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3990-4004. |
| [14] | WANG Yaogang, HAN Zishan, GAO Jiachen, WANG Xinyu, LI Siqi, YANG Quanhong, WENG Zhe. Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4043-4057. |
| [15] | LIU Yi, FANG Qiang, ZHONG Dazhong, ZHAO Qiang, LI Jinping. Cu facets regulation of Ag/Cu coupled catalysts for electrocatalytic reduction of carbon dioxide [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4136-4142. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||