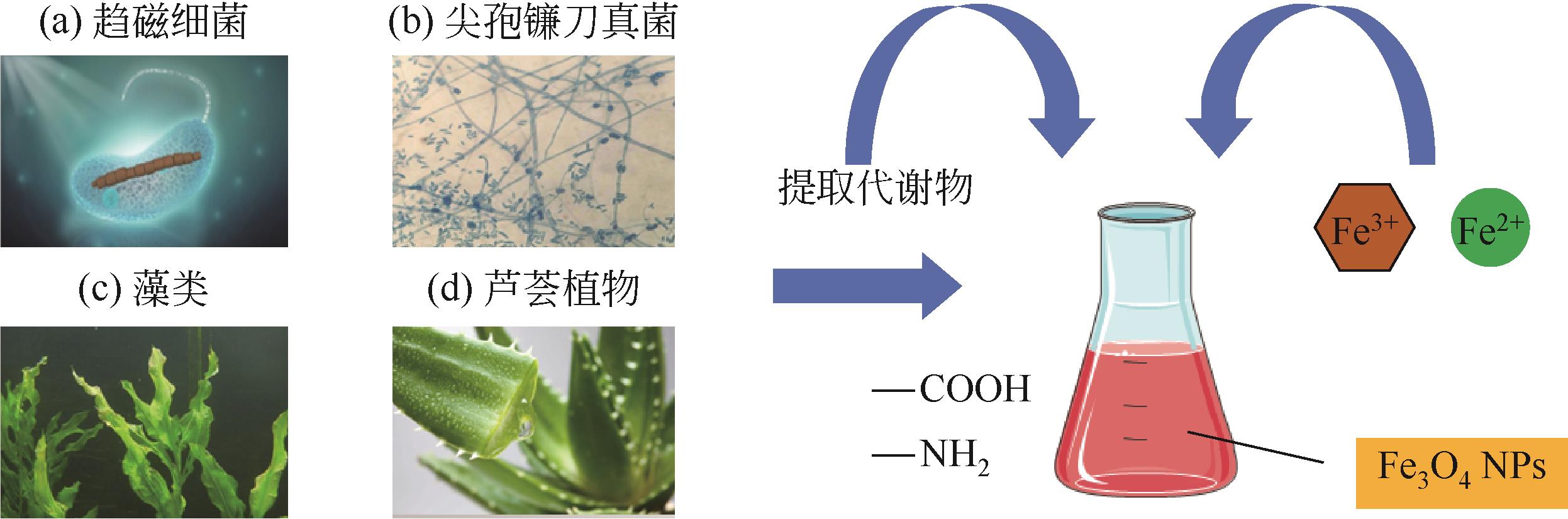
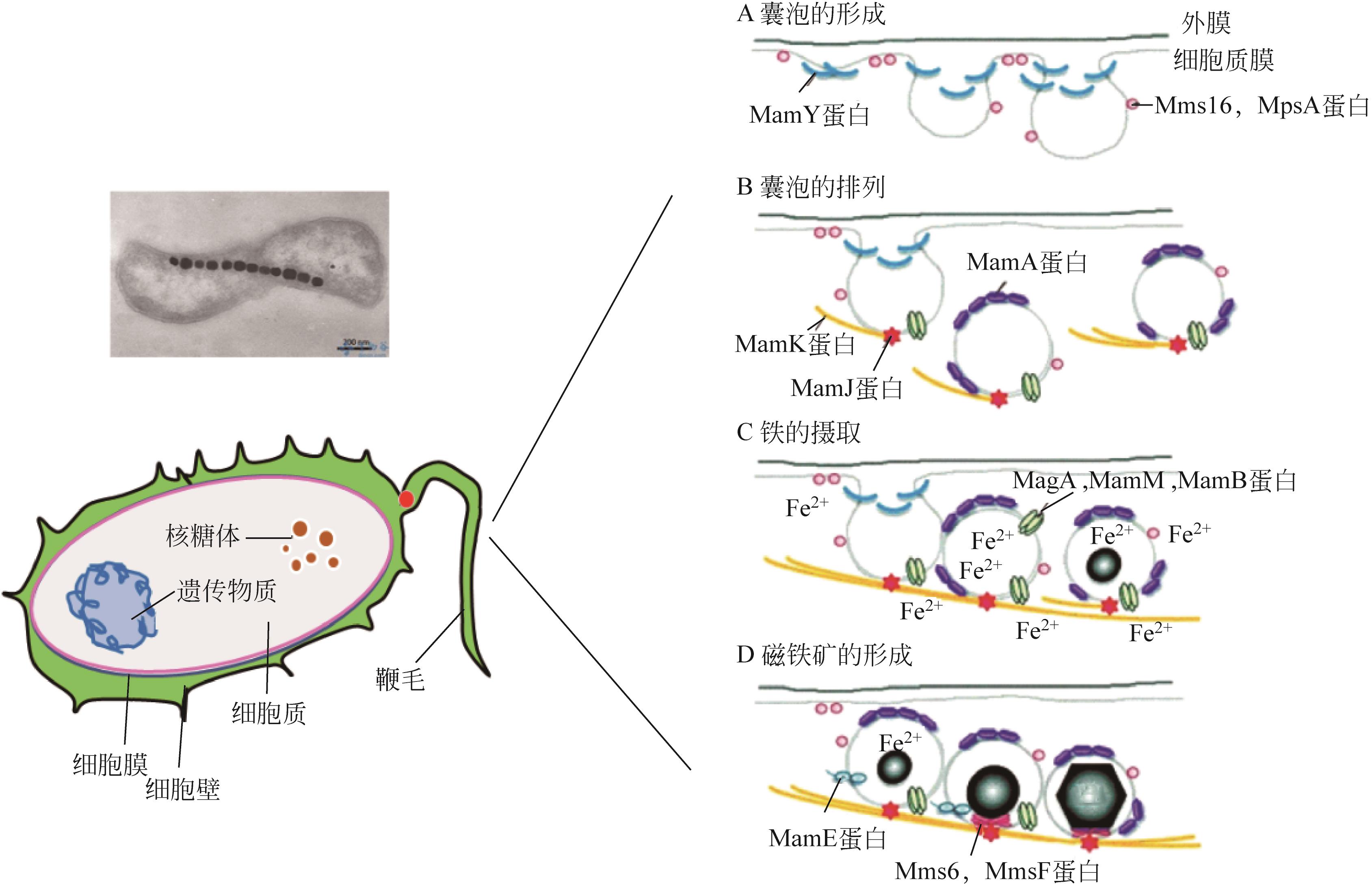
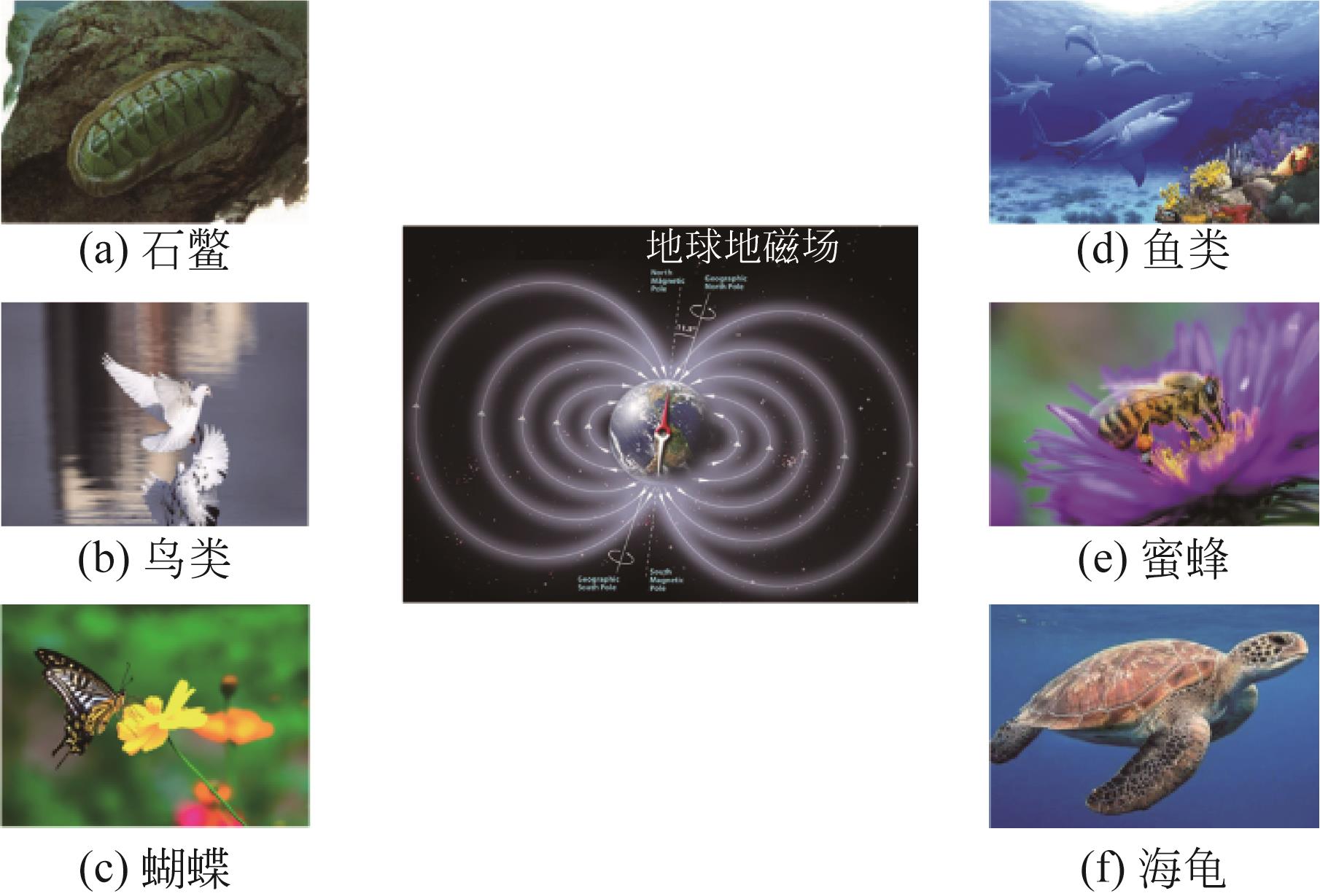
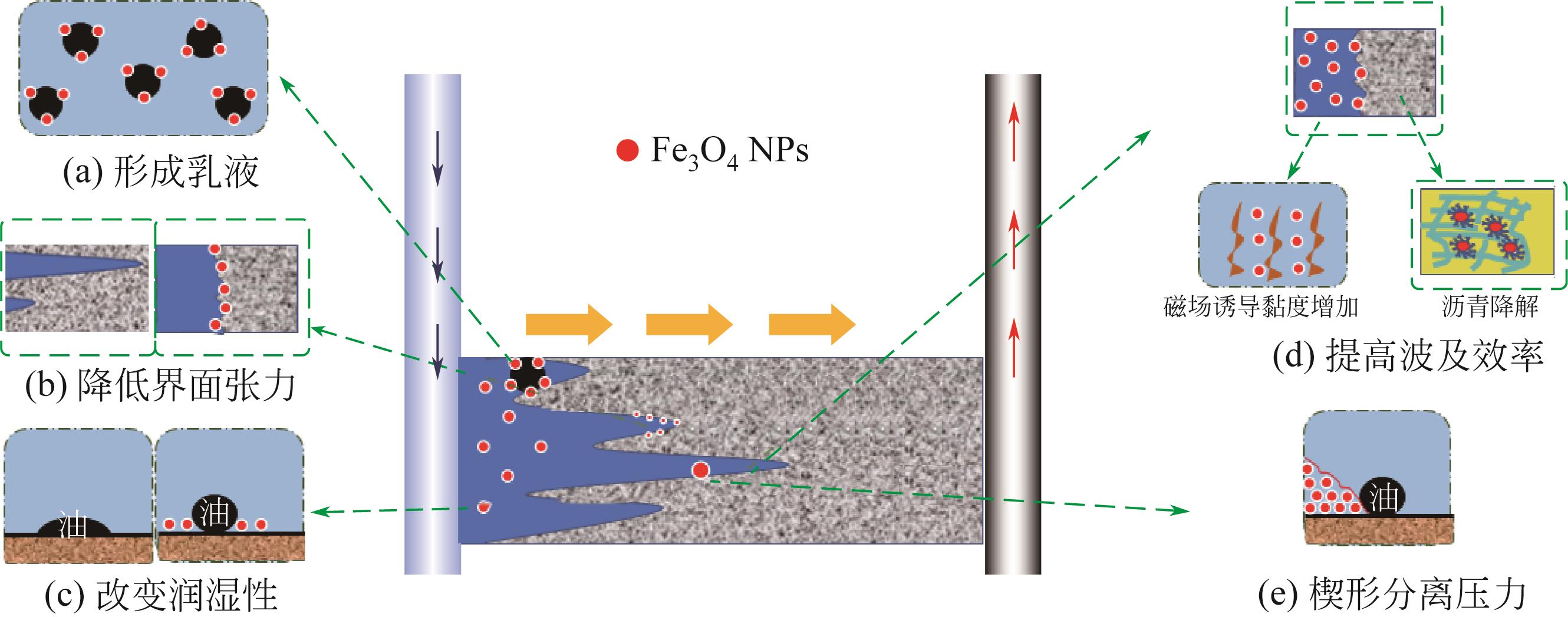
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (5): 2464-2474.DOI: 10.16085/j.issn.1000-6613.2022-1246
• Materials science and technology • Previous Articles Next Articles
Biosynthesis and EOR application of magnetic Fe3O4 NPs
LIU Yulong( ), YAO Junhu, SHU Chuangchuang, SHE Yuehui(
), YAO Junhu, SHU Chuangchuang, SHE Yuehui( )
)
- College of Petroleum Engineering, Yangtze University, Wuhan 430100, Hubei, China
-
Received:2022-07-04Revised:2022-08-23Online:2023-06-02Published:2023-05-10 -
Contact:SHE Yuehui
磁性Fe3O4纳米颗粒的生物合成及其在提高采收率中的应用
- 长江大学石油工程学院,湖北 武汉 430100
-
通讯作者:佘跃惠 -
作者简介:刘宇龙(1995—),男,博士研究生,研究方向为生物纳米解堵增注和提高采收率。E-mail:yulong13220@163.com。 -
基金资助:国家自然科学基金(51634008);国家重大科技专项(2017ZX05009-004)
CLC Number:
Cite this article
LIU Yulong, YAO Junhu, SHU Chuangchuang, SHE Yuehui. Biosynthesis and EOR application of magnetic Fe3O4 NPs[J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2464-2474.
刘宇龙, 姚俊虎, 舒闯闯, 佘跃惠. 磁性Fe3O4纳米颗粒的生物合成及其在提高采收率中的应用[J]. 化工进展, 2023, 42(5): 2464-2474.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-1246
| 细菌 | 铁源 | 尺寸/nm | 形貌 | 参考文献 |
|---|---|---|---|---|
| 放线杆菌 | K3Fe(CN)6/K4Fe(CN)6 | 50~150 | 均匀的立方颗粒 | [ |
| 硫还原地杆菌 | FeCl3 | 10.6~15 | 球形 | [ |
| 硝酸盐厌氧铁氧化菌BoFeN1 | FeCl2 | 55±15 | 球状 | [ |
| 铁还原菌:Thermoanaerobacter ethanolicus(TOR-39)和Shewanella loiha(PV-4) | FeCl3 | 35 | 八面体均匀晶体 | [ |
| 枯草芽孢杆菌 | Fe2O3 | 60~80 | 立方尖晶石,球形 | [ |
| 趋磁细菌:Magnetospirillum magneticum菌株AMB-1 | FeCl3和FeCl2 | 30~40 | 形态、尺寸均匀 | [ |
| 细菌 | 铁源 | 尺寸/nm | 形貌 | 参考文献 |
|---|---|---|---|---|
| 放线杆菌 | K3Fe(CN)6/K4Fe(CN)6 | 50~150 | 均匀的立方颗粒 | [ |
| 硫还原地杆菌 | FeCl3 | 10.6~15 | 球形 | [ |
| 硝酸盐厌氧铁氧化菌BoFeN1 | FeCl2 | 55±15 | 球状 | [ |
| 铁还原菌:Thermoanaerobacter ethanolicus(TOR-39)和Shewanella loiha(PV-4) | FeCl3 | 35 | 八面体均匀晶体 | [ |
| 枯草芽孢杆菌 | Fe2O3 | 60~80 | 立方尖晶石,球形 | [ |
| 趋磁细菌:Magnetospirillum magneticum菌株AMB-1 | FeCl3和FeCl2 | 30~40 | 形态、尺寸均匀 | [ |
| 分类 | 微生物 | 铁离子 | 尺寸/nm | 形貌 | 参考文献 |
|---|---|---|---|---|---|
| 真菌 | 黄萎病菌 | FeCl3 | 10~40 | 立方形 | [ |
| 黑曲霉真菌 | FeCl3 | 8 | 球形 | [ | |
| 尖孢镰刀菌 | FeCl3 | 26.78 | 形状不规则 | [ | |
| 藻类 | 褐藻水:Padina pavonica和Sargassum acinarium | FeCl3 | 10~19.5,21.6~27.4 | — | [ |
| 褐藻:尾藻水 | FeCl3 | 18±4 | 立方体 | [ | |
| 海藻Kappaphycus alvarezii | FeCl2·4H2O和FeCl3·6H2O | 14.7 | 球形 | [ | |
| 微藻(螺旋藻属) | FeCl2·4H2O和FeCl3·6H2O | 45 | 球形 | [ |
| 分类 | 微生物 | 铁离子 | 尺寸/nm | 形貌 | 参考文献 |
|---|---|---|---|---|---|
| 真菌 | 黄萎病菌 | FeCl3 | 10~40 | 立方形 | [ |
| 黑曲霉真菌 | FeCl3 | 8 | 球形 | [ | |
| 尖孢镰刀菌 | FeCl3 | 26.78 | 形状不规则 | [ | |
