Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (6): 3401-3410.DOI: 10.16085/j.issn.1000-6613.2020-1448
• Biochemical and pharmaceutical engineering • Previous Articles Next Articles
Research progress of recombinant human growth hormone delivery systems
LI Yao1( ), JU Xiaojie1,2(
), JU Xiaojie1,2( ), WANG Wei1,2, LIU Zhuang1,2, XIE Rui1,2, CHU Liangyin1,2
), WANG Wei1,2, LIU Zhuang1,2, XIE Rui1,2, CHU Liangyin1,2
- 1.School of Chemical Engineering, Sichuan University, Chengdu 610065, Sichuan, China
2.State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu 610065, Sichuan, China
-
Received:2020-07-27Revised:2020-09-01Online:2021-06-22Published:2021-06-06 -
Contact:JU Xiaojie
重组人生长激素给药系统的研究进展
李瑶1( ), 巨晓洁1,2(
), 巨晓洁1,2( ), 汪伟1,2, 刘壮1,2, 谢锐1,2, 褚良银1,2
), 汪伟1,2, 刘壮1,2, 谢锐1,2, 褚良银1,2
- 1.四川大学化学工程学院,四川 成都 610065
2.四川大学高分子材料工程国家重点实验室,四川 成都 610065
-
通讯作者:巨晓洁 -
作者简介:李瑶(1996—),女,硕士研究生。E-mail:1047176112@qq.com 。 -
基金资助:国家自然科学基金重大项目(21991101)
CLC Number:
Cite this article
LI Yao, JU Xiaojie, WANG Wei, LIU Zhuang, XIE Rui, CHU Liangyin. Research progress of recombinant human growth hormone delivery systems[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3401-3410.
李瑶, 巨晓洁, 汪伟, 刘壮, 谢锐, 褚良银. 重组人生长激素给药系统的研究进展[J]. 化工进展, 2021, 40(6): 3401-3410.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-1448
| 产品名称 | 生产企业 | rhGH修饰策略 | 给药频率 | 研发状态 |
|---|---|---|---|---|
| NNC126-0083[ | Novo Nordisk | 将43000 PEG分子附着于rhGH分子谷氨酰胺141上 | 7天1次 | 研发终止 |
| PHA-794428[ | Pfizer | 将40000分支型PEG修饰在rhGH分子的N末端上 | 7天1次 | 研发终止 |
| BBT-031[ | Bolder BioTechnology | 位点特异性PEG化rhGH | 7天1次 | 临床前研究 |
| ARX201[ | Ambrx | 在rhGH的氨基酸35处,对乙酰基苯丙氨酸取代天然酪氨酸后与30kg/mol的PEG连接 | 7天1次 | 研发终止 |
| Jintrolong?[ | GeneScience | 将40000支链亲水PEG残基连接到氨基末端 | 7天1次 | 在中国上市 |
| 产品名称 | 生产企业 | rhGH修饰策略 | 给药频率 | 研发状态 |
|---|---|---|---|---|
| NNC126-0083[ | Novo Nordisk | 将43000 PEG分子附着于rhGH分子谷氨酰胺141上 | 7天1次 | 研发终止 |
| PHA-794428[ | Pfizer | 将40000分支型PEG修饰在rhGH分子的N末端上 | 7天1次 | 研发终止 |
| BBT-031[ | Bolder BioTechnology | 位点特异性PEG化rhGH | 7天1次 | 临床前研究 |
| ARX201[ | Ambrx | 在rhGH的氨基酸35处,对乙酰基苯丙氨酸取代天然酪氨酸后与30kg/mol的PEG连接 | 7天1次 | 研发终止 |
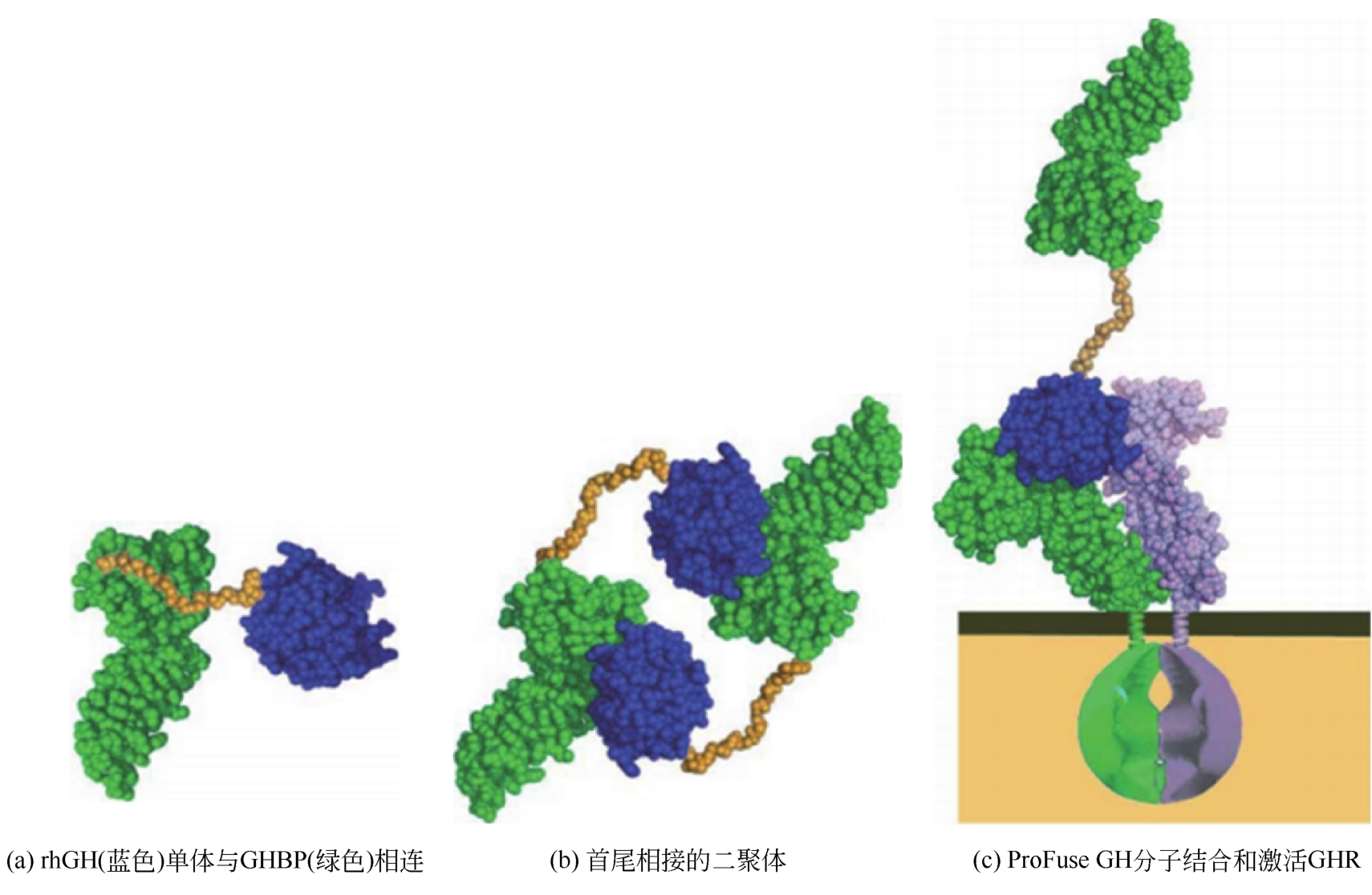
| Jintrolong?[ | GeneScience | 将40000支链亲水PEG残基连接到氨基末端 | 7天1次 | 在中国上市 |
| 名称 | 生产企业 | rhGH融合方式 | 给药频率 | 开发现状 |
|---|---|---|---|---|
| AG-B1512[ | Ahn-Gook | 与抗人血清白蛋白Fab抗体基因融合进行多肽连接 | 14或28天1次 | 临床前研究 |
| GX-H9[ | Genexine | 与IgD和IgG4的杂合免疫球蛋白Fc部分融合 | 7~14天1次 | 临床二期完成 |
| Albutropin(TV1106)[ | Teva | 人血清白蛋白融合到rhGH的N末端 | 7天1次 | 研发终止 |
| Somavaratan(VRS-317)[ | Versartis | 与天然产生的亲水氨基酸XTEN序列融合 | 7~30天1次 | 研发终止 |
| LAPS-rhGH(HM10560A)[ | Hanmi | 与同二聚体苷基化IgG4 Fc片段融合 | 7~14天1次 | 临床前研究 |
| JR-142[ | JCR | 工程rhGH与改良的人血清白蛋白C端在N端融合 | 7天1次 | 临床前研究 |
| ALT-P1(CJ-40002)[ | Alteogen | rhGH与重组α1-抗胰蛋白酶融合 | 未知 | 研发终止 |
| ProFuse GH[ | Asterion | rhGH与其受体胞外域结合蛋白片段融合 | 1月1次 | 临床前研究 |
| Somatrogon(MOD-4023)[ | OPKO and Pfizer | 与HCG的3个羧基末端B亚单位融合 | 7天1次 | 临床三期 |
