Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (11): 6167-6175.DOI: 10.16085/j.issn.1000-6613.2022-0063
• Resources and environmental engineering • Previous Articles Next Articles
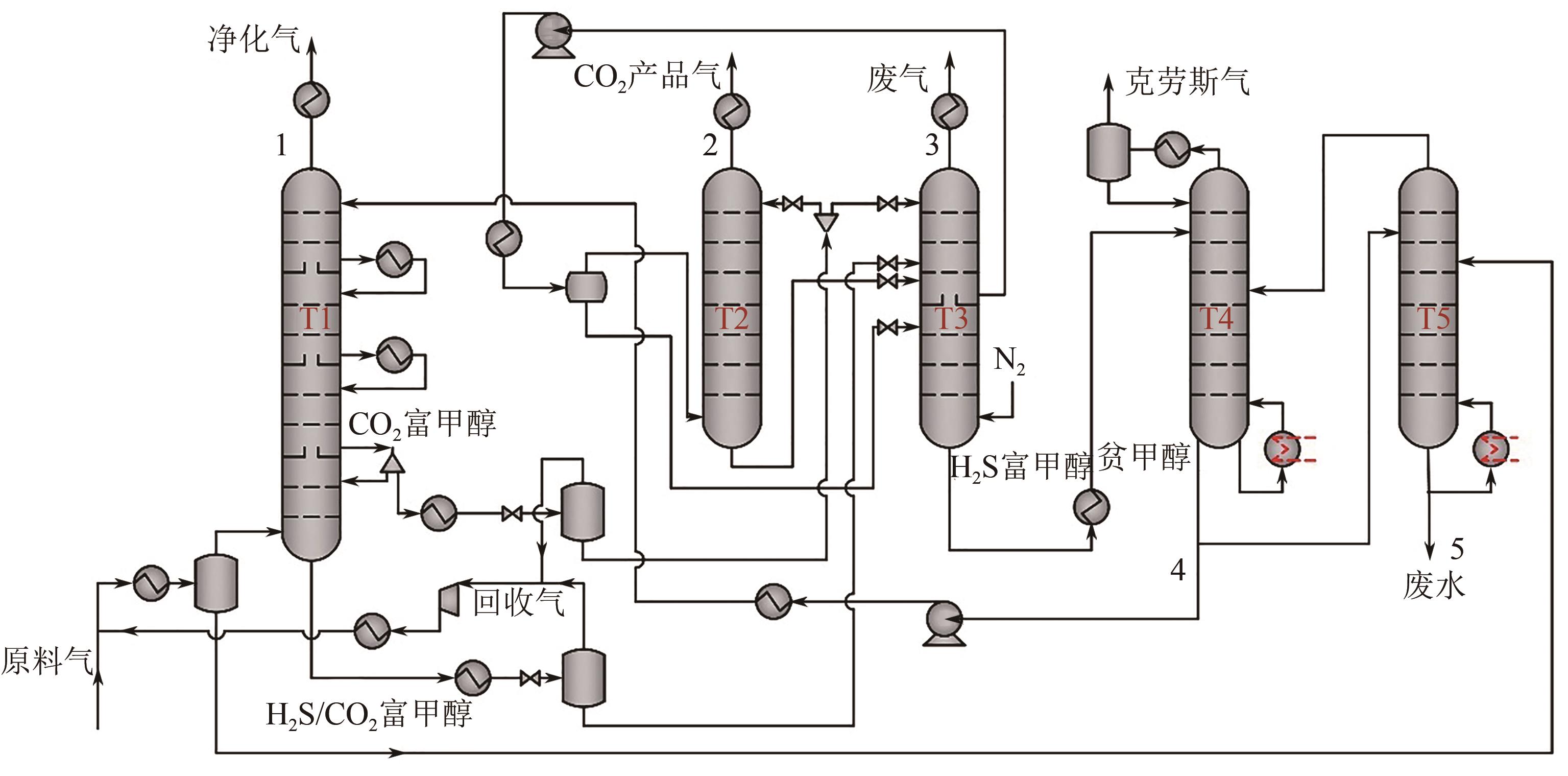
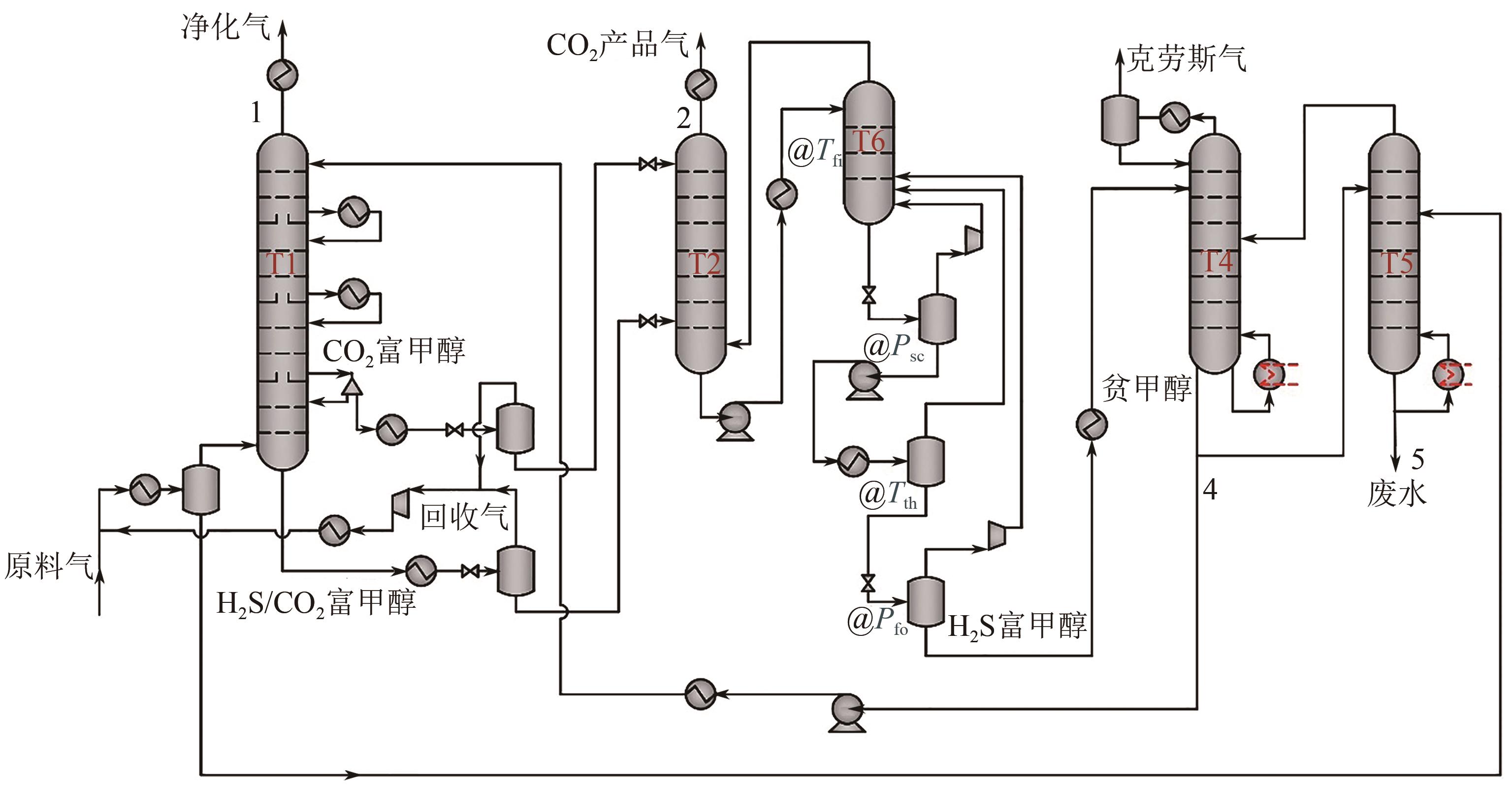
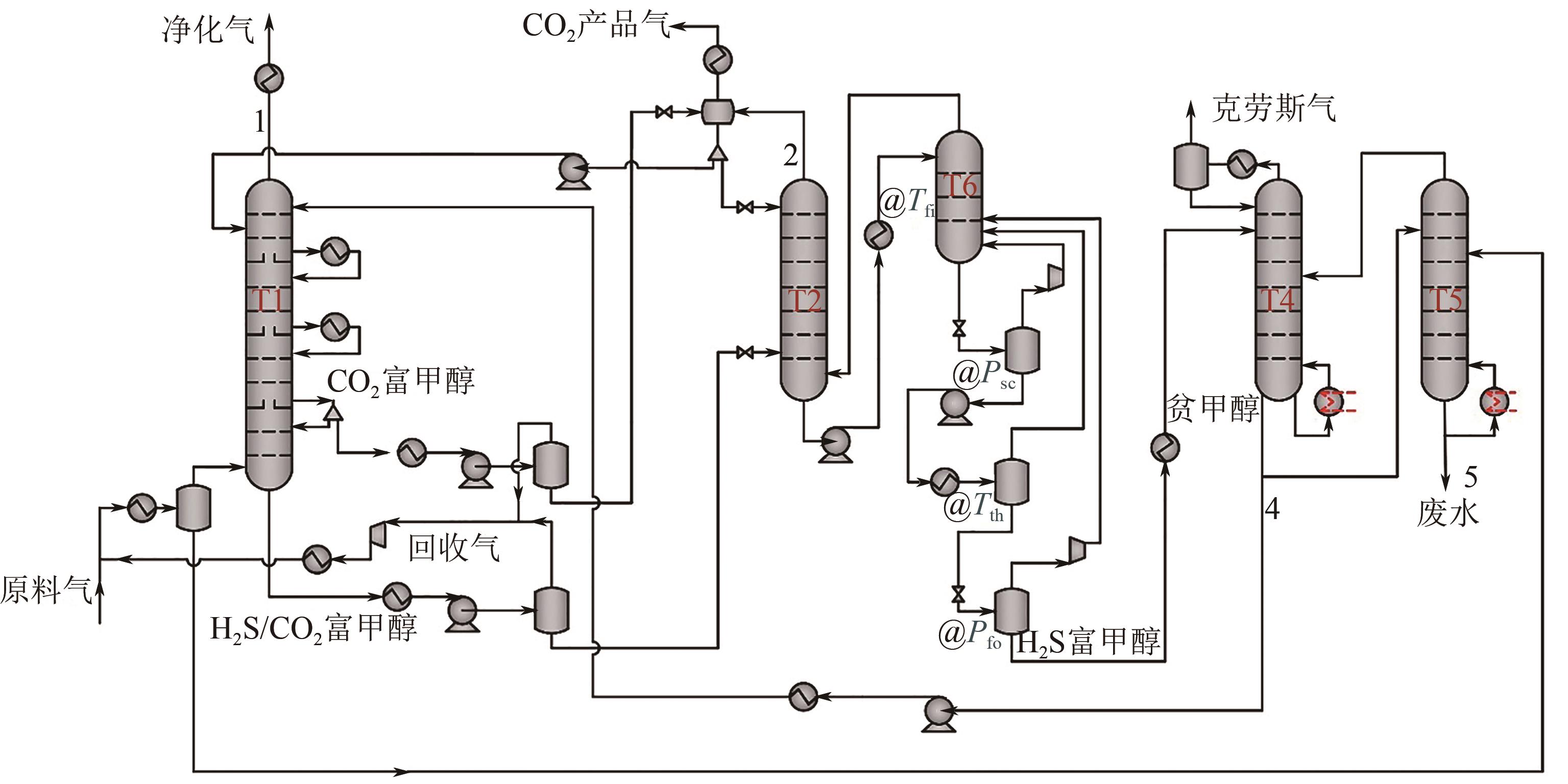
Improvement and optimization of carbon capture via Rectisol
- School of Energy Science and Engineering, Central South University, Changsha 410083, Hunan, China
-
Received:2022-01-09Revised:2022-04-02Online:2022-11-28Published:2022-11-25 -
Contact:YANG Sheng
低温甲醇洗碳捕集改进与优化
- 中南大学能源科学与工程学院,湖南 长沙 410083
-
通讯作者:杨声 -
作者简介:张陆(1996—),男,硕士研究生,研究方向为化工系统工程。E-mail:zhang-lu@csu.edu.cn。 -
基金资助:中南大学中央高校基本科研业务费专项(1053320212585);国家自然科学基金(22008265)
CLC Number:
Cite this article
ZHANG Lu, YANG Sheng. Improvement and optimization of carbon capture via Rectisol[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 6167-6175.
张陆, 杨声. 低温甲醇洗碳捕集改进与优化[J]. 化工进展, 2022, 41(11): 6167-6175.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0063
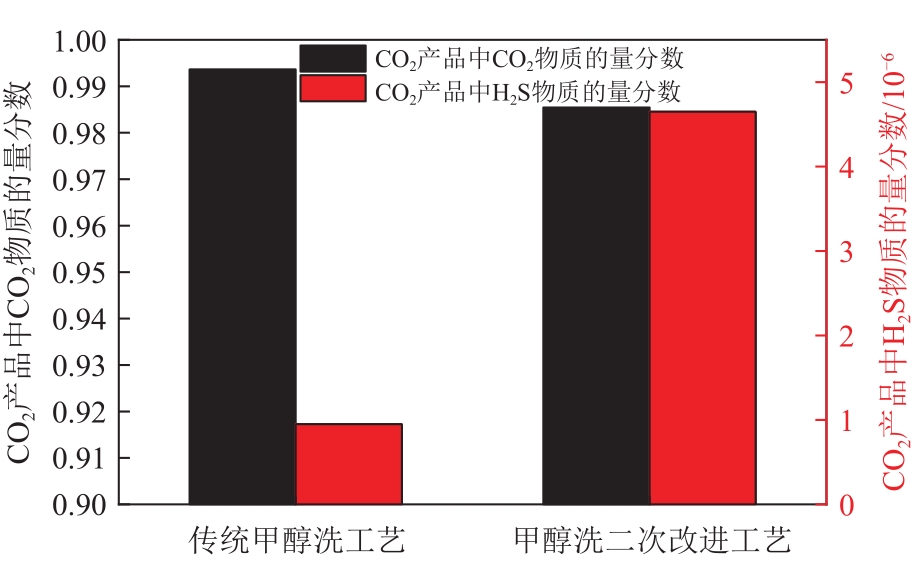
| 物流 | 组分 | 含量 |
|---|---|---|
| 净化气 | H2S | <0.1μL/L |
| CO2产品气 | CO2 | >98.5% |
| H2S | <5μL/L | |
| 废气 | H2S | <60μL/L |
| 贫甲醇 | H2S | <1μL/L |
| Claus气 | H2S | >35% |
| 废水 | CH3OH | <0.05% |
| 物流 | 组分 | 含量 |
|---|---|---|
| 净化气 | H2S | <0.1μL/L |
| CO2产品气 | CO2 | >98.5% |
| H2S | <5μL/L | |
| 废气 | H2S | <60μL/L |
| 贫甲醇 | H2S | <1μL/L |
| Claus气 | H2S | >35% |
| 废水 | CH3OH | <0.05% |
| 项目 | 组分 i | 组分 j | AIJ | BIJ |
|---|---|---|---|---|
| CPAKIJ | CH3OH | CO2 | -0.122174 | 0.205185 |
| CH3OH | H2S | 0.0381023 | -0.065128 | |
| CH3OH | H2 | 0.552763 | -1.79925 | |
| CPAVIJ | CH3OH | CO2 | 0.0147967 | 0.0818379 |
| CH3OH | H2S | -9.00×10-5 | -0.0199771 | |
| CH3OH | H2 | -0.348543 | 0.385507 |
