Chemical Industry and Engineering Progress ›› 2020, Vol. 39 ›› Issue (1): 145-151.DOI: 10.16085/j.issn.1000-6613.2019-0667
• Energy processes and technology • Previous Articles Next Articles
Electrochemical properties of Al-6061 and Al-7075 alloys as anode for aluminum air batteries
Yagang YANG1( ),Xiaohua YU1,Lei ZHANG1,Chunyang SHI1,Xiaodong ZHUANG1,Gang XIE1,2,3(
),Xiaohua YU1,Lei ZHANG1,Chunyang SHI1,Xiaodong ZHUANG1,Gang XIE1,2,3( )
)
- 1. Faculty of Metallurgy and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, Yunnan, China
2. Kunming Metallurgical Research Institute Co. , Ltd. , Kunming 650503, Yunnan, China
3. State Key Laboratory of Common Associated Non-ferrous Metal Resources Pressure Hydrometallurgy Technology, Kunming 650503, Yunnan, China
-
Received:2019-04-28Online:2020-01-14Published:2020-01-05 -
Contact:Gang XIE
铝空气电池用6061和7075铝合金阳极电化学性能
杨亚刚1( ),俞小花1,张磊1,史春阳1,庄晓东1,谢刚1,2,3(
),俞小花1,张磊1,史春阳1,庄晓东1,谢刚1,2,3( )
)
- 1. 昆明理工大学冶金与能源工程学院,云南 昆明 650093
2. 昆明冶金研究院有限公司,云南 昆明 650503
3. 共伴生有色金属资源加压湿法冶金技术国家重点实验室,云南 昆明650503
-
通讯作者:谢刚 -
作者简介:杨亚刚(1994—),男,硕士研究生,研究方向为铝空气电池。E-mail:1125037838@qq.com 。 -
基金资助:国家自然科学基金面上项目(51774160);云南省万人计划(YNWR-QNBJ-2018-327)
CLC Number:
Cite this article
Yagang YANG,Xiaohua YU,Lei ZHANG,Chunyang SHI,Xiaodong ZHUANG,Gang XIE. Electrochemical properties of Al-6061 and Al-7075 alloys as anode for aluminum air batteries[J]. Chemical Industry and Engineering Progress, 2020, 39(1): 145-151.
杨亚刚,俞小花,张磊,史春阳,庄晓东,谢刚. 铝空气电池用6061和7075铝合金阳极电化学性能[J]. 化工进展, 2020, 39(1): 145-151.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2019-0667
| 阳极 | Si/% | Cu/% | Mn/% | Zn/% | Fe/% | Ti/% | Mg/% | Cr/% |
|---|---|---|---|---|---|---|---|---|
| 纯铝 | — | — | — | — | — | — | — | — |
| 6061 | 1.08 | 0.27 | 1.00 | 1.82 | 0.60 | 0.15 | 1.00 | 0.20 |
| 7075 | 0.40 | 2.50 | 0.10 | 6.60 | 0.10 | 0.10 | 2.50 | 0.23 |
| 阳极 | Si/% | Cu/% | Mn/% | Zn/% | Fe/% | Ti/% | Mg/% | Cr/% |
|---|---|---|---|---|---|---|---|---|
| 纯铝 | — | — | — | — | — | — | — | — |
| 6061 | 1.08 | 0.27 | 1.00 | 1.82 | 0.60 | 0.15 | 1.00 | 0.20 |
| 7075 | 0.40 | 2.50 | 0.10 | 6.60 | 0.10 | 0.10 | 2.50 | 0.23 |
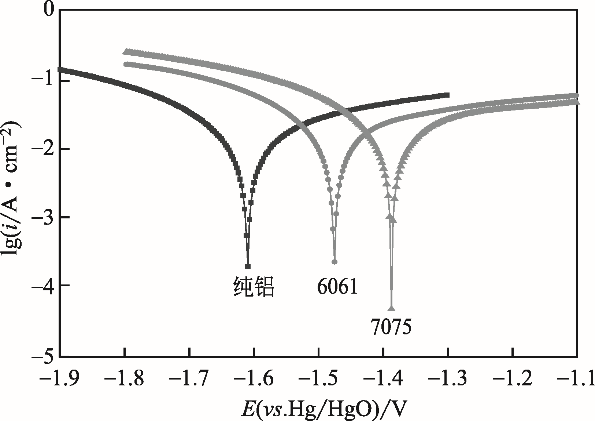
| 阳极 | 腐蚀电流 j corr /A?cm-2 | 腐蚀速率 CR/mm?y-1 | 极化电阻 R P/Ω?cm-2 | 腐蚀电位 E corr /V |