| 名称 | 生产企业 | rhGH融合方式 | 给药频率 | 开发现状 |
|---|---|---|---|---|
| AG-B1512[ | Ahn-Gook | 与抗人血清白蛋白Fab抗体基因融合进行多肽连接 | 14或28天1次 | 临床前研究 |
| GX-H9[ | Genexine | 与IgD和IgG4的杂合免疫球蛋白Fc部分融合 | 7~14天1次 | 临床二期完成 |
| Albutropin(TV1106)[ | Teva | 人血清白蛋白融合到rhGH的N末端 | 7天1次 | 研发终止 |
| Somavaratan(VRS-317)[ | Versartis | 与天然产生的亲水氨基酸XTEN序列融合 | 7~30天1次 | 研发终止 |
| LAPS-rhGH(HM10560A)[ | Hanmi | 与同二聚体苷基化IgG4 Fc片段融合 | 7~14天1次 | 临床前研究 |
| JR-142[ | JCR | 工程rhGH与改良的人血清白蛋白C端在N端融合 | 7天1次 | 临床前研究 |
| ALT-P1(CJ-40002)[ | Alteogen | rhGH与重组α1-抗胰蛋白酶融合 | 未知 | 研发终止 |
| ProFuse GH[ | Asterion | rhGH与其受体胞外域结合蛋白片段融合 | 1月1次 | 临床前研究 |
| Somatrogon(MOD-4023)[ | OPKO and Pfizer | 与HCG的3个羧基末端B亚单位融合 | 7天1次 | 临床三期 |
| 1 | MILLER F P. Growth hormone[M]. Saarbrücken Germany: Alphascript Publishing, 2010: 132. |
| 2 | STROBL J S, THOMAS M J. Human growth-hormone[J]. Pharmacological Reviews, 1994, 46: 1-34. |
| 3 | STEFANOVA E, KURTEV A, PENEVA L, et al. Threatment of Turner syndrome with rhGH[J]. Pediatriya, 2008, 48(1): 38-41. |
| 4 | 李丹, 陈红珊, 杜敏联, 等. 单用rhGH与rhGH联合小剂量司坦唑醇对改善Turner综合征患者生长速度的比较[J]. 实用医学杂志, 2016, 32(4): 559-562. |
| LI D, CHEN H S, DU M L, et al. Efficacies of rhGH alone and rhGH combined with stanozolol therapies on growth velocity of girls with Turner syndrome[J]. Journal of Practical Medicine, 2016, 32(4): 559-562. | |
| 5 | TRITOS N, DANIAS P. Growth hormone therapy in congestive heart failure due to left ventricular systolic dysfunction: a meta-analysis[J]. Endocrine Practice, 2008, 14(1): 40-49. |
| 6 | 包建华. 重组人生长激素的临床应用进展[J]. 药学服务与研究, 2008, 8(4): 272-275. |
| BAO J H. Advances in clinical application of recombinant human growth hormone[J]. Pharmaceutical Care & Research, 2008, 8(4): 272-275. | |
| 7 | FINE R N, KOHAUT E, BROWN D, et al. Long-term treatment of growth retarded children with chronic renal insufficiency, with recombinant human growth hormone[J]. Kidney International, 1996, 49(3): 781-785. |
| 8 | FINE R N. Recombinant human growth hormone (rhGH) treatment of children with chronic renal insufficiency (CRI)[J]. Clinical Pediatric Endocrinology, 1997, 19(S10): 69-72. |
| 9 | LOW J F A, BARROW R E, MITTENDORFER B, et al. The effect of short-term growth hormone treatment on growth and energy expenditure in burned children[J]. Burns, 2001, 27(5): 447-452. |
| 10 | 贺肖洁, 岑航辉, 韩春茂. rhGH对烧伤患者垂体-睾丸轴影响的文献对照研究[J]. 浙江创伤外科, 2014, 19(6): 880-882. |
| HE X J, CEN H H, HAN C M. rhGH effect on burn patients with pituitary-testicular axis with literature study[J]. Zhejiang Journal of Traumatic Surgery, 2014, 19(6): 880-882. | |
| 11 | ROSENFELD R G, BAKKER B. Compliance and persistence in pediatric and adult patients receiving growth hormone therapy[J]. Endocrine Practice, 2008, 14(2): 143-154. |
| 12 | ODDONE I, ARSICCIO A, DURU C, et al. Vacuum induced surface freezing for the freeze-drying of the human growth hormone: how does nucleation control affect protein stability?[J]. Journal of Pharmaceutical Sciences, 2020, 109(1): 254-263. |