| 项目 | 组分 i | 组分 j | AIJ | BIJ |
|---|---|---|---|---|
| CPAKIJ | CH3OH | CO2 | -0.122174 | 0.205185 |
| CH3OH | H2S | 0.0381023 | -0.065128 | |
| CH3OH | H2 | 0.552763 | -1.79925 | |
| CPAVIJ | CH3OH | CO2 | 0.0147967 | 0.0818379 |
| CH3OH | H2S | -9.00×10-5 | -0.0199771 | |
| CH3OH | H2 | -0.348543 | 0.385507 |
| 物流号 | 温度/℃ | 压力/bar | 摩尔流量/kmol·h-1 | 摩尔分数 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | N2 | CO | AR | CH4 | CO2 | H2S | COS | CH3OH | H2O | ||||
| 1 | -39.38 | 54.75 | 12206.00 | 0.6619 | 0.0033 | 0.3072 | 0.0014 | 0.0011 | 0.0250 | — | — | 100×10-6 | — |
| 2 | -51.28 | 3.00 | 1547.03 | 0.0008 | 0.0023 | 0.0044 | 25.06×10-6 | 68.04×10-6 | 0.9923 | 1.13×10-6 | — | 0.0002 | 0.12×10-6 |
| 3 | -61.35 | 1.85 | 4259.37 | 0.0013 | 0.1335 | 0.0072 | 41.43×10-6 | 0.0001 | 0.8577 | 1.27×10-6 | — | 0.0001 | — |
| 4 | 98.50 | 3.35 | 15067.00 | — | — | — | — | — | — | 0.39×10-6 | — | 0.9950 | 0.0050 |
| 5 | 139.88 | 3.60 | 709.55 | — | — | — | — | — | — | — | — | 100×10-6 | 0.9999 |
| 物流号 | 温度/℃ | 压力/bar | 摩尔流量/kmol·h-1 | 摩尔分数 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | N2 | CO | AR | CH4 | CO2 | H2S | COS | CH3OH | H2O | ||||
| 1 | -39.38 | 54.75 | 12206.00 | 0.6619 | 0.0033 | 0.3072 | 0.0014 | 0.0011 | 0.0250 | — | — | 100×10-6 | — |
| 2 | -51.28 | 3.00 | 1547.03 | 0.0008 | 0.0023 | 0.0044 | 25.06×10-6 | 68.04×10-6 | 0.9923 | 1.13×10-6 | — | 0.0002 | 0.12×10-6 |
| 3 | -61.35 | 1.85 | 4259.37 | 0.0013 | 0.1335 | 0.0072 | 41.43×10-6 | 0.0001 | 0.8577 | 1.27×10-6 | — | 0.0001 | — |
| 4 | 98.50 | 3.35 | 15067.00 | — | — | — | — | — | — | 0.39×10-6 | — | 0.9950 | 0.0050 |
| 5 | 139.88 | 3.60 | 709.55 | — | — | — | — | — | — | — | — | 100×10-6 | 0.9999 |
