Chemical Industry and Engineering Progress ›› 2022, Vol. 41 ›› Issue (11): 6053-6060.DOI: 10.16085/j.issn.1000-6613.2022-0153
• Fine chemicals • Previous Articles Next Articles
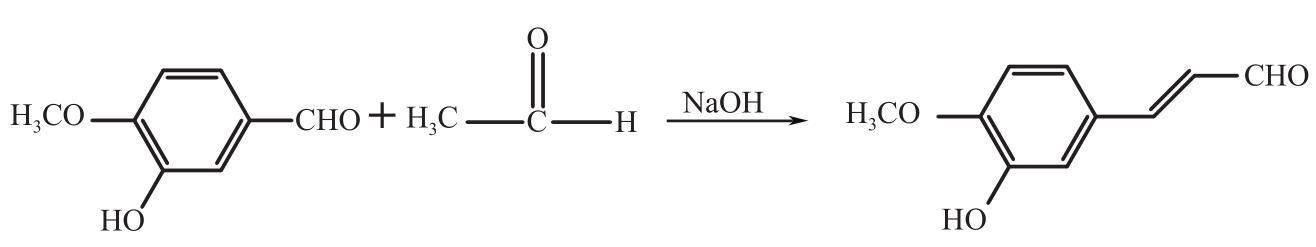
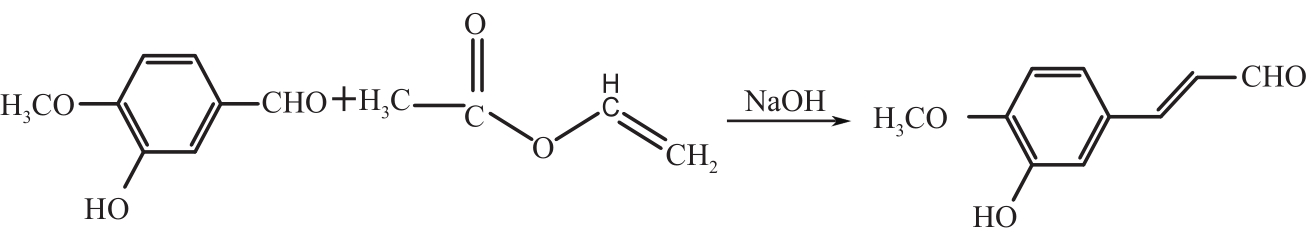
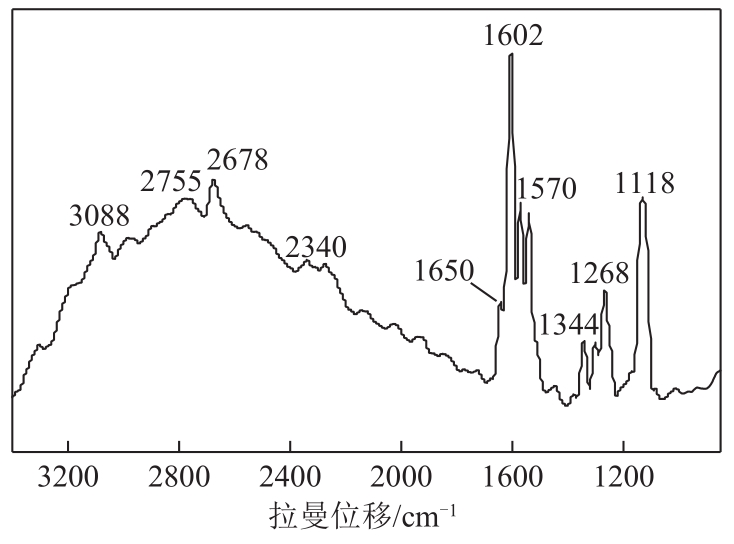
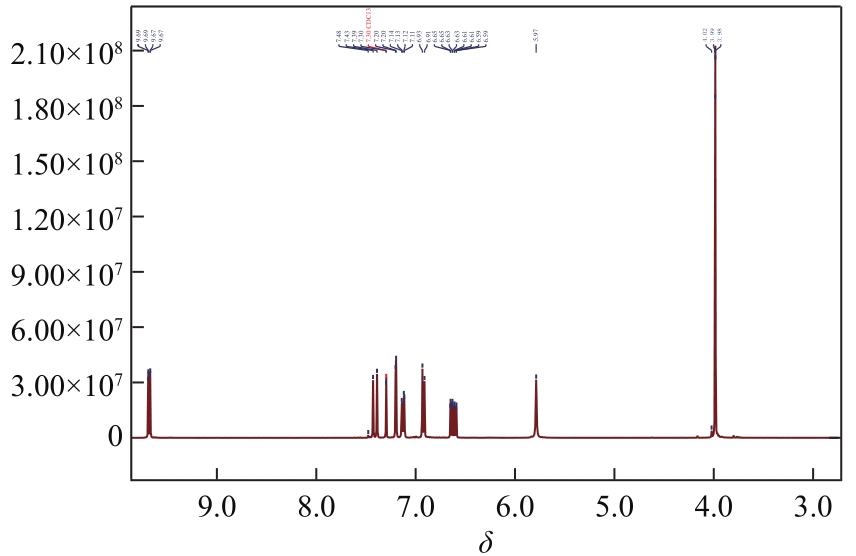
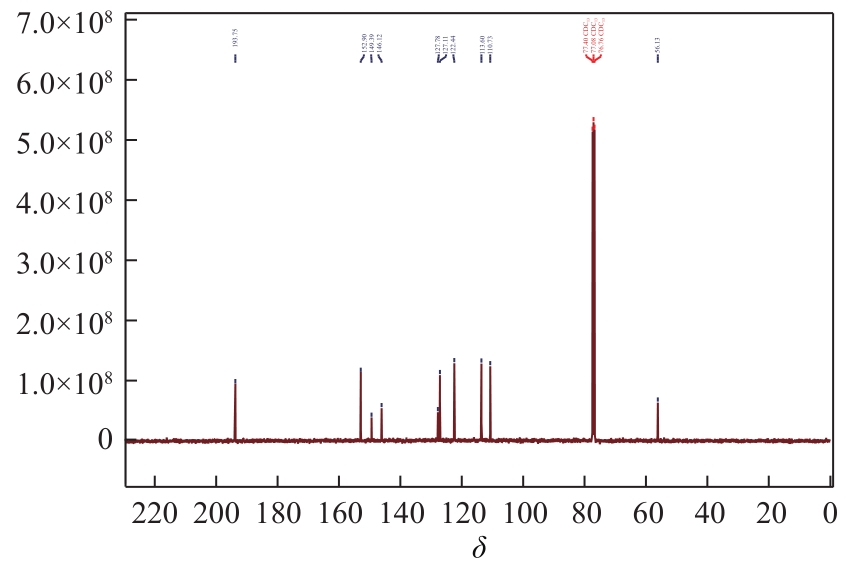
Synthesis of 3-hydroxy-4-methoxy cinnamaldehyde as intermediate of advantame
FANG Cong( ), LIU Yixue, LI Sifang(
), LIU Yixue, LI Sifang( )
)
- College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, Fujian, China
-
Received:2022-01-24Revised:2022-03-25Online:2022-11-28Published:2022-11-25 -
Contact:LI Sifang
爱德万甜中间体3-羟基-4-甲氧基肉桂醛的合成
- 厦门大学化学化工学院,福建 厦门 361005
-
通讯作者:黎四芳 -
作者简介:方聪(1993—),男,硕士研究生,研究方向为精细化工。E-mail:congfang@stu.xmu.edu.cn。
CLC Number:
Cite this article
FANG Cong, LIU Yixue, LI Sifang. Synthesis of 3-hydroxy-4-methoxy cinnamaldehyde as intermediate of advantame[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 6053-6060.
