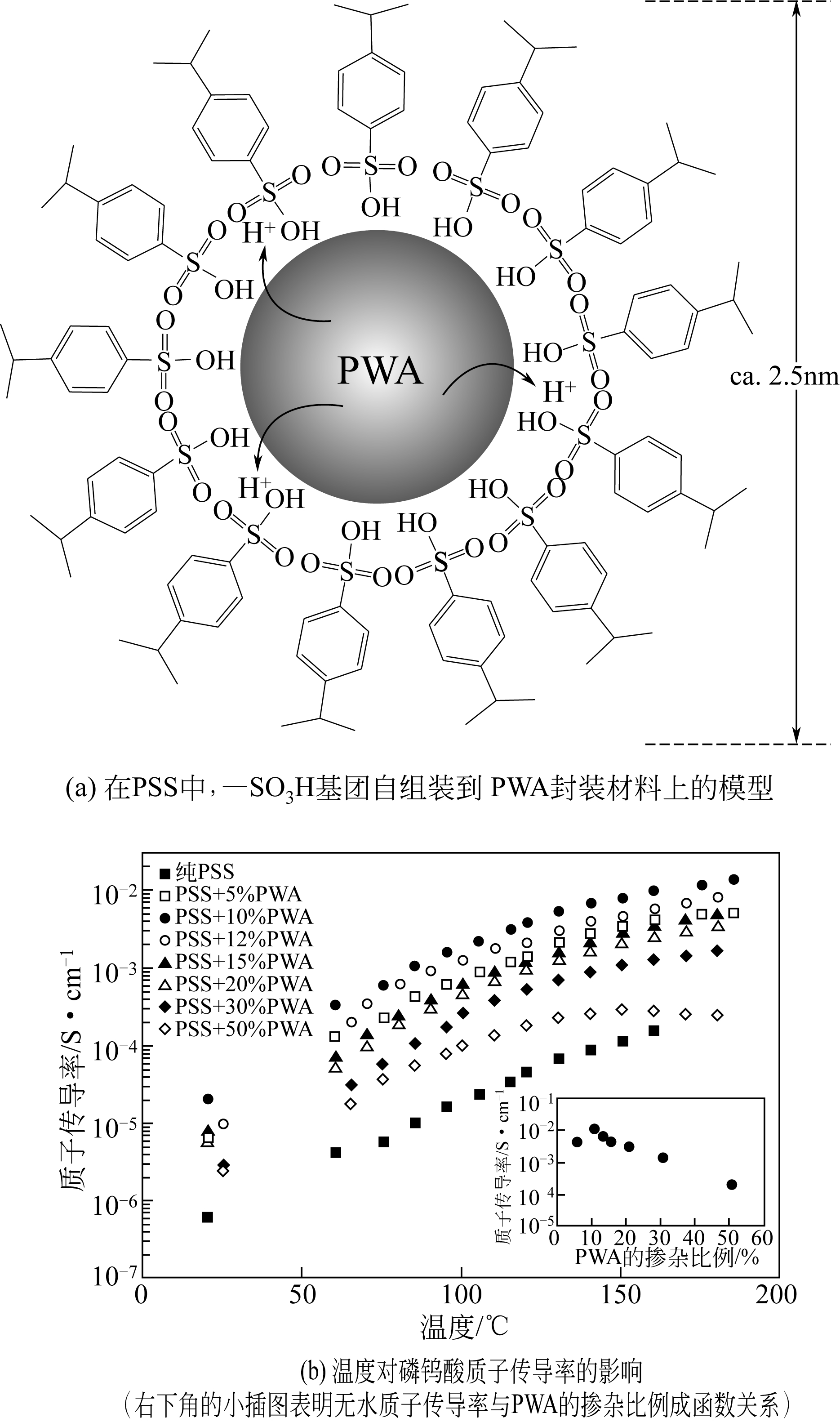
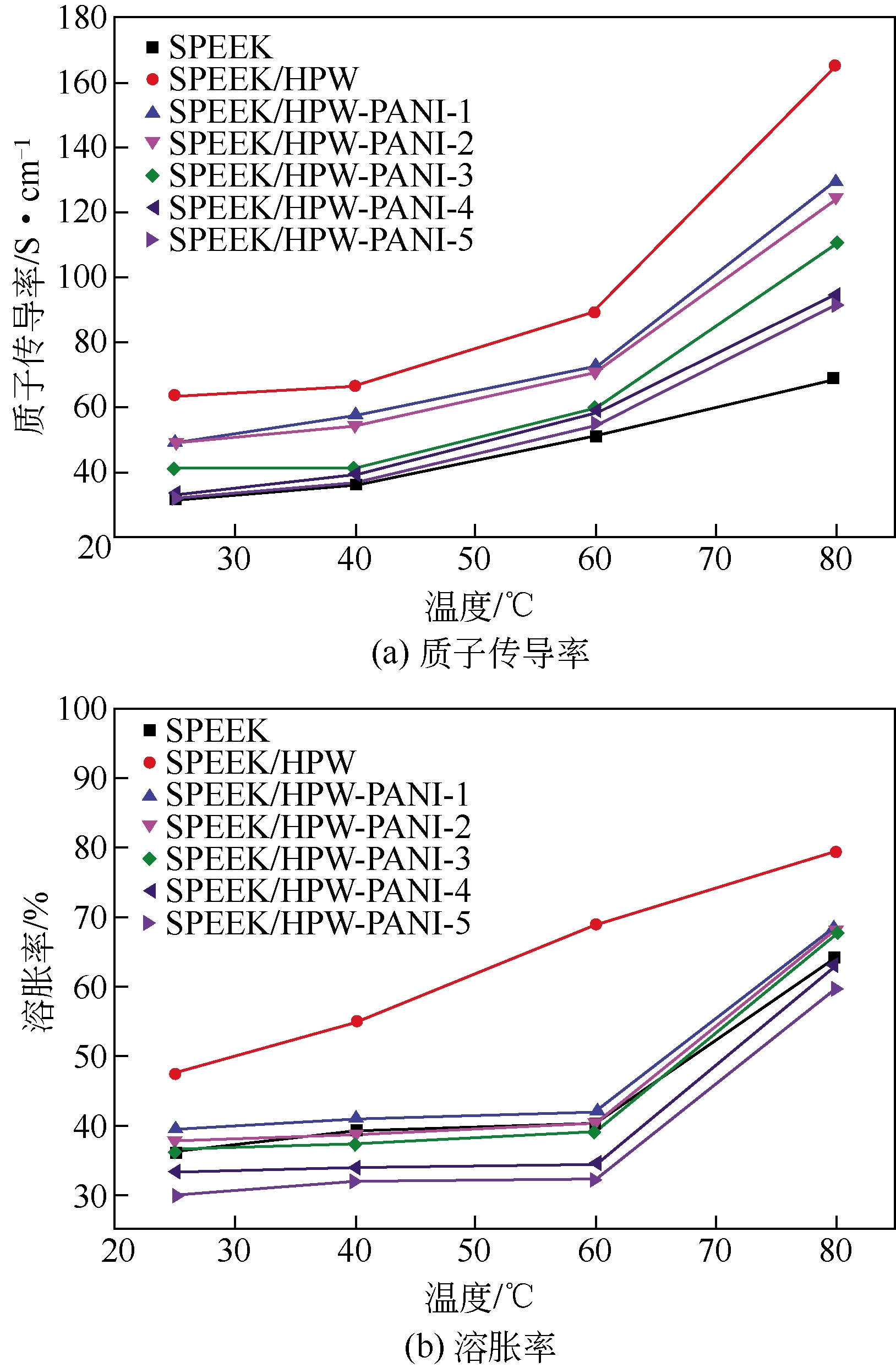
Chemical Industry and Engineering Progress ›› 2019, Vol. 38 ›› Issue (05): 2212-2221.DOI: 10.16085/j.issn.1000-6613.2018-1311
• Materials science and technology • Previous Articles Next Articles
Progress in high temperature inorganic proton conduction materials used for proton exchange membrane fuel cells
Yingfeng WANG( ),Kai LI,Shuirong LI,Duo WANG,Yueyuan YE,Yunquan LIU(
),Kai LI,Shuirong LI,Duo WANG,Yueyuan YE,Yunquan LIU( )
)
- College of Energy, Xiamen University, Xiamen 361102, Fujian, China
-
Received:2018-06-25Revised:2018-12-06Online:2019-05-05Published:2019-05-05 -
Contact:Yunquan LIU
用于质子交换膜燃料电池的高温无机质子传导材料研究进展
- 厦门大学能源学院,福建 厦门 361102
-
通讯作者:刘运权 -
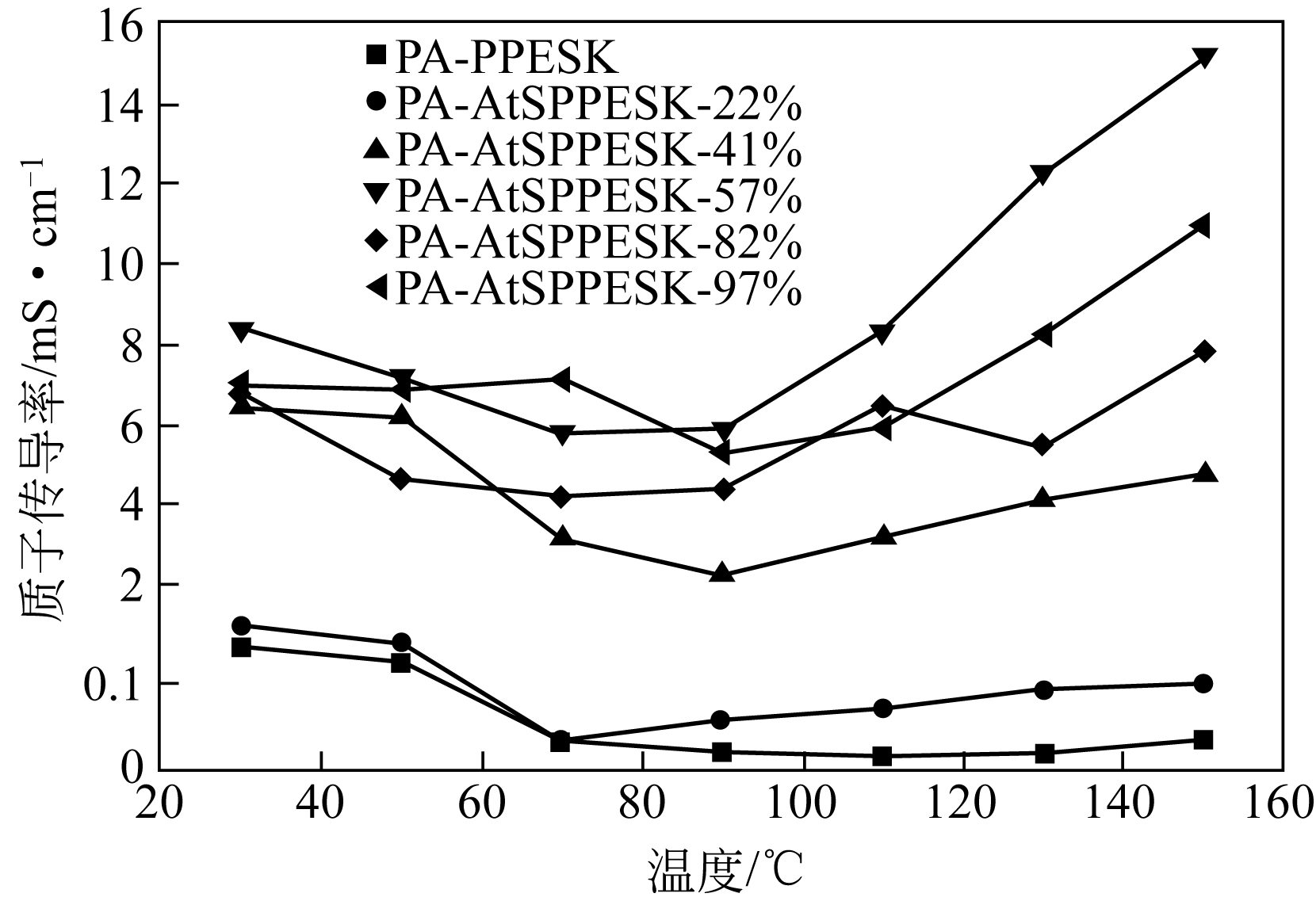
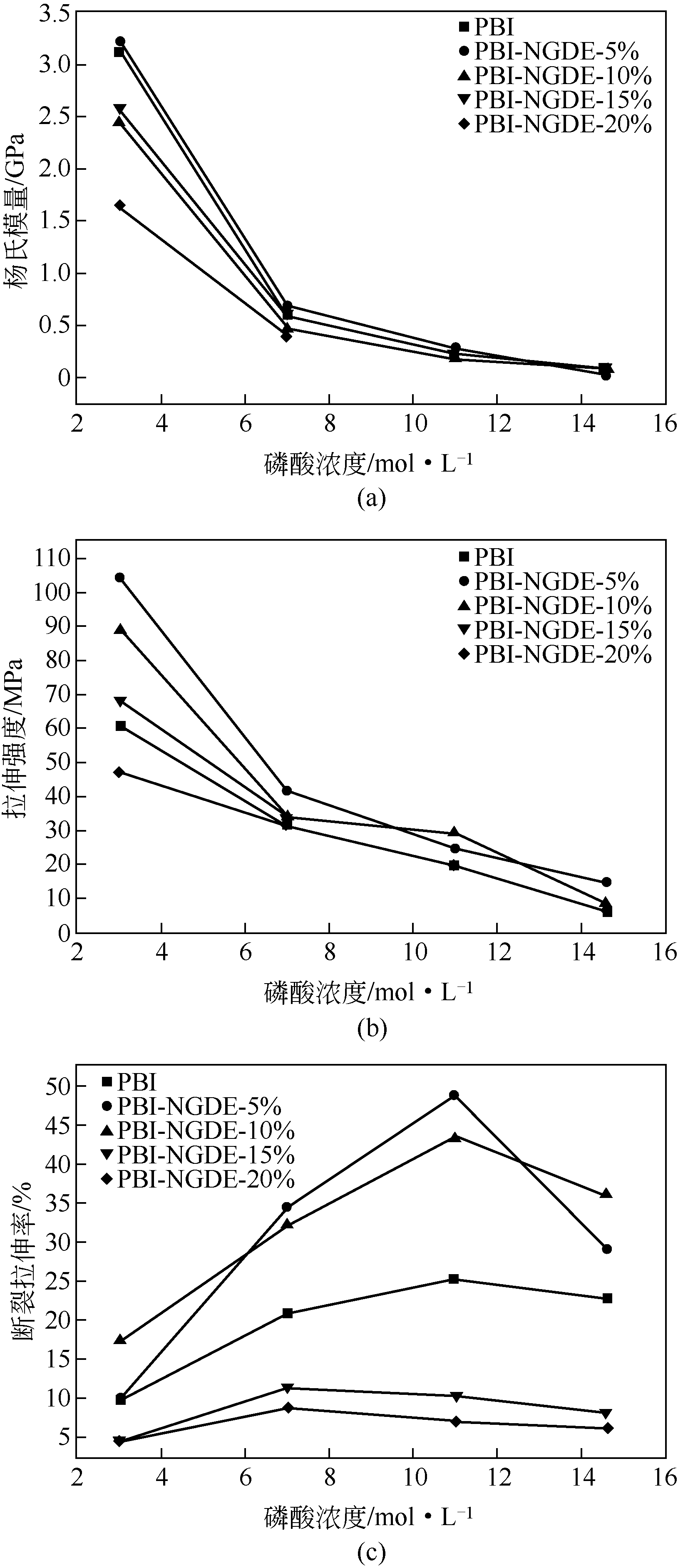
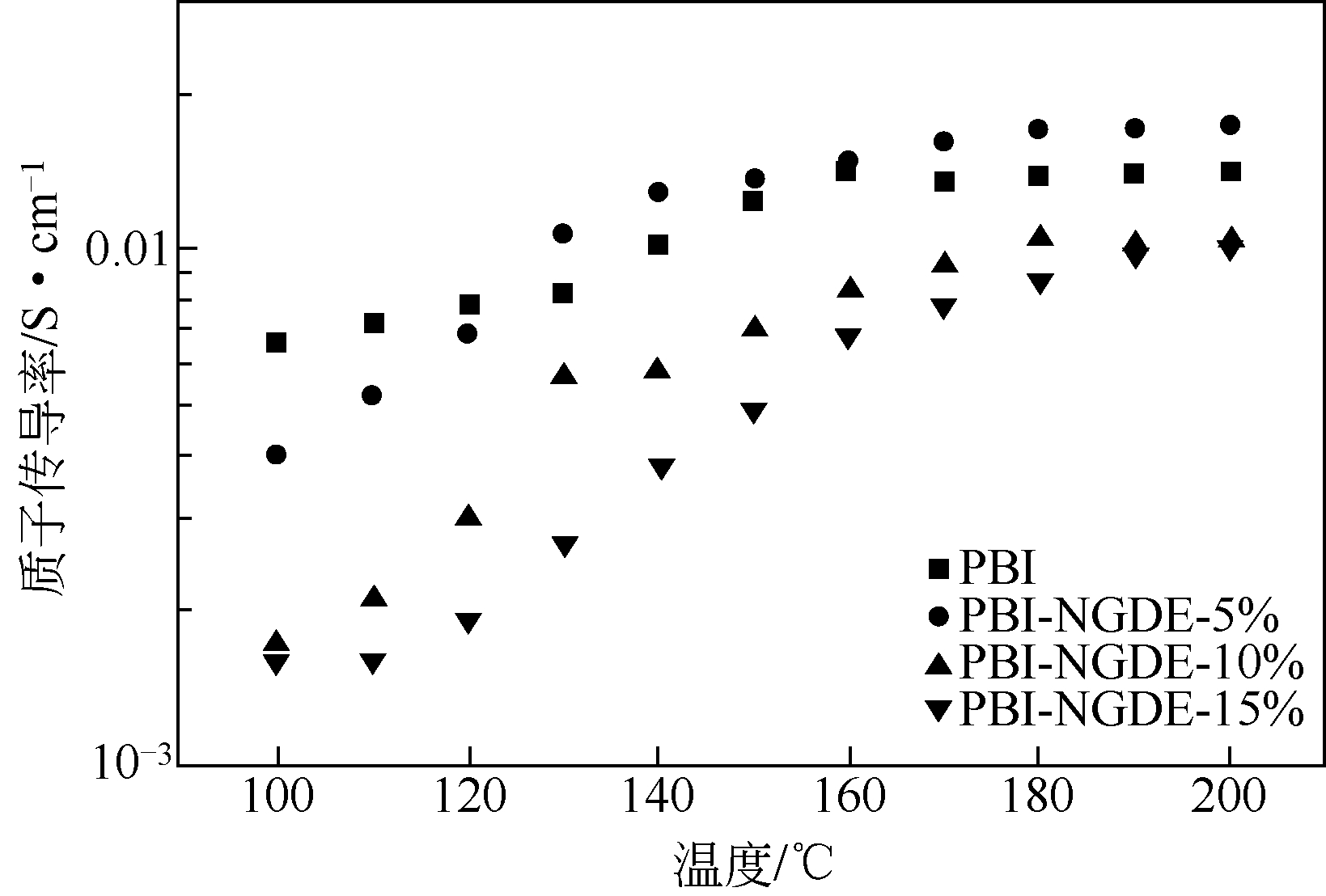
作者简介:<named-content content-type="corresp-name">王颖锋</named-content>(1994—),男,硕士研究生,研究方向为燃料电池。E-mail: <email>wangyingfeng666@163.com</email>。|刘运权,教授,博士生导师,研究方向为制氢与燃料电池。E-mail:<email>yq_liu@xmu.edu.cn</email>。 -
基金资助:福建省科技厅重点项目(2016H6024)
CLC Number:
Cite this article
Yingfeng WANG, Kai LI, Shuirong LI, Duo WANG, Yueyuan YE, Yunquan LIU. Progress in high temperature inorganic proton conduction materials used for proton exchange membrane fuel cells[J]. Chemical Industry and Engineering Progress, 2019, 38(05): 2212-2221.
王颖锋, 李凯, 李水荣, 王夺, 叶跃元, 刘运权. 用于质子交换膜燃料电池的高温无机质子传导材料研究进展[J]. 化工进展, 2019, 38(05): 2212-2221.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2018-1311
| 膜类型 | 拉伸强度/MPa | 断裂拉伸率/% |
|---|---|---|
| PBI | 133±7.09 | 65±17 |
| PBI/DBpX | 122±11.5 | 79±17 |
| PBI/BADGE | 114±2.25 | 26±13 |
| 膜类型 | 拉伸强度/MPa | 断裂拉伸率/% |
|---|---|---|
| PBI | 133±7.09 | 65±17 |
| PBI/DBpX | 122±11.5 | 79±17 |
| PBI/BADGE | 114±2.25 | 26±13 |
| 膜类型 | 质子传导率/S·cm-1 | 酸泄露率/% | 酸掺杂/% | 激活能量/kJ·mol-1 | ||
|---|---|---|---|---|---|---|
| 140℃ | 165℃ | 180℃ | ||||
| PBI | 0.0794 | 0.1030 | 0.1439 | 85 | 13.9±0.4 | 26 |
| PBI/BADGE | 0.0541 | 0.0590 | 0.0666 | 73 | 10.3±0.2 | 11 |
| PBI/EGDE | 0.0468 | 0.0682 | 0.0972 | 80 | 10.2±0.3 | 32 |
| PBI/TPA | 0.0631 | 0.0891 | 0.1172 | 82 | 12.7±0.5 | 28 |
| PBI/DBpX | 0.0711 | 0.1009 | 0.1513 | 80 | 15±0.4 | 32 |
| 膜类型 | 质子传导率/S·cm-1 | 酸泄露率/% | 酸掺杂/% | 激活能量/kJ·mol-1 | ||
|---|---|---|---|---|---|---|
| 140℃ | 165℃ | 180℃ | ||||
| PBI | 0.0794 | 0.1030 | 0.1439 | 85 | 13.9±0.4 | 26 |