|---|---|---|---|---|
| 纯铝 | 0.2602 | 8446.7 | 3.2827 | -1.5941 |
| 6061 | 0.2402 | 7857.0 | 2.7092 | -1.4575 |
| 7075 | 0.2372 | 7758.3 | 2.5766 | -1.3872 |
| 阳极 | 腐蚀电流 j corr /A?cm-2 | 腐蚀速率 CR/mm?y-1 | 极化电阻 R P/Ω?cm-2 | 腐蚀电位 E corr /V |
|---|---|---|---|---|
| 纯铝 | 0.2602 | 8446.7 | 3.2827 | -1.5941 |
| 6061 | 0.2402 | 7857.0 | 2.7092 | -1.4575 |
| 7075 | 0.2372 | 7758.3 | 2.5766 | -1.3872 |
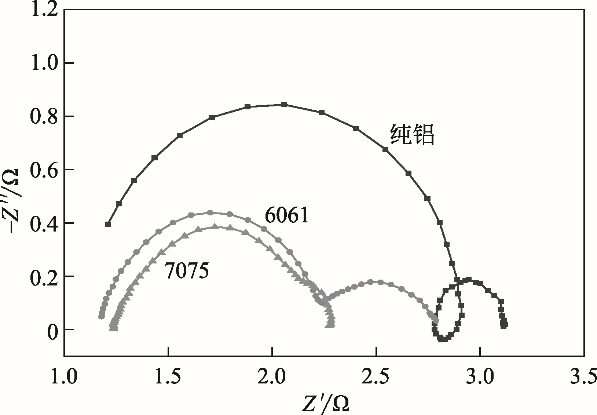
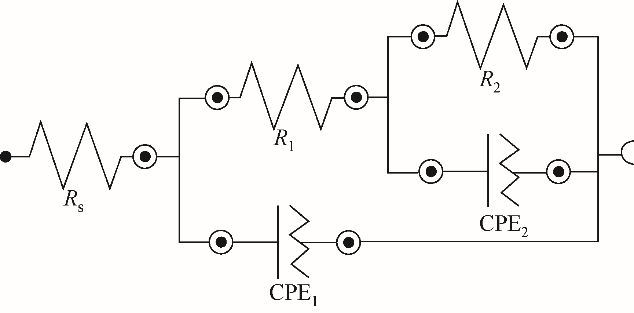
| 阳极 | R s/Ω?cm-2 | CEP1 | R 1/Ω?cm-2 | CEP2 | R 2/Ω?cm-2 | ||
|---|---|---|---|---|---|---|---|
| Y 0/10-4?Ω-1?cm-2?s n | n | Y 0/10-4?Ω-1?cm-2?s n | n | ||||
| 纯铝 | 1.121 | 0.175 | 0.99 | 1.725 | 1767 | 1.00 | 0.291 |
| 6061 | 1.171 | 5.659 | 0.87 | 1.093 | 1333 | 0.94 | 0.479 |
| 7075 | 1.081 | 287.8 | 0.23 | 0.166 | 50.49 | 0.81 | 1.101 |
| 阳极 | R s/Ω?cm-2 | CEP1 | R 1/Ω?cm-2 | CEP2 | R 2/Ω?cm-2 | ||
|---|---|---|---|---|---|---|---|
| Y 0/10-4?Ω-1?cm-2?s n | n | Y 0/10-4?Ω-1?cm-2?s n | n | ||||
| 纯铝 | 1.121 | 0.175 | 0.99 | 1.725 | 1767 | 1.00 | 0.291 |
| 6061 | 1.171 | 5.659 | 0.87 | 1.093 | 1333 | 0.94 | 0.479 |
| 7075 | 1.081 | 287.8 | 0.23 | 0.166 | 50.49 | 0.81 | 1.101 |
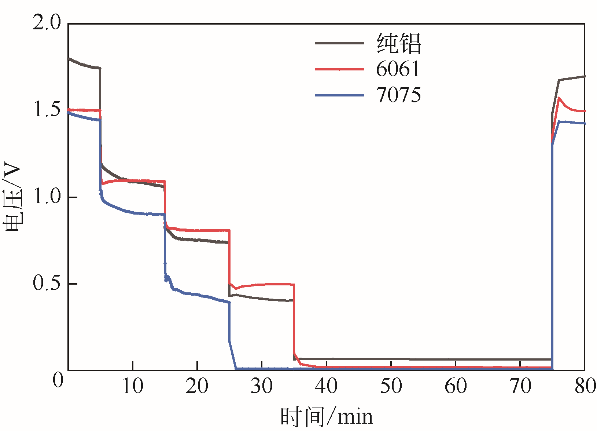
| 项目 | 纯铝 | 6061 | 7075 |
|---|---|---|---|
| 各电流密度下的平均放电电压/V | |||
| 静置 | 1.77 | 1.50 | 1.47 |
| 40mA·cm-2 | 1.17 | 1.22 | 1.00 |
| 60mA·cm-2 | 0.78 | 0.83 | 0.50 |
| 80mA·cm-2 | 0.42 | 0.50 | 0.01 |
| 100mA·cm-2 | 0.06 | 0.02 | 0.01 |
| 120mA·cm-2 | 0.06 | 0.02 | 0.01 |
| 静置 | 1.60 | 1.47 | 1.36 |
| W 0/g | 0.0332 | 0.0214 | 0.0184 |
| 综合放电过程腐蚀速率/g?cm-2?h-1 | 0.0249 | 0.0204 | 0.0176 |
| 综合放电过程中的能量密度/Wh·kg-1 | 771.08 | 901.16 | 531.30 |
| 项目 | 纯铝 | 6061 | 7075 |
|---|---|---|---|
| 各电流密度下的平均放电电压/V | |||
| 静置 | 1.77 | 1.50 | 1.47 |
| 40mA·cm-2 | 1.17 | 1.22 | 1.00 |
| 60mA·cm-2 | 0.78 | 0.83 | 0.50 |
| 80mA·cm-2 | 0.42 | 0.50 | 0.01 |
| 100mA·cm-2 | 0.06 | 0.02 | 0.01 |
| 120mA·cm-2 | 0.06 | 0.02 | 0.01 |
| 静置 | 1.60 | 1.47 | 1.36 |
| W 0/g | 0.0332 | 0.0214 | 0.0184 |
| 综合放电过程腐蚀速率/g?cm-2?h-1 | 0.0249 | 0.0204 | 0.0176 |
| 综合放电过程中的能量密度/Wh·kg-1 | 771.08 | 901.16 | 531.30 |
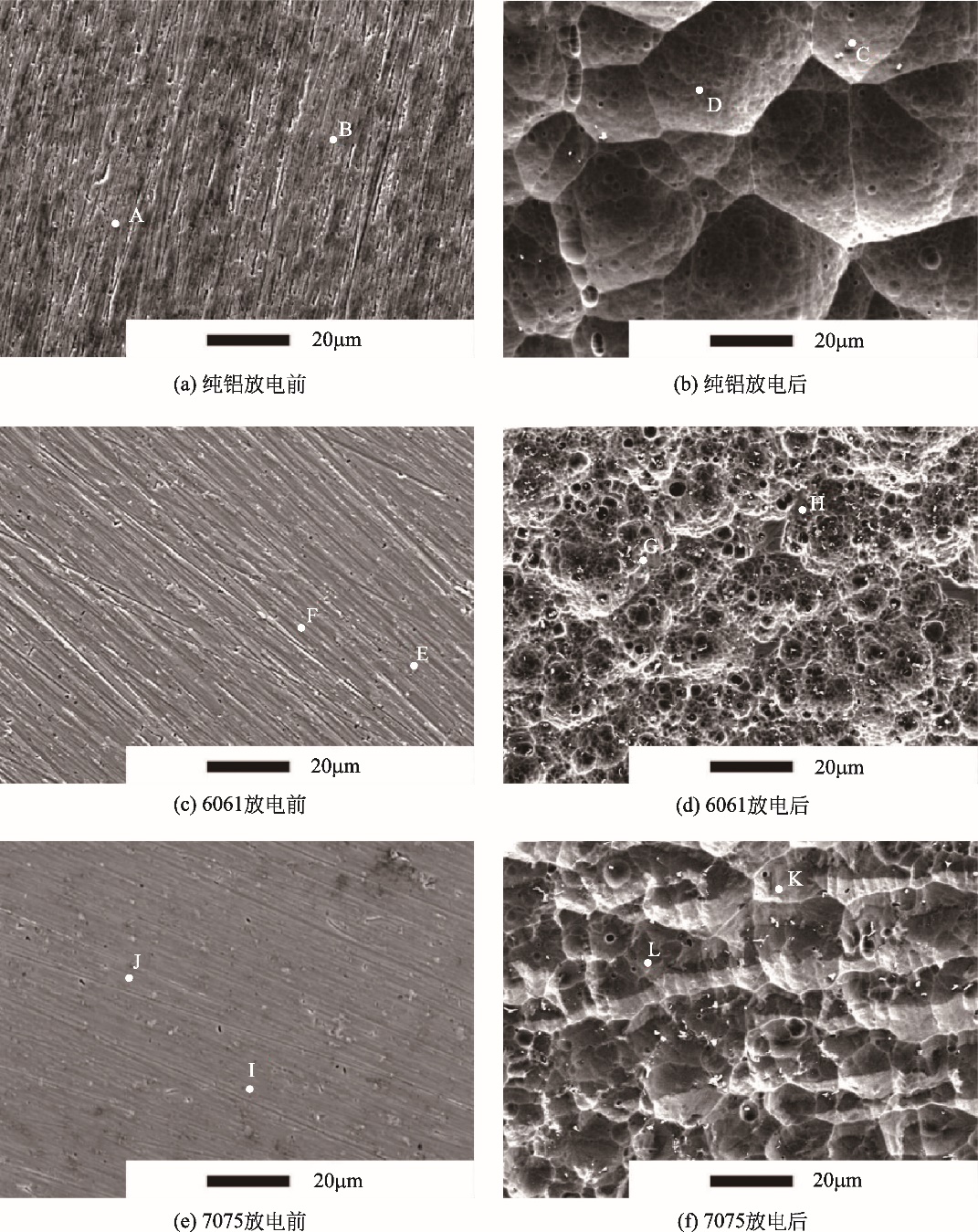
| 区域 | Al/% | Mg/% | Cu/% | Zn/% | Mn/% | Si/% | Ca/% | O/% | Fe/% |
|---|---|---|---|---|---|---|---|---|---|