方聪, 刘怡雪, 黎四芳. 爱德万甜中间体3-羟基-4-甲氧基肉桂醛的合成[J]. 化工进展, 2022, 41(11): 6053-6060.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-0153
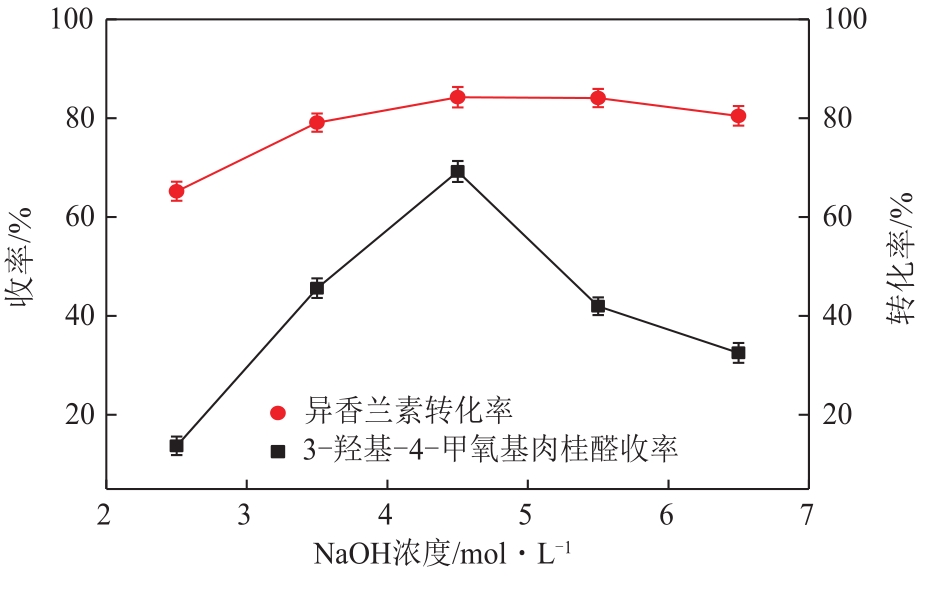
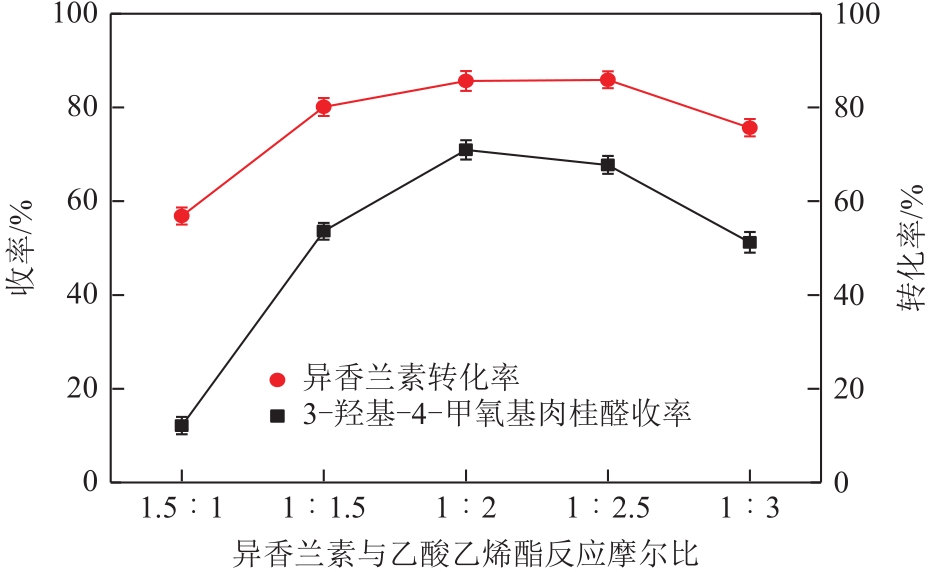
| 因素 | 水平 | ||
|---|---|---|---|
| -1 | 0 | 1 | |
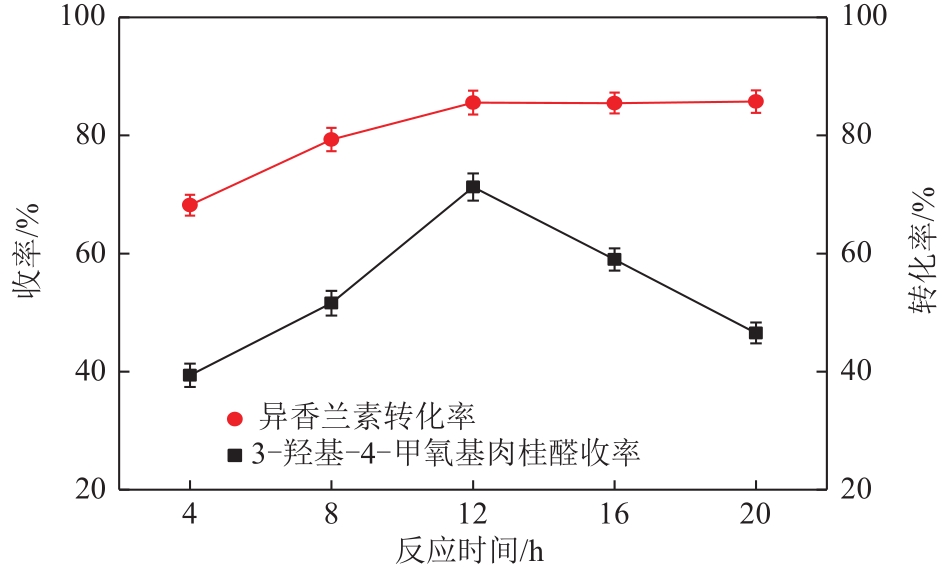
| A 反应时间/h | 8 | 12 | 16 |
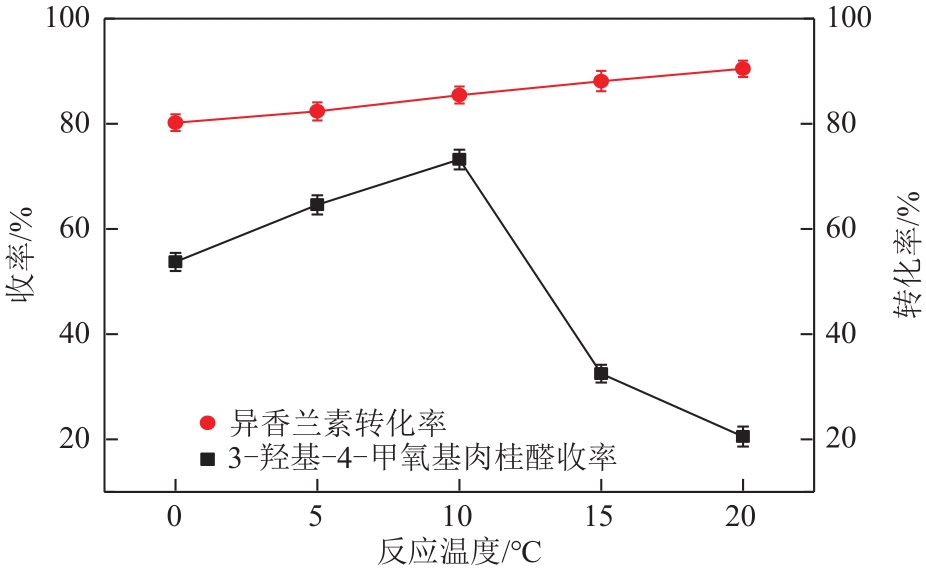
| B 反应温度/℃ | 5 | 10 | 15 |
| C NaOH浓度/mol·L-1 | 0.14 | 0.18 | 0.22 |
| D 异香兰素与乙酸乙烯摩尔比 | 1∶1.5 | 1∶2 | 1∶2.5 |
| 因素 | 水平 | ||
|---|---|---|---|
| -1 | 0 | 1 | |
| A 反应时间/h | 8 | 12 | 16 |
| B 反应温度/℃ | 5 | 10 | 15 |
| C NaOH浓度/mol·L-1 | 0.14 | 0.18 | 0.22 |
| D 异香兰素与乙酸乙烯摩尔比 | 1∶1.5 | 1∶2 | 1∶2.5 |
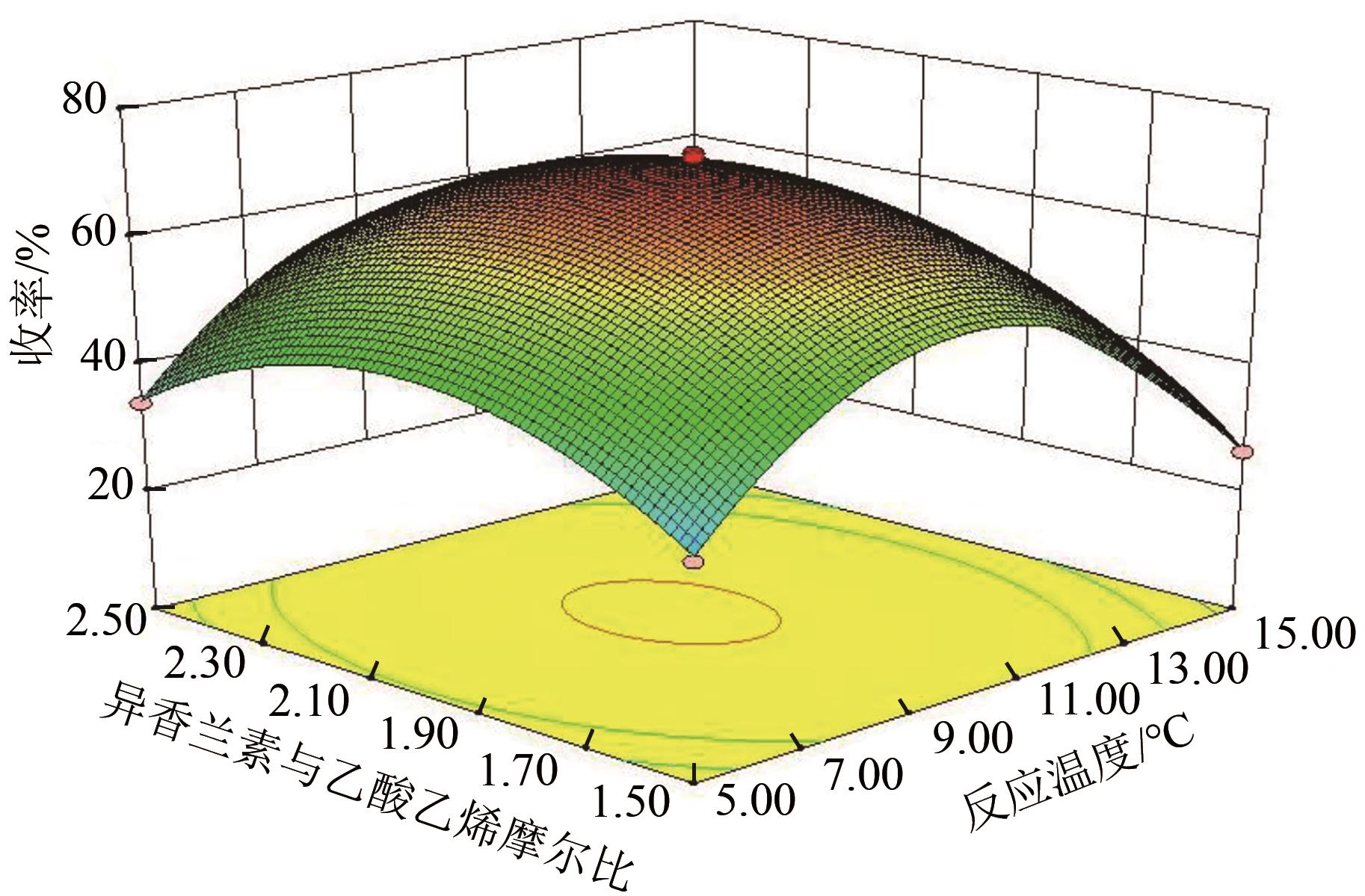
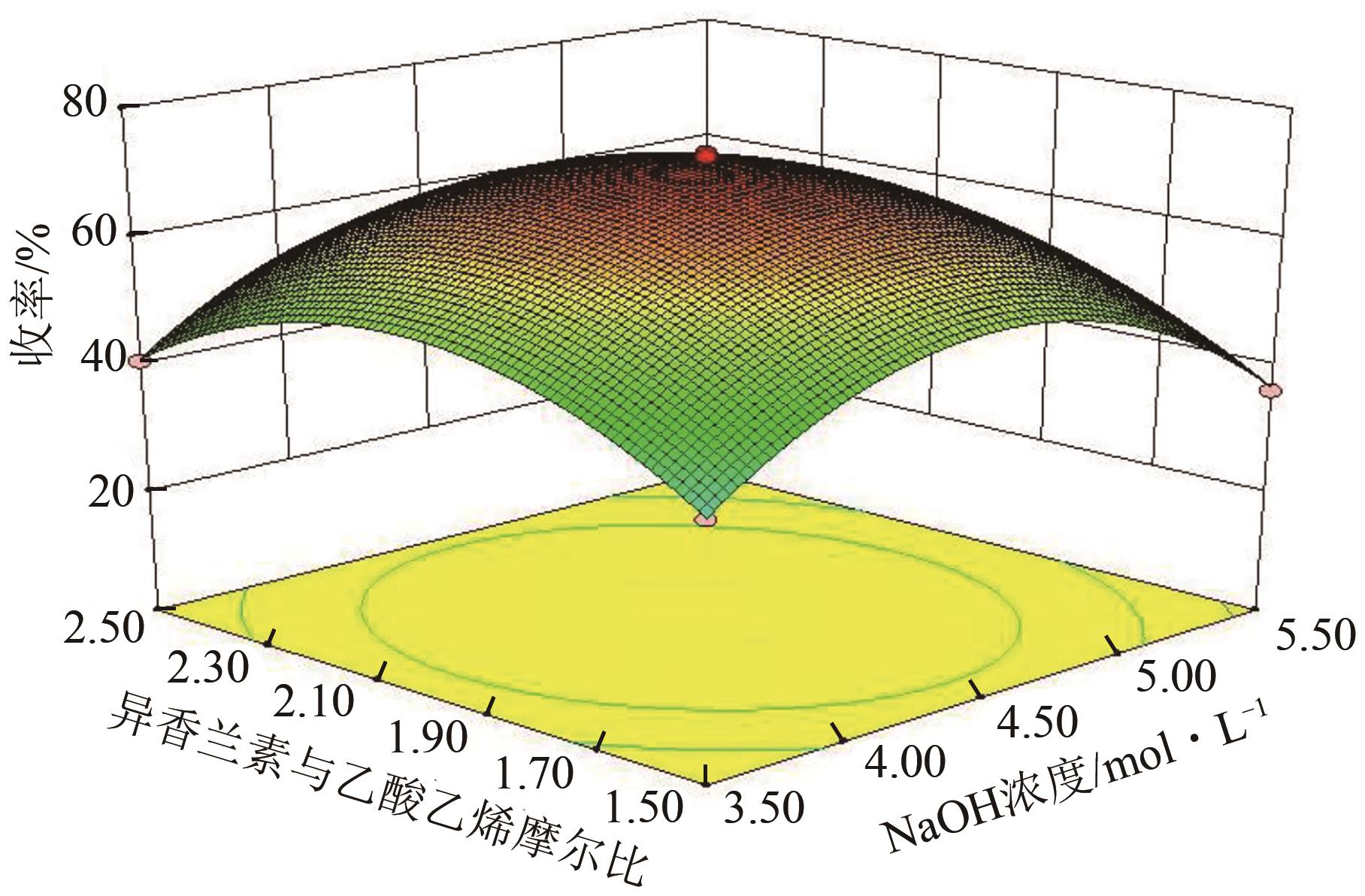
| 序列 | 因素 | 收率/% | ||||
|---|---|---|---|---|---|---|
| A:反应时间 | B:反应温度 | C:NaOH 浓度 | D:异香兰素与乙酸乙烯摩尔比 | 实际值 | 预测值 | |
| 1 | -1 | 0 | 1 | 0 | 37.94 | 38.92 |
| 2 | 0 | -1 | 1 | 0 | 31.55 | 29.23 |
| 3 | 1 | -1 | 0 | 0 | 36.61 | 37.56 |
| 4 | -1 | -1 | 0 | 0 | 34.17 | 35.31 |
| 5 | 1 | 1 | 0 | 0 | 30.57 | 30.59 |
| 6 | -1 | 1 | 0 | 0 | 28.88 | 29.09 |
| 7 | 0 | 1 | -1 | 0 | 25.36 | 25.66 |
| 8 | -1 | 0 | -1 | 0 | 41.13 | 40.71 |
| 9 | 0 | 0 | 0 | 0 | 69.23 | 71.44 |
| 10 | 0 | 0 | 0 | 0 | 71.25 | 71.44 |
| 11 | 0 | 0 | 0 | 0 | 70.98 | 71.44 |
| 12 | 1 | 0 | -1 | 0 | 43.95 | 43.83 |
| 13 | 0 | 1 | 1 | 0 | 23.25 | 22.44 |
| 14 | 0 | 0 | 0 | 0 | 72.54 | 71.44 |
| 15 | 0 | 0 | 0 | 0 | 73.21 | 71.44 |
| 16 | 0 | -1 | -1 | 0 | 33.29 | 32.07 |
| 17 | 1 | 0 | 1 | 0 | 39.59 | 40.21 |
| 18 | -1 | 0 | 0 | 1 | 45.82 | 44.61 |
| 19 | 0 | 1 | 0 | 1 | 28.79 | 28.75 |
| 20 | 0 | 0 | -1 | 1 | 40.77 | 41.27 |
| 21 | 0 | -1 | 0 | 1 | 33.98 | 34.52 |
| 22 | 1 | 0 | 0 | 1 | 46.78 | 46.12 |
| 23 | 0 | 0 | 1 | 1 | 37.02 | 37.89 |
| 24 | 0 | 0 | -1 | -1 | 39.11 | 39.40 |
| 25 | 0 | -1 | 0 | -1 | 32.93 | 33.83 |