| PBI/BADGE | 0.0541 | 0.0590 | 0.0666 | 73 | 10.3±0.2 | 11 |
| PBI/EGDE | 0.0468 | 0.0682 | 0.0972 | 80 | 10.2±0.3 | 32 |
| PBI/TPA | 0.0631 | 0.0891 | 0.1172 | 82 | 12.7±0.5 | 28 |
| PBI/DBpX | 0.0711 | 0.1009 | 0.1513 | 80 | 15±0.4 | 32 |
| 材料类型 | 质子传导率/S·cm-1 | 测试条件 | 最大功率密度/W·cm-2 | 测试温度/℃ | |
|---|---|---|---|---|---|
| PBI/DBpX[ | 0.1513 | 180℃ | 0.123 | 165 | |
| PA-doped PESB[ | 0.0730 | 160℃ | 0.427 | 160 | |
| PA-doped PBI[ | 0.1230 | 室温下,100%相对湿度 | N/A | N/A | |
| Phosphoric acid doped MTZPAEK[ | 0.0510 | 190℃ | 0.061 | 160 | |
| 材料类型 | 质子传导率/S·cm-1 | 测试条件 | 最大功率密度/W·cm-2 | 测试温度/℃ | |
|---|---|---|---|---|---|
| PBI/DBpX[ | 0.1513 | 180℃ | 0.123 | 165 | |
| PA-doped PESB[ | 0.0730 | 160℃ | 0.427 | 160 | |
| PA-doped PBI[ | 0.1230 | 室温下,100%相对湿度 | N/A | N/A | |
| Phosphoric acid doped MTZPAEK[ | 0.0510 | 190℃ | 0.061 | 160 | |
| 样品 | 厚度/nm | 拉伸强度/MPa | 延伸率/% | 杨氏模量/MPa |
|---|---|---|---|---|
| ANA/NF | 0.15 | 18.29±0.54 | 304.67±22.01 | 279.17±7.32 |
| MOR/NF | 0.18 | 16.60±0.32 | 225.00±17.44 | 311.57±2.94 |
| NF | 0.15 | 21.52±0.83 | 205.00±25.15 | 276.81±5.05 |
| NF117 | 0.18 | 26.65±1.22 | 359.33±34.21 | 236.42±7.66 |
| 样品 | 厚度/nm | 拉伸强度/MPa | 延伸率/% | 杨氏模量/MPa |
|---|---|---|---|---|
| ANA/NF | 0.15 | 18.29±0.54 | 304.67±22.01 | 279.17±7.32 |
| MOR/NF | 0.18 | 16.60±0.32 | 225.00±17.44 | 311.57±2.94 |
| NF | 0.15 | 21.52±0.83 | 205.00±25.15 | 276.81±5.05 |
| NF117 | 0.18 | 26.65±1.22 | 359.33±34.21 | 236.42±7.66 |
| 材料类型 | 质子传导率/S·cm-1 | 测试条件 | 最大功率密度/W·cm-2 | 测试条件 |
|---|---|---|---|---|
| 磺化介孔硅材料(S-MCM-41)[ | 6.94×10-3 | N/A | N/A | |
| SPVA-MOR[ | 5.20×10-2 | 80℃下100% 相对湿度 | 3.5×10-3 | 25℃/甲醇浓度1mol?L-1 |
| Tri@MS-PrNH2-1[ | 8.34×10-3 | 120℃ | N/A | N/A |
| MCM-48[ | 4.80×10-3 | 140℃ | N/A | N/A |
| 材料类型 | 质子传导率/S·cm-1 | 测试条件 | 最大功率密度/W·cm-2 | 测试条件 |
|---|---|---|---|---|
| 磺化介孔硅材料(S-MCM-41)[ | 6.94×10-3 | N/A | N/A | |
| SPVA-MOR[ | 5.20×10-2 | 80℃下100% 相对湿度 | 3.5×10-3 | 25℃/甲醇浓度1mol?L-1 |
| Tri@MS-PrNH2-1[ | 8.34×10-3 | 120℃ | N/A | N/A |
| MCM-48[ | 4.80×10-3 | 140℃ | N/A | N/A |
| 膜类型 | 25℃下质量 损失率/% | 80℃下质量 损失率/% |
|---|---|---|
| SPEEK | 1.68 | 2.38 |
| SPEEK/HPW | 2.07 | 28.93 |
| SPEEK/HPW-PANI-1 | 2.00 | 28.61 |
| SPEEK/HPW-PANI-2 | 1.94 | 28.41 |
| SPEEK/HPW-PANI-3 | 1.73 | 27.51 |
| SPEEK/HPW-PANI-4 | 1.55 | 21.45 |
| SPEEK/HPW-PANI-5 | 1.29 | 19.84 |
| 膜类型 | 25℃下质量 损失率/% | 80℃下质量 损失率/% |
|---|---|---|