| 摩尔分数 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 物流号 | 温度/℃ | 压力/bar | 摩尔流量 /kmol·h-1 | H2 | N2 | CO | AR | CH4 | CO2 | H2S | COS | CH3OH | H2O |
| 1 | -37.36 | 54.75 | 12200.84 | 0.6621 | 0.0033 | 0.3072 | 0.0014 | 0.0011 | 0.0249 | — | — | 76.60×10-6 | — |
| 2 | -51.77 | 3.00 | 1568.37 | 0.0009 | 0.0005 | 0.0046 | 38.77×10-6 | 70.23×10-6 | 0.9936 | 0.96×10-6 | — | 0.0003 | 0.20×10-6 |
| 3 | -62.90 | 1.85 | 4243.65 | 0.0015 | 0.1358 | 0.0077 | 65.56×10-6 | 0.0001 | 0.8547 | 1.23×10-6 | — | 0.0001 | — |
| 4 | 98.26 | 3.35 | 15066.00 | — | — | — | — | — | — | 0.40×10-6 | — | 0.9950 | 0.0050 |
| 5 | 140.06 | 3.60 | 709.55 | — | — | — | — | — | — | — | — | 77.06×10-6 | 0.9999 |
| 摩尔分数 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 物流号 | 温度/℃ | 压力/bar | 摩尔流量 /kmol·h-1 | H2 | N2 | CO | AR | CH4 | CO2 | H2S | COS | CH3OH | H2O |
| 1 | -37.36 | 54.75 | 12200.84 | 0.6621 | 0.0033 | 0.3072 | 0.0014 | 0.0011 | 0.0249 | — | — | 76.60×10-6 | — |
| 2 | -51.77 | 3.00 | 1568.37 | 0.0009 | 0.0005 | 0.0046 | 38.77×10-6 | 70.23×10-6 | 0.9936 | 0.96×10-6 | — | 0.0003 | 0.20×10-6 |
| 3 | -62.90 | 1.85 | 4243.65 | 0.0015 | 0.1358 | 0.0077 | 65.56×10-6 | 0.0001 | 0.8547 | 1.23×10-6 | — | 0.0001 | — |
| 4 | 98.26 | 3.35 | 15066.00 | — | — | — | — | — | — | 0.40×10-6 | — | 0.9950 | 0.0050 |
| 5 | 140.06 | 3.60 | 709.55 | — | — | — | — | — | — | — | — | 77.06×10-6 | 0.9999 |
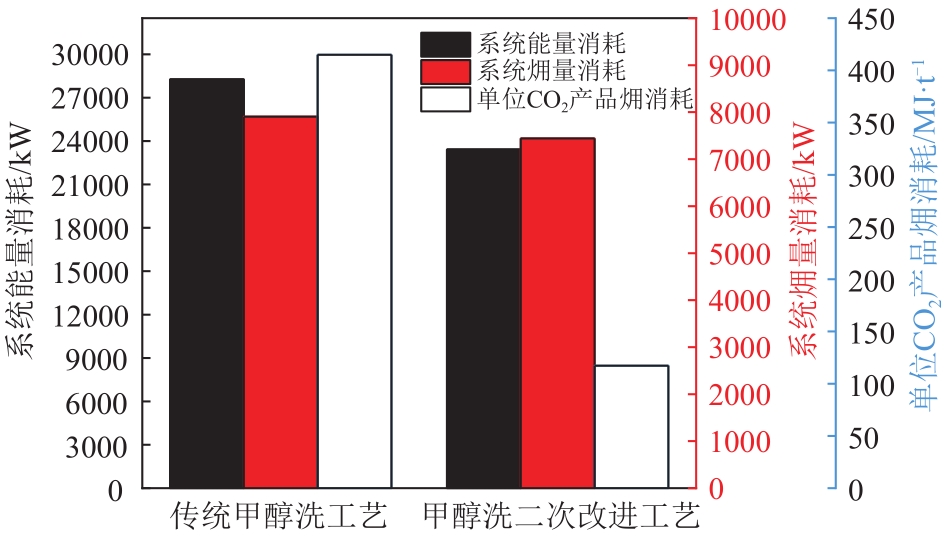
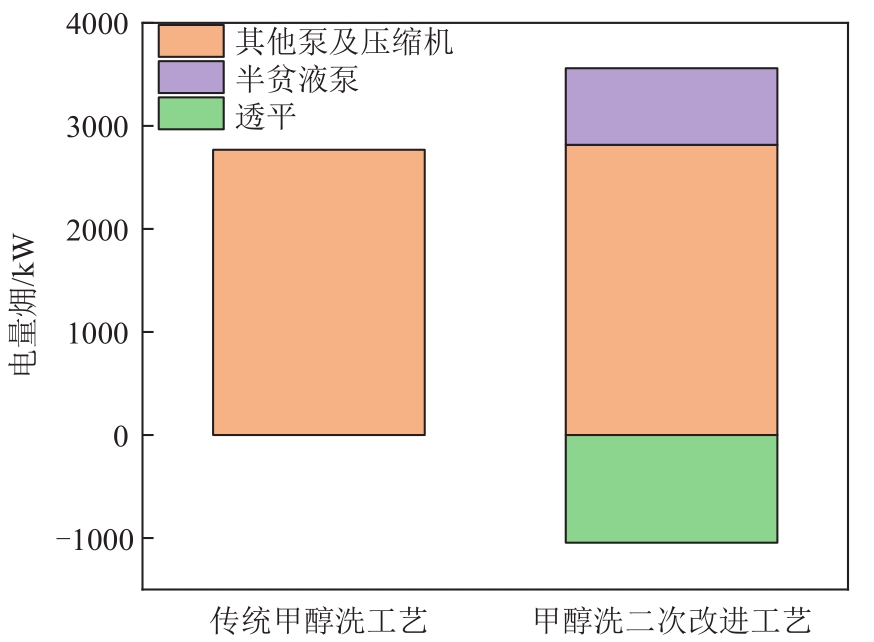
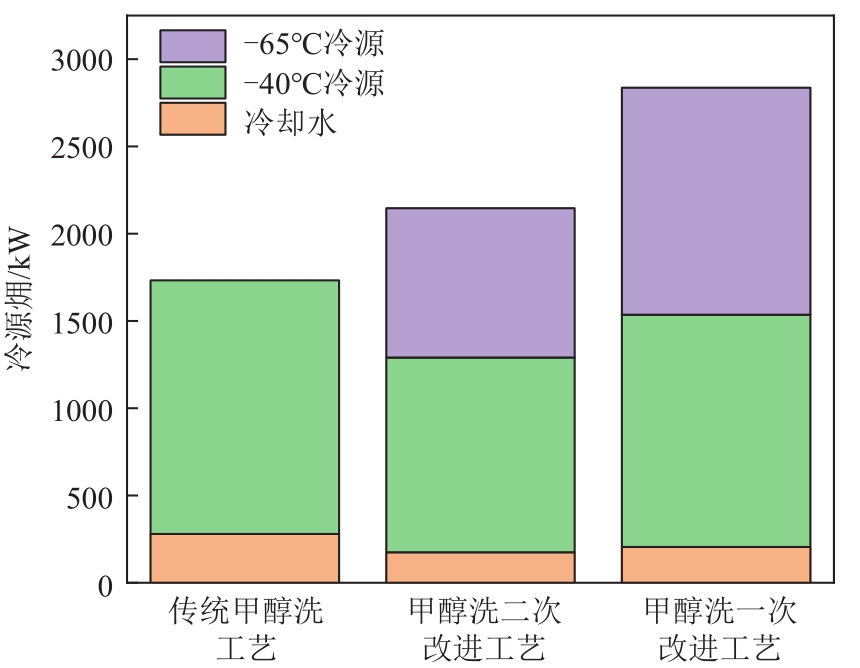
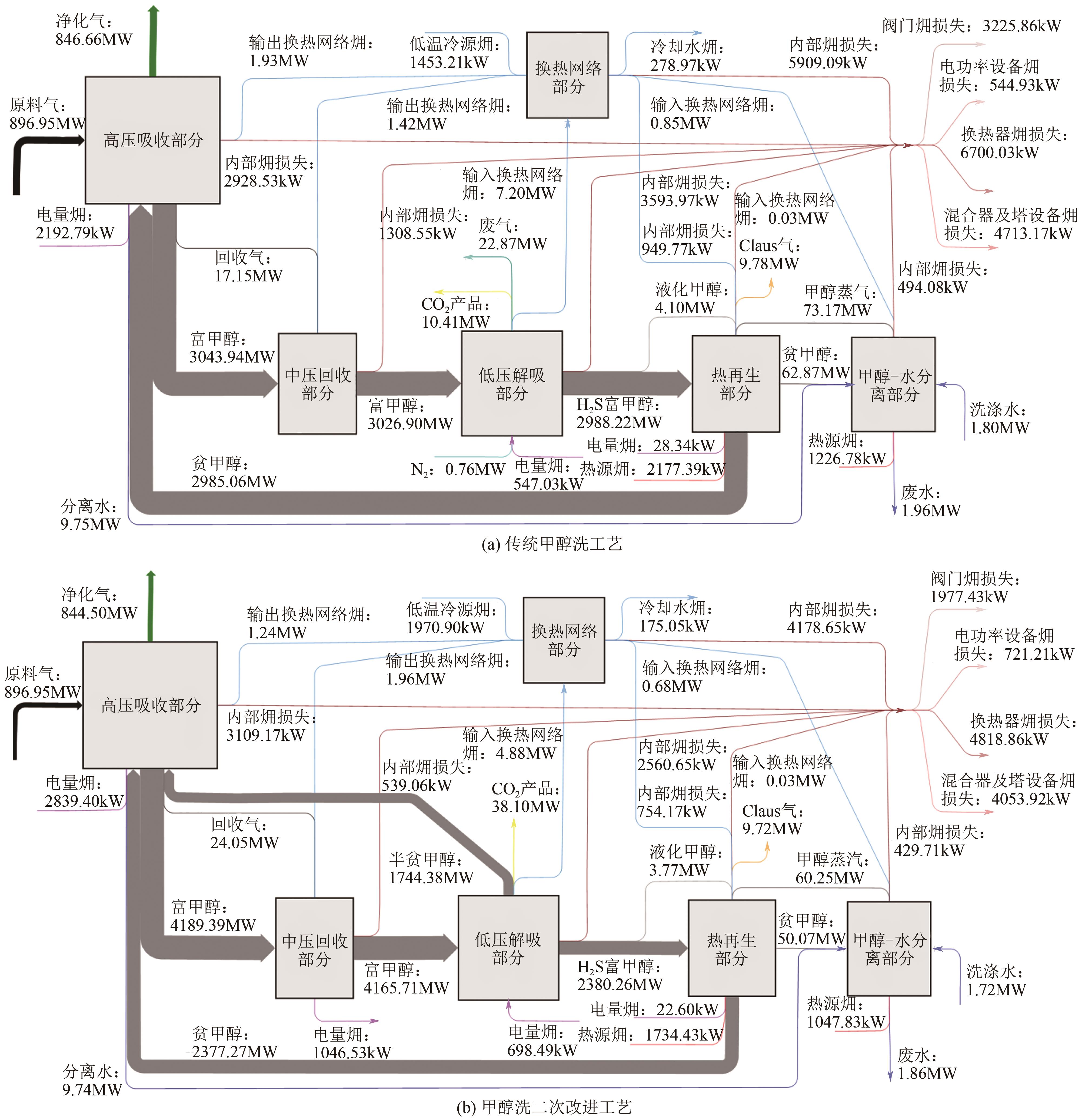
| 项目 | 传统甲醇洗工艺 | 甲醇洗一次改进工艺 |
|---|---|---|
| 电量/kW | 2768.16 | 3071.82 |
| 热量/kW | 11696.43 | 11696.43 |
| 冷量/kW | 13808.90 | 14103.26 |
| 系统能量消耗/kW | 28273.49 | 28871.50 |
| 电量㶲/kW | 2768.16 | 3071.82 |
| 热源㶲/kW | 3404.17 | 3404.17 |
| 冷源㶲/kW | 1732.18 | 2836.03 |