| 13 | WANG W. Instability, stabilization, and formulation of liquid protein pharmaceuticals[J]. International Journal of Pharmaceutics, 1999, 185(2): 129-188. |
| 14 | YUAN W, CAI Y, XU M, et al. Developments in human growth hormone preparations: sustained-release, prolonged half-life, novel injection devices, and alternative delivery routes[J]. International Journal of Nanomedicine, 2014, 2014: 3527. |
| 15 | 唐姝, 陈志祥, 陆伟根. 重组人生长激素长效制剂的研究进展[J]. 世界临床药物, 2014, 35(1): 36-41. |
| TANG S, CHEN Z X, LU W G. Research progress in the long-acting preparations of recombinant human growth hormone [J]. World Clinical Drugs, 2014, 35(1): 36-41. | |
| 16 | LAL R A, HOFFMAN A R. Perspectives on long-acting growth hormone therapy in children and adults[J]. Archives of Endocrinology and Metabolism, 2019, 63(6): 601-607. |
| 17 | GIAVOLI C, CAPPIELLO V, PORRETTI S, et al. Growth hormone therapy in GH-deficient adults: continuous vs. alternate-days treatment[J]. Hormone & Metabolic Research, 2003, 35(9): 557-561. |
| 18 | 马妍春, 杨秀云, 李磊姣, 等. 聚乙二醇修饰重组人生长激素的研究[J]. 吉林省教育学院学报, 2017, 33(12): 172-174. |
| MA Y C, YANG X Y, LI L J, et al. Study on the polyethylene glycol (PEG) modification of recombinant human growth hormone (rhGH)[J]. Journal of Educational Institute of Jilin Province, 2017, 33(12): 172-174. | |
| 19 | CLELAND J L, GEETHING N C, MOORE J A, et al. A novel long-acting human growth hormone fusion protein (VRS-317): enhanced in vivo potency and half-life[J]. Journal of Pharmaceutical Sciences, 2012, 101(8): 2744-2754. |
| 20 | YU Z Y, HUANG L, WEN R T, et al. Preparation and in vivo pharmacokinetics of rhGH-loaded PLGA microspheres[J]. Pharmaceutical Development & Technology, 2018, 24(4): 395-401. |
| 21 | WU L, CHEN J X, WU Y M, et al. Precise and combinatorial PEGylation generates a low-immunogenic and stable form of human growth hormone[J]. Journal of Controlled Release, 2017, 249: 84-93. |
| 22 | 李晶, 梁成罡. 聚乙二醇化重组人生长激素质控难点探讨[J]. 中国新药杂志, 2014, 23(3): 271-274. |
| LI J, LIANG C G. Difficulties in quality control of PEGylated rhGH[J]. Chinese Journal of New Drugs, 2014, 23(3): 271-274. | |
| 23 | CLARK R, OLSON K, FUH G, et al. Long-acting growth hormones produced by conjugation with poly ethylene glycol[J]. Journal of Biological Chemistry, 1996, 271(36): 21969-21977. |
| 24 | DE SCHEPPER J, RASMUSSEN M H, GUCEV Z, et al. Long-acting pegylated human GH in children with GH deficiency: a single-dose, dose-escalation trial investigating safety, tolerability, pharmacokinetics and pharmacodynamics[J]. European Journal of Endocrinology, 2011, 165(3): 401-409. |
| 25 | KEN K Y H. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency Ⅱ: A statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia[J]. European Journal of Endocrinology, 2007, 157(6): 695-700. |