| SPEEK | 1.68 | 2.38 |
| SPEEK/HPW | 2.07 | 28.93 |
| SPEEK/HPW-PANI-1 | 2.00 | 28.61 |
| SPEEK/HPW-PANI-2 | 1.94 | 28.41 |
| SPEEK/HPW-PANI-3 | 1.73 | 27.51 |
| SPEEK/HPW-PANI-4 | 1.55 | 21.45 |
| SPEEK/HPW-PANI-5 | 1.29 | 19.84 |
| 材料类型 | 质子传导率/ S·cm-1 | 测试条件 | 最大功率密度/ W·cm-2 | 测试条件 |
|---|---|---|---|---|
| SPEEK/HPW@MIL101[ | 0.2720 | 65℃,相对湿度100% | 0.383 | 相对湿度100%,60℃ |
| SPEEK/HPW/g-C3N4 [ | 0.2490 | 浸泡在60℃的水中48h | N/A | N/A |
| HPW/meso-SiO2/CSPEEK[ | 0.0019 | 相对湿度30%,120℃ | N/A | N/A |
| smpCTS/HPW[ | 0.0290 | 在80℃下 | 0.016 | 80℃/甲醇浓度2mol/L |
| 材料类型 | 质子传导率/ S·cm-1 | 测试条件 | 最大功率密度/ W·cm-2 | 测试条件 |
|---|---|---|---|---|
| SPEEK/HPW@MIL101[ | 0.2720 | 65℃,相对湿度100% | 0.383 | 相对湿度100%,60℃ |
| SPEEK/HPW/g-C3N4 [ | 0.2490 | 浸泡在60℃的水中48h | N/A | N/A |
| HPW/meso-SiO2/CSPEEK[ | 0.0019 | 相对湿度30%,120℃ | N/A | N/A |
| smpCTS/HPW[ | 0.0290 | 在80℃下 | 0.016 | 80℃/甲醇浓度2mol/L |
| 1 | AHADI M , TAM M, STUMPER J , et al . Thermal conductivity of catalyst layer of polymer electrolyte membrane fuel cells: part 1-experimental study[J]. J. Power Sources, 2017, 354: 207-214. |
| 2 | CHANG Guanjun , SHANG Zhenfang , YANG Li . Hydrogen bond cross-linked sulfonated poly(iminoether ether ketone) (PIEEK) for fuel cell membranes[J]. J. Power Sources,2015,282:401-408. |
| 3 | ARAYA S S , ZHOU F , LISO V , et al . A comprehensive review of PBI-based high temperature PEM fuel cells[J]. Int. J. Hydrogen. Energy, 2016, 41:21310-21344. |
| 4 | DANG Jingchuan , ZHAO Liping , ZHANG Jie , et al . Imidazole microcapsules toward enhanced phosphoric acid loading of polymer electrolyte membrane for anhydrous proton conduction[J]. J. Membrane Science,2018,545:88-89. |
| 5 | MELCHIOR J P , MAJER G , KREUER K D .Why do proton conducting polybenzimidazole phosphoric acid membranes perform well inhigh-temperature PEM fuel cells?[J]. Phys. Chem. Chem.Phys.,2017,19:601-612. |
| 6 | DIPPEL T , KREUER K D , LASSÈGUES J C , et al . Proton conductivity in fused phosphoric acid; A1H/31P PFG-NMR and QNS study[J]. Solid State Ionics,1993,61:41-46. |
| 7 | VILCIAUSKAS L , TUCKERMAN M E , BESTER G , et al . The mechanism of proton conduction in phosphoric acid[J]. Nat. Chem., 2012,4:461-466. |
| 8 | LI Qingfeng , David AILI , HJULER Hans Aage, et al . High temperature polymer electrolyte membrane fuel cells[M]. Switzerland: Springer Cham., 2016:169-215. |