| 26 | 0 | 0 | 1 | -1 | 36.07 | 36.73 |
| 27 | 1 | 0 | 0 | -1 | 45.78 | 44.97 |
| 28 | 0 | 1 | 0 | -1 | 26.07 | 26.40 |
| 29 | -1 | 0 | 0 | -1 | 44.10 | 42.73 |
| 序列 | 因素 | 收率/% | ||||
|---|---|---|---|---|---|---|
| A:反应时间 | B:反应温度 | C:NaOH 浓度 | D:异香兰素与乙酸乙烯摩尔比 | 实际值 | 预测值 | |
| 1 | -1 | 0 | 1 | 0 | 37.94 | 38.92 |
| 2 | 0 | -1 | 1 | 0 | 31.55 | 29.23 |
| 3 | 1 | -1 | 0 | 0 | 36.61 | 37.56 |
| 4 | -1 | -1 | 0 | 0 | 34.17 | 35.31 |
| 5 | 1 | 1 | 0 | 0 | 30.57 | 30.59 |
| 6 | -1 | 1 | 0 | 0 | 28.88 | 29.09 |
| 7 | 0 | 1 | -1 | 0 | 25.36 | 25.66 |
| 8 | -1 | 0 | -1 | 0 | 41.13 | 40.71 |
| 9 | 0 | 0 | 0 | 0 | 69.23 | 71.44 |
| 10 | 0 | 0 | 0 | 0 | 71.25 | 71.44 |
| 11 | 0 | 0 | 0 | 0 | 70.98 | 71.44 |
| 12 | 1 | 0 | -1 | 0 | 43.95 | 43.83 |
| 13 | 0 | 1 | 1 | 0 | 23.25 | 22.44 |
| 14 | 0 | 0 | 0 | 0 | 72.54 | 71.44 |
| 15 | 0 | 0 | 0 | 0 | 73.21 | 71.44 |
| 16 | 0 | -1 | -1 | 0 | 33.29 | 32.07 |
| 17 | 1 | 0 | 1 | 0 | 39.59 | 40.21 |
| 18 | -1 | 0 | 0 | 1 | 45.82 | 44.61 |
| 19 | 0 | 1 | 0 | 1 | 28.79 | 28.75 |
| 20 | 0 | 0 | -1 | 1 | 40.77 | 41.27 |
| 21 | 0 | -1 | 0 | 1 | 33.98 | 34.52 |
| 22 | 1 | 0 | 0 | 1 | 46.78 | 46.12 |
| 23 | 0 | 0 | 1 | 1 | 37.02 | 37.89 |
| 24 | 0 | 0 | -1 | -1 | 39.11 | 39.40 |
| 25 | 0 | -1 | 0 | -1 | 32.93 | 33.83 |
| 26 | 0 | 0 | 1 | -1 | 36.07 | 36.73 |
| 27 | 1 | 0 | 0 | -1 | 45.78 | 44.97 |
| 28 | 0 | 1 | 0 | -1 | 26.07 | 26.40 |
| 29 | -1 | 0 | 0 | -1 | 44.10 | 42.73 |
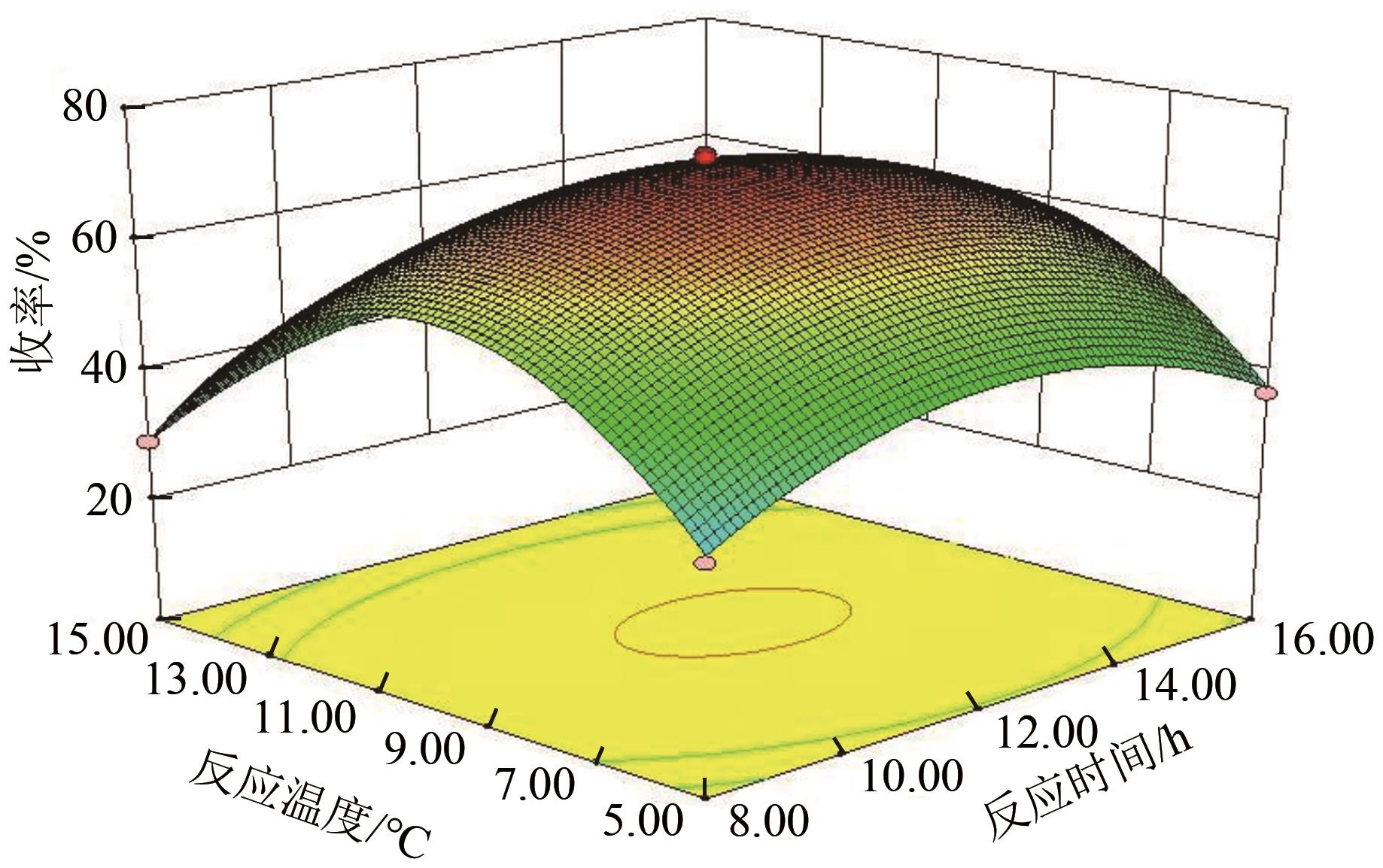
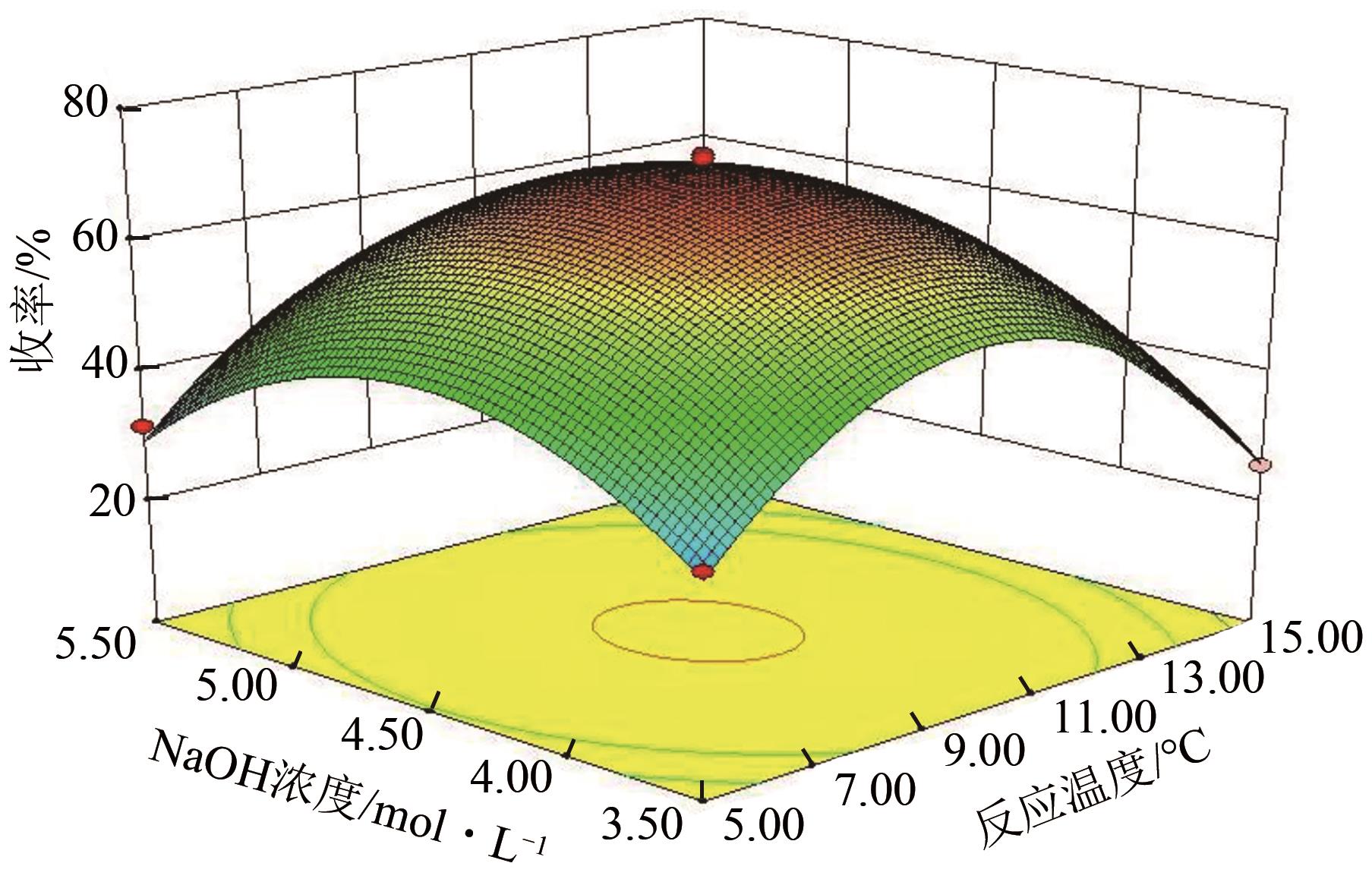
| 方差来源 | 平方和 | 自由度 | 均方 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 回归方程 | 6249.78 | 14 | 446.41 | 223.75 | < 0.0001 | + + |
| A | 10.53 | 1 | 10.53 | 5.28 | 0.0376 | |
| B | 130.75 | 1 | 130.75 | 65.53 | < 0.0001 | + + |
| C | 27.57 | 1 | 27.57 | 13.82 | 0.0023 | + |
| D | 6.90 | 1 | 6.90 | 3.46 | 0.0841 | |