| 系统㶲量消耗/kW | 7904.51 | 9312.02 |
| CO2产量/mol·s-1 | 432.89 | 1439.41 |
| 单位CO2产品㶲消耗/MJ·t-1 | 415 | 147 |
| N2消耗/kmol·h-1 | 580 | 0 |
| 克劳斯气中H2S浓度/% | 40.82 | 40.53 |
| 项目 | 传统甲醇洗工艺 | 甲醇洗一次改进工艺 |
|---|---|---|
| 电量/kW | 2768.16 | 3071.82 |
| 热量/kW | 11696.43 | 11696.43 |
| 冷量/kW | 13808.90 | 14103.26 |
| 系统能量消耗/kW | 28273.49 | 28871.50 |
| 电量㶲/kW | 2768.16 | 3071.82 |
| 热源㶲/kW | 3404.17 | 3404.17 |
| 冷源㶲/kW | 1732.18 | 2836.03 |
| 系统㶲量消耗/kW | 7904.51 | 9312.02 |
| CO2产量/mol·s-1 | 432.89 | 1439.41 |
| 单位CO2产品㶲消耗/MJ·t-1 | 415 | 147 |
| N2消耗/kmol·h-1 | 580 | 0 |
| 克劳斯气中H2S浓度/% | 40.82 | 40.53 |
| 1 | DOTTORI F, SZEWCZYK W, CISCAR J C, et al. Increased human and economic losses from river flooding with anthropogenic warming[J]. Nature Climate Change, 2018, 8(9): 781-786. |
| 2 | LI Y, SHANG J H, ZHANG C, et al. The role of freshwater eutrophication in greenhouse gas emissions: a review[J]. Science of the Total Environment, 2021, 768: 144582. |
| 3 | MICHAELIDES E E. Thermodynamic analysis and power requirements of CO2 capture, transportation, and storage in the ocean[J]. Energy, 2021, 230: 120804. |
| 4 | BUI M, ADJIMAN C S, BARDOW A, et al. Carbon capture and storage (CCS): the way forward[J]. Energy & Environmental Science, 2018, 11(5): 1062-1176. |
| 5 | 王明坛, 谢圣林, 许子通. 二氧化碳捕集技术的现状与最新进展[J]. 当代化工, 2016, 45(5): 1002-1005. |
| WANG Mingtan, XIE Shenglin, XU Zitong. Present state and latest development of CO2 capture technology[J]. Contemporary Chemical Industry, 2016, 45(5): 1002-1005. | |
| 6 | 张娜. 低温甲醇洗技术及其在煤化工中的应用[J]. 化工设计通讯, 2021, 47(3): 11-12. |
| ZHANG Na. Low-temperature methanol washing technology and its application in coal chemical industry[J]. Chemical Engineering Design Communications, 2021, 47(3): 11-12. | |