| 26 | HOU L, CHEN Z H, LIU D, et al. Comparative pharmacokinetics and pharmacodynamics of a PEGylated recombinant human growth hormone and daily recombinant human growth hormone in growth hormone-deficient children[J]. Drug Design Development & Therapy, 2016, 10(1): 13-21. |
| 27 | GUAN Y, HE F, WU J, et al. A long-acting pegylated recombinant human growth hormone (Jintrolong) in healthy adult subjects: two single-dose trials evaluating safety, tolerability and pharmacokinetics[J]. Journal of Clinical Pharmacy and Therapeutics, 2018, 43(5): 640-646. |
| 28 | LUO X, HOU L, LIANG L, et al. Long-acting PEGylated recombinant human growth hormone (Jintrolong) for children with growth hormone deficiency: phase Ⅱ and phase Ⅲ multicenter, randomized studies[J]. European Journal of Endocrinology, 2017, 177(2): 195-205. |
| 29 | TOURAINE P, D'SOUZA G, KOURIDES I, et al. Lipoatrophy in growth hormone deficient patients treated with a long-acting pegylated growth hormone[J]. Hormone Research, 2009, 72: 199-200. |
| 30 | GRUNFELD C, THOMPSON M, BROWN S J, et al. Recombinant human growth hormone to treat HIV-associated adipose redistribution syndrome: 12-week induction and 24-week maintenance therapy[J]. Journal of Acquired Immune Deficiency Syndromes, 2007, 45(3): 286-297. |
| 31 | CHRISTIANSEN J S, BACKELJAUW P F, BIDLINGMAIER M, et al. Growth hormone research society perspective on the development of long-acting growth hormone preparations[J]. European Journal of Endocrinology, 2016, 174(6): C1-C8. |
| 32 | QIN X, LI J, LI Y, et al. Isoform separation and structural identification of mono-PEGylated recombinant human growth hormone (PEG-rhGH) with pH gradient chromatography[J]. Journal of Chromatography B, 2017, 1044/1045: 206-213. |
| 33 | YANG Y Y, BAI X, YUAN X X, et al. Efficacy and safety of long-acting growth hormone in children with short stature: a systematic review and meta-analysis[J]. Endocrine, 2019, 65(1): 25-34. |
| 34 | Y K S J J B. Preclinical pharmacokinetic and pharmacodynamic studies of a novel, long acting growth hormone (AG-B1512)[Z]. Orlando:2017. |
| 35 | KU C R, BRUE T, SCHILBACH K, et al. Long-acting FC-fusion rhGH (GX-H9) shows potential for up to twice-monthly administration in GH-deficient adults[J]. European Journal of Endocrinology, 2018, 179(3): 169-179. |
| 36 | COHEN-BARAK O, BARKAY H, RASAMOELISOLO M, et al. Assessment of the pharmacokinetics, pharmacodynamics, and safety of single doses of TV-1106, a long-acting growth hormone, in healthy Japanese and Caucasian subjects[J]. Clinical Pharmacology in Drug Development, 2017, 6(4): 331-342. |
| 37 | MOORE W V, NGUYEN H J, KLETTER G B, et al. A randomized safety and efficacy study of somavaratan (VRS-317), a long-acting rhGH, in pediatric growth hormone deficiency[J]. Journal of Clinical Endocrinology & Metabolism, 2016, 101(3): 1091-1097. |