| AB | 0.14 | 1 | 0.14 | 0.070 | 0.7945 | |
| AC | 0.34 | 1 | 0.34 | 0.17 | 0.6850 | |
| AD | 0.13 | 1 | 0.13 | 0.065 | 0.8025 | |
| BC | 0.034 | 1 | 0.034 | 0.017 | 0.8977 | |
| BD | 0.70 | 1 | 0.70 | 0.35 | 0.5638 | |
| CD | 0.13 | 1 | 0.13 | 0.063 | 0.8052 | |
| A2 | 979.04 | 1 | 979.04 | 490.72 | < 0.0001 | + + |
| B2 | 4391.39 | 1 | 4391.39 | 2201.09 | < 0.0001 | + + |
| C2 | 2118.43 | 1 | 2118.43 | 1061.82 | < 0.0001 | + + |
| D2 | 1372.84 | 1 | 1372.84 | 688.11 | < 0.0001 | + + |
| 残差 | 27.93 | 14 | 2.00 | — | — | |
| 失拟项 | 18.46 | 10 | 1.85 | 0.78 | 0.6603 | |
| 纯误差 | 9.47 | 4 | 2.37 | — | — | |
| 总和 | 6277.71 | 28 | — | — | — |
| 方差来源 | 平方和 | 自由度 | 均方 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 回归方程 | 6249.78 | 14 | 446.41 | 223.75 | < 0.0001 | + + |
| A | 10.53 | 1 | 10.53 | 5.28 | 0.0376 | |
| B | 130.75 | 1 | 130.75 | 65.53 | < 0.0001 | + + |
| C | 27.57 | 1 | 27.57 | 13.82 | 0.0023 | + |
| D | 6.90 | 1 | 6.90 | 3.46 | 0.0841 | |
| AB | 0.14 | 1 | 0.14 | 0.070 | 0.7945 | |
| AC | 0.34 | 1 | 0.34 | 0.17 | 0.6850 | |
| AD | 0.13 | 1 | 0.13 | 0.065 | 0.8025 | |
| BC | 0.034 | 1 | 0.034 | 0.017 | 0.8977 | |
| BD | 0.70 | 1 | 0.70 | 0.35 | 0.5638 | |
| CD | 0.13 | 1 | 0.13 | 0.063 | 0.8052 | |
| A2 | 979.04 | 1 | 979.04 | 490.72 | < 0.0001 | + + |
| B2 | 4391.39 | 1 | 4391.39 | 2201.09 | < 0.0001 | + + |
| C2 | 2118.43 | 1 | 2118.43 | 1061.82 | < 0.0001 | + + |
| D2 | 1372.84 | 1 | 1372.84 | 688.11 | < 0.0001 | + + |
| 残差 | 27.93 | 14 | 2.00 | — | — | |
| 失拟项 | 18.46 | 10 | 1.85 | 0.78 | 0.6603 | |
| 纯误差 | 9.47 | 4 | 2.37 | — | — | |
| 总和 | 6277.71 | 28 | — | — | — |
| 1 | AMINO Yusuke, MORI Kenich, TOMIYAMA Yasuyuki, et al. Development of new, low calorie sweetener: new aspartame derivative[M]//WEERASINGHE Deepthi K, DUBOIS Grant E. Sweetness and sweeteners: biology, chemistry, and psychophysics. Washington D C: ACS, 2008: 463-480. |
