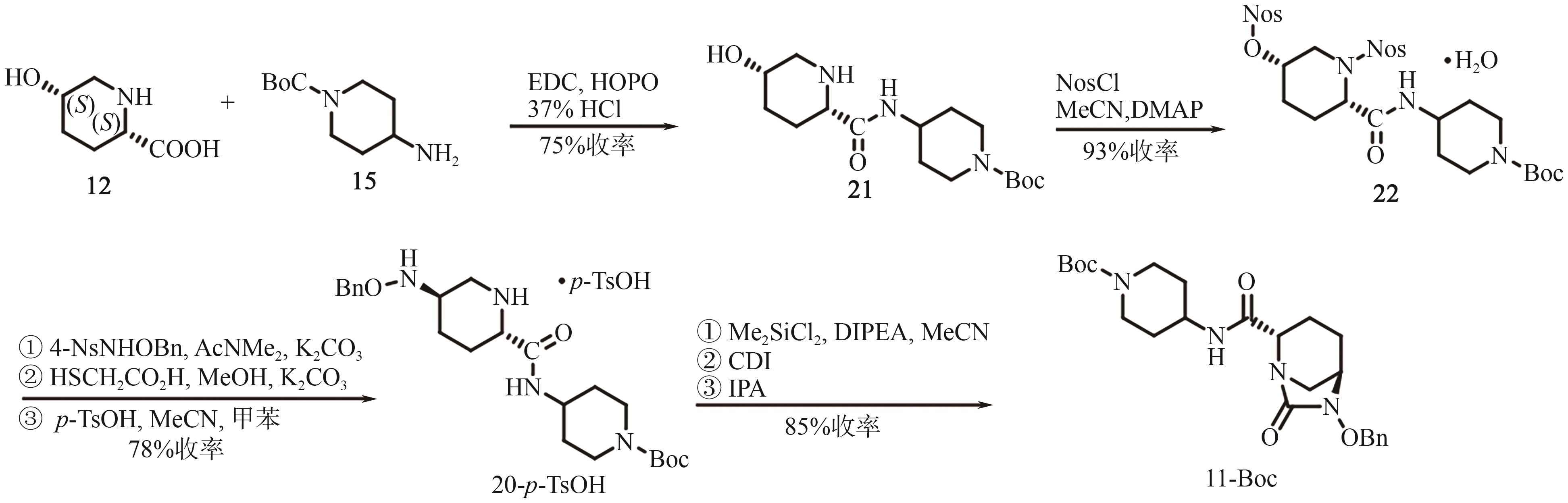
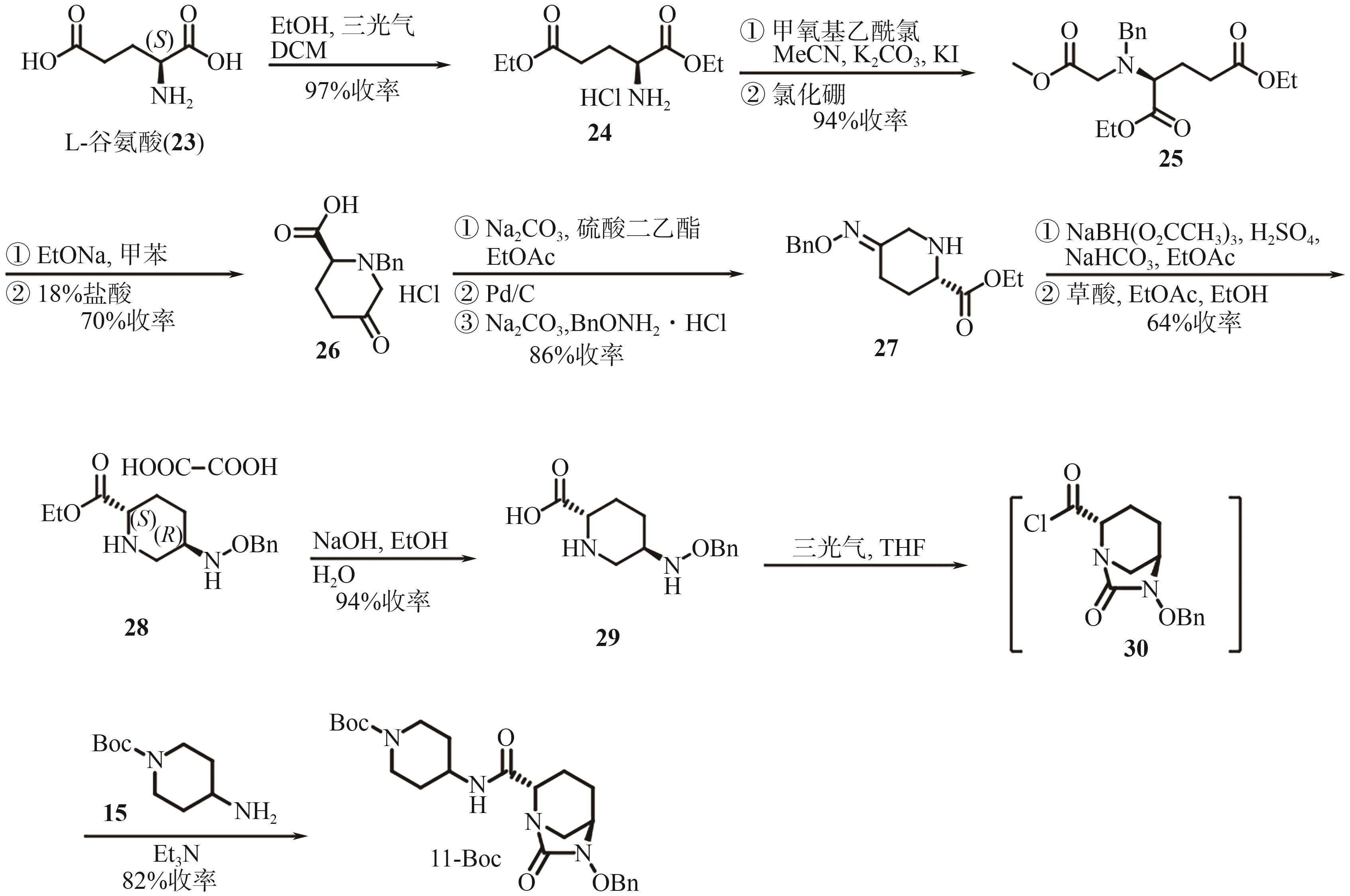
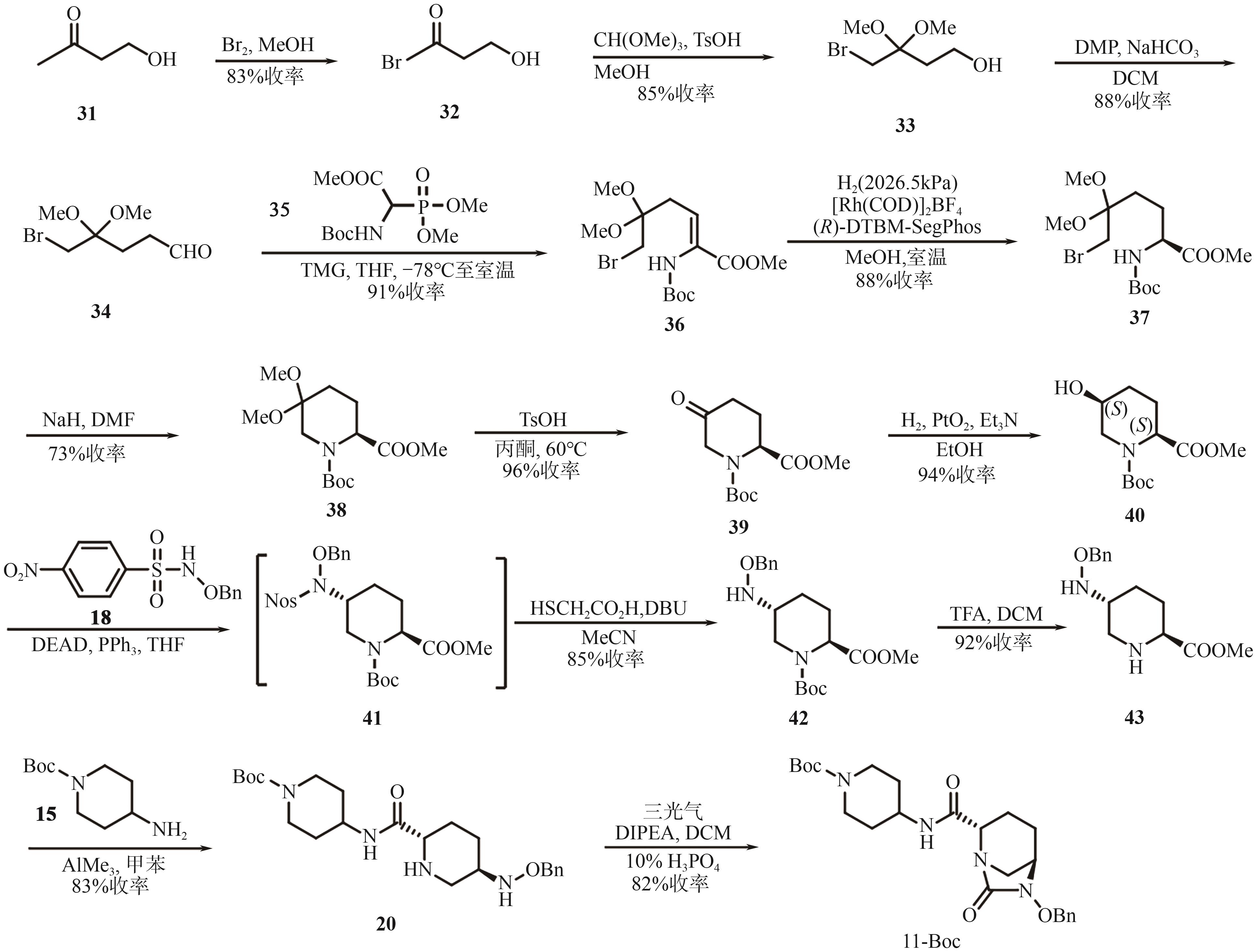
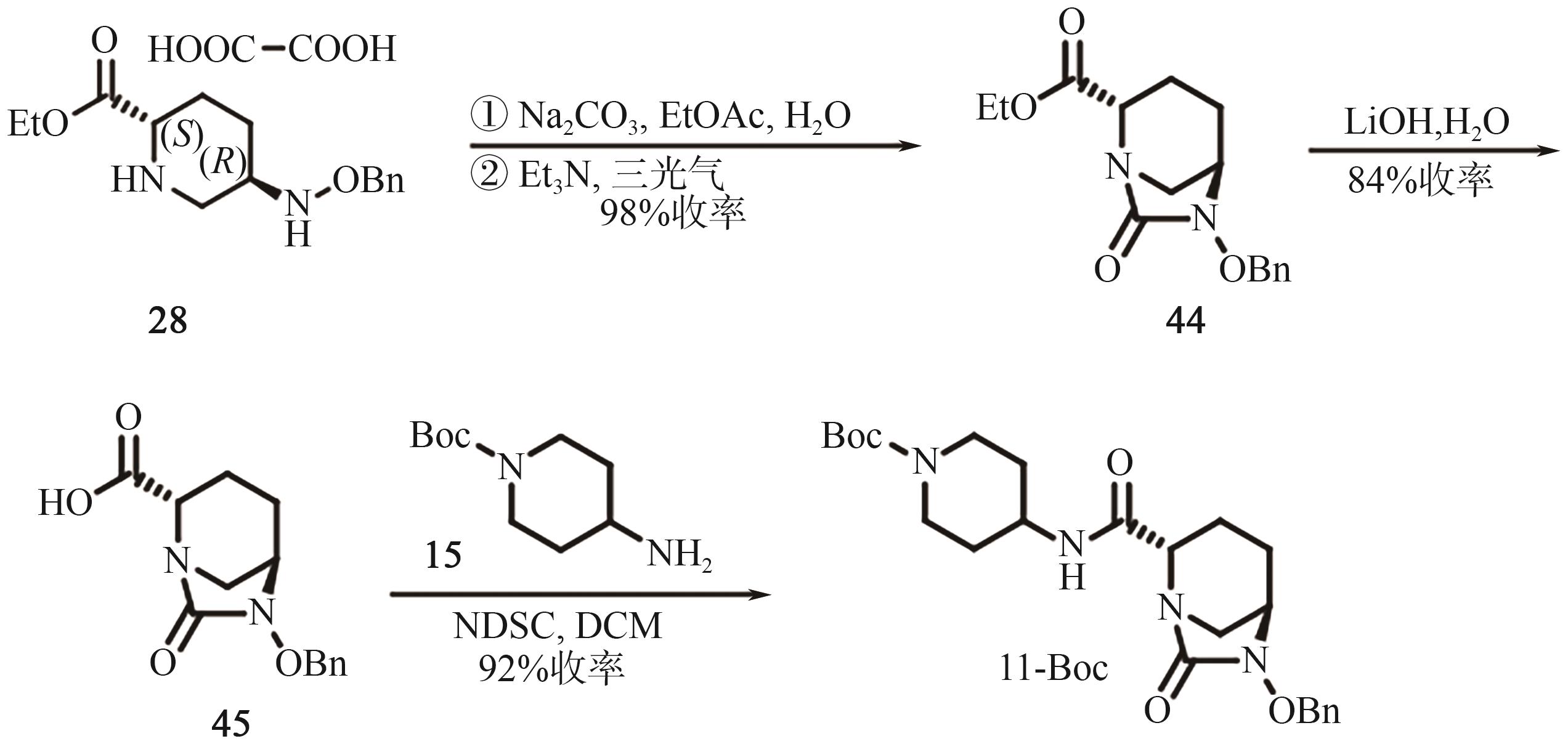
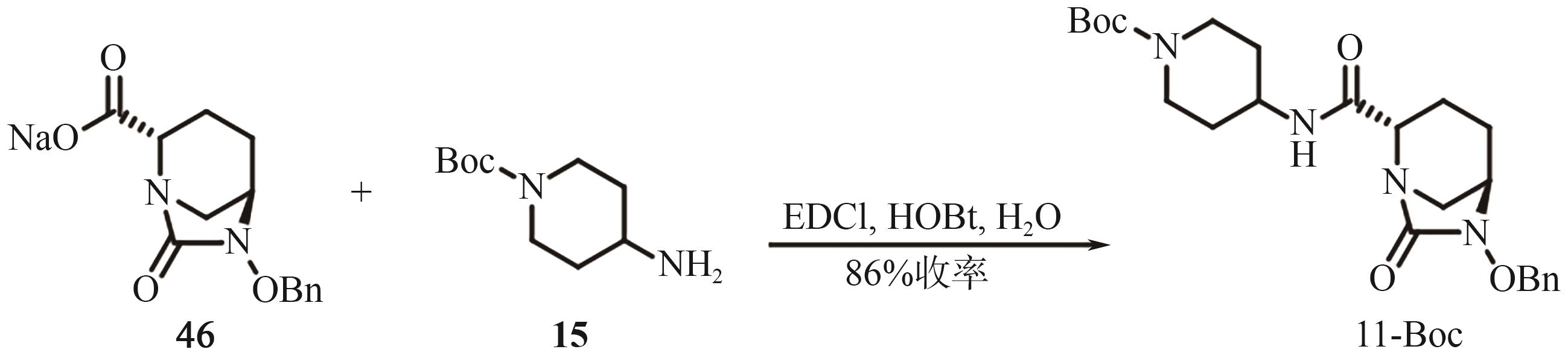
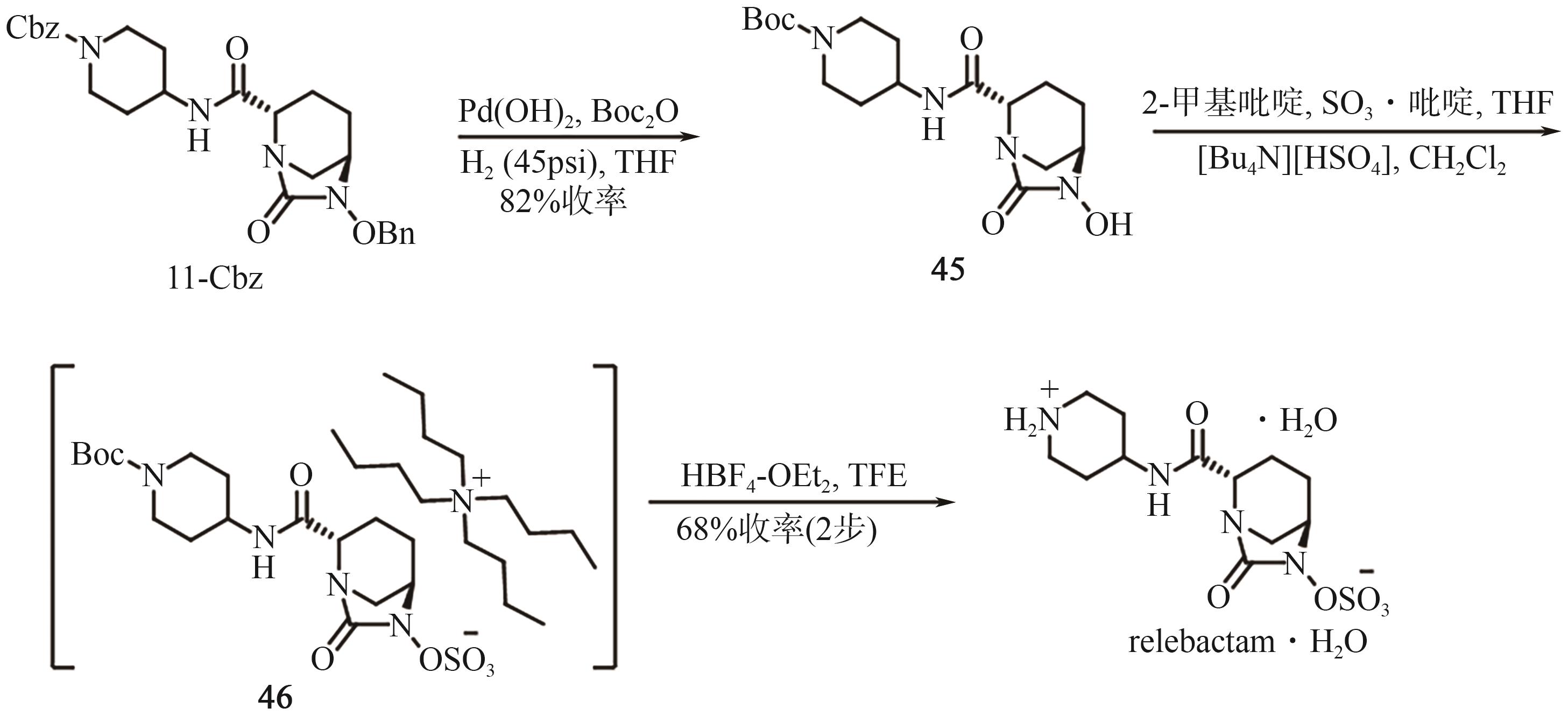