| 38 | KANG J. 6 month results of a phase Ⅱ, randomized, active controlled, open label study of safety and efficacy of HM10560A a long acting rHgh-HMC001 conjugate in adult patients with growth hormone deficiency (AGHD)[Z]. San Diego Convention Center: Endocrine Reviews, 2015. |
| 39 | Announces completion of phase 1 clinical trial notification of JR-142, a long-acting growth hormone[R]. 2019. |
| 40 | LEE S M, CHO J S, CHUNG H S, et al. Human phase 1 clinical data of ALT-P1 (hGH-NexP) by healthy Korean males[J]. Horm. Res. Paediatr., 2016, 86: 598. |
| 41 | WILKINSON I R, FERRANDIS E, ARTYMIUK P J, et al. A ligand-receptor fusion of growth hormone forms a dimer and is a potent long-acting agonist[J]. Nature Medicine, 2007, 13(9): 1108-1113. |
| 42 | KRAMER W G, JARON MENDELSON M, KOREN R, et al. Pharmacokinetics, pharmacodynamics, and safety of a long-acting human growth hormone (MOD-4023) in healthy Japanese and Caucasian adults[J]. Clinical Pharmacology in Drug Development, 2018, 7(5): 554-563. |
| 43 | KIM S J, KWAK H H, CHO S Y, et al. Pharmacokinetics, pharmacodynamics, and efficacy of a novel long-acting human growth hormone-Fc fusion protein[J]. Molecular Pharmaceutics, 2015, 12(10): 3759-3765. |
| 44 | FOSS S, GREVYS A, SAND K M K, et al. Enhanced FcRn-dependent transepithelial delivery of IgG by Fc-engineering and polymerization[J]. Journal of Controlled Release, 2016, 223: 42-52. |
| 45 | RASMUSSEN M H, OLSEN M W B, ALIFRANGIS L, et al. A reversible albumin-binding growth hormone derivative is well tolerated and possesses a potential once-weekly treatment profile[J]. Journal of Clinical Endocrinology & Metabolism, 2014, 99(10): 1819-1829. |
| 46 | BATTELINO T, RASMUSSEN M H J, De SCHEPPER J, et al. Somapacitan, a once-weekly reversible albumin-binding GH derivative, in children with GH deficiency: a randomized dose-escalation trial[J]. Clinical Endocrinology, 2017, 87: 350-358. |
| 47 | JOHANNS SONG, GORDON MB, HϕJBYRM, et al. Once-weekly Somapacitan is effective and well tolerated in adults with GH deficiency: a randomized phase 3 trial[J]. The Journal of Clinical Endocrinology & Metabolism, 2020, 105(4): e1358-e1376. |
| 48 | 王晨晖. 装载蛋白药物的PCADK/PLGA混合微球研究及在重组人生长激素中的应用[D]. 长春: 吉林大学, 2016. |
| WANG C H. Study on protein-loaded PCADK/PLGA microspheres and the application in rhGH delivery[D]. Changchun: Jilin University, 2016. | |
| 49 | CLELAND J L, JOHNSON O L, PUTNEY S. Recombinant human growth hormone poly (lactic-co-glycolic acid) microsphere formulation development[J]. Advanced Drug Delivery Reviews, 1997, 28(1): 71-84. |
| 50 | ROHOLLAH G, MAHDI A, ELAHEH E Z, et al. mPEG-PLA and PLA-PEG-PLA nanoparticles as new carriers for delivery of recombinant human growth hormone (rhGH)[J]. Scientific Reports, 2018, 8(1): 9854. |
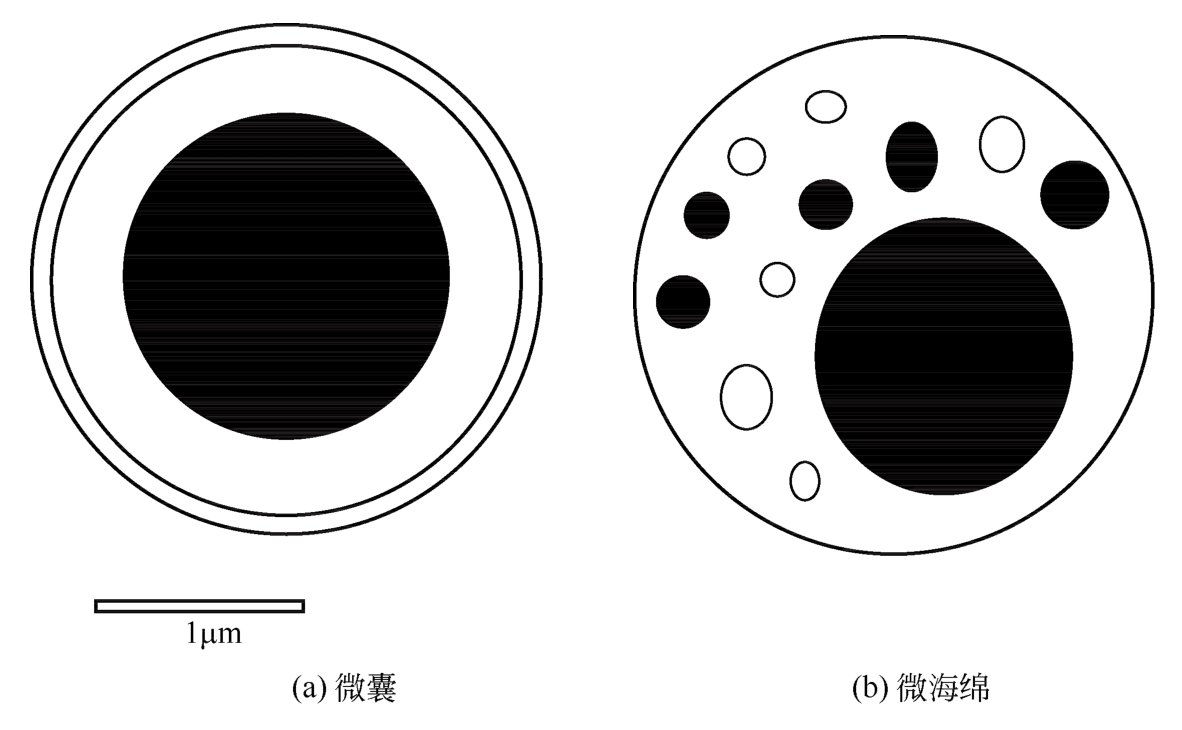
| 51 | KIM S J, HAHN S K, KIM M J, et al. Development of a novel sustained release formulation of recombinant human growth hormone using sodium hyaluronate microparticles[J]. Journal of Controlled Release, 2005, 104(2): 323-335. |