| 7 | SCHOLES C A, ANDERSON C J, STEVENS G W, et al. Membrane gas separation-physical solvent absorption combined plant simulations for pre-combustion capture[J]. Energy Procedia, 2013, 37: 1039-1049. |
| 8 | RANKE G, WEISS H. Process for separating and recovering gaseous components from a gas mixture by physical washing: EP 79105255.8[P]. 1980-07-09. |
| 9 | YANG S, QIAN Y, YANG S Y. Development of a full CO2 capture process based on the rectisol wash technology[J]. Industrial & Engineering Chemistry Research, 2016, 55(21): 6186-6193. |
| 10 | SHARMA I, HOADLEY A F A, MAHAJANI S M, et al. Multi-objective optimisation of a Rectisol™ process for carbon capture[J]. Journal of Cleaner Production, 2016, 119: 196-206. |
| 11 | GATTI M, MARTELLI E, MARECHAL F, et al. Review, modeling, Heat Integration, and improved schemes of Rectisol®-based processes for CO2 capture[J]. Applied Thermal Engineering, 2014, 70(2): 1123-1140. |
| 12 | LIU X, YANG S Y, HU Z G, et al. Simulation and assessment of an integrated acid gas removal process with higher CO2 capture rate[J]. Computers & Chemical Engineering, 2015, 83: 48-57. |
| 13 | 佘红梅, 尹广华, 贾春临. 变压吸附技术提纯回收甲醇洗放空气中二氧化碳[J]. 大氮肥, 2009, 32(2): 129-132. |
| SHE Hongmei, YIN Guanghua, JIA Chunlin. Using PSA technology to recover CO2 from rectisol vented gas[J]. Large Scale Nitrogenous Fertilizer Industry, 2009, 32(2): 129-132. | |
| 14 | 刘都现, 韩振生. 浅析低温甲醇洗尾气中二氧化碳的回收利用[J]. 山东工业技术, 2016(11): 43. |
| LIU Duxian, HAN Zhensheng. Discussion on recover and utilization of CO2 from rectisol tail gas[J]. Shandong Industrial Technology, 2016(11): 43. | |
| 15 | 解寅珑. 基于某厂低温甲醇洗工艺装置模拟与优化改进方案研究[D]. 西安: 西北大学, 2020. |
| XIE Yinlong. The study on process simulation and optimization improvement scheme of rectisol equipment of on A plant[D]. Xi’an: Northwest University, 2020. | |