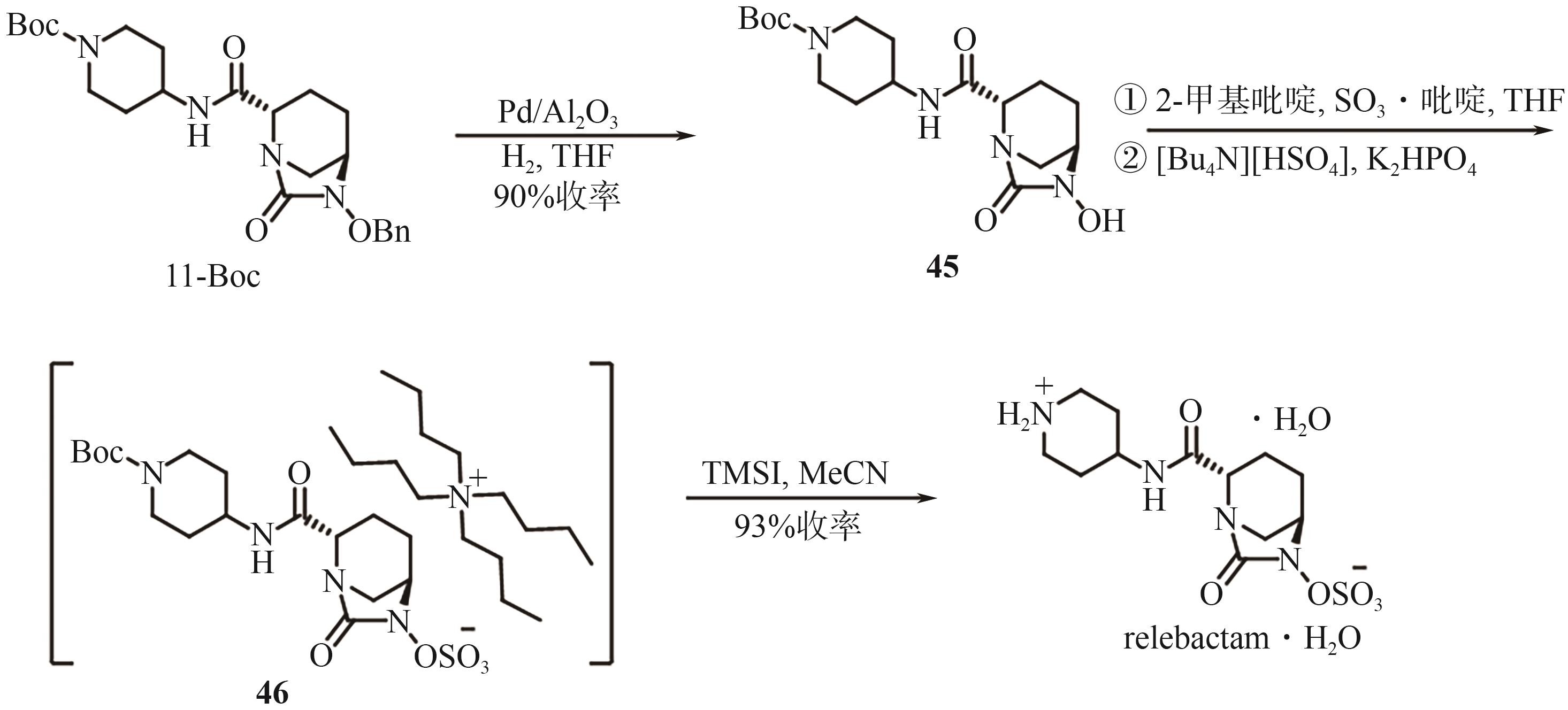
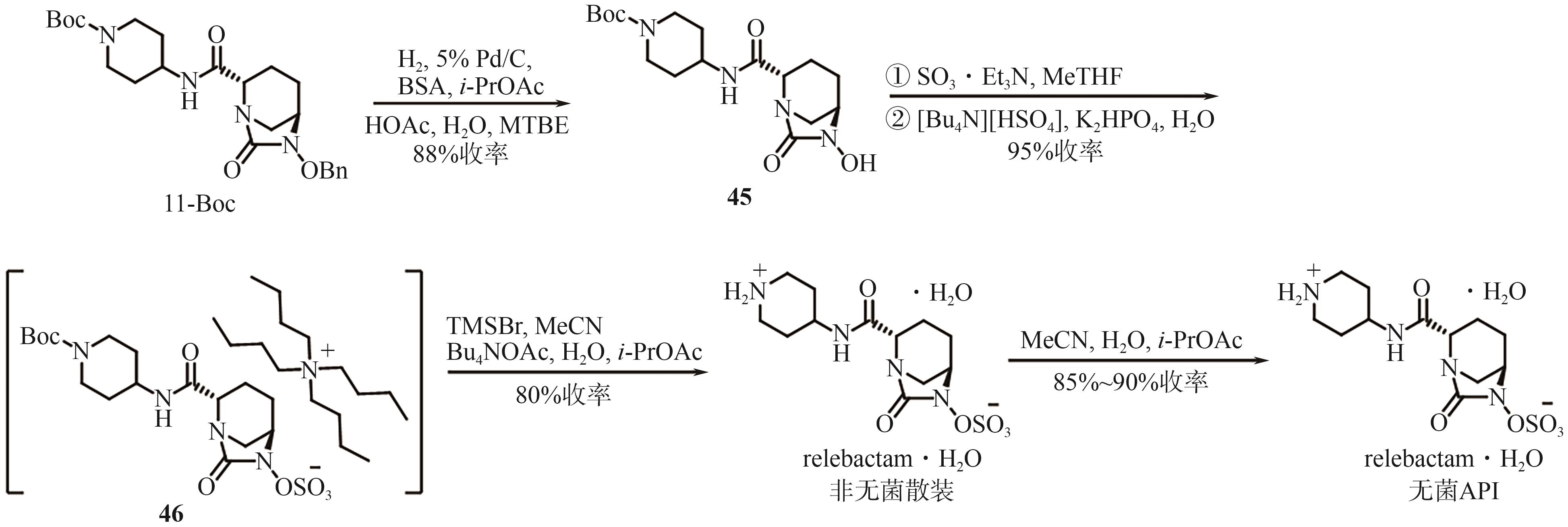
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (9): 5224-5233.DOI: 10.16085/j.issn.1000-6613.2024-1151
• Biochemical and pharmaceutical engineering • Previous Articles