| A | 100.00 | — | — | — | — | — | — | — | — |
| B | 100.00 | — | — | — | — | — | — | — | — |
| C | 30.61 | 0.12 | — | — | — | — | 34.23 | 35.05 | — |
| D | 100.00 | — | — | — | — | — | — | — | — |
| E | 95.68 | 0.86 | — | 1.80 | 0.68 | 0.99 | — | — | — |
| F | 93.49 | 0.68 | — | 1.88 | 1.01 | 1.09 | — | — | — |
| G | 59.13 | 0.63 | 0.20 | 1.23 | — | 38.25 | — | — | — |
| H | 93.49 | 0.68 | 0.89 | 1.88 | — | 1.09 | — | — | — |
| I | 56.10 | 2.09 | 8.71 | 4.71 | 0.88 | — | — | — | 29.31 |
| J | 86.78 | 2.46 | 2.98 | 7.78 | 0 | — | — | — | 0 |
| K | 81.73 | 2.06 | 3.42 | 8.67 | — | — | — | — | 4.09 |
| L | 86.27 | 2.33 | 2.98 | 8.42 | — | — | — | — | 0 |
| 区域 | Al/% | Mg/% | Cu/% | Zn/% | Mn/% | Si/% | Ca/% | O/% | Fe/% |
|---|---|---|---|---|---|---|---|---|---|
| A | 100.00 | — | — | — | — | — | — | — | — |
| B | 100.00 | — | — | — | — | — | — | — | — |
| C | 30.61 | 0.12 | — | — | — | — | 34.23 | 35.05 | — |
| D | 100.00 | — | — | — | — | — | — | — | — |
| E | 95.68 | 0.86 | — | 1.80 | 0.68 | 0.99 | — | — | — |
| F | 93.49 | 0.68 | — | 1.88 | 1.01 | 1.09 | — | — | — |
| G | 59.13 | 0.63 | 0.20 | 1.23 | — | 38.25 | — | — | — |
| H | 93.49 | 0.68 | 0.89 | 1.88 | — | 1.09 | — | — | — |
| I | 56.10 | 2.09 | 8.71 | 4.71 | 0.88 | — | — | — | 29.31 |
| J | 86.78 | 2.46 | 2.98 | 7.78 | 0 | — | — | — | 0 |
| K | 81.73 | 2.06 | 3.42 | 8.67 | — | — | — | — | 4.09 |
| L | 86.27 | 2.33 | 2.98 | 8.42 | — | — | — | — | 0 |
| 1 | CHAUBY N , YADAV D K , SINGH V K , et al . A comparative study of leaves extracts for corrosion inhibition effect on aluminium alloy in alkaline medium[J]. Ain. Shams. Eng. J., 2015, 22: 38-44. |
| 2 | 王诚, 邱平达, 蔡克迪, 等 . 铝空气电池关键技术研究进展[J]. 化工进展, 2016, 35(5): 1396-1403. |
| WANG C , QIU P D , CAI K D , et al . Research progress on key technologies of aluminum air batteries[J]. Chemical Industry and Engineering Progress, 2016, 35(5): 1396-1403. | |
| 3 | WANG L , WANG W , YANG G , et al . A hybrid aluminum/hydrogen/air cell system[J]. Int. J. Hydrogen Energy, 2013, 38: 14801-4809. |
| 4 | YANG S , KNICKLE H . Design and analysis of aluminum/air battery system for electric vehicles[J]. J. Power Sources, 2002, 112: 162-173. |
| 5 | YANG S , YANG W , SUN G , et al . Secondary current density distribution analysis of an aluminumeair cell[J]. J. Power Sources, 2006, 161: 1412-1419. |