| 9 | YUAN Sen , GUO Xiaoxia , AILI D , et al . Poly(imide benzimidazole)s for high temperature polymer electrolyte membrane fuel cells[J]. J. Membranes Science, 2014, 454(59):351-358. |
| 10 | PAN Jiefeng , XU Tongwen , WU liang , et al . Proton exchange membrane from tetrazole-based poly(phthalazinone ether sulfone ketone) for high-temperature fuel cells[J]. International Journal of Hydrogen Energy,2016,41(28):12337-12346. |
| 11 | WANG Shuang , ZHANG Gang , NA Hui ,et al . Novel epoxy-based cross-linked polybenzimidazole for high temperature proton exchange membrane fuel cells[J]. Int.J. Hydrogen Energy, 2011,36(14):8412-8421. |
| 12 | CHE Quantong , YUE Jie . Polymerized imidazolium ionic liquids crosslinking sulfonated poly(ether ether ketone)(SPEEK) for high-temperature proton exchange membrane[J]. RSC Advances,2016,6(113):111729-111738. |
| 13 | OZDEMIR Y , OZKAN N , DEVRIM Y . Fabrication and characterization of cross-linked polybenzimidazole based membranes for high temperature pem fuel cells[J]. Electrochimica Acta,2017,245:1-13. |
| 14 | WANG Kaili , CHANG Guanjun , YANG Li , et al . Phosphoric acid-doped poly(ether sulfone benzotriazole) for high-temperature proton exchange membrane fuel cell applications[J]. Journal of Membrane Science,2018,549(74):23-27. |
| 15 | JAHANGIRI S , ARAVI I , OZDEN-YENIGUN E , et al . Fabrication and optimization of proton conductive polybenzimidazole electrospun nanofiber membranes[J]. Polym. Adv. Technol.,2018,29(1):594-602. |
| 16 | ZHAO Chengji , BU Fanzhe , ZHANG Yurong , et al . 1,2,4-Triazole functionalized poly(arylene ether ketone) for high temperature proton exchange membrane with enhanced oxidative stability[J]. Journal of Membrane Science,2018,545(43):167-175. |
| 17 | YOON M , KIM K, NATARAJAN S , et al . Proton conduction in metal-organic frameworks and related modularly built porous solids[J]. Angew. Chem. Int. Ed.,2013,52(10):2688-2700. |
| 18 | XU Feng , MU Shichun . Nanoceramic oxide hybrid electrolyte membranes for proton exchange membrane fuel cells[J]. Journal of Nanoscience and Nanotechnology,2014,14(2):1169-1180. |
| 19 | SHENDEROVICH I G , BUNTKOWSKY G , SCHREIBER A , et al . Pyridine-15N-A mobile NMR sensor for surface acidity and surface defects of mesoporous silica[J]. J.Phys.Chem.B, 2003,107(43):11924-11939. |
| 20 | FRANKE M E , SIMON U . Solvate-supported proton transport in zeolites[J]. Chem. Phys. Chem.,2004,5(4):465-472. |