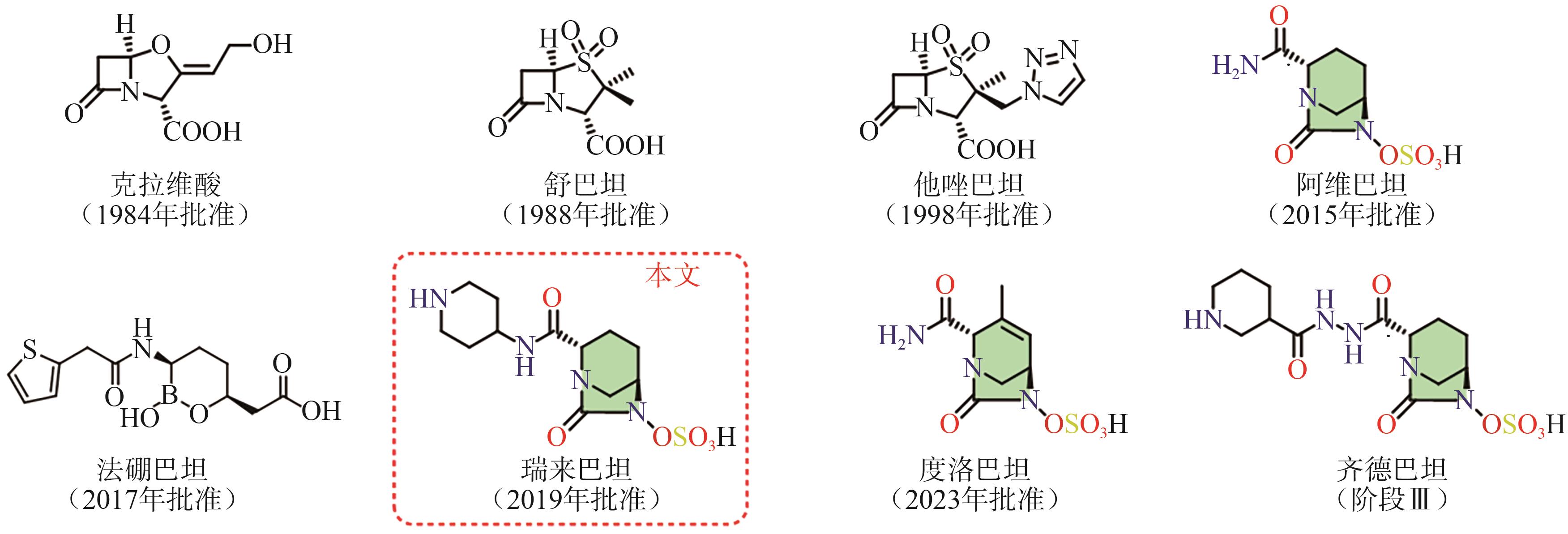
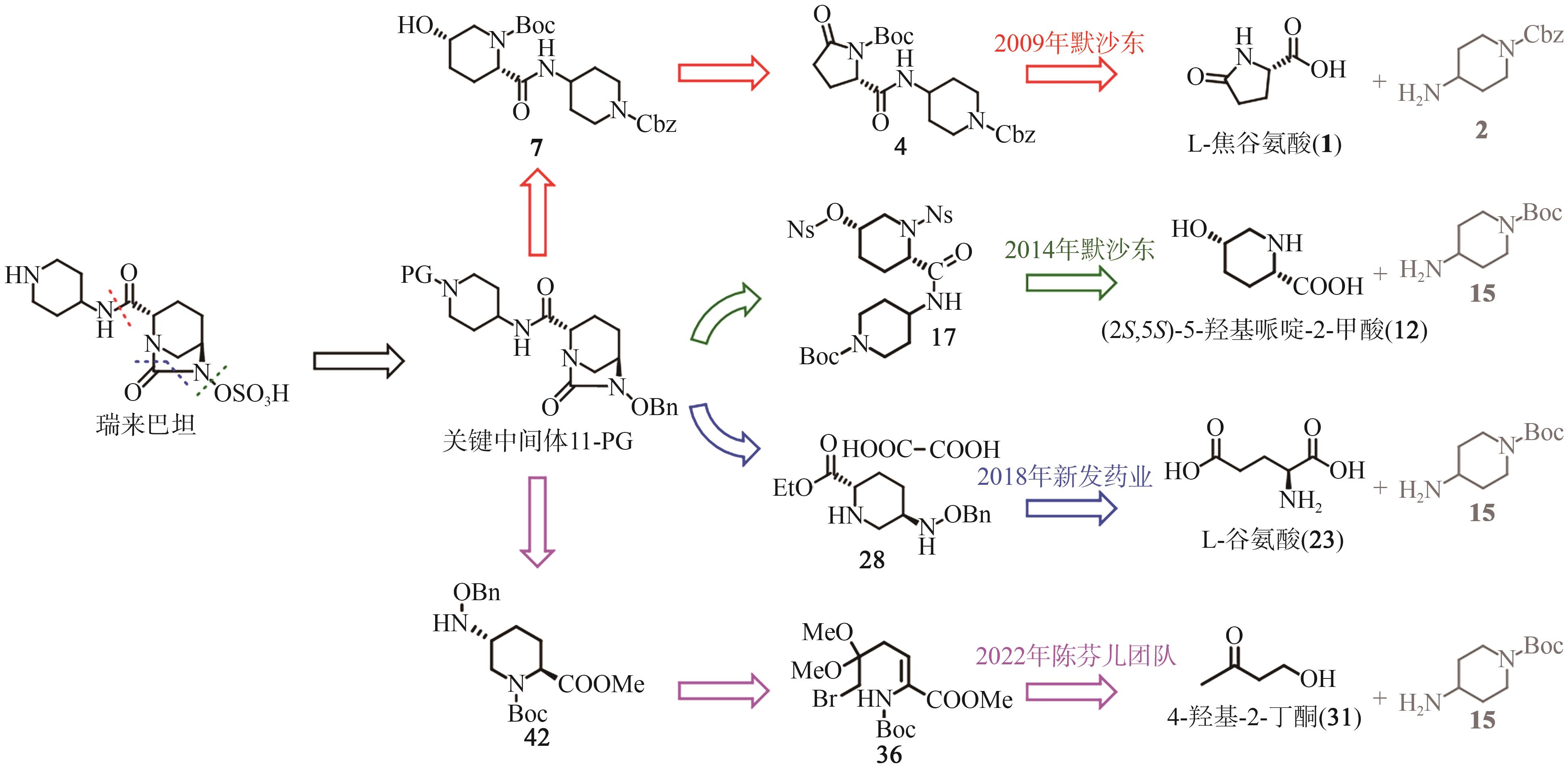
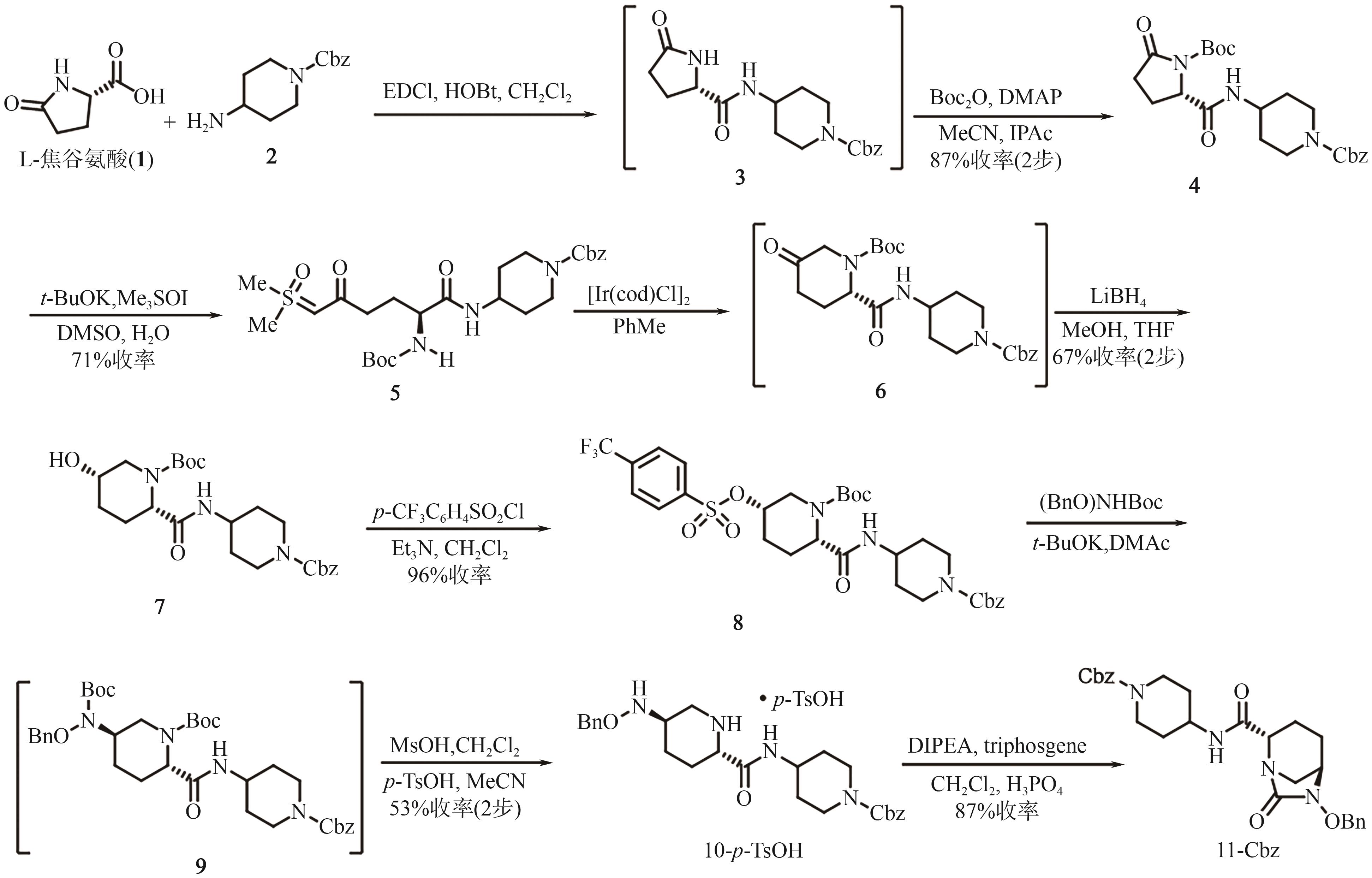
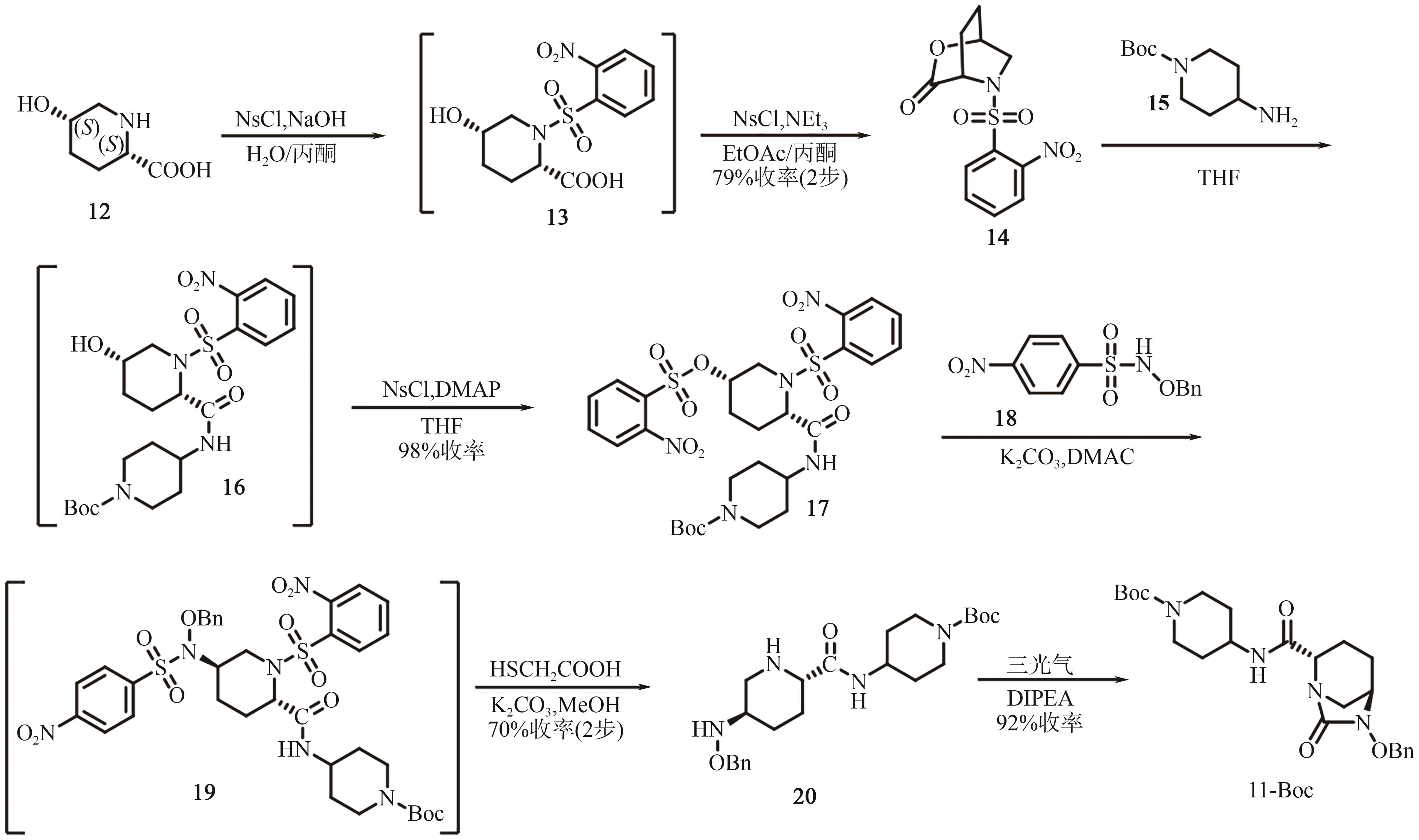
Progress on synthesis of relebactam and its key intermediates
WANG Shuai( ), ZHANG Deyi, LI Chao(
), ZHANG Deyi, LI Chao( ), QIAO Renzhong(
), QIAO Renzhong( )
)
- College of Life Science and Technology, Beijing University of Chemical Technology, Beijing 100029, China
-
Received:2024-07-18Revised:2024-08-20Online:2025-09-30Published:2025-09-25 -
Contact:LI Chao, QIAO Renzhong
瑞来巴坦及其中间体的合成研究进展
- 北京化工大学生命科学与技术学院,北京 100029
-
通讯作者:李超,乔仁忠 -
作者简介:王帅(1994—),男,博士,研究方向为药物合成工艺。E-mail:13553190576@163.com。 -
基金资助:国家自然科学基金(22177011);国家自然科学基金(21977012);国家自然科学基金(21672021)
CLC Number:
Cite this article
WANG Shuai, ZHANG Deyi, LI Chao, QIAO Renzhong. Progress on synthesis of relebactam and its key intermediates[J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5224-5233.