| 16 | MOORTGAT J. Reservoir simulation with the cubic plus (cross-) association equation of state for water, CO2, hydrocarbons, and tracers[J]. Advances in Water Resources, 2018, 114: 29-44. |
| 17 | WANG W G, WANG J F, LU Z J, et al. Exergoeconomic and exergoenvironmental analysis of a combined heating and power system driven by geothermal source[J]. Energy Conversion and Management, 2020, 211: 112765. |
| 18 | LIU J, ZHANG X S, LIN Z, et al. Exergy and energy analysis of a novel dual-chilling-source refrigerating system applied to temperature and humidity independent control[J]. Energy Conversion and Management, 2019, 197: 111875. |
| 19 | HINDERINK A P, KERKHOF F P J M, LIE A B K, et al. Exergy analysis with a flowsheeting simulator—I. Theory; calculating exergies of material streams[J]. Chemical Engineering Science, 1996, 51(20): 4693-4700. |
| 20 | 张旭. 基于㶲分析的焦炉煤气甲烷化工艺优化[D]. 马鞍山: 安徽工业大学, 2019. |
| ZHANG Xu. Optimization of coke oven gas methanation process based on exergy analysis[D]. Ma’anshan: Anhui Universit of Technology, 2019. | |
| 21 | 傅秦生. 能量系统的热力学分析方法[M]. 西安: 西安交通大学出版社, 2005. |
| FU Qinsheng. Methods for thermodynamic analysis of energy systems[M]. Xi’an: Xi’an Jiaotong University Press, 2005. | |
| 22 | YU M X, CUI P Z, WANG Y Let al. Advanced exergy and exergoeconomic analysis of cascade absorption refrigeration system driven by low-grade waste heat[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(19): 16843-16857. |
| 23 | 全国能源基础与管理标准化技术委员会. 能量系统㶲分析技术导则: [S]. 北京: 中国标准出版社,2005. |
| Energy Fundamentals and Management. Technical guides for exergy analysis in energy system: [S]. Beijing: Standards Press of China, 2005. | |
| 24 | 刘振学, 王力. 实验设计与数据处理[M]. 2版.北京: 化学工业出版社, 2015. |