| 21 | ARICO A S , ANTONUCCI P L , GIORDANO N , et al . Ionic conductivity in heteropolyacid-tin mordenite composite electrolytes[J]. Materials Letters, 1995,24(6):399-405. |
| 22 | BORDUIN R , LI W . Fabrication of foamed polyethersulfone-zeolite mixed matrix membranes for polyethsulfone-zeolite mixed marixmembranes for polymer electrolyte membrane fuel cell humidification[J]. Journal of Manufactuing Science and Engineering-Transactions of the Asme,2017,139:021004(1-7). |
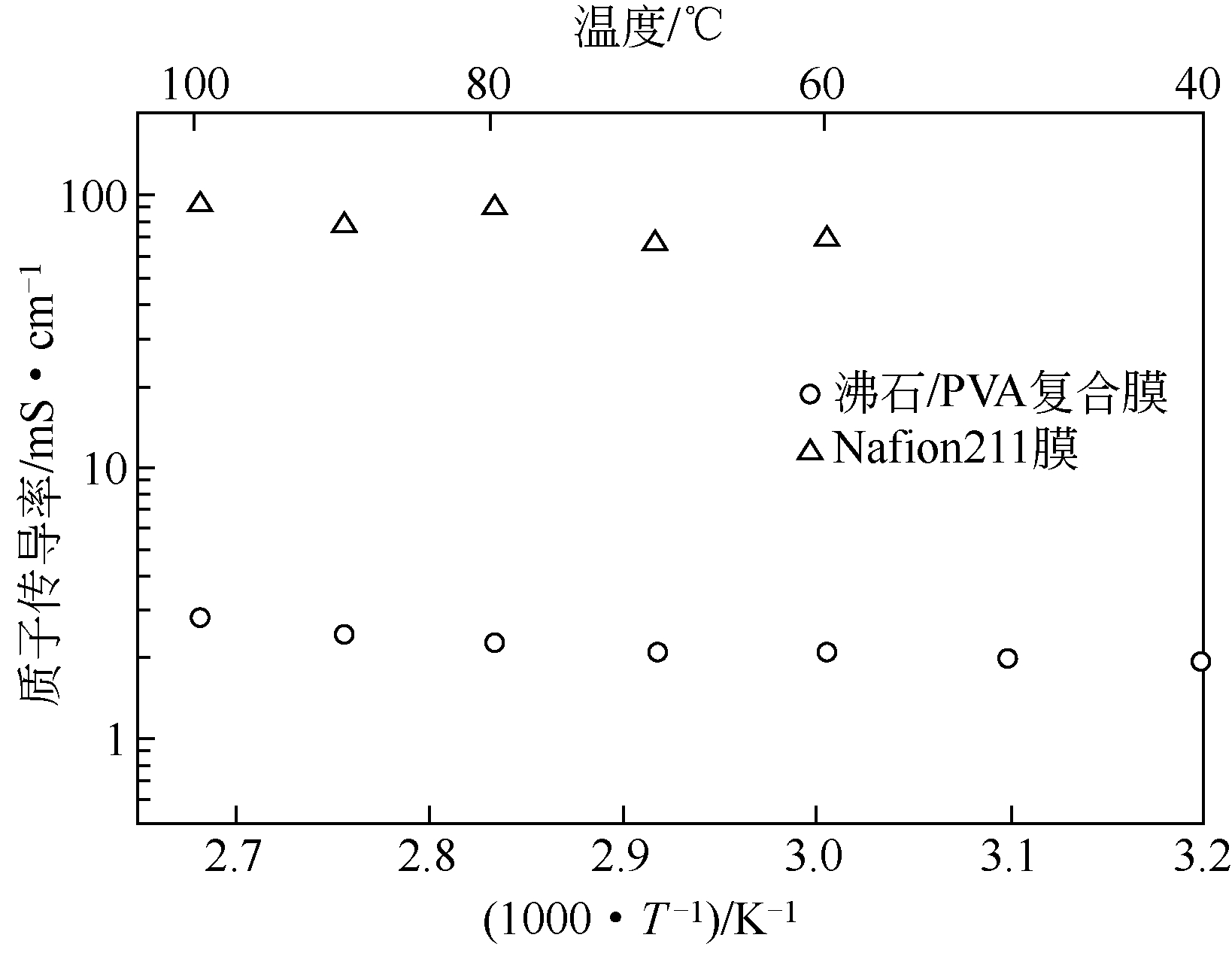
| 23 | NISHIHARA M , TERAYAMA Y , HAJI T , et al . Proton-conductive nano zeolite-PVA composite film as a new water-absorbing electrolyte for water electrolysis[J]. Express Polymer Letters ,2018,12(3):256-264. |
| 24 | DATRINDADE L G , PEREIRA E C . SPEEK/zeolite/ionic-liquid anhydrous polymer membranes for fuel-cell applications[J]. Eur. J. Inor. Chem, 2017,17(17):2369-2376. |
| 25 | PRAPAINAINAR P , DU Z H , KONGKACHUICHAY P , et al . Mordenite/nafion and analcime/nafion composite membranes prepared by spray method for improved direct methanol fuel cell performance[J]. Applied Surface Science,2017,421(Part A):24-41. |
| 26 | GAHLOT S , SHARMA P P , KULSHRESTHA V , et al . Nanoporous composite proton exchange membranes: high conductivity and thermal stability[J]. Colloids and Surfaces A,2018,542:8-14. |
| 27 | UCTUG F G , NIJEM J . Effect of polymer sulfonation on the proton conductivity and fuel cell performance of polyvinylalcohol-mordenite direct methanol fuel cell membranes[J]. Asia-Pac. J.Chem. Eng.,2017,12(5):682-693. |
| 28 | WU Zhenzhen , LI Juan , ZHANG Xianming . Heterogeneous hybrid of propyl amino functionalized MCM-41 and 1H-1,2,4-triazole for high efficient intermediate temperature proton conductor[J]. RSC Advances,2017,7(82):52321-52326. |
| 29 | DVOYASHKINA N , WARK M , FREUDE D , et al . Proton mobility in sulfonic acid functionalized mesoporous materials studied by MAS PFG NMR diffusometry and impedance spectroscopy[J]. Microporous and Mesoporous Materials,2018,255:140-147. |
| 30 | Mohammad NORSYAHIDA , Bakar Mohamad ABU , Amir H Kadhum ABDUL , et al . A review on synthesis and characterization of solid acid materials for fuel cell applications[J]. Journal of Power Sources,2016,322:77-92. |
| 31 | A Boysen DANE , Uda TETSUYA , R I Chisholm CALUM , et al . High-performance solid acid fuel cells through humidity stabilization[J]. Science,2004,303(5654):68-70. |