王帅, 张得意, 李超, 乔仁忠. 瑞来巴坦及其中间体的合成研究进展[J]. 化工进展, 2025, 44(9): 5224-5233.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1151
| [1] | BAHR Guillermo, GONZÁLEZ Lisandro J, VILA Alejandro J. Metallo-β-lactamases in the age of multidrug resistance: From structure and mechanism to evolution, dissemination, and inhibitor design[J]. Chemical Reviews, 2021, 121(13): 7957-8094. |
| [2] | MURRAY Christopher J L, IKUTA Kevin Shunji, SHARARA Fablina, et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis[J]. The Lancet, 2022, 399(10325): 629-655. |
| [3] | YANG Yongqiang, YAN Yu-Hang, SCHOFIELD Christopher J, et al. Metallo-β-lactamase-mediated antimicrobial resistance and progress in inhibitor discovery[J]. Trends in Microbiology, 2023, 31(7): 735-748. |
| [4] | CHEN Cheng, OELSCHLAEGER Peter, WANG Dongmei, et al. Structure and mechanism-guided design of dual serine/metallo-carbapenemase inhibitors[J]. Journal of Medicinal Chemistry, 2022, 65(8): 5954-5974. |
| [5] | Jürgen BREM, PANDUWAWALA Tharindi, HANSEN Jon Ulf, et al. Imitation of β-lactam binding enables broad-spectrum metallo-β- lactamase inhibitors[J]. Nature Chemistry, 2022, 14(1): 15-24. |
| [6] | COLEMAN Ken. Diazabicyclooctanes (DBOs): A potent new class of non-β-lactam β-lactamase inhibitors[J]. Current Opinion in Microbiology, 2011, 14(5): 550-555. |
| [7] | CRASS Ryan L, Manjunath P PAI. Pharmacokinetics and pharmacodynamics of β-lactamase inhibitors[J]. Pharmacotherapy, 2019, 39(2): 182-195. |
| [8] | DRAWZ Sarah M, BONOMO Robert A. Three decades of β-lactamase inhibitors[J]. Clinical Microbiology Reviews, 2010, 23(1): 160-201. |
| [9] | 周子腾. β-内酰胺酶抑制剂他唑巴坦的合成研究[D]. 济南: 山东大学, 2018. |
| ZHOU Ziteng. Synthesis of β-lactamase inhibitor tazobactam[D]. Jinan: Shandong University, 2018. | |
| [10] | 李丽,刘宝树,郑学明,等. 舒巴坦钠溶析结晶工艺优化[J]. 化工进展, 2019, 38(6): 2905-2914. |
| LI Li, LIU Baoshu, ZHENG Xueming, et al. Optimization of anti-solvent crystallization process of sulbactam sodium[J]. Chemical Industry and Engineering Progress, 2019, 38(6): 2905-2914. | |
| [11] | DOUAFER Hana, ANDRIEU Véronique, PHANSTIEL Otto 4th, et al. Antibiotic adjuvants: Make antibiotics great again![J]. Journal of Medicinal Chemistry, 2019, 62(19): 8665-8681. |
| [12] | 吕训磊, 周伟澄, 林快乐. 2019年美国FDA批准上市的新药简介[J]. 中国医药工业杂志, 2020, 51(1): 1-29. |
| Xunlei Lyu, ZHOU Weicheng, LIN Kuaile. Overviews of the new drugs in 2019 approved by U.S. FDA[J]. Chinese Journal of Pharmaceuticals, 2020, 51(1): 1-29. | |
| [13] | LI Rong, CHEN Xi, ZHOU Cong, et al. Recent advances in β-lactamase inhibitor chemotypes and inhibition modes[J]. European Journal of Medicinal Chemistry, 2022, 242: 114677. |
| [14] | 闫江坤, 赵冬梅. Imipenem/cilastatin/relebactam(recarbrio)[J]. 中国药物化学杂志, 2020, 30(3): 191. |
| YAN Jiangkun, ZHAO Dongmei. Imipenem/cilastatin/relebactam(recarbrio)[J]. Chinese Journal of Medicinal Chemistry, 2020, 30(3): 191. | |
| [15] | MCVICKER Rebecca U, O’BOYLE Niamh M. Chirality of new drug approvals (2013—2022): Trends and perspectives[J]. Journal of Medicinal Chemistry, 2024, 67(4): 2305-2320. |
| [16] | CHUNG John Y L, MENG Dongfang, SHEVLIN Michael, et al. Diastereoselective FeCl3·6H2O/NaBH4 reduction of oxime ether for the synthesis of β-lactamase inhibitor relebactam[J]. The Journal of Organic Chemistry, 2020, 85(2): 994-1000. |
| [17] | MINOSHIMA Masafumi, UMENO Taro, KADOOKA Kohei, et al. Development of a versatile protein labeling tool for live-cell imaging using fluorescent β-lactamase inhibitors[J]. Angewandte Chemie International Edition, 2023, 62(18): e202301704. |
| [18] | LANG Pauline A, LEISSING Thomas M, PAGE Malcolm G P, et al. Structural investigations of the inhibition of Escherichia coli AmpC β-lactamase by diazabicyclooctanes[J]. Antimicrobial Agents and Chemotherapy, 2021, 65(2): e02073-20. |
| [19] | MANGION Ian K, RUCK Rebecca T, RIVERA Nelo, et al. A concise synthesis of a β-lactamase inhibitor[J]. Organic Letters, 2011, 13(20): 5480-5483. |
| [20] | MILLER Steven P, ZHONG Yongli, LIU Zhijian, et al. Practical and cost-effective manufacturing route for the synthesis of a β-lactamase inhibitor[J]. Organic Letters, 2014, 16(1): 174-177. |
| [21] | 王保林, 戚聿新, 徐欣, 等. 一种瑞来巴坦的简便制备方法: CN111072660B[P]. 2021-05-18. |
| WANG Baolin, QI Yuxin, XU Xin, et al. Simple preparation method of relebactam: CN111072660B[P]. 2021-05-18. | |
| [22] | YIN Jianguo, WEISEL Mark, JI Yining, et al. Improved preparation of a key hydroxylamine intermediate for relebactam: Rate enhancement of benzyl ether hydrogenolysis with DABCO[J]. Organic Process Research & Development, 2018, 22(3): 273-277. |
| [23] | KIM Jungchul, ITOH Tetsuji, XU Feng, et al. Development of a practical manufacturing process to relebactam via thorough understanding of the origin and control of oligomeric impurities[J]. Organic Process Research & Development, 2021, 25(10): 2249-2259. |
| [24] | YANG Zhi, CHEN Yu, WAN Linxi, et al. Catalytic asymmetric total synthesis of diazabicyclooctane β-lactamase inhibitors avibactam and relebactam[J]. Chemical Communications, 2022, 58(77): 10869-10872. |
| [25] | BLIZZARD Timothy A, CHEN Helen, GUDE Candido, et al. Beta-lactamase inhibitors: US20110294777[P]. 2011-12-01. |
| [26] | Liu Zhijian, YASUDA Nobuyoshi, YANG Lu, et al. Process for the preparation of tert-butyl 4-((2, 5)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamido)piperidine-1-carboxylate and analogs there of: US10053460[P]. 2018-08-21. |