| 6 | KRAYTSBERG A , EIN-ELI Y . The impact of nano-scaled materials on advanced metaleair battery systems[J]. Nano Energy, 2013, 2:468-480. |
| 7 | FAN L , LU H . The effect of grain size on aluminum anodes for aleair batteries in alkaline electrolytes[J]. J. Power Sources, 2015, 284:409-415. |
| 8 | 邵海洋, 文九巴, 马景灵,等 . Al-Ga-Mg-Sn-xSi阳极合金电化学性能研究[J]. 电源技术, 2015, 39(3): 502-505. |
| SHAO H H , WEN K B , MA J L , et al . Electrochemical properties of Al-Ga-Mg-Sn-xSi anode alloy[J]. Power Technology, 2015, 39(3):502-505. | |
| 9 | 屈钧娥,齐公台,张磊 .温度对A1-Zn-In-Sn-Mg阳极性能影响的探讨[J]. 华中科技大学学报, 2001, 29(8): 102-104. |
| QU J E , QI G T , ZHANG L . Discussion on the effect of temperature on the performance of A1-Zn-In-Sn-Mg anode[J]. Journal of Huazhong University of Science and Technology, 2001, 29(8): 102-104. | |
| 10 | 马景灵,郝星辰 .铝空气电池Al5Zn0.02In0.1Mg0.05Ti阳极材料性能研究[J]. 腐蚀科学与防护技术, 2014, 26(1): 25-29. |
| MA J L , HAO X C . Study on the properties of Al5Zn0.02In0.1Mg0.05Ti anode materials[J]. Science and Protection of Corrosion, 2014, 26(1):25-29. | |
| 11 | FAN L , LU H , LENG J . Performance of fifine structured aluminum anodes in neutral and alkaline electrolytes for Al-air batteries[J]. Electrochim. Acta, 2015,165: 22-28. |
| 12 | TANG Y , LU L , ROESKY H W , et al . The effect of zinc on the aluminum anode of the aluminum-air battery[J]. Journal of Power Sources, 2004, 138(1): 313-318. |
| 13 | MA J , WEN J , GAO J , et al . Performance of Al0.5Mg0.02Ga0.1Sn0.5Mn as anode for aleair battery in NaCl solutions[J]. J. Power Sources, 2014, 253: 419-423. |
| 14 | MA J , WEN J , GAO J , et al . Performance of 6061Mg1Zn0.1Ga0.1Sn as anode for Al-air battery[J]. Electrochim. Acta, 2014,129: 69-75. |
| 15 | RASHVAND A M , JAFARIAN M , MOGHANNI B O H , et al . Study of the alloying additives and alkaline zincate solution effects on the commercial aluminum as galvanic anode for use in alkaline batteries[J]. Mater. Chem. Phys., 2013, 143:133-142. |
| 16 | TANG Y , LU L , ROESKY H W , et al . The effect of zinc on the aluminum anode of the aluminumeair battery[J]. J. Power Sources, 2004,138:313-318. |