| 32 | SOSSINA M H , DANE A B , CALUM R I C , et al . Solid acids as fuel cell electrolytes[J]. Nature,2001,410(6831):910-913. |
| 33 | OH S Y, KAWAMURA G , MUTO H , et al . Anhydrous protic conduction of mechanochemically synthesized CsHSO4-azole-derived composites[J]. Electrochimica Acta,2012,75:11-19. |
| 34 | DANG Dai , ZHAO Bote , CHEN Dongchang , et al . A durable polyvinyl butyral-CsH2PO4 composite electrolyte for solid acid fuel cells[J]. Journal of Power Sources,2017,359:1-6. |
| 35 | CHAI Zhanli , SUO Quanyu , WANG Hui , et al . Mesoporous lanthanum phosphate nanostructurescontaining H3PO4 as superior electrolyte for PEM fuelcells[J]. RSC Advances, 2013,3(44):21928-21935. |
| 36 | NAKAMURA O , KODAMA T , OGINO I , et al . High-conductivity solid proton conductors: dodecamolybdophosphoric acid and dodecatungstophosphoric acid crystals[J]. Chemistry Letters,1979,8(1):17-18. |
| 37 | 孙偲祎,魏梅林 .基于杂多酸和菲咯啉/联喹啉类金属配合物的复合物的结构及性能研究[D].新乡:河南师范大学,2017. |
| SUN Siwei , WEI Meilin . Structures and properties of the composites based on heteropoly acids and phenanthroline/biquinoline metal coordination compounds[D]. Xinxiang:Henan Normal University,2017. | |
| 38 | YAMADA M , HONMA I . Heteropolyacid-encapsulated self-assembled materials for anhydrous proton-conducting electrolytes[J]. J.Phys.Chem.B, 2006,110(41):20486-20490. |
| 39 | LU Shanfu , XU Xin , XIANG Yan , et al . A self-anchored phosphotungstic acid hybrid proton exchange membrane achieved via one-step synthesis[J]. Adv. Energy Mater.,2014,4(17):1400842. |
| 40 | BOSE A B , GOPU S , LI W . Enhancement of proton exchange membrane fuel cells performance at elevated temperatures and lower humidities by incorporating immobilized phosphotungstic acid in electrodes[J]. Journal of Power Source,2014,263:217-222. |
| 41 | REN Suzhen , XU Meiling , HAO Ce , et al . Effects of microstructural functional polyaniline layers on SPEEK/HPW proton exchange membranes[J]. Journal of Applied Polymer Science,2014,131:41033(1-8). |
| 42 | ZHANG Bei , CAO Ying , WU Hong , et al . Proton exchange nanohybrid membranes with high phosphotungstic acid loading within metal-organic frameworks for PEMFC applications[J]. Electrochimica Acta,2017,240:186-194. |
| 43 | ZHOU Qiong , DONG Cuicui , WANG Qian , et al . Influence of alkaline 2D carbon nitride nanosheets as fillers for anchoring HPW and improving conductivity of SPEEK nanocomposite membranes[J]. Int.J. of Hydrogen Energy,2017,42:10317-10328. |