| 52 | OLUFUNMI J L, JEFFREY C L, LEE H J, et al. A month-long effect from a single injection of microencapsulated human growth hormone[J]. Nature Medicine, 1996, 2(7): 795-799. |
| 53 | WREN A. How best to approach endocrine evaluation in patients with HIV in the era of combined antiretroviral therapy?[J]. Clinical Endocrinology, 2013, 79(3): 310-313. |
| 54 | WANG C, YU C, LIU J, et al. Preparation and in vivo evaluation of PCADK/PLGA microspheres for improving stability and efficacy of rhGH[J]. International Journal of Pharmaceutics, 2015, 495(2): 924-931. |
| 55 | HUANG G L, HUANG H L. Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system[J]. Journal of Controlled Release, 2018, 278: 122-126. |
| 56 | PÉTER F, BIDLINGMAIER M, SAVOY C, et al. Three-year efficacy and safety of LB03002, a once-weekly sustained-release growth hormone (GH) preparation, in prepubertal children with GH deficiency (GHD)[J]. Journal of Clinical Endocrinology & Metabolism, 2011, 97(2): 400-407. |
| 57 | HWANG J S, LEE H S, LEE K, et al. Once-weekly administration of sustained-release growth hormone in Korean prepubertal children with idiopathic short stature: a randomized, controlled phase Ⅱ study[J]. Hormone Research in Paediatrics, 2018, 90(1): 54-63. |
| 58 | COSTANTINO H R, JOHNSON O L, ZALE S E. Relationship between encapsulated drug particle size and initial release of recombinant human growth hormone from biodegradable microspheres[J]. Journal of Pharmaceutical Sciences, 2004, 93(10): 2624-2634. |
| 59 | REN T T, YUAN W E, ZHAO Z, et al. Sustained-release polylactide-co-glycolide microspheres loaded with pre-formulated protein polysaccharide nanoparticles[J]. Micro & Nano Letters, 2011, 6(2): 70-74. |
| 60 | LEVIN G, GERSHONOWITZ A, SACKS H, et al. Transdermal delivery of human growth hormone through RF-microchannels[J]. Pharmaceutical Research, 2005, 22(4): 550-555. |
| 61 | FOLKESSON H G, HEDIN L, WESTROM B R. Lung to blood passage of human growth hormone after intratracheal instillation: stimulation of growth in hypophysectomized rats[J]. Journal of Endocrinology, 1992, 134(2): 197-203. |
| 62 | ILLUM L, FARRAJ F N, DAVIS S S, et al. Investigation of the nasal absorption of biosynthetic human growth hormone in sheep—Use of a bioadhesive microsphere delivery system[J]. International Journal of Pharmaceutics, 1990, 63(3): 207-211. |
| 63 | 蒲兴群, 巨晓洁, 谢锐, 等. 聚合物阵列微针及其在透皮给药系统的应用[J]. 化工学报, 2020(1): 43-53. |
| PU X Q, JU X J, XIE R, et al. Polymeric microneedle arrays for applications in transdermal drug delivery systems[J]. CIESC Journal, 2020(1): 43-53. | |
| 64 | AMERI M, KADKHODAYAN M, NGUYEN J, et al. Human growth hormone delivery with a microneedle transdermal system: preclinical formulation, stability, delivery and PK of therapeutically relevant doses[J]. Pharmaceutics, 2014, 6(2): 220-234. |