| 17 | LIU Y , LI J , LI W , et al . Spinel LiMn2O4 nanoparticles dispersed on nitrogen-doped reduced graphene oxide nanosheets as an effificient electrocatalyst for aluminium-air battery[J]. Int. J. Hydrogen Energy, 2015, 40: 9225-9234. |
| 18 | LIU Y , LI J , LI W , et al . Exploring the nitrogen species of nitrogen doped graphene as electrocatalysts for oxygen reduction reaction in aleairbatteries[J]. Int. J. Hydrogen Energy, 2016, 41:10354-10365. |
| 19 | WANG M , LAI Y , FANG J , et al . N-doped porous carbon derived from biomass as an advanced electrocatalyst for aqueous aluminium/air battery[J]. Int. J. Hydrogen Energy, 2015, 40: 16230-16237. |
| 20 | DAVYDOVA E S , ATAMANYUK I N , ILYUKHIN A S , et al . Nitrogen-doped carbonaceous catalysts for gas-diffusion cathodes for alkaline aluminum-air batteries[J]. J. Power Sources, 2016, 306:329-336. |
| 21 | OZCAN S , TOKUR M , CETINKAYA T , et al . Free standing flexible graphene oxide + a-MnO2 composite cathodes for Li-air batteries[J]. Solid State Ion., 2016, 286: 34-39. |
| 22 | FU Z , YAN L , LI K , et al . The performance and mechanism of modifified activated carbon air cathode by nonstoichiometric nano Fe3O4 in the microbial fuel cell[J]. Biosens Bioelectron, 2015, 74:989-995. |
| 23 | PARSONS R . Atlas of electrochemical equilibria in aqueous solutions[J]. Journal of Electroanalytical Chemistry & Interfacial Electrochemistry, 1967, 13(4): 471-471. |
| 24 | 施高杰, 薛济来, 高兴宇, 等 .铝-空气电池用石墨烯掺杂复合催化颗粒性能实验研究[J]. 炭素技术, 2017(2) :16-20. |
| SHI G J , XUE J L , GAO X Y , et al . Experimental study on performance of graphene-doped composite catalytic particles for aluminium-air batteries[J]. Carbon Technology, 2017(2): 16-20. | |
| 25 | YUAN J , WANG J , SHE Y , et al . BiOCl microassembles consisting of ultrafifine nanoplates: a highperformance electro-catalyst for air electrode of aleair batteries[J]. J. Power Sources, 2014, 263: 37-45. |
| 26 | FAN L , LU H , LENG J , et al . The effect of crystal orientation on the aluminum anodes of the aluminum-air batteries in alkaline electrolytes[J]. Journal of Power Sources, 2015, 299: 66-69. |
| 27 | YANG C C , HSU S T, CHIEN W C . All solid-state electric double-layer capacitors based on alkaline polyvinyl alcohol polymer electrolytes[J]. Journal of Power Sources, 2005, 152(1): 303-310. |