| 2 | BISHAY I E, BURSEY R G. Advantame[M]//O’BRIEN-Nabors Lyn. Alternative sweeteners. 4th edition. Boca Raton: CRC Press, 2012: 31-45. |
| 3 | OTABE A, FUJIEDA T, MASUYAMA T, et al. Advantame-an overview of the toxicity data[J]. Food and Chemical Toxicology: an International Journal Published for the British Industrial Biological Research Association, 2011, 49(S1): S2-S7. |
| 4 | RENWICK A G. Postscript on advantame: a novel high-potency low-calorie sweetener[J]. Food and Chemical Toxicology, 2011, 49: S1. |
| 5 | Food Safety Commission of Japan. Advantame: summary[R]. Tokyo: FSCJ, 2013. |
| 6 | EFSA Panel on Food Additives and Nutrient Sources added to Food ANS). Scientific opinion on the safety of advantame for the proposed uses as a food additive[J]. EFSA Journal, 2013, 11(7): 3301-3368. |
| 7 | ZHANG Jiyue, ZHANG Jianbo, YU Hangyu, et al. Theoretical risk assessment of dietary exposure to advantame among the Chinese population[J]. Biomedical and Environmental Sciences: BES, 2019, 32(12): 930-933. |
| 8 | DUBOIS Grant E, PRAKASH Indra. Non-caloric sweeteners, sweetness modulators, and sweetener enhancers[J]. Annual Review of Food Science and Technology, 2012, 3: 353-380. |
| 9 | Kay O’DONNELL. Aspartame, neotame and advantame[M]// Sweeteners and sugar alternatives in food technology. Oxford: John Wiley & Sons Inc., 2012: 117-136. |
| 10 | 黎四芳. 高甜度甜味剂的生产与应用[M]. 厦门: 厦门大学出版社, 2021. |
| LI Sifang. Production and application of high potency sweeteners[M]. Xiamen: Xiamen University Press, 2021. | |
| 11 | 方聪, 刘怡雪, 黎四芳. 新型超高甜度二肽甜味剂爱德万甜的研究进展[J]. 中国食品添加剂, 2021, 32(2): 128-136. |
| FANG Cong, LIU Yixue, LI Sifang. Research progress in new ultra-high potency dipeptide sweetener advantame[J]. China Food Additives, 2021, 32(2): 128-136. | |