| [27] | CHUNG John Y L, ITOH Tetsuji, KIM Jungchul, et al. Process for the preparation of intermediates useful for making ( 2,5)-7-oxo--piperidin-4-yl-6-(sulfoxy)-1,6-diazabicyclo[3.2.1]octane-2-carboxamide: US12162883[P]. 2024-12-10. |
| [28] | 戚聿新, 李新发, 徐欣, 等. 一种 5-苄氧氨基哌啶-2S-甲酸或其衍生物的制备方法: CN109970625B[P]. 2021-02-26. |
| QI Yuxin, LI Xinfa, XU Xin, et al. Method for preparing 5R-benzyloxyaminopiperidin-2S-carboxylic acid or derivative thereof: CN109970625B[P]. 2021-02-26. | |
| [29] | BALL Matthew, BOYD Alistair, ENSOR Gareth J, et al. Development of a manufacturing route to avibactam, a β-lactamase inhibitor[J]. Organic Process Research & Development, 2016, 20(10): 1799-1805. |
| [30] | 王帅. 阿维巴坦的合成工艺研究[D]. 北京: 北京化工大学, 2020. |
| WANG Shuai. Synthesis and optimization of avibactam[D]. Beijing: Beijing University of Chemical Technology, 2020. | |
| [31] | 龚杰, 周忠波, 胡涛, 等. 一种瑞利巴坦中间体的制备方法: CN111606844A[P]. 2020-09-01. |
| GONG Jie, ZHOU Zhongbo, HU Tao, et al. Preparation of piperidine derivatives as intermediate of relebactam: CN111606844A[P]. 2020-09-01. | |
| [32] | 彭坤, 朱晓斐, 杨庆坤, 等. 一种瑞来巴坦的制备方法: CN111943950B[P]. 2022-03-29. |
| PENG Kun, ZHU Xiaofei, YANG Qingkun, et al. Preparation method of relebactam with high purity and yield: CN111943950B[P]. 2022-03-29. | |
| [33] | PEILLERON Laure, CARIOU Kevin. Synthetic approaches towards avibactam and other diazabicyclooctane β-lactamase inhibitors[J]. Organic & Biomolecular Chemistry, 2020, 18(5): 830-844. |
| [34] | TAMBOLI Yasinalli, KILBILE Jaydeo T, MERWADE Arvind Y. Large-scale amide coupling in aqueous media: Process for the production of diazabicyclooctane β-lactamase inhibitors[J]. Organic Process Research & Development, 2023, 27(1): 120-128. |
| [35] | WANKHEDE Karuna Suresh, SURWASE Mahesh Manikrao, BHAWSAR Satish, et al. A process for preparation of ( 2,5 R)- sulfuric acid mono-{[(4-aminopiperidin-4-yl) carbonyl]-7-oxo-1,6-diazabicyclo[3.2.1]-oct-6-yl}ester: WO2015033191[P]. 2015-03-12. |
| [36] | BLIZZARD Timothy A, CHEN Helen, KIM Seongkon, et al. Discovery of MK-7655, a β-lactamase inhibitor for combination with Primaxin® [J]. Bioorganic & Medicinal Chemistry Letters, 2014, 24(3): 780-785. |
| [37] | CHEN Yefeng, XU Minqiu, XU Weipan, et al. Highly selective and wash-free visualization of resistant bacteria with a relebactam-derived fluorogenic probe[J]. Chemical Communications, 2019, 55(67): 9919-9922. |
| [38] | Montserrat MORA-OCHOMOGO, LOHANS Christopher T. β-Lactam antibiotic targets and resistance mechanisms: From covalent inhibitors to substrates[J]. RSC Medicinal Chemistry, 2021, 12(10): 1623-1639. |
| [39] | WANG Shuai, CHEN Zhaohang, ZHAO Jincheng, et al. Separation of avibactam by gemini quaternary ammonium salt: Extraction, precipitation, and application in large-scale production[J]. Journal of Cleaner Production, 2023, 428: 139420. |
| [40] | WANG Shuai, ZHAO Xinpeng, CHANG Hao, et al. A new avibactam Gemini quaternary ammonium salt: Synthesis, self-assembly, vibrational spectra, crystal structures and DFT calculations[J]. Journal of Molecular Structure, 2023, 1293: 136214. |
| [41] | NIGAMA Satish C, MANN André, TADDEI Maurizio, et al. Selective removal of the tert-butoxycarbonyl group from secondary amines: ZnBr2 as the deprotecting reagent[J]. Synthetic Communications, 1989, 19(18): 3139-3142. |
| [1] | Chengjun JIANG, Guilin CHENG. Progress in co-crystallization as a separation technology [J]. Chemical Industry and Engineering Progress, 2020, 39(1): 311-319. |
| [2] | ZHAO Xinyu, XU Mingchao, GUO Qingmei, LI Xiaomei, TENG Mingyu. Research progress on the synthetic technology of Lapatinib [J]. Chemical Industry and Engineering Progress, 2017, 36(03): 1018-1032. |
| [3] | JIANG Chengjun, XU Guoming, YUAN Shenfeng. Research on the dynamic kinetic resolution of 1,1,1-trifluoroisopropylamine by Pd/Al2O3 and Novozyme 435 [J]. Chemical Industry and Engineering Progree, 2017, 36(01): 324-328. |
| [4] | LI Zhenzhen, HUANG Huaying, REN Changjing, ZHANG Qiyi, ZHAO Qiang. Preparation and property of multi-function composite doxorubicin-loading lposomes [J]. Chemical Industry and Engineering Progree, 2016, 35(S2): 279-282. |
| [5] | ZHU Mengqin, GE Lu, QIU Lipeng. Preparation and characterization of hyaluronic acid copolymeric micelles [J]. Chemical Industry and Engineering Progree, 2016, 35(S2): 283-286. |
| [6] | WU Chengjun, MA Yang, LI Na, LÜ Haining, SUN Tiemin. Study on the synthesis of Nebivolol, a-aderenergic blocker [J]. Chemical Industry and Engineering Progree, 2016, 35(S2): 295-300. |
| [7] | LIN Furong, QIN Liang, JIANG Ming. A novel process for synthesizing mitiglinide calcium [J]. Chemical Industry and Engineering Progree, 2016, 35(08): 2537-2541. |
| [8] | FENG Juanjuan, ZHU Nannan, DENG Yangmin, HAN Xiaoling, ZHANG Xiaoli, ZHAO Binxia. Increasing productivity of phosphatidyserine by repeated batch reaction [J]. Chemical Industry and Engineering Progree, 2016, 35(04): 1180-1183. |
| [9] | JIANG Chengjun, LÜ Liqiong, HUANG Jun. Recent progress in dynamic kinetic resolution by metal catalysis cooperate with enzymes [J]. Chemical Industry and Engineering Progree, 2016, 35(03): 807-814. |
| [10] | CHEN Lianfeng, ZHANG Ding, WANG Fengyun, XIA Mingzhu, LEI Wu, ZHU Qijun. Improved synthesis of flunarizine dihydrochloride [J]. Chemical Industry and Engineering Progree, 2015, 34(04): 1104-1108. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||