| 28 | WANG D , GAO L , ZHANG D , et al . Experimental and theoretical investigation on corrosion inhibition of AA5052 aluminium alloy by l-cysteine in alkaline solution[J]. Mater. Chem. Phys., 2016, 169:142-151. |
| 29 | EGAN D R , PONCE D E , LEON C , et al . Developments in electrode materials andelectrolytes for aluminiumeair batteries[J]. J. Power Sources, 2013, 236: 293-310. |
| 30 | NESTORIDI M , PLETCHER D , WOOD R J , et al . The study of aluminium anodes for high power density Al/air batteries with brine electrolytes[J]. J. Power Sources, 2008, 178: 445-455. |
| 31 | ROSALBINO F , DELSANTE S , BORZONE G , et al . Influence of rare earth metals on the characteristics of anodic oxide films on aluminium and their dissolution behaviour in NaOH solution[J]. Corros. Sci., 2010, 52: 322-326. |
| 32 | SOLMAZ R . Investigation of the inhibition effect of 5-((E)-4-phenylbuta-1,3-dienylideneamino)-1,3,4-thiadiazo-le-2-thiol Schiff base on mild steel corrosion in hydrochloric acid[J]. Corros. Sci., 2010, 52: 3321-3330. |
| 33 | 洪刚, 程旭东, 张海兵,等 . Al-Sn-Ga-Bi-Pb-Cd合金电极低温海水电化学性能研究[J]. 电镀与精饰, 2018, 40(4): 37-41. |
| HONG G , CHENG X D , ZHANG H B , et Al . Electrochemical properties of low-temperature seawater with Al-Sn-Ga-Bi-Pb-Cd alloy electrode[J]. Electroplating and Finishing, 2018, 40(4): 37-41. | |
| 34 | 马正青, 黎文献, 余琨, 等 .新型铝合金阳极的腐蚀行为[J].表面技术, 2002(4): 17-20. |
| MA Z Q , LI W X , YU K , et al . Corrosion behavior of new aluminum alloy anodes[J]. Surface Technology, 2002(4): 17-20. | |
| 35 | MUTLU R N , ATES S , YAZIC I , et al . Al-6013-T6 and Al-7075-T7351 alloy anodes for aluminium-air battery[J]. International Journal of Hydrogen Energy, 2017, 42(36): 23315-23325. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | GAO Yufei, LU Jinfeng. Mechanism of heterogeneous catalytic ozone oxidation:A review [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 430-438. |
| [3] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [4] | WANG Fu'an. Consumption and emission reduction of the reactor of 300kt/a propylene oxide process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 213-218. |