| LIU Zhenxue, WANG Li. Experimental design and data processing[M]. 2nd ed. Beijing: Chemical Industry Press, 2015. | |
| 25 | DENG J Q, CAO Z, ZHANG D B, et al. Integration of energy recovery network including recycling residual pressure energy with pinch technology[J]. Chinese Journal of Chemical Engineering, 2017, 25(4): 453-462. |
| [1] | ZHANG Ruijie, LIU Zhilin, WANG Junwen, ZHANG Wei, HAN Deqiu, LI Ting, ZOU Xiong. On-line dynamic simulation and optimization of water-cooled cascade refrigeration system [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 124-132. |
| [2] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [3] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [4] | DAI Huantao, CAO Lingyu, YOU Xinxiu, XU Haoliang, WANG Tao, XIANG Wei, ZHANG Xueyang. Adsorption properties of CO2 on pomelo peel biochar impregnated by lignin [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 356-363. |
| [5] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [6] | BAI Yadi, DENG Shuai, ZHAO Ruikai, ZHAO Li, YANG Yingxia. Exploration on standardized test scheme and experimental performance of temperature swing adsorption carbon capture unit [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3834-3846. |
| [7] | LU Shijian, ZHANG Yuanyuan, WU Wenhua, YANG Fei, LIU Ling, KANG Guojun, LI Qingfang, CHEN Hongfu, WANG Ning, WANG Feng, ZHANG Juanjuan. Health risk assessment of nitrosamine pollutant diffusion in a million ton CO2 capture project [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3209-3216. |
| [8] | WANG Jiuheng, RONG Nai, LIU Kaiwei, HAN Long, SHUI Taotao, WU Yan, MU Zhengyong, LIAO Xuqing, MENG Wenjia. Enhanced CO2 capture performance and strength of cellulose-templated CaO-based pellets with steam reactivation [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3217-3225. |