| 65 | PILLAI O, PANCHAGNULA R. Transdermal iontophoresis of insulin[J]. Skin Pharmacology and Physiology, 2004, 17(6): 289-297. |
| 66 | TOUITOU E, GODIN B, KARL Y, et al. Oleic acid, a skin penetration enhancer, affects langerhans cells and corneocytes[J]. Journal of Controlled Release, 2002, 80(1/2/3): 1-7. |
| 67 | HAN Y, ZHAO Q Y, YU D P, et al. Treatment of chest wall tuberculosis with transdermal ultrasound-mediated drug delivery[J]. Experimental & Therapeutic Medicine, 2015, 9(4): 1433-1437. |
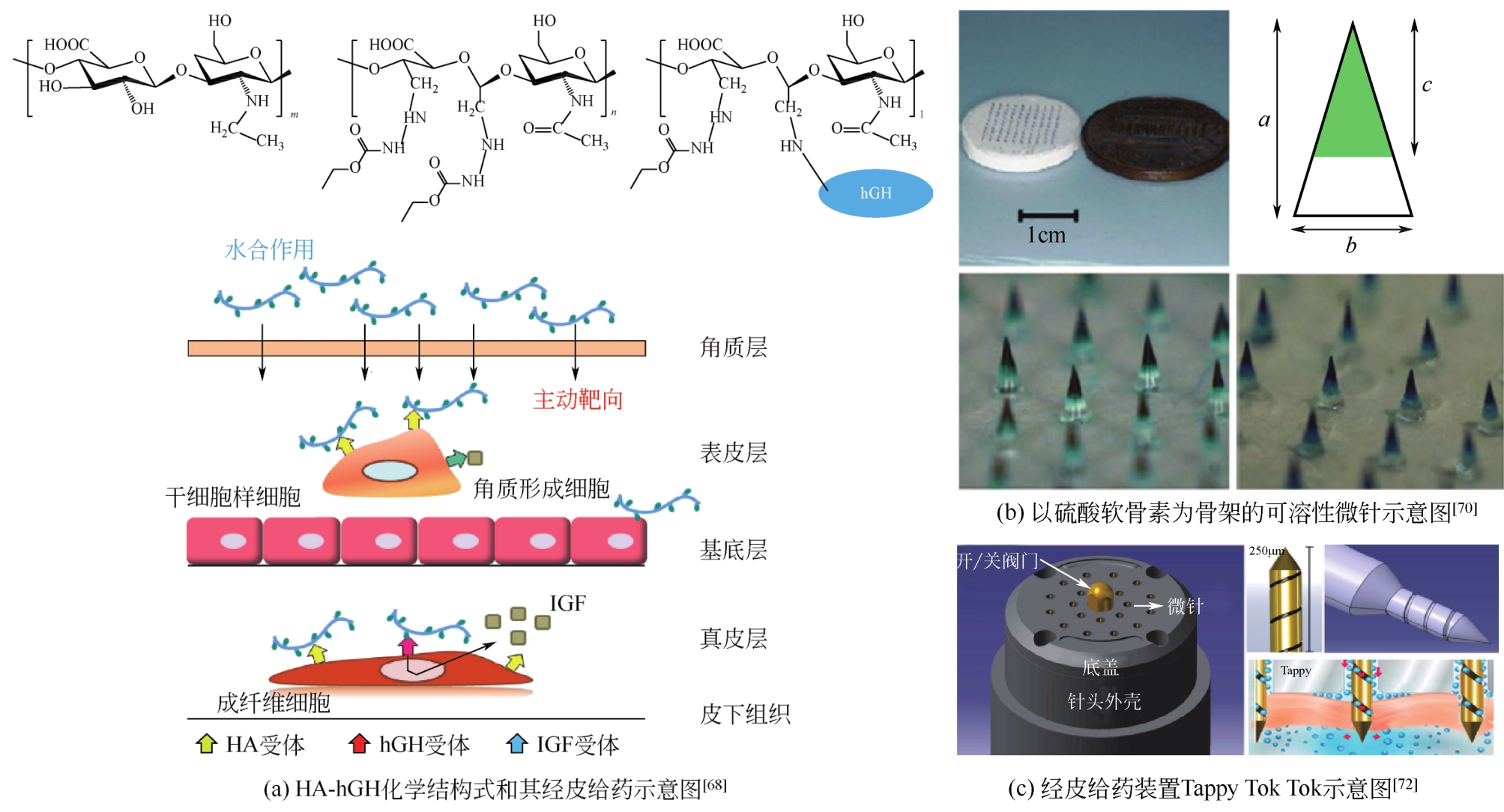
| 68 | YANG J A, KIM E S, KWON J H, et al. Transdermal delivery of hyaluronic acid-human growth hormone conjugate[J]. Biomaterials, 2012, 33(25): 5947-5954. |
| 69 | YE Y Q, YU J C, WEN D, et al. Polymeric microneedles for transdermal protein delivery[J]. Advanced Drug Delivery Reviews, 2018, 127(1): 106-118. |
| 70 | LEE J W, CHOI S O, FELNER E I, et al. Dissolving microneedle patch for transdermal delivery of human growth hormone[J]. Small, 2011, 7(4): 531-539. |
| 71 | FUKUSHIMA K, ISE A, MORITA H, et al. Two-layered dissolving microneedles for percutaneous delivery of peptide/protein drugs in rats[J]. Pharm. Res., 2011, 28(1): 7-21. |
| 72 | GYUBIN N, TAEKWANG K, JO-EUN S, et al. Iontophoretic transdermal delivery of human growth hormone (hGH) and the combination effect of a new type microneedle, Tappy Tok Tok®[J]. Pharmaceutics, 2018, 10(3): 153. |
| 73 | PATTON J S, MCCABE J G, HANSEN S E, et al. Absorption of human growth hormone from the rat lung[J]. Biotechnology Therapeutics, 1989, 1(3): 213-228. |
| 74 | COLTHORPE P, FAIR S J, SMITH I J, et al. The influence of regional deposition on the pharmacokinetics of pulmonary-delivered human growth hormone in rabbits[J]. Pharmaceutical Research, 1995, 12(3): 356-359. |
| 75 | BOSQUILLON C, PRÉAT V, VANBEVER R. Pulmonary delivery of growth hormone using dry powders and visualization of its local fate in rats[J]. Journal of Controlled Release, 2004, 96(2): 233-244. |
| 76 | JALALIPOUR M, GILANI K, ESMAILY H, et al. Effect of dimethyl-β-cyclodextrin concentrations on the pulmonary delivery of recombinant human growth hormone dry powder in rats[J]. Journal of Pharmaceutical Sciences, 2010, 97(12): 5176-5185. |
| 77 | WALVOORD E C, DE LA PEÑA A, PARK S, et al. Inhaled growth hormone (GH) compared with subcutaneous GH in children with GH deficiency: pharmacokinetics, pharmacodynamics, and safety[J]. Journal of Clinical Endocrinology & Metabolism, 2009, 94(6): 2052-2059. |
| 78 | RIJT S H VAN, BEIN T, MEINERS S. Medical nanoparticles for next generation drug delivery to the lungs[J]. European Respiratory Journal, 2014, 44(3): 765-774. |
| 79 | WEERS J G, TARARA T E, CLARK A R. Design of fine particles for pulmonary drug delivery[J]. Expert Opinion on Drug Delivery, 2007, 4(3): 297-313. |