| 44 | SONG J-M , H-S WOO ,SOHNJ-Y, et al . 12HPW/meso-SiO2 nanocomposite CSPEEK membranes for proton exchange membrane fuel cells[J]. Journal of Industrial and Engineering Chemistry,2016,36263:132-138. |
| 45 | LU Shanfu , WU Qiuxia , WANG Haining , et al . Novel methanol-blocking proton exchange membrane achieved via self-anchoring phosphotungstic acid into chitosan membrane with submicro-pores[J]. Journal of Membrane Science,2016,500:203-210. |
| 46 | 伍志鲲 .铌酸催化剂在有机合成中的应用[D].长沙:湖南 师范大学,2001. |
| WU Zhikun . Application of niobic acids in organic synthesis [D]. Changsha:Hunan Normal University, 2001. | |
| 47 | 王一荻,臧宏瑛 .基于铌氢氧化合物复合材料的制备及其质子传导性能研究[D].长春:东北师范大学,2017. |
| WANG Yidi , ZANG Hongying . Preparation of niobium hydroxides-based composites and their proton conductivity[D]. Changchun:Northeast Normal University,2017. | |
| 48 | CHAI Zhanli , DONG Dehua , WANG Cheng , et al . Nanoporous niobium phosphate electrolyte membrane for low temperature fuel cell[J]. Journal of Membrane Science, 2010,356(1/2):147-153. |
| 49 | DUAN Chuancheng , TONG Jianhua , SHANG Meng , et al . Readily processed protonic ceramic fuel cells with high performance at low temperatures[J]. Science,2015,349(6254):1321-1326. |
| 50 | DUAN C C , KEE R J, ZHU HY , et al . Highly durable, coking and sulfur tolerant fuel-flexible protonic ceramic fuel cells[J]. Nature,2018,557(7704):217-221. |
| [1] | CHEN Kuangyin, LI Ruilan, TONG Yang, SHEN Jianhua. Structure design of gas diffusion layer in proton exchange membrane fuel cell [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 246-259. |
| [2] | XU Jiaheng, LI Yongsheng, LUO Chunhuan, SU Qingquan. Optimization of methanol steam reforming process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 41-46. |
| [3] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [4] | JIANG Bolong, CUI Yanyan, SHI Shunjie, CHANG Jiacheng, JIANG Nan, TAN Weiqiang. Synthesis of transition metal Co3O4/ZnO-ZIF oxygen reduction catalyst by Co/Zn-ZIF template method and its electricity generation performance [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3066-3076. |
| [5] | MA Zhejie, ZHANG Wenli, ZHAO Xuankai, LI Ping. Progress on the influence of oxygen mass transfer resistance in PEMFC cathode catalyst layer [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2860-2873. |