| 12 | NAGASHIMA Kazutaka, AOKI Yuuichi, TAKEMOTO Tadashi, et al. Process for production of aspartyl dipeptide ester derivative, novel production intermediate therefor, and process for production thereof: US6794531[P]. 2004-09-21. |
| 13 | MORI Kenichi, FUJITA Shinji, FUNAKOSHI Nao, et al. Process for producing cinnamaldehyde derivatives, use thereof and the like: US7141263[P]. 2006-11-28. |
| 14 | 陈博儒, 陈良, 朱思明. 甜味剂中间体3-羟基-4-甲氧基苯丙烯醛的合成工艺优化及其结构表征[J]. 食品工业科技, 2018, 39(22): 144-149. |
| CHEN Boru, CHEN Liang, ZHU Siming. Optimization of synthesis process and structural characterization of 3-hydroxy-4-methoxy benzal acrolein—A aweetener intermediate[J]. Science and Technology of Food Industry, 2018, 39(22): 144-149. | |
| 15 | CHEN Boru, LIU Qiang, WANG Huan, et al. Purification, characterization, and identification of 3-hydroxy-4-methoxy benzal acrolein—An intermediate of synthesizing advantame[J]. Food Science & Nutrition, 2020, 8(2): 744-753. |
| 16 | 吴美红, 郑建仙. 超高倍甜味剂N-[3-(3-羟基-4-甲氧基苯基)丙基]-阿斯巴甜的合成研究[J]. 食品与发酵工业, 2009, 35(6): 1-5. |
| WU Meihong, ZHENG Jianxian. Synthesis of high potency sweetener N-[3-(3-hydroxy-4-methoxyphenyl) propyl]-aspartyl-L-henylalaninel-methylester[J]. Food and Fermentation Industries, 2009, 35(6): 1-5. | |
| 17 | 刘怡雪. Advantame的合成[D]. 厦门: 厦门大学, 2018. |
| LIU Yixue. The synthesis of advantame[D]. Xiamen: Xiamen University, 2018. | |
| 18 | DIVI M K P, RAO M A N, NOWSHUDDIN S. Process for the preparation of advantame:US9512063[P]. 2016-12-06. |
| 19 | Gardner SWAIN C, POWELL Arnet L, SHEPPARD William A, et al. Mechanism of the cannizzaro reaction[J]. Journal of the American Chemical Society, 1979, 101(13): 3576-3583. |
| 20 | J J Vanden EYNDE, MUTONKOLE K, VAN HAVERBEKE Y. Surfactant-assisted organic reactions in water. Effect of ultrasound on condensation reactions between active methylene compounds and arylaldehydes[J]. Ultrasonics Sonochemistry, 2001, 8(1): 35-39. |