| 藻类 | 褐藻水:Padina pavonica和Sargassum acinarium | FeCl3 | 10~19.5,21.6~27.4 | — | [ |
| 褐藻:尾藻水 | FeCl3 | 18±4 | 立方体 | [ | |
| 海藻Kappaphycus alvarezii | FeCl2·4H2O和FeCl3·6H2O | 14.7 | 球形 | [ | |
| 微藻(螺旋藻属) | FeCl2·4H2O和FeCl3·6H2O | 45 | 球形 | [ |
| 植物 | 部位 | 铁源 | 粒径/nm | 参考文献 |
|---|---|---|---|---|
大麦 酸模 | 叶子 | FeCl3·6H2O | 30,10~40 | [ |
| 安第斯黑莓 | 叶子 | FeSO4·7H2O | 54.5±24.6 | [ |
| 西番莲 | 果实 | FeCl3·6H2O | 22.3±3 | [ |
| 苜蓿 | — | FeNH4(SO4)2·12H2O | <5 | [ |
| 车前草皮 | 叶子 | FeCl3·6H2O | 50 | [ |
| 芦荟植物提取液 | 叶子 | Fe(C5H8O2)3 | 6~35 | [ |
| 西瓜 | 果皮 | FeCl3·6H2O | <20 | [ |
| 桉树 | 叶子 | FeCl3·6H2O | 80 | [ |
| 柑橘 | 果皮 | FeCl3,FeCl2·4H2O | 50~200 | [ |
| 大豆 | 豆芽 | Fe(NH4)2(SO4)2·6H2O,FeCl3·6H2O | 8 | [ |
| 植物 | 部位 | 铁源 | 粒径/nm | 参考文献 |
|---|---|---|---|---|
大麦 酸模 | 叶子 | FeCl3·6H2O | 30,10~40 | [ |
| 安第斯黑莓 | 叶子 | FeSO4·7H2O | 54.5±24.6 | [ |
| 西番莲 | 果实 | FeCl3·6H2O | 22.3±3 | [ |
| 苜蓿 | — | FeNH4(SO4)2·12H2O | <5 | [ |
| 车前草皮 | 叶子 | FeCl3·6H2O | 50 | [ |
| 芦荟植物提取液 | 叶子 | Fe(C5H8O2)3 | 6~35 | [ |
| 西瓜 | 果皮 | FeCl3·6H2O | <20 | [ |
| 桉树 | 叶子 | FeCl3·6H2O | 80 | [ |
| 柑橘 | 果皮 | FeCl3,FeCl2·4H2O | 50~200 | [ |
| 大豆 | 豆芽 | Fe(NH4)2(SO4)2·6H2O,FeCl3·6H2O | 8 | [ |
| 类型 | 尺寸 /nm | 浓度 | 驱替介质 | EOR机制 | RF/% | 参考 文献 | ||
|---|---|---|---|---|---|---|---|---|
黏度 /mPa·s | IFT /mN·m-1 | 接触角 | ||||||
| Fe3O4@十二烷基三甲基溴化铵(CTAB) | — | — | 碳酸钙颗粒 | — | 30→1 | 90°→<30° | 35 | [ |
| Fe3O4@SiO2@黄原胶 | — | 1500mg/L | 碳酸岩 | 0.89→1.93 | 28.3→8.6 | 134°→34° | — | [ |
| Fe3O4@柠檬酸盐聚合物 | 47 | 400mg/L | 砂岩 | 0.99→1.08 | 11.23→7.92 | 160°→114° | 26 | [ |
| Fe3O4@柠檬酸 | 20~26 | 0.8%~2%(质量分数) | 微模型 | — | 37.47→16.71 | 106°→41° | 22 | [ |
| Fe3O4@C | 60 | 100mg/L | 砂岩 | — | 24.3→1×10-4 | 54°→10° | 30 | [ |
| Fe3O4@SiO2 | 30 | 0.05%(质量分数) | 碳酸岩砂 | 1.09→1.19 | 39→17.5 | 137°→40° | 24 | [ |
| Fe3O4@壳聚糖 | — | 0.03%(质量分数) | 填砂管 | 264→161 | 22.49→14.47 | 145°→90° | 10.8 | [ |
| Fe3O4 | <80 | 0.8%(质量分数) | 砂岩 | — | — | 50.44°→29.7° | 15.38 | [ |
| Fe3O4@SiO2 | 25~30 | 0.1%(质量分数) | 玻璃微模型 | 1.09→1.19 | 25→21 | 120°→106° | 13.2 | [ |
| Pd功能化磁铁矿 | — | — | — | 催化降黏 | 90 | [ | ||
| 类型 | 尺寸 /nm | 浓度 | 驱替介质 | EOR机制 | RF/% | 参考 文献 | ||
|---|---|---|---|---|---|---|---|---|
黏度 /mPa·s | IFT /mN·m-1 | 接触角 | ||||||
| Fe3O4@十二烷基三甲基溴化铵(CTAB) | — | — | 碳酸钙颗粒 | — | 30→1 | 90°→<30° | 35 | [ |
| Fe3O4@SiO2@黄原胶 | — | 1500mg/L | 碳酸岩 | 0.89→1.93 | 28.3→8.6 | 134°→34° | — | [ |
| Fe3O4@柠檬酸盐聚合物 | 47 | 400mg/L | 砂岩 | 0.99→1.08 | 11.23→7.92 | 160°→114° | 26 | [ |
| Fe3O4@柠檬酸 | 20~26 | 0.8%~2%(质量分数) | 微模型 | — | 37.47→16.71 | 106°→41° | 22 | [ |
| Fe3O4@C | 60 | 100mg/L | 砂岩 | — | 24.3→1×10-4 | 54°→10° | 30 | [ |
| Fe3O4@SiO2 | 30 | 0.05%(质量分数) | 碳酸岩砂 | 1.09→1.19 | 39→17.5 | 137°→40° | 24 | [ |