| [5] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| [6] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [7] | LEI Wei, JIANG Weijia, WANG Yugao, HE Minghao, SHEN Jun. Synthesis of N,S co-doped coal-based carbon quantum dots by electrochemical oxidation and its application in Fe3+ detection [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4799-4807. |
| [8] | LYU Chengyuan, ZHANG Han, YANG Mingwang, DU Jianjun, FAN Jiangli. Recent advances of dioxetane-based afterglow system for bio-imaging [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4108-4122. |
| [9] | LI Runlei, WANG Ziyan, WANG Zhimiao, LI Fang, XUE Wei, ZHAO Xinqiang, WANG Yanji. Efficient catalytic performance of CuO-CeO2/TiO2 for CO oxidation at low-temperature [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4264-4274. |
| [10] | ZHANG Yaojie, ZHANG Chuanxiang, SUN Yue, ZENG Huihui, JIA Jianbo, JIANG Zhendong. Application of coal-based graphene quantum dots in supercapacitors [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4340-4350. |
| [11] | ZHAO Jian, ZHUO Zewen, DONG Hang, GAO Wenjian. A new method for observation of microstructure of waxy crude oil and its emulsion system [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4372-4384. |
| [12] | XUE Kai, WANG Shuai, MA Jinpeng, HU Xiaoyang, CHONG Daotong, WANG Jinshi, YAN Junjie. Planning and dispatch of distributed integrated energy systems for industrial parks [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3510-3519. |
| [13] | WANG Zhicai, LIU Weiwei, ZHOU Cong, PAN Chunxiu, YAN Honglei, LI Zhanku, YAN Jingchong, REN Shibiao, LEI Zhiping, SHUI Hengfu. Synthesis and performance of a superplasticizer based on coal-based humic acid [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3634-3642. |
| [14] | JIANG Bolong, CUI Yanyan, SHI Shunjie, CHANG Jiacheng, JIANG Nan, TAN Weiqiang. Synthesis of transition metal Co3O4/ZnO-ZIF oxygen reduction catalyst by Co/Zn-ZIF template method and its electricity generation performance [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3066-3076. |
| [15] | ZHAN Yong, WANG Hui, WEI Tingting, ZHU Xingyu, WANG Xiankai, CHEN Sisi, DONG Bin. In situ reduction effect of Mn2+ enhanced ozone conditioning on sludge in biological treatment process [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3253-3260. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||