| 21 | 丁秋平. 胶束中氨基酸直接催化Aldol反应研究[D]. 南昌: 江西师范大学, 2003. |
| DING Qiuping. Study of the aldol reactions directly catalyzed by amino acids in aqueous micelles[D]. Nanchang: Jiangxi Normal University, 2003. | |
| 22 | VASHISHTHA Manu, MISHRA Manish, SHAH Dinesh O. A novel approach for selective cross aldol condensation using reusable NaOH-cationic micellar systems[J]. Applied Catalysis A: General, 2013, 466: 38-44. |
| 23 | 张媛媛, 郎万中, 褚联峰, 等. PVP相转移催化合成辛烯醛[J]. 广州化工, 2009, 37(9): 155-157, 160. |
| ZHANG Yuanyuan, LANG Wanzhong, CHU Lianfeng, et al. Synthesis of 2-ethylhexenal with phase transfer catalyst PVP[J]. Guangzhou Chemical Industry, 2009, 37(9): 155-157, 160. | |
| 24 | 陈逸. 表面活性剂及其在相转移催化方面的应用研究[J]. 当代化工研究, 2019(1): 105-106. |
| CHEN Yi. Surfactant and its application in phase transfer catalysis[J]. Modern Chemical Research, 2019(1): 105-106. |
| [1] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [2] | ZHENG Qian, GUAN Xiushuai, JIN Shanbiao, ZHANG Changming, ZHANG Xiaochao. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. |
| [3] | ZHAO Wei, ZHAO Deyin, LI Shihan, LIU Hongda, SUN Jin, GUO Yanqiu. Synthesis and application of triazine drag reducing agent for nature gas pipeline [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 391-399. |
| [4] | WANG Zhengkun, LI Sifang. Green synthesis of gemini surfactant decyne diol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 400-410. |
| [5] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [6] | LI Mengyuan, GUO Fan, LI Qunsheng. Simulation and optimization of the third and fourth distillation columns in the recovery section of polyvinyl alcohol production [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 113-123. |