| Fe3O4@壳聚糖 | — | 0.03%(质量分数) | 填砂管 | 264→161 | 22.49→14.47 | 145°→90° | 10.8 | [ |
| Fe3O4 | <80 | 0.8%(质量分数) | 砂岩 | — | — | 50.44°→29.7° | 15.38 | [ |
| Fe3O4@SiO2 | 25~30 | 0.1%(质量分数) | 玻璃微模型 | 1.09→1.19 | 25→21 | 120°→106° | 13.2 | [ |
| Pd功能化磁铁矿 | — | — | — | 催化降黏 | 90 | [ | ||
| 1 | 李杨. 铁强化厌氧水解酸化微生物种间氢传递及其调控[D]. 大连: 大连理工大学, 2017. |
| LI Yang. Interspecific hydrogen transfer and its regulation in Fe-enhanced anaerobic hydrolysis and acidification microorganisms[D]. Dalian : Dalian University of Technology, 2017. | |
| 2 | 秦曦. 微生物电催化耦合磁铁矿负载型生物炭强化污泥厌氧消化机理研究[D]. 上海: 华东师范大学, 2022. |
| QIN Xi. Study on mechanism of microbial electrocatalysis coupled with magnetite supported biochar to enhance anaerobic digestion of sludge[D]. Shanghai: East China Normal University, 2022. | |
| 3 | 冯阳阳, 赵众从, 杨文博, 等. 微生物合成金属纳米颗粒及在稠油催化降黏中的应用研究进展[J]. 化工进展, 2021, 40(4): 2215-2226. |
| FENG Yangyang, ZHAO Zhongcong, YANG Wenbo, et al. Microbial natural synthetic metal nanoparticles and the application in heavy oil catalytic viscosity reduction[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2215-2226. | |
| 4 | 翟旭东, 徐政. 利用生物技术制备纳米材料的研究新进展[J]. 中国粉体技术, 2010, 16(2): 76-82. |
| ZHAI Xudong, XU Zheng. New development of nano-sized materials synthesis by bio-technology[J]. China Powder Science and Technology, 2010, 16(2): 76-82. | |
| 5 | 刘清, 邓真宁, 滑熠龙, 等. 纳米铁的绿色合成及其在环境中的应用研究进展[J]. 化工进展, 2020, 39(5): 1950-1963. |
| LIU Qing, DENG Zhenning, HUA Yilong, et al. Green synthesis of Fe nanoparticles and their environmental applications[J]. Chemical Industry and Engineering Progress,2020,39(5): 1950-1963. | |
| 6 | ZHONG Xun, CHEN Jiating, AN Ran, et al. A state-of-the-art review of nanoparticle applications with a focus on heavy oil viscosity reduction[J]. Journal of Molecular Liquids, 2021, 344: 117845. |
| 7 | 刘清, 张美, 韩科昌, 等. 生物法合成纳米铁的研究进展[J]. 环境工程, 2015, 33(3): 163-167. |
| LIU Qing, ZHANG Mei, HAN Kechang, et al. Research progress on biosynthesis of iron nanoparticles[J]. Environmental Engineering, 2015, 33(3): 163-167. | |
| 8 | MAROUZI Somayeh, SABOURI Zahra, DARROUDI Majid. Greener synthesis and medical applications of metal oxide nanoparticles[J]. Ceramics International, 2021, 47(14): 19632-19650. |
| 9 | ABHILASH, REVATI K, PANDEY B D. Microbial synthesis of iron-based nanomaterials—A review[J]. Bulletin of Materials Science, 2011, 34(2): 191-198. |
| 10 | ARAKAKI Atsushi, SHIMIZU Katsuhiko, Mayumi ODA, et al. Biomineralization-inspired synthesis of functional organic/inorganic hybrid materials: organic molecular control of self-organization of hybrids[J]. Organic & Biomolecular Chemistry, 2015, 13(4): 974-989. |