| [6] | GAO Weitao, YIN Qinan, TU Ziqiang, GONG Fan, LI Yang, XU Hong, WANG Cheng, MAO Zongqiang. Proton transport in metal-organic frameworks and their applications in proton exchange membranes [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 260-268. |
| [7] | HU Bing, XU Lijun, HE Shan, SU Xin, WANG Jiwei. Researching progress of hydrogen production by PEM water electrolysis under the goal of carbon peak and carbon neutrality [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4595-4604. |
| [8] | GAO Weitao, LEI Yijie, ZHANG Xun, HU Xiaobo, SONG Pingping, ZHAO Qing, WANG Cheng, MAO Zongqiang. An overview of proton exchange membrane fuel cell [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1539-1555. |
| [9] | ZHANG Dong, ZHANG Rui, ZHANG Bin, AN Zhoujian, LEI Che. Research progress of combined cooling-heat-and-power systems based on PEMFC [J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1608-1621. |
| [10] | FENG Zhanxiong, WANG Yun, MA Qiang, ZHANG Chuang, WANG Cheng. Preparation of Pt/C catalyst by continuous pipeline microwave technology and its oxygen reduction performance [J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6377-6384. |
| [11] | WAN Nianfang. Research progress of membrane electrode assembly of proton exchange membrane water electrolysis for hydrogen production [J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6385-6394. |
| [12] | DU Xin, FAN Jinwei, GUO Lijun, WANG Jinlong. Simulation of fuel cell aging process with heterogeneous agglomerate model [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5755-5760. |
| [13] | LI Zhenghan, TU Zhengkai. Research progress of simulation models of proton exchange membrane fuel cell [J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5272-5296. |
| [14] | WANG Meng, LIU Lili, LI Na, HU Zhaoxia, CHEN Shouwen. Preparation and properties of sulfonate modification nano-diamonds doped sulfonated poly(aryl ether sulfone) proton exchange membranes [J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5645-5652. |
| [15] | XING Yijing, LIU Fang, ZHANG Yalin, LI Haibin. Research progress on preparation methods of membrane electrode assemblies for proton exchange membrane fuel cells [J]. Chemical Industry and Engineering Progress, 2021, 40(S1): 281-290. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||