| [9] | GU Shiya, DONG Yachao, LIU Linlin, ZHANG Lei, ZHUANG Yu, DU Jian. Design and optimization of pipeline system for carbon capture considering intermediate nodes [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2799-2808. |
| [10] | LING Shan, LIU Juming, ZHANG Qiancheng, LI Yan. Research progress on simulated moving bed separation process and its optimization methods [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2233-2244. |
| [11] | WANG Dong, YU Pinhua, CHEN Bin, XIAO Ang, CHEN Feng, JIANG Yangyang. Energy saving optimization of cyclohexane three-effect distillation in cyclohexanone production [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2245-2251. |
| [12] | SANG Wei, TANG Jianfeng, HUA Yihuai, CHEN Jie, SUN Peiyuan, XU Yifei. Effects of physical solvent and amine properties on the performance of biphasic solvent [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2151-2159. |
| [13] | SHANG Yu, XIAO Man, CUI Qiufang, TU Te, YAN Shuiping. Recovery characteristics of PVDF/BN-OH flat composite membrane for waste heat of hot stripped gas in CO2 capture process [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1618-1628. |
| [14] | KONG Xiangru, ZHANG Xiaoyang, SUN Pengxiang, CUI Lin, DONG Yong. Research progress of solid porous materials for direct CO2 capture from air [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1471-1483. |
| [15] | LU Shijian, LIU Miaomiao, LIU Ling, KANG Guojun, MAO Songbai, WANG Feng, ZHANG Juanjuan, GONG Yuping. Progress and future development trend of amine method of CO2 capture technology from flue gas [J]. Chemical Industry and Engineering Progress, 2023, 42(1): 435-444. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||