| 11 | BAZYLINSKI D A, FRANKEL R B, KONHAUSER K O. Modes of biomineralization of magnetite by microbes[J]. Geomicrobiology Journal, 2007, 24(6): 465-475. |
| 12 | BHARDE Atul, WANI Aijaz, SHOUCHE Yogesh, et al. Bacterial aerobic synthesis of nanocrystalline magnetite[J]. Journal of the American Chemical Society, 2005, 127(26): 9326-9327. |
| 13 | SAIF S, ADIL S F, CHAUDHRY A, et al. Microbial synthesis of magnetic nanomaterials[M]. Agri-Waste and Microbes for Production of Sustainable Nanomaterials. Amsterdam: Elsevier, 2022: 323-356. |
| 14 | BYRNE J M, MUHAMADALI H, COKER V S, et al. Scale-up of the production of highly reactive biogenic magnetite nanoparticles using Geobacter sulfurreducens [J]. Journal of the Royal Society Interface, 2015, 12(107): 20150240. |
| 15 | MIOT Jennyfer, LI Jinhua, BENZERARA Karim, et al. Formation of single domain magnetite by green rust oxidation promoted by microbial anaerobic nitrate-dependent iron oxidation[J]. Geochimica et Cosmochimica Acta, 2014, 139: 327-343. |
| 16 | ROH Y, LAUF R J, MCMILLAN A D, et al. Microbial synthesis and the characterization of metal-substituted magnetites[J]. Solid State Communications, 2001, 118(10): 529-534. |
| 17 | SUNDARAM P A, AUGUSTINE R, KANNAN M. Extracellular biosynthesis of iron oxide nanoparticles by Bacillus subtilis strains isolated from rhizosphere soil[J]. Biotechnology and Bioprocess Engineering, 2012, 17(4): 835-840. |
| 18 | PROZOROV T, MALLAPRAGADA S K, NARASIMHAN B, et al. Protein-mediated synthesis of uniform superparamagnetic magnetite nanocrystals[J]. Advanced Functional Materials, 2007, 17(6): 951-957. |
| 19 | BHARDE Atul, RAUTARAY Debabrata, BANSAL Vipul, et al. Extracellular biosynthesis of magnetite using fungi[J]. Small, 2006, 2(1): 135-141. |
| 20 | ABDEEN Mai, SABRY Soraya, GHOZLAN Hanan, et al. Microbial-physical synthesis of Fe and Fe3O4 magnetic nanoparticles using Aspergillus niger YESM1 and supercritical condition of ethanol[J]. Journal of Nanomaterials, 2016, 2016: 1-7. |
| 21 | BALAKRISHNAN G S, RAJENDRAN K, KALIRAJAN J. Microbial synthesis of magnetite nanoparticles for arsenic removal[J]. Journal of Applied Biology & Biotechnology, 2020, 8(3): 7-5. |
| 22 | EL-KASSAS H Y, ALY-ELDEEN M A, GHARIB S M. Green synthesis of iron oxide (Fe3O4) nanoparticles using two selected brown seaweeds: characterization and application for lead bioremediation[J]. Acta Oceanologica Sinica, 2016, 35(8): 89-98. |
| 23 | MAHDAVI M, NAMVAR F, AHMAD M B, et al. Green biosynthesis and characterization of magnetic iron oxide (Fe₃O₄) nanoparticles using seaweed (Sargassum muticum) aqueous extract[J]. Molecules, 2013, 18(5): 5954-5964. |