| 80 | 杨林, 贾梅, 甄英丽. 鼻腔给药系统研究进展[J]. 西北国防医学杂志, 2016, 37(10): 671-673. |
| YANG L, JIA M, ZHEN Y L, Research progress of nasal drug delivery system[J]. Medical Journal of National Defeding Forces in Northwestern China, 2016, 37(10): 671-673. | |
| 81 | BALDWIN P A, KLINGBEIL C K, GRIMM C J, et al. The effect of sodium tauro-24,25-dihydrofusidate on the nasal absorption of human growth hormone in three animal models[J]. Pharmaceutical Research, 1990, 7(5): 547-552. |
| 82 | HEDIN L, OLSSON B, DICZFALUSY M, et al. Intranasal administration of human growth hormone (hGH) in combination with a membrane permeation enhancer in patients with GH deficiency: a pharmacokinetic study[J]. Journal of Clinical Endocrinology & Metabolism, 1993, 76(4): 962-967. |
| 83 | AGERHOLM C, BASTHOLM L, JOHANSEN P B, et al. Epithelial transport and bioavailability of intranasally administered human growth hormone formulated with the absorption enhancers didecanoyl-L-α-phosphatidylcholine and α-cyclodextrin in rabbits[J]. Journal of Pharmaceutical Sciences, 1994, 83(12): 1706-1711. |
| 84 | ZYL L V, PREEZ J D, GERBER M, et al. Essential fatty acids as transdermal penetration enhancers[J]. Journal of Pharmaceutical Sciences, 2016, 105(1): 188-193. |
| 85 | DU PLESSIS L H, LUBBE J, STRAUSS T, et al. Enhancement of nasal and intestinal calcitonin delivery by the novel PheroidTM fatty acid based delivery system, and by N-trimethyl chitosan chloride[J]. International Journal of Pharmaceutics, 2010, 385(1/2): 181-186. |
| 86 | STEYN D, DU PLESSIS L, KOTZÉ A. Nasal delivery of recombinant human growth hormone: in vivo evaluation with Pheroid technology and N-trimethyl chitosan chloride[J]. Journal of Pharmacy & Pharmaceutical Sciences, 2010, 13(2): 263-273. |
| 87 | DUPLESSIS Lissinda H, KOTZÉ Awie F. Pheroid™ vesicles and microsponges for nasal delivery of biopharmaceuticals[M]. DAS NEVES Jośe, SARMENTP Bruno. Mucosal delivery of biopharmaceuticals. New York: Springer Science+Business Media, 2014: 333-343. |
| [1] | ZHAO Jing, WANG Pan, LIU Yannan, FU Rongzhan, DUAN Zhiguang, FAN Daidi. Recent advances in biotransformation of ginsenosides [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1238-1247. |
| [2] | Weihong YIN, Xiaojie JU, Rui XIE, Wei WANG, Zhuang LIU, Liangyin CHU. Dual drug-loaded core-shell microspheres for programmed sequential release [J]. Chemical Industry and Engineering Progress, 2021, 40(2): 998-1007. |
| [3] | WANG Xiaoxue, JU Xiaojie, CHU Liangyin, XIE Rui, WANG Wei, LIU Zhuang. Preparation of chitosan microparticles with acid-induced burst release property via electrospraying [J]. Chemical Industry and Engineering Progree, 2015, 34(10): 3712-3718. |
| [4] | Yao /Li XiaoJie Ju Rui XIE Liang-Yin CHU. Research progress of recombinant human growth hormone delivery systems [J]. Chemical Industry and Engineering Progress, , (): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||