| [7] | ZHANG Ruijie, LIU Zhilin, WANG Junwen, ZHANG Wei, HAN Deqiu, LI Ting, ZOU Xiong. On-line dynamic simulation and optimization of water-cooled cascade refrigeration system [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 124-132. |
| [8] | WANG Fu'an. Consumption and emission reduction of the reactor of 300kt/a propylene oxide process [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 213-218. |
| [9] | LI Chunli, HAN Xiaoguang, LIU Jiapeng, WANG Yatao, WANG Chenxi, WANG Honghai, PENG Sheng. Research progress of liquid distributors in packed columns [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4479-4495. |
| [10] | GENG Yuanze, ZHOU Junhu, ZHANG Tianyou, ZHU Xiaoyu, YANG Weijuan. Homogeneous/heterogeneous coupled combustion of heptane in a partially packed bed burner [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4514-4521. |
| [11] | GAO Yanjing. Analysis of international research trend of single-atom catalysis technology [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4667-4676. |
| [12] | LI Dongze, ZHANG Xiang, TIAN Jian, HU Pan, YAO Jie, ZHU Lin, BU Changsheng, WANG Xinye. Research progress of NO x reduction by carbonaceous substances for denitration in cement kiln [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4882-4893. |
| [13] | WANG Chen, BAI Haoliang, KANG Xue. Performance study of high power UV-LED heat dissipation and nano-TiO2 photocatalytic acid red 26 coupling system [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4905-4916. |
| [14] | WU Haibo, WANG Xilun, FANG Yanxiong, JI Hongbing. Progress of the development and application of 3D printing catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3956-3964. |
| [15] | XIANG Yang, HUANG Xun, WEI Zidong. Recent progresses in the activity and selectivity improvement of electrocatalytic organic synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4005-4014. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||