| 24 | YEW Y P, SHAMELI K, MIYAKE M, et al. Green synthesis of magnetite (Fe3O4) nanoparticles using seaweed (kappaphycus alvarezii) extract[J]. Nanoscale Research Letters, 2016, 11(1): 276. |
| 25 | HAWEZY H J S, SDIQ K H, QADR V A, et al. Biosynthesis of magnetite-nanoparticles using microalgae (Spirulina sp. and Spirogyra sp.) [J]. Indian Journal of Public Health Research & Development, 2020, 11(7): 1023-1027. |
| 26 | 孙文涛, 李春. 微生物合成植物天然产物的细胞工厂设计与构建[J]. 化工进展, 2021, 40(3): 1202-1214. |
| SUN Wentao, LI Chun. Design and construction of microbial cell factory for biosynthesis of plant natural products[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1202-1214. | |
| 27 | MAKAROV V V, MAKAROVA S S, LOVE A J, et al. Biosynthesis of stable iron oxide nanoparticles in aqueous extracts of Hordeum vulgare and Rumex acetosa plants[J]. Langmuir, 2014, 30(20): 5982-5988. |
| 28 | KUMAR Brajesh, SMITA Kumari, CUMBAL Luis, et al. Phytosynthesis and photocatalytic activity of magnetite (Fe3O4) nanoparticles using the Andean blackberry leaf[J]. Materials Chemistry and Physics, 2016, 179: 310-315. |
| 29 | KUMAR Brajesh, SMITA Kumari, CUMBAL Luis, et al. Biogenic synthesis of iron oxide nanoparticles for 2-arylbenzimidazole fabrication[J]. Journal of Saudi Chemical Society, 2014, 18(4): 364-369. |
| 30 | HERRERA-BECERRA R, ZORRILLA C, ASCENCIO J A. Production of iron oxide nanoparticles by a biosynthesis method: An environmentally friendly route[J]. The Journal of Physical Chemistry C, 2007, 111(44): 16147-16153. |
| 31 | VENKATESWARLU S, RAO Y S, BALAJI T, et al. Biogenic synthesis of Fe3O4 magnetic nanoparticles using plantain peel extract[J]. Materials Letters, 2013, 100: 241-244. |
| 32 | PHUMYING Santi, LABUAYAI Sarawuth, THOMAS Chunpen, et al. Aloe vera plant-extracted solution hydrothermal synthesis and magnetic properties of magnetite (Fe3O4) nanoparticles[J]. Applied Physics A, 2013, 111(4): 1187-1193. |
| 33 | PRASAD C, GANGADHARA S, VENKATESWARLU P. Bio-inspired green synthesis of Fe3O4 magnetic nanoparticles using watermelon rinds and their catalytic activity[J]. Applied Nanoscience, 2016, 6(6): 797-802. |
| 34 | CAO Dan, JIN Xiaoying, GAN Li, et al. Removal of phosphate using iron oxide nanoparticles synthesized by eucalyptus leaf extract in the presence of CTAB surfactant[J]. Chemosphere, 2016, 159: 23-31. |
| 35 | 柳检. 典型富集植物对铅的吸收和耐受机制研究[D]. 北京: 中国地质科学院, 2019. |
| LIU Jian. Mechanism of lead uptake and tolerance in typical accumulator plants[D]. Beijing: Chinese Academy of Geological Sciences, 2019. | |
| 36 | EHRAMPOUSH M H, MIRIA M, SALMANI M H, et al. Cadmium removal from aqueous solution by green synthesis iron oxide nanoparticles with tangerine peel extract[J]. Journal of Environmental Health Science and Engineering, 2015, 13(1): 84. |
| 37 | CAI Yan, SHEN Yuhua, XIE Anjian, et al. Green synthesis of soya bean sprouts-mediated superparamagnetic Fe3O4 nanoparticles[J]. Journal of Magnetism and Magnetic Materials, 2010, 322(19): 2938-2943. |
| 38 | 钱霞. 蜜蜂中磁铁矿纳米粒子的合成[J]. 原子与分子物理学报, 2021, 38(5): 77-81. |
| QIAN Xia. Synthesis of nano-magnetite particles in the honeybees[J]. Journal of Atomic and Molecular Physics, 2021, 38(5): 77-81. | |
| 39 | HSU C Y, KO F Y, LI C W, et al. Magnetoreception system in honeybees (Apis mellifera) [J]. PlosOne, 2007, 2(4): e395. |
| 40 | 尧德中,熊茜桃. 生物合成磁铁矿及其应用[J]. 大自然探索, 1995(2): 63-66. |
| YAO Dezhong, XIONG Qiantao. Biogenic magnetite and its application[J]. Exploration of Nature, 1995(2): 63-66. | |
| 41 | 刘小峰, 史远. 鸟类磁感受的生物物理机制研究进展[J]. 生物物理学报, 2009, 25(4): 247-254. |
| LIU Xiaofeng, SHI Yuan. Progress in the research on the biophysical mechanism underlying avian magnetoreception[J]. Acta Biophysica Sinica, 2009, 25(4): 247-254. | |
| 42 | ZHOU Kaibo, ZHOU Xiang, LIU Jie, et al. Application of magnetic nanoparticles in petroleum industry: a review[J]. Journal of Petroleum Science and Engineering, 2020, 188: 106943. |
| 43 | PEREIRA M L, MAIA K C B, SILVA W C, et al. Fe3O4 nanoparticles as surfactant carriers for enhanced oil recovery and scale prevention[J]. ACS Applied Nano Materials, 2020, 3(6): 5762-5772. |
| 44 | YAKASAI F, JAAFAR M Z, BANDYOPADHYAY S, et al. Application of iron oxide nanoparticles in oil recovery—A critical review of the properties, formulation, recent advances and prospects[J]. Journal of Petroleum Science and Engineering, 2022, 208: 109438. |
| 45 | SUN Xiaofei, ZHANG Yanyu, CHEN Guangpeng, et al. Application of nanoparticles in enhanced oil recovery: a critical review of recent progress[J]. Energies, 2017, 10(3): 345. |
| 46 | BINKS B P, YIN D. Pickering emulsions stabilized by hydrophilic nanoparticles: in situ surface modification by oil[J]. Soft Matter, 2016, 12(32): 6858-6867. |
| 47 | KAZEMZADEH Y, DEHDARI B, ETEMADAN Z, et al. Experimental investigation into Fe3O4/SiO2 nanoparticle performance and comparison with other nanofluids in enhanced oil recovery[J]. Petroleum Science, 2019, 16(3): 578-590. |
| 48 | DIVANDARI H, HEMMATI-SARAPARDEH A, SCHAFFIE M, et al. Integrating functionalized magnetite nanoparticles with low salinity water and surfactant solution: Interfacial tension study[J]. Fuel, 2020, 281: 118641. |
| 49 | KONDIPARTY Kirti, NIKOLOV Alex, WU Stanley, et al. Wetting and spreading of nanofluids on solid surfaces driven by the structural disjoining pressure: Statics analysis and experiments[J]. Langmuir, 2011, 27(7): 3324-3335. |
| 50 | AMROUCHE F, GOMARI S R, ISLAM M, et al. A novel hybrid technique to enhance oil production from oil-wet carbonate reservoirs by combining a magnetic field with alumina and iron oxide nanoparticles[J]. Journal of Cleaner Production, 2021, 281: 124891. |
| 51 | ZHANG H, RAMAKRISHNAN T S, NIKOLOV A, et al. Enhanced oil displacement by nanofluid's structural disjoining pressure in model fractured porous media[J]. Journal of Colloid and Interface Science, 2018, 511: 48-56. |
| 52 | ESMAEILNEZHAD E, CHOI H J, SCHAFFIE M, et al. Characteristics and applications of magnetized water as a green technology[J]. Journal of Cleaner Production, 2017, 161: 908-921. |
| 53 | ADIL M, LEE K, MOHD Z H, et al. Experimental study on electromagnetic-assisted ZnO nanofluid flooding for enhanced oil recovery (EOR)[J]. PLoS One, 2018, 13(2): e0193518. |
| 54 | ESMAEILNEZHAD E, VAN I E, CHON O H, et al. An experimental study on enhanced oil recovery utilizing nanoparticle ferrofluid through the application of a magnetic field[J]. Journal of Industrial and Engineering Chemistry, 2018, 58: 319-327. |
| 55 | KOTHARI Nikita, RAINA Bhavna, CHANDAK Krishna, et al. Application of ferrofluid for enhanced surfactant flooding in EOR[C]//SPE EUROPEC/EAGE Annual Conference and Exhibition. OnePetro, 2010. |
| 56 | OMAJALI J B, HART A, WALKER M, et al. In-situ catalytic upgrading of heavy oil using dispersed bionanoparticles supported on gram-positive and gram-negative bacteria[J]. Applied Catalysis B: Environmental, 2017, 203: 807-819. |
| 57 | 杨方源, 赵尉伶, 孙英, 等. 表面活性剂修饰磁性纳米颗粒的除砷效果研究[J]. 新疆农业大学学报, 2021, 44(3): 188-195. |
| YANG Fangyuan, ZHAO Yuling, SUN Ying, et al. Study on the arsenic removal effect of surfactant-modified magnetic nanoparticles[J]. Journal of Xinjiang Agricultural University, 2021, 44(3): 188-195. | |
| 58 | ALI J, MANSHAD A K, IMANI I, et al. Greenly synthesized Magnetite@SiO2@Xanthan nanocomposites and its application in enhanced oil recovery: IFT reduction and wettability alteration[J]. Arabian Journal for Science and Engineering, 2020, 45(9): 7751-7761. |
| 59 | IZADI N, KOOCHI M M, AMROLLAHI A, et al. Investigation of functionalized polyelectrolyte polymer-coated Fe3O4 nanoparticles stabilized in high salinity brine at high temperatures as an EOR agent[J]. Journal of Petroleum Science and Engineering, 2019, 178: 1079-1091. |
| 60 | DIVANDARI Hassan, Abdolhossein HEMMATI-SARAPARDEH, SCHAFFIE Mahin, et al. Integrating synthesized citric acid-coated magnetite nanoparticles with magnetic fields for enhanced oil recovery: experimental study and mechanistic understanding[J]. Journal of Petroleum Science and Engineering, 2019, 174: 425-436. |
| 61 | BETANCUR S, CARRASCO-MARÍN F, PÉREZ-CADENAS A F, et al. Effect of magnetic iron core–carbon shell nanoparticles in chemical enhanced oil recovery for ultralow interfacial tension region[J]. Energy & Fuels, 2019, 33(5): 4158-4168. |
| 62 | KAZEMZADEH Yousef, SHARIFI Mohammad, RIAZI Masoud, et al. Potential effects of metal oxide/SiO2 nanocomposites in EOR processes at different pressures[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 559: 372-384. |
| 63 | REZVANI Hosein, RIAZI Masoud, TABAEI Morteza, et al. Experimental investigation of interfacial properties in the EOR mechanisms by the novel synthesized Fe3O4@Chitosan nanocomposites[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 544: 15-27. |
| [1] | SI Yinfang, HU Yujie, ZHANG Fan, DONG Hao, SHE Yuehui. Biosynthesis of zinc oxide nanoparticles and its application to antibacterial [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2013-2023. |
| [2] | FENG Yangyang, ZHAO Zhongcong, YANG Wenbo, HU Linqi, ZHANG Wenda, SHE Yuehui. Microbial natural synthetic metal nanoparticles and the application in heavy oil catalytic viscosity reduction [J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2215-2226. |
| [3] | Wenya ZHONG, Wenjia YU, Yiming GU, Jing GUO, Bo FAN, Zhiqiang CAI. Progress of ansamitocins by biosynthesis [J]. Chemical Industry and Engineering Progress, 2021, 40(2): 990-997. |
| [4] | CHENG Shen, ZHANG Songhong, YUN Junxian. Recent advances in microbial synthesis of α-ketoisocaproate [J]. Chemical Industry and Engineering Progress, 2018, 37(12): 4821-4829. |
| [5] | NI Zheng, GUAN Jintao, SHEN Shaochuan, YUN Junxian. An overview of recent advances in microbial synthesis and separation of phenyllactic acid [J]. Chemical Industry and Engineering Progress, 2016, 35(11): 3627-3633. |
| [6] | HUANG Jinbiao1,SHANG Long’an2. Advance in biosynthesis of polyhydroxyalkanoate [J]. Chemical Industry and Engineering Progree, 2011, 30(9): 2041-. |
| [7] | LI Jinjuan,ZHAO Lin,TAN Xin,HUANG Yu,LIU Tingyi. Synthesis and characterization of PHA produced by acclimated activated sludge from acidified starchy wastewater [J]. Chemical Industry and Engineering Progree, 2011, 30(7): 1618-. |
| [8] | WANG Haisheng,ZHANG Xiaoxia,LU Yuan,RUAN Zhiyong,XING Xinhui,JIANG Ruibo. Recent research progress of bacterial violacein [J]. Chemical Industry and Engineering Progree, 2008, 27(3): 315-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||